Abstract
Mammalian platelets are small, anuclear circulating cells that form tightly adherent, shear-resistant thrombi to prevent blood loss after vessel injury. Platelet thrombi that form in coronary and carotid arteries also underlie common vascular diseases such as myocardial infarction and stroke and are the target of drugs used to treat these diseases. Birds have high-pressure cardiovascular systems like mammals but generate nucleated thrombocytes rather than platelets. Here, we show that avian thrombocytes respond to many of the same activating stimuli as mammalian platelets but are unable to form shear-resistant aggregates ex vivo. Avian thrombocytes are larger than mammalian platelets, spread less efficiently on collagen, and express much lower levels of the α2bβ3 integrin required for aggregate formation, features predicted to make thrombocyte aggregates less resistant than platelets are to the high fluid shear forces of the arterial vasculature. In vivo carotid vessel injury stimulates the formation of occlusive platelet thrombi in mice but not in the size- and flow-matched carotid artery of the Australian budgerigar. These studies indicate that unique physical and molecular features of mammalian platelets enable them to form shear-resistant arterial thrombi, an essential element in the pathogenesis of human cardiovascular diseases.
Introduction
The formation of occlusive thrombi overlying small areas of arterial vessel injury is a primary step in the pathogenesis of myocardial infarction and other common cardiovascular diseases.1 Such thrombi typically arise after rupture of an atherosclerotic plaque and must resist extremely high shear forces exerted by flowing blood to grow on the arterial vessel wall.2 Angiographic studies of coronary arteries after thrombolytic therapy for acute myocardial infarction have revealed that the coronary thrombi responsible for occluding blood flow frequently form over atherosclerotic plaques that do not reduce blood flow.3 Thus, the formation of shear-resistant thrombi underlies common cardiovascular diseases.
Studies in human patients and animal models have shown that shear-resistant arterial thrombi are formed by platelets,4,5 and antiplatelet therapies are first-line therapies for acute coronary syndromes such as myocardial infarction.6 Platelets are small anuclear blood cells that function specifically in hemostasis and are found only in mammals.7 Whether the ability of platelets to form shear-resistant thrombi reflects a universal hemostatic requirement in animals with high-pressure vascular systems, or whether it is a unique consequence of mammalian evolution is unknown.
Birds have blood pressures greater than those of mammals, and the hemodynamic forces in avian arteries match or exceed those in mammals.8,9 Thus, the hemostatic requirement of birds is predicted to be very similar to that of mammals. Birds generate larger, nucleated blood cells known as thrombocytes that are believed to function like platelets,10-13 but extensive molecular and functional studies of avian thrombocytes have not been performed. In the present study, we use molecular and cellular approaches to compare human platelets and avian thrombocytes and in vivo thrombosis assays to determine whether birds form arterial vaso-occlusive thrombi as mammals do. Our findings reveal that the fundamental molecular and cellular responses of platelets are shared with avian thrombocytes but that avian thrombocytes are incapable of forming shear-resistant aggregates ex vivo; in addition, birds do not form vaso-occlusive thrombi after arterial vessel wall injury in vivo. These findings suggest that platelets evolved in mammals to provide a hemostatic advantage that in modern humans has become a primary mechanism of cardiovascular disease.
Methods
Antibodies and reagents
Mouse AP-2 mAb was a generous gift from Thomas Kunicki (CHOC Children's Hospital, Orange County, CA).14 Mouse K55 mAb was a generous gift from Hyun Lillehoj (US Department of Agriculture, Beltsville, MD).15 Type-I fibrillar collagen derived from equine tendon was purchased from Chronolog. Thrombin from bovine plasma (40-300 NIH units/mg protein) was purchased from Sigma-Aldrich. Protein phosphatase 2 (PP2) and D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK) were purchased from Calbiochem. Eptifibatide was purchased from Merck. Alexa-Fluor 488 and 647 Monoclonal Antibody Labeling Kits and Alexa-Fluor 594–conjugated phalloidin were purchased from Molecular.
Blood collection and preparation
Chicken studies.
Blood was collected from the wing vein of healthy female Rhode Island red pullet chickens ∼ 6 months of age from a commercial source with the use of a 19-gauge butterfly needle into 10-mL or 30-mL syringes anticoagulated with either 1:10 (vol/vol) 93μM PPACK or 1:6 (vol/vol) acid/citrate/dextrose (65mM Na3citrate·2H2O, 70mM citric acid·H2O, 100mM dextrose, pH 4.4). Whole blood was diluted 1:1 in modified Tyrode buffer (137mM NaCl, 20mM HEPES, 2.7mM KCL, 3.3mM NaH2PO4, 5.6mM glucose, 1 g/L BSA, pH 7.4) containing 1mM MgCl2, and 12-mL aliquots were spun at 50g for 5 minutes. The thrombocyte-rich plasma layer was isolated by aspiration pipetting. Preparation of washed thrombocytes was performed on blood samples collected in acid/citrate/dextrose. Then, 1 U/mL apyrase and 1μM prostacyclin were added to thrombocyte-rich plasma, and samples were washed once in platelet wash buffer (10mM HEPES, 1mM EDTA, 150mM NaCl, pH 6.0) before they were resuspended in modified Tyrode buffer. Cells were counted with a hemocytometer and the larger, ovaloid red blood cells were deliberately not counted. All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Human studies.
Blood was collected from 6 healthy donors by the use of a 19-gauge butterfly needle into 10-mL or 30-mL syringes anticoagulated as described previously. Washed platelets were prepared as described previously for thrombocytes.
[3H] 5-HT secretion assays
Thrombocyte-rich plasma or platelet-rich plasma was incubated with [3H] 5-HT (2 μCi [0.074 MBg]/mL) at 37°C for 30 minutes. Thrombocyte or platelet suspensions were washed and resuspended to a final concentration of 2 × 107 cells/mL or 2 × 108 platelets/mL, respectively, in modified Tyrode buffer plus 1μM imiprimine and 1mM CaCl2. Cell aliquots of 500 mL were stimulated under nonstirring conditions at 37°C for 10 minutes. In certain cases, inhibitors were added and cells were incubated at 37°C for 10 minutes before stimulation. Reactions were stopped by the addition of equal volumes 2% paraformaldehyde/0.1mM EDTA followed by centrifugation at 10 000g for 5 minutes. [3H] counts were measured on a scintillation counter (Beckman-Coulter LS6500) from the supernatants of stimulated samples and unstimulated controls, which were used as background. To determine total loading, [3H] counts were measured in an equal volume of loaded cells after lysis in an equal volume of fixing buffer plus 2% Triton X-100. Percent serotonin release was represented as ([3H] sample − [3H] background)/([3H] total − [3H] background) × 100. Percent inhibition was represented as (% release with inhibitor)/(% release without inhibitor) × 100.
Whole-blood flow assays
The tapered-wall parallel plate flow chamber was assembled as previously described.16,17 The assembled chamber was then placed on the stage of an inverted microscope (Nikon Eclipse TE2000-U). Blood collected in PPACK was warmed at 37°C for 10 minutes and 2.5 μg/mL fluorescently labeled Alexa-Fluor 488–conjugated AP-2 mAb was added, along with inhibitors, which binds both human and chicken α2bβ3 to label platelets or thrombocytes.14 Whole blood was perfused over the collagen-coated glass slide at a flow rate of 0.428 mL/min by the use of a syringe pump (Model ‘11′ Plus; Harvard Apparatus) for 5 or 15 minutes. After whole-blood perfusion, chambers were rinsed with modified Tyrode buffer at the same flow rate for 5 minutes before static image collection.
Whole-blood flow image capture and analysis
During whole-blood flow, platelet or thrombocyte adhesion was monitored continuously by epifluorescence microscopy. Images were captured with a CCD camera (C9300-201 Hamamatsu) mounted on an inverted microscope with a 300-W Xenon lamp (Perkin Elmer Optoelectronics) through a Lambda DG-4 high-speed filter changer (Sutter Instruments) used at 470 nm Ex/525 nm Em. For real-time fluorescent microscopy during blood flow, images were captured continuously with a 300-ms exposure time at an interval of 100 ms with Slidebook Version 5 software (Intelligent Imaging Innovations) as previously described.18 Static image collection was obtained by the use of epifluorescence microscopy at different axial positions along the flow chamber that correspond to a given shear rate. Platelet or thrombocyte percent surface coverage and average aggregate size were quantified by the use of ImageJ Version 1.44 software (National Institutes of Health). Both results were reported as the mean ± SEM.
Fluorescence microscopy of collagen slides after whole-blood flow
Slides were removed from the flow chamber and fixed in 1% paraformaldehyde for 5 minutes and washed 3 times in PBS. Cells on the slide were permeabilized in 0.02% Triton X-100 in PBS with BSA 1 mg/mL in for 5 minutes followed by 3 washes in PBS. Filamentous actin was stained with Alexa-Fluor 594–conjugated phalloidin (25 μL/mL) for 45 minutes at room temperature. Slides were imaged with fluorescence microscopy as previously described.19
Scanning electron microscopy of collagen slides after whole-blood flow
Permanox (Thermo Fisher Scientific) slides were coated with bovine type-I collagen or fibrillar chicken collagen as described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Permanox was used to facilitate slide cutting for microscopy preparation. Results were similar for both bovine and chicken collagen. After whole-blood perfusion, slides were removed from the flow chamber, washed 3 times in PBS, and fixed in 2% glutaraldehyde followed by preparation for microscopy with a Philips XL20 scanning electron microscope.
Flow cytometric analysis
Washed platelets or thrombocytes were diluted to a concentration of 2.5 × 107 platelets/mL or 2 × 107 cells/mL, respectively and 2.5 × 106 platelets or 2.5 × 106 thrombocytes were stained with Alexa-Fluor 488–conjugated AP-2 mAb at room temperature for 1 hour and analyzed by flow cytometry. Mean fluorescent intensity of AP-2–positive cells was quantified for platelets and thrombocytes.
Thrombocyte spreading assay
Washed chicken thrombocytes or human platelets were collected as described previously. Glass coverslips (no. 1.5) were coated with 100 μg/mL acid soluble type-I collagen (PureCol; Inamed Biomaterials) overnight at 4°C and then rinsed with PBS. Platelet spreading was visualized by reflection interference contrast microscopy (RICM) by the use of an inverted microscope (Axiovert 200; Karl Zeiss) with an antiflex 63Í oil immersion objective and a reflector cube. Images were recorded with a charge-coupled device camera (Retiga Exi Fast Cooled Mono 12-bit camera, 32-0082B-128 QImaging) every 10 seconds for 10 minutes.
In vivo thrombosis assays
A modification of the FeCl3-induced thrombosis assay originally described by Kurz et al20 was performed on the carotid arteries of mice and Australian budgerigars. Mice were C57Bl/6 either male or female 8-10 weeks of age (The Jackson Laboratory); Australian budgerigars were from a commercial source. In brief, mice or birds were anesthetized by inhaling isoflurane, a midline incision was made in the neck, and the carotid artery was exposed by blunt dissection. Blood flow in the artery was measured with a Doppler flow probe (Transonic Systems Inc). After a baseline flow measurement, a 1 × 1-mm piece of filter paper soaked with various concentrations of FeCl3 (10%-40%) was placed on the outside of the blood vessel for 5-20 minutes. The filter paper was then removed, and blood flow was monitored for an additional 20 minutes. The time to complete vessel occlusion was recorded. At the conclusion of the 20-minute observation period, the artery was excised, rinsed in PBS, and fixed 4% paraformaldehyde for histologic analysis.
Results
Chicken thrombocytes express genes encoding orthologs for most of the major platelet signaling pathways
To determine how closely avian thrombocytes resemble platelets, we compared the gene expression and functional responses of chicken thrombocytes with those of human platelets. Pure populations of thrombocytes and lymphocytes were sorted from whole chicken blood by use of the AP-2 monoclonal antibody that binds human and chicken α2b integrins14 and the K55 monoclonal antibody that binds chicken lymphocytes but not thrombocytes.21 Examination of the genes that are expressed selectively in thrombocytes by the use of both quantitative PCR and microarray analysis revealed orthologs for many of the genes known to be required for platelet synthesis and function, including those encoding the MPL receptor that stimulates platelet production, the protease-activated receptors that mediate thrombin activation of platelets, the β3 and α2 integrins that mediate platelet adhesion to fibrinogen and collagen, respectively, and the glycoprotein Ib/V/IX (GP9) receptor complex that binds von Willebrand Factor (VWF) to mediate adhesion under shear (Table 1, Figure 1A). One notable exception was the lack of thrombocyte-specific up-regulation of the gene encoding the ADP receptor P2RY12 in thrombocytes. Thrombocytes and lymphocytes express roughly equal amounts of p2ry12, and high cycle thresholds (30.3 and 28.0 for thrombocytes and lymphocytes, respectively) suggest a low level of expression in both cell types. This result is consistent with previous studies in which the authors indicated that avian thrombocytes neither contain nor respond strongly to ADP.13,22,23 Avian thrombocytes therefore express most of the molecular machinery known to be essential for platelet function, indicating that platelets evolved in mammals after the primary mechanisms of cellular hemostasis had already been established.
Detection of platelet-specific gene expression in thrombocytes by transcriptional profiling
| Human gene . | Log2 ratio (T/L) . | P . |
|---|---|---|
| MPL | 8.72 | < .001 |
| GP9 | 9.80 | < .001 |
| ITGB3 | 9.25 | < .001 |
| ITGB1 | 1.13 | .002 |
| ITGA6 | 2.37 | .004 |
| ITGA2 | 7.75 | < .001 |
| ITGAV | 4.60 | < .001 |
| F2R | 4.72 | .001 |
| F2RL3 | 2.80 | .007 |
| P2RX1 | 9.67 | < .001 |
| P2RY1 | 3.91 | < .001 |
| P2RY12 | 0 | .58 |
| HTR2A | 8.86 | < .001 |
| CD36 | 10.01 | < .001 |
| TBXAS1 | 6.22 | < .001 |
| GP1BA | 8.99 | < .001 |
| Human gene . | Log2 ratio (T/L) . | P . |
|---|---|---|
| MPL | 8.72 | < .001 |
| GP9 | 9.80 | < .001 |
| ITGB3 | 9.25 | < .001 |
| ITGB1 | 1.13 | .002 |
| ITGA6 | 2.37 | .004 |
| ITGA2 | 7.75 | < .001 |
| ITGAV | 4.60 | < .001 |
| F2R | 4.72 | .001 |
| F2RL3 | 2.80 | .007 |
| P2RX1 | 9.67 | < .001 |
| P2RY1 | 3.91 | < .001 |
| P2RY12 | 0 | .58 |
| HTR2A | 8.86 | < .001 |
| CD36 | 10.01 | < .001 |
| TBXAS1 | 6.22 | < .001 |
| GP1BA | 8.99 | < .001 |
Thrombocytes and lymphocytes were sorted by positive and negative selection for cell-specific markers, and gene expression analysis was performed by use of the Affymetrix chicken gene chip. The log2 of the gene expression ratio of thrombocytes vs lymphocytes (T L) and P values for genes of known platelet function are shown. All gene names used are those approved by the HUGO gene nomenclature committee (HGNC). F2R and F2RL3 refer to PAR1 and PAR3, respectively.
Avian thrombocytes express genes associated with platelet function and signal in response to platelet agonists collagen and thrombin. (A) Real-time quantitative PCR in thrombocytes and lymphocytes of select genes with a known role in platelet function. Shown is the log2 difference in gene expression between chicken thrombocytes and lymphocytes. (B-E) 5-HT release from platelets or thrombocytes was measured after loading with [3H] 5-HT. Cells were stimulated with increasing doses of bovine thrombin (B) or equine type-I collagen (C). (D) Chicken thrombocytes were stimulated with collagen (3 μg/mL) in the presence of increasing doses of PP2. (E) PP2 (3μM) was used to inhibit 1.0 U/mL thrombin- or 3.0 μg/mL collagen-induced 5-HT release in either human platelets or chicken thrombocytes. An equivalent volume of DMSO solvent was added in no inhibitor controls. Mean ± SD is shown for all panels; n = 3 experiments per condition.
Avian thrombocytes express genes associated with platelet function and signal in response to platelet agonists collagen and thrombin. (A) Real-time quantitative PCR in thrombocytes and lymphocytes of select genes with a known role in platelet function. Shown is the log2 difference in gene expression between chicken thrombocytes and lymphocytes. (B-E) 5-HT release from platelets or thrombocytes was measured after loading with [3H] 5-HT. Cells were stimulated with increasing doses of bovine thrombin (B) or equine type-I collagen (C). (D) Chicken thrombocytes were stimulated with collagen (3 μg/mL) in the presence of increasing doses of PP2. (E) PP2 (3μM) was used to inhibit 1.0 U/mL thrombin- or 3.0 μg/mL collagen-induced 5-HT release in either human platelets or chicken thrombocytes. An equivalent volume of DMSO solvent was added in no inhibitor controls. Mean ± SD is shown for all panels; n = 3 experiments per condition.
Chicken thrombocytes degranulate in response to collagen and thrombin in a manner similar to platelets
Thrombus formation after arterial vessel wall injury is believed to involve deceleration of rapidly moving platelets on the injured vessel wall by interaction between platelet GPIb and vessel wall VWF, the primary activation of platelet integrins by thrombin and/or collagen to mediate firm adhesion to the vessel wall, and growth of a 3-dimensional platelet aggregate supported by integrin α2bβ3 cross-linking of platelets.24 To compare the primary activation responses of human platelets and chicken thrombocytes, both cell types were loaded with 3H-5-HT and stimulated with increasing doses of bovine α thrombin and equine type-I collagen. Consistent with previous studies,11,23 thrombocytes exhibited robust signaling responses to both thrombin and collagen (Figure 1B-C). The thrombocyte thrombin dose-response was right-shifted compared with that of platelets, but this finding may reflect the less-efficient cleavage of chicken thrombin receptors by bovine thrombin. Although an orthologue for the platelet collagen receptor glycoprotein VI (GPVI) could not be identified in the chicken genome, the activation of both chicken thrombocytes and human platelets by collagen was inhibited by PP2, a src-family kinase inhibitor (Figure 1D-E). Thus, thrombocyte collagen responses are likely mediated by an immune-type receptor homologous to GPVI. This observation is consistent with the fact that GPVI is encoded by a gene in the leukocyte receptor cluster that evolved after the evolutionary separation of mammals and birds.25 These studies demonstrate that avian thrombocytes respond to the same primary agonists that activate platelets.
Chicken thrombocytes adhere to collagen under flow but do not form shear-resistant aggregates
An ex vivo assay that reproduces many of the platelet responses to arterial vessel injury is perfusion of whole blood over a collagen-coated surface under high fluid shear. In this assay, platelets first roll on the collagen-coated surface, then adhere firmly to collagen, and adherent platelets subsequently recruit additional platelets to develop 3-dimensional aggregates.26,27 The findings that avian thrombocytes express the GPIb receptor required for platelet rolling on collagen-bound VWF, exhibit robust signaling in response to collagen that activates surface integrins, and express the α2β1 integrin required for platelet adhesion to collagen under shear suggested that these cells should respond in a manner similar to platelets when they flowed over a collagen surface. However, the shear forces exerted on cells by flowing blood are proportional to the square of the cell diameter, and thrombocytes (diameter 6-7 μm10 ) are subject to shear forces 9-25 times greater than those experienced by platelets (diameter 1-2 μm).
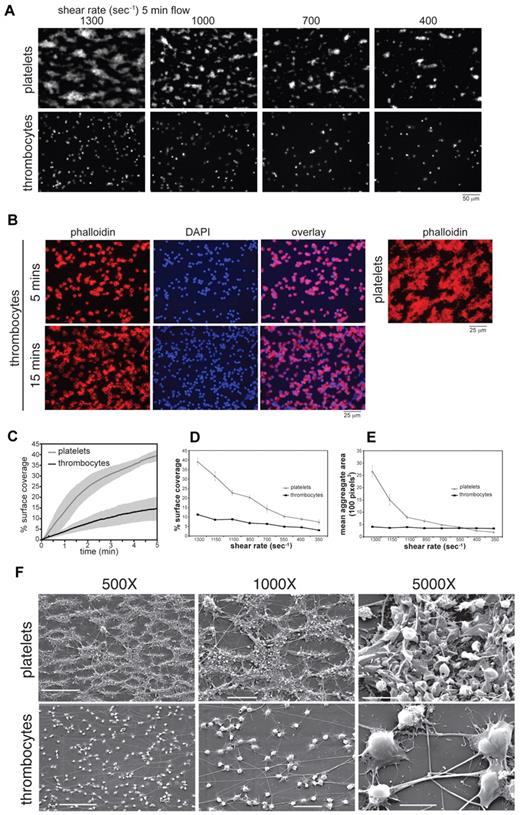
To compare the responses of human platelets and chicken thrombocytes to collagen under flow, whole human and chicken blood was treated with the thrombin inhibitor PPACK and perfused over a slide coated with type-I and -III chicken collagen. Platelets and thrombocytes were visualized in real time by detection of bound FITC-AP-2 antibody or after flow by phalloidin staining of cellular actin. Although AP-2 antibody has been reported to inhibit platelet aggregation,28 it does not affect thrombocyte aggregation,29 and similar responses were observed for platelets and thrombocytes with and without AP-2 treatment (eg, Figure 2A vs B). During flow over collagen, human platelets rolled, adhered firmly, and rapidly formed large, 3-dimensional aggregates on the collagen surface (Figure 2A, supplemental Video 1). Chicken thrombocytes also rolled slowly before firmly adhering to the collagen surface, but after 5 minutes of perfusion, adherent thrombocytes covered a smaller area compared with platelets, and thrombocytes did not form any 3-dimensional aggregates on the collagen surface (Figure 2A-E, supplemental Video 2). A longer perfusion time (15 minutes) resulted in nearly complete surface coverage by thrombocytes, but 3-dimensional aggregates were still not observed when either phase-contrast microscopy or 4′-6-diamidino-2-phenylindole staining to detect overlapping thrombocyte nuclei were used (Figure 2B).
Avian thrombocytes adhere to collagen but fail to form 3-dimensional aggregates under flow. PPACK-anticoagulated human or chicken whole blood was perfused through a tapered-wall parallel plate flow chamber for 5 minutes over a collagen-coated glass slide. (A) Representative fluorescent images captured at areas corresponding to shear rates of 1300, 1100, 700, and 400 seconds−1 after 5 minutes perfusion of whole blood labeled with AP-2 mAb. (B) Representative images of thrombocyte or platelet adhesion to collagen after whole blood flow for 5 or 15 minutes at 1300 seconds−1. Slides were fixed, permeabilized, and stained with Alex-Fluor 594–conjugated phalloidin to detect actin filaments. Nuclear staining of thrombocytes with DAPI is also shown. (C) Time traces of percent surface coverage of collagen surface by platelets or thrombocytes. Shown is mean ± SD (gray zone); n = 7-10 experiments for each condition. Percent collagen surface area coverage (D) and mean aggregate area (E) of human platelets and chicken thrombocytes after perfusion of whole blood for 5 minutes was determined by analysis of fluorescent images. Shown are the mean ± SEM, n = 7-10 experiments for each condition. (F) Scanning electron microscopy is shown after perfusion of human and chicken blood over collagen at a shear rate of approximately 1300 seconds−1. Scale bars indicate 50 μm (500×), 20 μm (1000×), and 5 μm (5000×). Shown are representative images from 3 and 5 human and chicken flow experiments, respectively.
Avian thrombocytes adhere to collagen but fail to form 3-dimensional aggregates under flow. PPACK-anticoagulated human or chicken whole blood was perfused through a tapered-wall parallel plate flow chamber for 5 minutes over a collagen-coated glass slide. (A) Representative fluorescent images captured at areas corresponding to shear rates of 1300, 1100, 700, and 400 seconds−1 after 5 minutes perfusion of whole blood labeled with AP-2 mAb. (B) Representative images of thrombocyte or platelet adhesion to collagen after whole blood flow for 5 or 15 minutes at 1300 seconds−1. Slides were fixed, permeabilized, and stained with Alex-Fluor 594–conjugated phalloidin to detect actin filaments. Nuclear staining of thrombocytes with DAPI is also shown. (C) Time traces of percent surface coverage of collagen surface by platelets or thrombocytes. Shown is mean ± SD (gray zone); n = 7-10 experiments for each condition. Percent collagen surface area coverage (D) and mean aggregate area (E) of human platelets and chicken thrombocytes after perfusion of whole blood for 5 minutes was determined by analysis of fluorescent images. Shown are the mean ± SEM, n = 7-10 experiments for each condition. (F) Scanning electron microscopy is shown after perfusion of human and chicken blood over collagen at a shear rate of approximately 1300 seconds−1. Scale bars indicate 50 μm (500×), 20 μm (1000×), and 5 μm (5000×). Shown are representative images from 3 and 5 human and chicken flow experiments, respectively.
Both platelet and thrombocyte adhesion to collagen under flow was abolished by chelation of the cations required for integrin function, suggesting that shear-resistant adhesion in thrombocytes is integrin dependent (supplemental Figure 1A-B). Scanning electron microscopy of bovine type I collagen-coated slides after perfusion of human blood revealed large 3-dimensional platelet aggregates on top of collagen fibrils, but thrombocytes were bound individually to the collagen surface, and thrombocyte aggregates were not observed (Figure 2F). Collagen-bound thrombocytes exhibited cell spreading, accompanied by filopodia and lamellipodia formation and cell vacuolization, findings consistent with cellular activation and degranulation (Figure 2F). These studies reveal that chicken thrombocytes and platelets adhere individually to collagen under flow and become activated but that thrombocytes do not form large, shear-resistant aggregates as platelets do.
Chicken thrombocytes spread less efficiently than platelets and express low levels of the α2bβ3 integrin required for aggregation
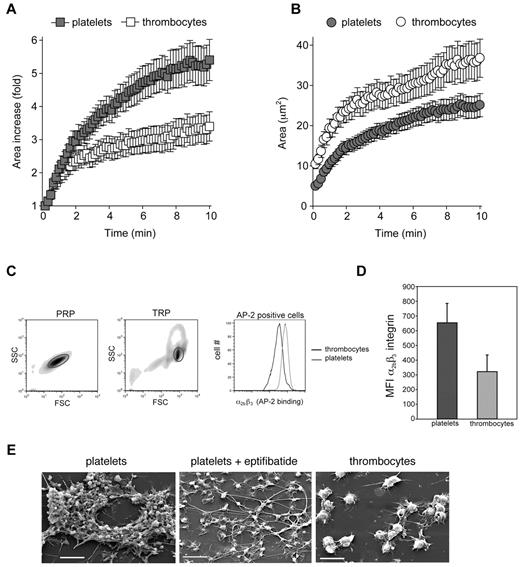
Three-dimensional platelet aggregates form under flow by adhesion of circulating platelets to a platelet monolayer that is bound to collagen ex vivo or to the vessel wall in vivo.5 Thus, strong platelet-platelet adhesion and dramatic shape change to reduce effective shear forces are likely to contribute to the growth and stability of the platelet aggregate. To determine why thrombocytes are able to form an adherent cell monolayer but not 3-dimensional aggregates under flow, we examined the ability of these cells to spread on the collagen surface and measured the density of α2bβ3 integrins that mediate aggregate formation. Platelets and thrombocytes were allowed to settle on a collagen-coated surface and the adherent cell surface area was measured over the course of 10 minutes by the use of RICM.30 Platelets exhibited a 3-fold increase in surface area within 2 minutes of contact with collagen and a 5-fold increase at 10 minutes (Figure 3A and supplemental Video 3). Thrombocytes covered an area 30% greater than that of platelets at 10 minutes (Figure 3B, supplemental Video 3), but the increase was only 2-fold at 2 minutes and 3.5-fold at 10 minutes (Figure 3A; P < .0001 vs platelets).
Avian thrombocytes spread less efficiently than platelets and express low levels of α2bβ3 integrins. Platelet and thrombocyte spreading on collagen was measured over 10 minutes with RICM. (A) The fold increase in surface area covered by adherent platelets and thrombocytes is shown. (B) The absolute area covered by adherent platelets and thrombocytes is shown. Mean ± SEM is shown; n = 25 for each group. (C) Levels of α2bβ3 integrin expression on washed human platelets and washed chicken thrombocytes were determined by staining with FITC-conjugated AP-2 mAb staining and analyzed by flow cytometry. The forward and side scatter of values of cells in platelet-rich plasma (PRP) and thrombocyte-rich plasma (TRP) are shown, and gates denote platelets and thrombocytes, respectively. Staining of human platelets and chicken thrombocytes for the α2bβ3 integrin with the use of AP-2 mAb binding is shown. (D) The histogram depicts the mean fluorescence intensity (MFI) of AP-2 binding for platelets and thrombocytes. Error bars indicate SD; n = 3 in each group. (E) Scanning electron microscopy images are shown after perfusion of human and chicken blood over collagen at a shear rate of approximately 1300 seconds−1. The α2bβ3 integrin antagonist eptifibatide was added at 10μM. Scale bars indicate 10 μm. Shown are representative images from 3 and 5 human and chicken flow experiments, respectively.
Avian thrombocytes spread less efficiently than platelets and express low levels of α2bβ3 integrins. Platelet and thrombocyte spreading on collagen was measured over 10 minutes with RICM. (A) The fold increase in surface area covered by adherent platelets and thrombocytes is shown. (B) The absolute area covered by adherent platelets and thrombocytes is shown. Mean ± SEM is shown; n = 25 for each group. (C) Levels of α2bβ3 integrin expression on washed human platelets and washed chicken thrombocytes were determined by staining with FITC-conjugated AP-2 mAb staining and analyzed by flow cytometry. The forward and side scatter of values of cells in platelet-rich plasma (PRP) and thrombocyte-rich plasma (TRP) are shown, and gates denote platelets and thrombocytes, respectively. Staining of human platelets and chicken thrombocytes for the α2bβ3 integrin with the use of AP-2 mAb binding is shown. (D) The histogram depicts the mean fluorescence intensity (MFI) of AP-2 binding for platelets and thrombocytes. Error bars indicate SD; n = 3 in each group. (E) Scanning electron microscopy images are shown after perfusion of human and chicken blood over collagen at a shear rate of approximately 1300 seconds−1. The α2bβ3 integrin antagonist eptifibatide was added at 10μM. Scale bars indicate 10 μm. Shown are representative images from 3 and 5 human and chicken flow experiments, respectively.
To compare the levels of α2bβ3 integrins, human platelets and chicken thrombocytes were stained with FITC-conjugated AP-2 monoclonal antibody. Despite the large difference in cell size, the number of α2bβ3 receptors on the platelet surface was approximately double that on the thrombocyte surface (MFI 653 on platelets vs 322 on thrombocytes; P = .018; Figure 3C-D). When conservative estimates of platelet and thrombocyte diameters of 2 μm and 6-7 μm were used, respectively,10 the thrombocyte was predicted to have a surface area at least 9-12 times that of a platelet, a value consistent with the difference in forward scatter observed between the 2 cell types when flow cytometry was used (Figure 3C, Leytin et al31 ). Thus, the density of α2bβ3 receptors on platelets was at least 18-25 times greater than that on thrombocytes.
Consistent with these findings, treatment of platelets with the α2bβ3 integrin blocking agent eptifibatide32 before perfusion of whole blood over a collagen surface at arterial shear rates resulted in a cellular monolayer that appeared very similar to that of untreated chicken thrombocytes when examined with scanning electron microscopy (Figure 3E, supplemental Figure 2, supplemental Video 4). These studies suggest the following: (1) that the lower level of primary thrombocyte adhesion to collagen under flow relative to that of platelets can be in part attributed to a reduced ability of the nucleated thrombocyte to reduce effective shear forces by spreading on the collagen surface, and (2) that the inability of thrombocytes to form secondary aggregates may be because of the low density of the α2bβ3 integrin required for cell-cell adhesion. The relatively low level of α2bβ3 integrin receptor expression on chicken thrombocytes is also consistent with a lack of up-regulation of thrombocyte P2RY12, an ADP receptor that is required to maintain α2bβ3 integrins in the active state during formation of shear-resistant platelet aggregates.33 Thus, α2bβ3 and P2RY12, the major targets of drugs used to treat human coronary thrombosis, are expressed at high levels in platelets but not in avian thrombocytes.
Carotid vessel wall injury results in vaso-occlusive thrombosis in mice but not in birds
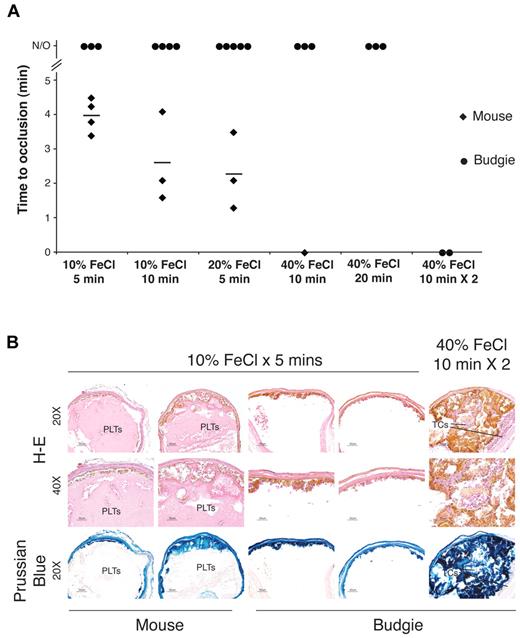
The inability of chicken thrombocytes to form shear-resistant aggregates under flow suggested that the vaso-occlusive arterial thrombi formed by platelets in response to vessel injury in mammals may not arise in birds, despite their similar vascular anatomy and hemodynamics. A model of arterial thrombosis in response to vessel injury that is platelet dependent and believed to stimulate the platelet responses that underlie pathogenic arterial thrombus formation in humans is ferric chloride (FeCl3) injury of the mouse carotid. In this assay, FeCl3 placed on the outside of the carotid artery results in damage to the inside of the vessel wall that stimulates platelet adhesion at the site of injury and progresses to complete vessel occlusion because of formation of a shear-resistant platelet thrombus.34 Exposure of the mouse carotid artery to increasing doses of FeCl3 resulted in progressively rapid loss of blood flow indicative of vessel occlusion (Figure 4A). Histologic analysis confirmed the presence of large, occlusive platelet thrombi overlying the site of injury in vessels without flow (Figure 4B, supplemental Figure 3). Prussian blue staining to detect FeCl3 demonstrated that formation of an occlusive platelet thrombus in the mouse carotid arose with FeCl3 penetration through the vessel wall and in the absence of FeCl3 in the thrombus itself (Figure 4B, supplemental Figure 3).
Arterial vessel wall injury results in thrombotic occlusion in mice but not in birds. The carotid arteries of anesthetized mice and Australian budgerigars were exposed to filter pads soaked in the indicated concentrations of FeCl3 for 5-20 minutes. (A) Time to occlusion after removal of the FeCl3 pad was determined by measuring arterial blood flow using a Doppler flow probe. N/O indicates no occlusion, defined as normal blood flow after 20 minutes afterFeCl3 pad removal, the termination point of the experiment. Zero minutes indicates occlusion before the time of Doppler probe measurement. Diamonds and circles represent individual mouse and budgerigar carotids, respectively. Horizontal bars indicate the mean time to occlusion for mouse carotid vessels at a given concentration and time of FeCl3 administration. Budgerigar carotid occlusion was observed only after repeated administration of the highest concentration of FeCl3 (40% FeCl3 for 10 minutes × 2). (B) Histologic examination of thrombus formation in FeCl3-injured mouse and budgerigar carotid arteries. Transverse sections of injured carotid arteries were stained with H&E or Prussian blue (to detect FeCl3). The occlusive mass of eosin-staining anuclear cells that occlude the mouse arterial lumen is composed of platelets (PLTs). Budgerigar thrombocytes (TCs) are elongated nucleated cells that do not stain strongly for Prussian blue. Occlusive thrombi failed to form in injured budgerigar carotids in the absence of FeCl3 penetration into the vessel lumen. Scale bar = 50 μm. n = 1-5 experiments for each condition, as indicated by the number of diamonds and circles in panel A.
Arterial vessel wall injury results in thrombotic occlusion in mice but not in birds. The carotid arteries of anesthetized mice and Australian budgerigars were exposed to filter pads soaked in the indicated concentrations of FeCl3 for 5-20 minutes. (A) Time to occlusion after removal of the FeCl3 pad was determined by measuring arterial blood flow using a Doppler flow probe. N/O indicates no occlusion, defined as normal blood flow after 20 minutes afterFeCl3 pad removal, the termination point of the experiment. Zero minutes indicates occlusion before the time of Doppler probe measurement. Diamonds and circles represent individual mouse and budgerigar carotids, respectively. Horizontal bars indicate the mean time to occlusion for mouse carotid vessels at a given concentration and time of FeCl3 administration. Budgerigar carotid occlusion was observed only after repeated administration of the highest concentration of FeCl3 (40% FeCl3 for 10 minutes × 2). (B) Histologic examination of thrombus formation in FeCl3-injured mouse and budgerigar carotid arteries. Transverse sections of injured carotid arteries were stained with H&E or Prussian blue (to detect FeCl3). The occlusive mass of eosin-staining anuclear cells that occlude the mouse arterial lumen is composed of platelets (PLTs). Budgerigar thrombocytes (TCs) are elongated nucleated cells that do not stain strongly for Prussian blue. Occlusive thrombi failed to form in injured budgerigar carotids in the absence of FeCl3 penetration into the vessel lumen. Scale bar = 50 μm. n = 1-5 experiments for each condition, as indicated by the number of diamonds and circles in panel A.
To determine whether arterial injury confers a similar thrombotic response in birds, we performed this study on the Australian budgerigar (Melopsittacus undulatus), a bird similar in size and weight to the mouse. The budgerigar carotid has a luminal diameter very close in size to that of the mouse (0.48 ± 0.001 mm for the mouse vs 0.46 ± 0.012 mm for the budgerigar), and volumetric blood flow rate in the 2 vessels was also closely matched (1.2 ± 0.12 mL/min for the mouse vs 1.3 ± 0.18 mL/min for the budgerigar; n = 15 budgerigars and n = 11 mice). In contrast to the mouse, however, FeCl3 injury of the budgerigar carotid did not result in cessation of blood flow unless the greatest dose (40% FeCl3) was repeatedly administered for a total of 20 minutes (Figure 4A). At lower doses of FeCl3, histologic examination revealed only small mural thrombi overlying areas of FeCl3 injury of the budgerigar carotid vessel wall (Figure 4B, supplemental Figure 3). At greater doses of FeCl3, histologic examination revealed larger thrombi that contained FeCl3 throughout the thrombus (Figure 4B, supplemental Figure 3). These findings indicate that arterial thrombus formation in the budgerigar is dependent on repetitive FeCl3 injury (eg, of circulating blood cells) and does not propagate from injury restricted to the vessel wall as it does in the mouse because of platelet-platelet adhesion. These in vivo results are consistent with the failure of thrombocytes to generate shear-resistant aggregates on collagen ex vivo and reveal that arterial vessel injury results in the rapid formation of occlusive thrombi in mice but not in birds.
Discussion
The biology of platelets has been studied in great detail in the context of human disease and experimentally by the use of genetically modified mice, but little is known regarding the extent to which the hemostatic function of platelets is conserved with that of analogous cell types in other vertebrate species. We focused on avian thrombocytes and arterial thrombosis because platelets are thought to be causal for pathologic thrombus formation in human arteries and birds have blood pressures and cardiovascular physiologies that closely resemble those of mammals. Our studies reveal that the basic molecular machinery of cellular hemostasis (eg, activation by thrombin and collagen) was established before the evolution of mammals and platelets but that mammalian platelets exhibit molecular and cellular differences from thrombocytes that have significant effects on the ability to form arterial thrombi. The 2 most important of these appear to be enhanced adhesion and spreading under shear, perhaps associated with loss of the cell nucleus, and the ability to form platelet aggregates that are highly stable and shear resistant. These platelet responses are likely to reflect high expression and/or increased affinity of the α2bβ3 integrin and the function of receptors such as P2RY12 that maintain the activation state of that integrin.35 These findings suggest a potential connection between the development of platelets in mammals and the pathogenesis of common human cardiovascular diseases.
Anuclear platelets are a distinguishing characteristic of all mammals, but why platelets evolved has not been clear. It has been proposed that platelets arose in association with placentation and live birth,36 but platelets are present in egg-laying monotremes and therefore appeared early in mammalian evolution and many millions of years before live birth. The fossil record suggests that during the first 140 million years of mammalian evolution, when dinosaurs (the ancestors of modern birds) were the dominant land animals, mammals were small, burrowing animals.37,38 Our studies identify important functional differences between mammalian platelets and avian thrombocytes, eg, their small size, ability to change shape unimpeded by a cell nucleus, and molecular refinements such as a greater density of α2bβ3 integrins, that are consistent with more efficient hemostatic responses by platelets compared with thrombocytes. Although this prediction cannot be tested in all contexts, the finding that equivalent degrees of arterial vessel wall injury in vessels of similar size and equal hemodynamic forces result in the occlusion in mammals but not in birds is consistent with the hypothesis that platelets mediate more efficient thrombotic responses than nucleated thrombocytes. Thus, although the reason for platelet evolution in mammals can never be known with certainty, it is tempting to speculate that platelets may have allowed early mammals to better survive trauma and thereby provided a survival advantage.
The finding that chicken thrombocytes adhere to collagen under flow but are unable to form 3-dimensional aggregates like platelets do suggested that the shear-resistant platelet thrombi that form in human coronary arteries might not reflect a universal hemostatic response to arterial vessel wall injury. Indeed, FeCl3 injury of the carotid vessel wall of the mouse resulted in the formation of an occlusive platelet thrombus, even when the injury is limited to the area at the vessel wall. In contrast, identical injury of the size and flow-matched Australian budgerigar resulted in formation of a small mural thrombus that failed to propagate unless the FeCl3 injury extended to cells throughout the thrombus and vessel lumen. Finally, it is important to note that our studies address cell-mediated arterial thrombosis and do not compare the relative contribution of platelets and thrombocytes to all aspects of hemostasis in vivo. The small mural thrombi formed by avian thrombocytes may be sufficient to prevent excess blood loss after traumatic injury; alternatively, birds may use other mechanisms to support hemostasis.
Regardless, these studies suggest that the development of shear-resistant, vaso-occlusive arterial thrombi, a key step in the pathogenesis of myocardial infarction, is not a universal hemostatic response but instead depends on molecular and cellular features that evolved solely in mammalian platelets. Two of these features, high levels of the α2bβ3 integrin and expression of the P2RY12 receptor, are indeed the targets of primary antiplatelet drugs used to treat acute myocardial infarction. Thus, cardiovascular disease in modern man may be linked to evolutionary changes that augmented hemostatic responses in early mammals.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rick Shannon, Gary Koretzky, and the University of Pennsylvania Department of Medicine for their support of these studies.
These studies were also supported by National Institute of Health training grant T32HL07439-30 (to A.A.S.) and by an American Heart Association (AHA) postdoctoral fellowship 09POST2140195 (to D.L.).
National Institutes of Health
Authorship
Contribution: A.A.S., T.J.S., J.R.R., D.L., C.N., P.M., M.C., K.R., and M.L.K. performed experiments; A.A.S., T.J.S., L.F.B., D.A.H., J.W., K.R., and M.L.K. interpreted data and wrote the manuscript; and S.C. and C.G. generated chicken collagen, an essential reagent for these studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark L. Kahn, Department of Medicine, University of Pennsylvania, Translational Research Center, Rm 11-123, 3400 Civic Center Blvd, Bldg 421, Philadelphia, PA 19104; e-mail: markkahn@mail.med.upenn.edu.

![Figure 1. Avian thrombocytes express genes associated with platelet function and signal in response to platelet agonists collagen and thrombin. (A) Real-time quantitative PCR in thrombocytes and lymphocytes of select genes with a known role in platelet function. Shown is the log2 difference in gene expression between chicken thrombocytes and lymphocytes. (B-E) 5-HT release from platelets or thrombocytes was measured after loading with [3H] 5-HT. Cells were stimulated with increasing doses of bovine thrombin (B) or equine type-I collagen (C). (D) Chicken thrombocytes were stimulated with collagen (3 μg/mL) in the presence of increasing doses of PP2. (E) PP2 (3μM) was used to inhibit 1.0 U/mL thrombin- or 3.0 μg/mL collagen-induced 5-HT release in either human platelets or chicken thrombocytes. An equivalent volume of DMSO solvent was added in no inhibitor controls. Mean ± SD is shown for all panels; n = 3 experiments per condition.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/13/10.1182_blood-2011-02-338244/4/m_zh89991178590001.jpeg?Expires=1767752037&Signature=G3OfHmdY~9sCAyW5Cp1JOJYcmHYs4blekpvYINVE-6JvKn9ut1xCeQItpyiUWjcNoyXTTLP2kio1ftWkJwyYTOEGXjuOj-Zi6rr8IJXEN67EG4htXUL~yc8x5iGBYE544t2xYFnNEjNsSy9sSdI-0PntKFR-zYCsDBZH6Bm4tobNg~p5~AzRVn3KE7U1u376v76wUO-sYZmY0KRNEr6RuD1SZRniUdHTbOkWIaw1d8vKi8IawCyIAys3Du2llMZWxoXuGxUzS-IKQGHX-r3l4wCZCB63IqwBxPlaRd8heZh~LYaTdfMXsPF6RLVrV~Mxdm~-7HHDiPbFnt55UePkKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal