Abstract
The ubiquitin-editing enzyme A20/TNFAIP3 is essential for controlling signals inducing the activation of nuclear factor-κB transcription factors. Polymorphisms and mutations in the TNFAIP3 gene are linked to various human autoimmune conditions, and inactivation of A20 is a frequent event in human B-cell lymphomas characterized by constitutive nuclear factor-κB activity. Through B cell-specific ablation in the mouse, we show here that A20 is required for the normal differentiation of the marginal zone B and B1 cell subsets. However, loss of A20 in B cells lowers their activation threshold and enhances proliferation and survival in a gene-dose–dependent fashion. Through the expression of proinflammatory cytokines, most notably interleukin-6, A20-deficient B cells trigger a progressive inflammatory reaction in naive mice characterized by the expansion of myeloid cells, effector-type T cells, and regulatory T cells. This culminates in old mice in an autoimmune syndrome characterized by splenomegaly, plasma cell hyperplasia, and the presence of class-switched, tissue-specific autoantibodies.
Introduction
B cells play essential roles during protective immune responses to invading pathogens. On encounter of foreign antigen and with cognate T-cell help, B lymphocytes proliferate and form distinct histologic structures, termed germinal center (GC). In the GC, they undergo somatic hypermutation and class-switch recombination. During somatic hypermutation, they introduce random mutations into their immunoglobulin variable regions while they exchange the heavy chain constant region during class-switch recombination to allow for different effector functions. After a selection process by antigen, B cells differentiate into memory B cells and plasma cells (PCs), which secrete antibodies.1 The deregulation of this process is heavily implicated in human disease. Production of class-switched antibodies against self-antigens causes or contributes to various autoimmune syndromes and unrestrained B-cell proliferation and survival can result in lymphomas.1,2 It is thought that the majority of human lymphomas derive from the GC, probably because the DNA damage inherent to the GC reaction facilitates mutations and chromosomal translocations.1,3
Recently, the ubiquitin-editing enzyme A20, encoded by the tumor necrosis factor-α-inducible gene 3 (TNFAIP3), has been associated with both autoimmunity and lymphomagenesis. Polymorphisms and mutations in or near the TNFAIP3 genomic locus have been linked with various human autoimmune syndromes with a strong humoral component, such as systemic lupus erythematosus (SLE),4,5 rheumatoid arthritis,6,7 and celiac disease.8 Loss of A20 function through mutations, chromosomal deletions, and/or promoter methylation is a frequent event in several human lymphomas,9-12 all of which are characterized by constitutive activation of nuclear factor-κB (NF-κB).13 These factors regulate a plethora of genes encoding for proinflammatory mediators, antiapoptotic proteins, cell adhesion molecules and, for negative feedback control, inhibitory proteins, such as p100, IκBα, and A20.14,15
During the transmission of NF-κB activating signals from cell-surface receptors such as the B-cell receptor (BCR), CD40, or Toll-like receptors (TLRs), signal transduction occurs via the attachment of polyubiquitin chains to key proteins, including MALT1 or TRAF6. Polyubiquitin chains, linked via K63 or linear assembly, serve to recruit different kinase complexes. In the case of canonical NF-κB, induced proximity allows the upstream kinase TAK1 to phosphorylate its target IKK2, which then effects NF-κB activation. A20, whose transcription is induced by NF-κB, dampens signaling through 2 main activities. First, as deubiquitinase A20 removes K63-linked polyubiquitin chains from essential signaling intermediates, such as TRAF6. Second, A20 induces, in concert with other proteins, degradation of some of its molecular targets, through addition of K48-linked ubiquitin chains.14,16 Degradation of RIP1 limits TNF-induced signaling,14 whereas degradation of the K63-chain-specific E2 ligases Ubc13/UbcH5c generally affects the assembly of K63-linked polyubiquitin chains.17
To date, the main molecular action of A20 is to prevent prolonged NF-κB activation in response to stimulation of TNF-R, TLR-, or NOD-like receptors and the TCR.14,16 Signal con-tainment by A20 is crucial for immune homeostasis because A20-deficient mice die early on due to an uncontrolled inflammatory disease. The inflammation is triggered via MyD88-dependent TLRs by the commensal intestinal flora.18 To study the cell-type-specific and cell-intrinsic roles of A20 in the mouse, we recently generated a conditional Tnfaip3 allele (A20F).19 Given the implication of A20/TNFAIP3 in B-cell lymphomas and autoimmune diseases, we used B lineage-specific ablation of A20 to study its role in B-cell development, function, and disease.
Methods
Genetically modified mice
All mice described in this study are published and were originally generated using C57BL/6 ES cells, or backcrossed to C57BL/6 at least 6 times. Mice were housed in the specific pathogen-free animal facility of the Max Planck Institute. All animal procedures were approved by the Regierung of Oberbayern.
Flow cytometry
Single-cell suspensions were prepared20 and stained with monoclonal antibodies: AA4.1(AA4.1), B220(RA3-6B2), CD1d(1B1), CD19(eBio1D3), CD22(2D6), CD23(B3B4), CD25(PC61.5), CD3(17A2), CD38(90) CD4(RM4-5), CD44(IM7), CD5(53-7.3), CD62L(MEL-14), CD69(H1.2F3), CD8(53-6.7), FoxP3(FJK-16s), GR-1(RB6-8C5), IgD(11-26), IgM(II/41), IL-10(JES5-16E3), IL-17A(eBio17B7), IL-4(11B11), IL-6(MP5-20F3), INF-γ(XMG1.2), Mac-1(M1/70), TCR-β(H57-597), TNF-α(MP6-XT22), CD95(15A7), CD21(7G6), CD86(GL-1), CD80(16-10A1), c-kit(2B8) (eBioscience), Ly-6G(1A8), Siglec-F(E50-2440), CD138(281-2) (BD Biosciences), and PNA (Vector Laboratories).
For intranuclear FoxP3 stainings (eBioscience) according to the manufacturer's instructions, dead cells were excluded with ethidium monoazide bromide. Samples were acquired on FACSCalibur and FACSCantoII (BD Biosciences) machines and analyzed with FlowJo software (TreeStar). For intracellular cytokine stainings, cells were stimulated for 5 hours at 37°C with 10nM phorbol myristate acetate (Calbiochem), 1mM ionomycin (Calbiochem), and 10nM brefeldin-A (Applichem). Cells were treated with Fc-block (eBioscience), and washed and surface-stained before fixation with 2% paraformaldehyde and permeabilization with 0.5% saponin. For in vitro culture, cells were purified by MACS (Miltenyi Biotec; > 85%-90% pure). Final concentrations of the activating stimuli: 2.5 μg/mL αCD40 (HM40–3, eBioscience), 10 μg/mL αIgM (Jackson ImmunoResearch Laboratories), 0.1μM CpG (InvivoGen), 20 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich), and 4 ng/mL IL-4 (R&D Systems). Enzyme-linked immunosorbent assays were conducted using antibody pairs to IL-6 (BD Biosciences) and TNF (BD Biosciences). Cell-cycle analyses by propidium iodide (PI) and carboxyfluorescin diacetate succimidyl ester (CFDASE) were conducted as described.20
Real-time PCR
RNA was isolated (QIAGEN RNeasy Mini Kit) and reverse transcribed (Promega) for quantitative real-time polymerase chain reaction (PCR) using probes and primers from the Universal Probe Library (Roche Diagnostics) according to the manufacturer's instructions.
Biochemistry
B-cell whole-cell, cytoplasmic, and nuclear lysates were essentially prepared as published.20 PVDF membranes were blotted with the following antibodies: p-IκBα, p-ERK, ERK, p-JNK, JNK, p-Akt, Akt, p-p38, p38, p100/p52 (Cell Signaling); IκBα, PLCγ2, RelB, RelA, c-Rel, Lamin B (Santa Cruz Biotechnology); tubulin (Upstate Biotechnology); p105/50 (Abcam); and A20.19
Immunofluorescence and immunohistochemistry
For immunofluorescence stainings, frozen 10-μm sections were thawed, air-dried, methanol-fixed, and stained for 1 hour at room temperature in a humidified chamber with B220-fluorescein isothiocyanate (eBioscience), biotinylated rat anti-CD3 (BD Biosciences), biotinylated rat anti-CD138 (BD Biosciences), and rabbit anti–laminin (gift from Michael Sixt) followed by Cy3-streptavidin and Cy5-conjugated anti–rabbit antibodies (Jackson ImmunoResearch). Immunohistochemically, MZB cells were identified by anti-CD1d (eBioscience) and metallophilic macrophages by anti-MOMA1 (Serotec). Detection of IgG immune complexes in paraformaldehyde-fixed kidney sections was performed using peroxidase-labeled anti–mouse IgG antibodies and 3-amino-9-ethylcarbazole staining (Vector Laboratories). For the detection of tissue-specific autoantibodies, frozen sections from organs of Rag2−/− mice were incubated with sera of aged and control mice and an anti–mouse IgG-Cy3 conjugate (Jackson ImmunoResearch Laboratories).
Images were acquired on a Zeiss Axiophot microscope (10×/0.3 or 20×/0.75 objectives; Carl Zeiss) using a Zeiss AxioCamMRm camera (Carl Zeiss) for monochromatic pictures and a Zeiss AxioCamMRC5 for RGB pictures. The following software programs were employed: Axiovision release 4.8 (Carl Zeiss), Photoshop CS4 and Illustrator CS4 (Adobe Systems).
Immunizations, ELISA
Mice were immunized intraperitoneally with 10 μg NP-Ficoll (Biosearch Technologies) and bled by the tail vein. Serum immunoglobulin concentrations and NP-specific antibodies were determined by ELISA.21 The detection of antinuclear and anticardiolipin autoantibodies was performed using ELISA kits (Varelisa). Rheumatoid factor: ELISA plates were coated with rabbit IgG (Jackson ImmunoResearch Laboratories).
Results
Loss of A20 leads to defects in the generation and/or localization of mature B-cell subsets
To inactivate A20 specifically in B lineage cells at different developmental time points and to distinguish the specific effects of A20 ablation from potential artifacts inherent to individual cre-transgenic strains,22 we initially investigated the consequences of CD19cre- and Mb1cre-mediated ablation of A20 in parallel (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Because we did not observe any significant differences between CD19cre/A20F/F and Mb1cre/A20F/F mice in our experiments, we refer to them collectively as BA20−/− mice (BA20+/− for heterozygous deletion). Most of the experiments presented here were conducted using CD19cre. Efficient loss of the NF-κB–inducible A20 protein in B cells of these mice was confirmed by Western blot in both resting conditions and after treatment with LPS (Figure 1A).
Conditional knockout of A20 in B cells induces severe defects in B-cell development and differentiation. (A) A20 protein expression in CD43-depleted B cells after 4-hour culture with or without 10 μg/mL LPS. (B-E) Absolute cell numbers were calculated from 5 to 7 age-matched mice per genotype. Data are mean ± SD. (B) Absolute splenocyte and B-cell numbers of the indicated genotypes. (C) Absolute cell numbers of splenic mature (B220+AA4.1−), marginal zone/marginal zone precursor (MZ; MZ-P: B220+CD1dhighCD21high), follicular (B220+AA4.1−CD1d−CD23+), and transitional (B220+AA4.1+) B cells. (D) Representative proportions of transitional (Trans: B220+AA4.1+) and mature (B220+AA4.1−) B cells of total lymphocytes (top panels) and of follicular (FO: CD1dintCD21int) and marginal zone/marginal zone precursor (MZ: CD1dhighCD21high) B cells of CD19+ B cells (bottom panels) in the spleen. (E) CD23 expression on B220+CD1dhiCD21hi B cells (left panel). Absolute cell numbers of marginal zone (B220+CD1dhiCD21hiCD23lo) and marginal zone precursor (B220+CD1dhiCD21hiCD23hi) B cells (right panel). (F) Top panels: Immunofluorescence of spleen sections: green represents αB220, B cells; red, αCD3, T cells; and blue, laminin. Bottom panels: Immunohistochemistry of spleen sections: blue represents MOMA-1, metallophilic macrophages; and brown, CD1d-expressing cells. Bar represents 100 μm. (G) Proportions of B220loCD138hi plasma blasts in splenic B cells 3 days after LPS treatment. (H) Blimp1 mRNA expression relative to porphobilinogen deaminase was determined by real-time PCR in splenic B cells 3 days after LPS treatment. (I) Antigen-specific IgM and IgG3 serum titers in response to T-independent immunizations with 10 μg NP-Ficoll determined by ELISA. Lines represent geometric means for 5 mice per experimental group. *P < .05 (1-way analysis of variance). **P < .001 (1-way analysis of variance).
Conditional knockout of A20 in B cells induces severe defects in B-cell development and differentiation. (A) A20 protein expression in CD43-depleted B cells after 4-hour culture with or without 10 μg/mL LPS. (B-E) Absolute cell numbers were calculated from 5 to 7 age-matched mice per genotype. Data are mean ± SD. (B) Absolute splenocyte and B-cell numbers of the indicated genotypes. (C) Absolute cell numbers of splenic mature (B220+AA4.1−), marginal zone/marginal zone precursor (MZ; MZ-P: B220+CD1dhighCD21high), follicular (B220+AA4.1−CD1d−CD23+), and transitional (B220+AA4.1+) B cells. (D) Representative proportions of transitional (Trans: B220+AA4.1+) and mature (B220+AA4.1−) B cells of total lymphocytes (top panels) and of follicular (FO: CD1dintCD21int) and marginal zone/marginal zone precursor (MZ: CD1dhighCD21high) B cells of CD19+ B cells (bottom panels) in the spleen. (E) CD23 expression on B220+CD1dhiCD21hi B cells (left panel). Absolute cell numbers of marginal zone (B220+CD1dhiCD21hiCD23lo) and marginal zone precursor (B220+CD1dhiCD21hiCD23hi) B cells (right panel). (F) Top panels: Immunofluorescence of spleen sections: green represents αB220, B cells; red, αCD3, T cells; and blue, laminin. Bottom panels: Immunohistochemistry of spleen sections: blue represents MOMA-1, metallophilic macrophages; and brown, CD1d-expressing cells. Bar represents 100 μm. (G) Proportions of B220loCD138hi plasma blasts in splenic B cells 3 days after LPS treatment. (H) Blimp1 mRNA expression relative to porphobilinogen deaminase was determined by real-time PCR in splenic B cells 3 days after LPS treatment. (I) Antigen-specific IgM and IgG3 serum titers in response to T-independent immunizations with 10 μg NP-Ficoll determined by ELISA. Lines represent geometric means for 5 mice per experimental group. *P < .05 (1-way analysis of variance). **P < .001 (1-way analysis of variance).
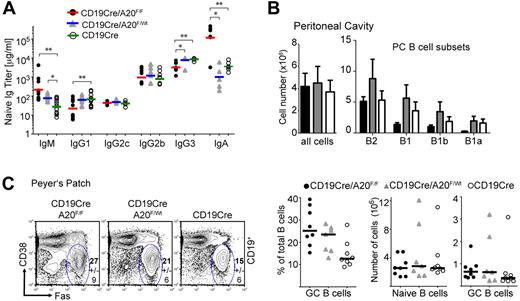
Loss of A20 does not affect early B-cell development in the bone marrow, except for a minor increase in immature B cells. In contrast, the number of mature recirculating B cells is significantly reduced (supplemental Figure 1C-E). BA20−/− mice have enlarged spleens, coinciding with a minor increase in total B-cell numbers (supplemental Figure 1B; Figure 1B), suggesting that other cell types are also expanded. Mature follicular B-cell numbers are unaffected by A20 deficiency, but immature transitional B cells accumulate (Figure 1C-D) without any apparent block within the transitional compartment (supplemental Figure 2A). The numbers of marginal zone B (MZB) cells, defined by CD1d and CD21 expression (Figure 1C), are elevated because of an expansion of CD23+ MZB precursor cells (Figure 1E; supplemental Figure 2B). Inspection of the splenic follicular organization by immunofluorescence revealed normal B- and T-cell compartments in BA20−/− spleens (Figure 1F). However, in BA20−/− spleen sections, CD1d-expressing MZB cells are mostly located inside the follicle, whose border is defined by MOMA-1-expressing metallophilic macrophages located adjacent to the marginal sinus. In contrast, on BA20+/− and control spleen sections, CD1d-expressing cells are properly located in the marginal zone (Figure 1F). Immunofluorescent detection of laminin and B cells indicates that the BA20−/− splenic marginal zone is essentially devoid of B cells (supplemental Figure 2B), further demonstrating that A20-deficient MZB cells are not at their proper location. To test MZB cell function, we treated splenocyte cultures with LPS for 3 days and monitored the differentiation of short-lived plasma cells. At early times after activation of splenic B cells by LPS MZB cells, and to much lesser extent follicular B cells, differentiate into short-lived plasma cells.23 As opposed to control B cells, A20-deficient splenic B cells show a profound defect in their ability to differentiate into Blimp1-expressing plasmablasts after LPS stimulation in vitro (Figure 1G-H), indicating the absence or paucity of functional MZB cells. Further underscoring this notion, BA20−/− mice display a significant reduction in the production of class-switched, antigen-specific IgG3 after immunizations with the TI-II antigen NP-Ficoll (Figure 1I), a response exquisitely dependent on the presence of MZB cells.24 These results are in line with a significant reduction of total IgG3 serum levels in naive BA20−/− mice. IgG1 levels are also lower in these mice, whereas IgG2c and IgG2b levels are unchanged and IgM and IgA serum levels are strongly elevated (Figure 2A; supplemental Table 1). Supporting a role for A20 in B-cell differentiation and/or proper localization, peritoneal B1, especially B1a cells, are reduced in BA20−/− mice (Figure 2B; supplemental Figure 2E). In addition, thymic B cells are reduced in BA20−/− mice, although they appear elevated in BA20+/− mice (supplemental Figure 2D). Together, our results uncover several surprising deficiencies in the gen-eration, differentiation, and/or maintenance of mature B-cell subsets in absence of A20. The affected subsets include MZB and B1 cells, which are thought to mediate the innate functions of the B lineage.25
A20-deficiency impairs B1 cell generation but enhances GC formation in the GALT. (A) Titers of immunoglobulin isotypes were determined by ELISA; n = 6 to 12 per genotype. (B) Absolute cell numbers of B-cell subsets in the peritoneal cavity: B2 (CD19+B220+), B1 (CD19highB220low), B1a (CD19highB220lowCD5+), and B1b (CD19highB220lowCD5−) cell numbers were calculated from 5 to 7 age-matched mice per genotype. Data are mean ± SD. (C) Left panel: Proportions of GC (CD19+PNAhiFashiCD38lo) of total B cells in Peyer patches. Data are mean ± SD of 8 mice per genotype. Right panel: Proportions of GC B cells depicted as individual data points (left chart), absolute cell numbers for naive B cells (mantle zone B cells: CD19+PNA− Fas−CD38hi; middle chart), and GC B cells (right chart). Bars represent medians of 8 mice per group (same as in the left panel). *P < .05 (1-way analysis of variance). **P < .001 (1-way analysis of variance).
A20-deficiency impairs B1 cell generation but enhances GC formation in the GALT. (A) Titers of immunoglobulin isotypes were determined by ELISA; n = 6 to 12 per genotype. (B) Absolute cell numbers of B-cell subsets in the peritoneal cavity: B2 (CD19+B220+), B1 (CD19highB220low), B1a (CD19highB220lowCD5+), and B1b (CD19highB220lowCD5−) cell numbers were calculated from 5 to 7 age-matched mice per genotype. Data are mean ± SD. (C) Left panel: Proportions of GC (CD19+PNAhiFashiCD38lo) of total B cells in Peyer patches. Data are mean ± SD of 8 mice per genotype. Right panel: Proportions of GC B cells depicted as individual data points (left chart), absolute cell numbers for naive B cells (mantle zone B cells: CD19+PNA− Fas−CD38hi; middle chart), and GC B cells (right chart). Bars represent medians of 8 mice per group (same as in the left panel). *P < .05 (1-way analysis of variance). **P < .001 (1-way analysis of variance).
A20-deficiency enhances GC B-cell formation in gut-associated lymphoid tissues
Given A20's role as negative regulator of NF-κB signaling, we expected A20-deficient B cells to be hyper-reactive to stimulation. We did not observe significant spontaneous GC formation in the spleens of the mice analyzed, indicating that spontaneous B-cell activation, if present in these mice, is not strong enough to induce GCs. In naive mice, gut-associated lymphoid tissue (GALT) is the place where B cells are constantly triggered by bacterial antigens to enter the GC.26 The percentages and, to a lesser extent, absolute cell numbers of GC B cells in mesenteric lymph nodes and Peyer patches are increased in BA20−/− and BA20+/− mice (Figure 2C; and data not shown). This shows that, during B-cell activation by bacterial antigens in the GALT, strong hemizygous effects of A20 deficiency become apparent.
B cell–specific lack of A20 induces the spontaneous expansion of regulatory T, effector-type T, and myeloid cells
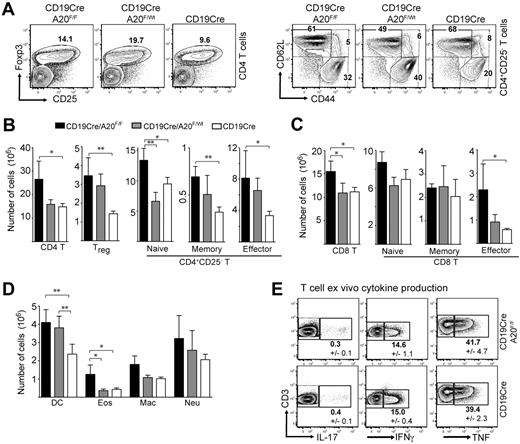
The increase in transitional and MZB precursor cell numbers is not sufficient to explain the higher splenocyte numbers in young BA20−/− mice (Figure 1B). Further detailed analyses showed that homozygous and heterozygous ablation of A20 in B cells induces elevated numbers of T and myeloid cells. The sizes of many splenic T-cell compartments are increased in BA20−/− mice, especially the regulatory (Figure 3A-B), memory-type and effector-type CD4 (Figure 3B), and effector-type CD8 T cells (Figure 3C). Similar effects on T cells were observed in the lymph nodes (data not shown). The analysis of ex vivo T-cell cytokine production by intracellular staining revealed comparable proportions of IL-17–, interferon-γ– and TNF-producing cells between BA20−/− and control mice (Figure 3E). We were unable to detect any IL-4-producing cells (not shown). These analyses suggest the absence of detectable T-cell differentiation into specific T-helper lineages. The cell numbers of all splenic myeloid subsets analyzed were higher in BA20−/− compared with control mice, with significant increases in dendritic cells and eosinophils (Figure 3D). Importantly, heterozygous loss of A20 in B cells induces an intermediate phenotype (Figures 3A-D) in most aspects of this immune deregulation. BA20+/− mice contain even higher proportions of splenic regulatory and effector T cells than BA20−/− mice (Figure 3A). However, the higher absolute cell numbers of total CD4 T cells in BA20−/− mice leads to the greatest absolute cell numbers also in these subsets (Figure 3B). BA20−/− and BA20+/− mice contain elevated proportions of IL-10–producing B cells (supplemental Figure 2C), excluding the possibility that absence of this immune-regulatory B-cell subset27 causes the perturbation in immune homeostasis. To validate that CD19cre mediates inactivation of A20 alleles only in B cells, we performed PCR on DNA purified from splenic cell subsets of BA20−/− mice. The purified T cells, dendritic cells, macrophages, and granulocytes contain less than 0.2% A20-knockout cells, which could be contaminating B cells (supplemental Figure 3). Our findings therefore indicate that the absence or gene-dose reduction of A20, specifically in B cells, induces an immune deregulation reminiscent of sterile inflammation, possibly held in check by regulatory lymphocyte subsets.
Ablation of A20 in the B-lineage has B cell-extrinsic effects on immune homeostasis. (A) Dot-plots showing percentages of CD4+Foxp3+ regulatory T cells and CD4+CD25− T cells (naïve indicates CD44intCD62Lhi; memory, CD44hiCD62Lhi; and effector, CD44hiCD62Llo) in the spleen. Numbers indicate the mean of 4 to 6 mice for each genotype. (B-D) Absolute cell numbers of CD4 T (B), CD8 T (C), and myeloid cell (D) subsets in BA20−/−, BA20+/−, and CD19cre mice (n = 6 per group; 8-12 weeks old). Data are mean ± SD. Treg indicates Foxp3+; naive, CD44intCD62Lhi; memory, CD44hiCD62Lhi; effector/memory, CD44hiCD62Llo; DC, dendritic cell (CD11c+); Eos, eosinophils (Gr1intSiglecF+); Mac, macrophages (Mac1+Gr1lo); and Neu, neutrophils (Gr1hiLy6G+). (E) Intracellular cytokine staining of ex vivo isolated splenocytes (gated on T cells). Numbers represent mean plus or minus SD of 3 mice per genotype. *P < .05 (1-way analysis of variance). **P < .001 (1-way analysis of variance).
Ablation of A20 in the B-lineage has B cell-extrinsic effects on immune homeostasis. (A) Dot-plots showing percentages of CD4+Foxp3+ regulatory T cells and CD4+CD25− T cells (naïve indicates CD44intCD62Lhi; memory, CD44hiCD62Lhi; and effector, CD44hiCD62Llo) in the spleen. Numbers indicate the mean of 4 to 6 mice for each genotype. (B-D) Absolute cell numbers of CD4 T (B), CD8 T (C), and myeloid cell (D) subsets in BA20−/−, BA20+/−, and CD19cre mice (n = 6 per group; 8-12 weeks old). Data are mean ± SD. Treg indicates Foxp3+; naive, CD44intCD62Lhi; memory, CD44hiCD62Lhi; effector/memory, CD44hiCD62Llo; DC, dendritic cell (CD11c+); Eos, eosinophils (Gr1intSiglecF+); Mac, macrophages (Mac1+Gr1lo); and Neu, neutrophils (Gr1hiLy6G+). (E) Intracellular cytokine staining of ex vivo isolated splenocytes (gated on T cells). Numbers represent mean plus or minus SD of 3 mice per genotype. *P < .05 (1-way analysis of variance). **P < .001 (1-way analysis of variance).
A20 regulates B-cell responses in vitro in a gene-dose–dependent fashion
Our observations of enhanced GC B-cell formation in the GALT in BA20−/− and BA20+/− mice suggested the possibility that their B cells are more easily activated in this context. To analyze this in more detail, we first quantified the relative A20 mRNA expression at different time points after activation with αIgM, αCD40, LPS, CpG DNA, and TNF. All these stimuli induced a strong increase in A20 mRNA, peaking at approximately 1 hour (supplemental Figure 4A), prompting us to test the in vitro responses of purified A20-deficient and A20+/− B cells to these mitogens. B cells up-regulate certain cell surface molecules (activation markers) upon activation, among them B7.1/CD80, B7.2/CD86, MHC II, CD25, and Fas. The expression of these markers was already slightly higher in unstimulated B cells purified from BA20−/− and BA20+/− mice compared with control B cells (Figure 4A; supplemental 4B). This is probably because of the latent immune activation we observed in these mice. Upon activation with the indicated mitogens, A20-deficient B cells produced higher surface levels of many activation markers (Figure 4A; supplemental Figure 3B). B cells carrying one functional A20 allele showed an intermediate phenotype regarding expression of these activation markers (Figure 4A). We then monitored αIgM-, αCD40-, LPS-, and CpG DNA-induced proliferation by 3 assays: Both [3H]thymidine incorporation (Figure 4B) and cell cycle analysis (Figure 4C) showed increased proliferative activity in the A20-deficient B-cell cultures in response to all stimuli, and quantification of live cells revealed increased survival after all stimuli, except for CpG DNA (Figure 4C). By CFSE dilution assay (Figure 4D), we determined that the absence of A20 increased the proportion of B cells that initially start to divide (% divided) in response to αCD40 and LPS. Only CpG DNA and BCR crosslinking enhanced the proliferation of dividing A20-deficient B cells (Proliferation Index; Figure 4D). Combination of stimuli, such as LPS and αIgM, or the addition of IL-4, did not yield additive effects (supplemental Figure 4C). In all stimulations, the magnitude of the A20+/− B-cell response was in between A20−/− and control B cells (Figure 4B-D). Taken together, our studies show that reduction of A20 function magnifies B-cell responses through 3 mechanisms, depending on the activating stimulus: lowering the threshold for initial activation (αCD40, LPS), protecting activated B cells against apoptosis (αIgM, αCD40, LPS), and enhancing the proliferation of cells that were activated to divide (αIgM, CpG).
A20-deficiency amplifies B-cell responses. (A) Expression levels of the respective B-cell activation marker after overnight stimulation with LPS or αCD40. The dot-plots are representative of 3 to 6 independent experiments. (B) [3H]Thymidine incorporation during a 10-hour pulse 48 hours after stimulation of B-cell cultures with αIgM, αCD40, LPS, or CpG. Data are mean ± SD of triplicate measurements. The experiment was repeated with similar results. (C) Cell cycle profile analysis by propidium iodide staining of B cells 2 days after stimulation with the indicated mitogens. Percentages of dead (sub G0) and live cells are indicated at the top of each histogram. The distribution within the cell cycle was calculated with the FlowJo software Version 8.7.3 using the Watson model (values do not add up to 100%). Data are means of 2 independent experiments. (D) Assessment of proliferation by the carboxyfluorescein succinimidyl ester dilution assay: histograms represent carboxyfluorescein succinimidyl ester intensities 3 days after stimulation. The tables under each histogram show the proliferation index (Prol. Index: average number of divisions of the proliferating cells), the percentage of dividing cells (% Divided: the proportion of cells that initially started to divide), and the division index (Div. Index: average number of divisions of all cells); values were calculated with the FlowJo software. Data represent the means of 5 independent experiments, and bold values are significantly different (P < .05) from wild-type according to 1-way analysis of variance analysis. (E) Evaluation of IL-6 production in overnight activated B cells by ELISA (top panels), intracellular FACS (middle panels), and real-time PCR (bottom panels). For intracellular FACS, B cells were stimulated for 3 days. cDNA was quantified relative to porphobilinogen deaminase. Data are mean ± SD of 3 independent experiments. (F) Quantification of TNF production by overnight-stimulated B cells via ELISA. Data are mean ± SD of 3 independent experiments.
A20-deficiency amplifies B-cell responses. (A) Expression levels of the respective B-cell activation marker after overnight stimulation with LPS or αCD40. The dot-plots are representative of 3 to 6 independent experiments. (B) [3H]Thymidine incorporation during a 10-hour pulse 48 hours after stimulation of B-cell cultures with αIgM, αCD40, LPS, or CpG. Data are mean ± SD of triplicate measurements. The experiment was repeated with similar results. (C) Cell cycle profile analysis by propidium iodide staining of B cells 2 days after stimulation with the indicated mitogens. Percentages of dead (sub G0) and live cells are indicated at the top of each histogram. The distribution within the cell cycle was calculated with the FlowJo software Version 8.7.3 using the Watson model (values do not add up to 100%). Data are means of 2 independent experiments. (D) Assessment of proliferation by the carboxyfluorescein succinimidyl ester dilution assay: histograms represent carboxyfluorescein succinimidyl ester intensities 3 days after stimulation. The tables under each histogram show the proliferation index (Prol. Index: average number of divisions of the proliferating cells), the percentage of dividing cells (% Divided: the proportion of cells that initially started to divide), and the division index (Div. Index: average number of divisions of all cells); values were calculated with the FlowJo software. Data represent the means of 5 independent experiments, and bold values are significantly different (P < .05) from wild-type according to 1-way analysis of variance analysis. (E) Evaluation of IL-6 production in overnight activated B cells by ELISA (top panels), intracellular FACS (middle panels), and real-time PCR (bottom panels). For intracellular FACS, B cells were stimulated for 3 days. cDNA was quantified relative to porphobilinogen deaminase. Data are mean ± SD of 3 independent experiments. (F) Quantification of TNF production by overnight-stimulated B cells via ELISA. Data are mean ± SD of 3 independent experiments.
A20-deficient B cells exhibit spontaneous IL-6 secretion and highly elevated IL-6 secretion on activation
In light of the T effector and myeloid cell expansion, we wondered whether the higher excitability of A20-deficient B cells leads to secretion of proinflammatory cytokines, which in turn affect the differentiation and expansion of immune cells. IL-6 is a pleiotropic cytokine with inflammatory activity, and its levels are up-regulated in various autoimmune diseases, such as rheumatoid arthritis and SLE.28 Activated A20-deficient B cells produced dramatically more IL-6 mRNA and protein than control B cells in response to all mitogens (Figure 4E). We could again detect an intermediate phenotype in A20+/− B cells (supplemental Figure 4D). Indeed, A20-deficient B cells spontaneously produced and released IL-6 into the cell culture medium in the absence of stimulation in comparable amounts to the IL-6 secretion in αIgM-stimulated control B cells (Figure 4E). A20-deficient B cells also produced slightly more TNF in response to all stimuli (Figure 4F). In accord with these findings, we detected more IL-6 and TNF-producing cells in ex vivo isolated A20-deficient compared with control B cells (supplemental Figure 4E). The evaluation of IL-6 production in immune cell subsets of 3- to 4-month-old BA20−/− mice by intracellular flow cytometry and real-time PCR revealed that, in addition to B cells, dendritic cells and, to a lesser extent, macrophages also produce IL-6 (supplemental Figure 5). This suggests that secondary activation of myeloid cells by A20-deficient B cells exacerbates the immune deregulation in BA20−/− mice.
A20 negatively regulates canonical NF-κB, but not MAPK or AKT activation, in response to engagement of the BCR, CD40, and TLRs
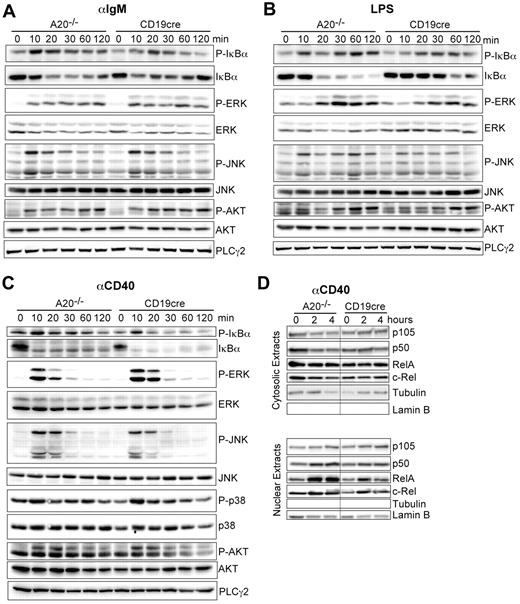
We established that A20 is a gene-dose–dependent negative regulator of B-cell activation in response to triggering of the BCR, CD40, and TLRs. A20 was reported to limit the activation of canonical NF-κB in response to various stimuli in other cell types.14,16 Therefore, we wanted to confirm that the hyper-reactivity of A20-deficient B cells also correlates with enhanced canonical NF-κB activity in response to the stimuli used in our study. Indeed, the absence of A20 selectively enhances the activation of the canonical NF-κB pathway, evidenced by prolonged increased IκBα phosphorylation and degradation (Figure 5A-C). The activation of MAPK pathways or AKT phosphorylation was unaltered in response to αIgM, LPS, and αCD40 (Figure 5A-C). The increased IκBα phosphorylation correlated well with an increase in nuclear translocation of p50 and RelA 2 and 4 hours after activation with αCD40 (Figure 5D). We detected elevated expression of p100 and RelB, substrates of the alternative NF-κB pathway and transcriptional targets of canonical NF-κB in whole cell extracts of A20-deficient B cells (data not shown) in accord with the higher canonical NF-κB activity in these cells. Taken together, although we do not rule out that A20 has additional functions, we demonstrate that deficiency for A20 in B cells strongly enhances activation of the canonical NF-κB pathway in response to BCR cross-linking (antigen), T-cell costimulation (αCD40), and microbial components (TLRs).
A20 controls canonical NF-κB activation in response to B-cell mitogens. (A-C) Western blot of whole cell lysates stimulated for the indicated time points with (A) 10 μg/mL αIgM, (B) 20 μg/mL LPS, and (C) 10 μg/mL αCD40. (D) Western blots on cytoplasmic and nuclear extracts after stimulation with αCD40. Results are representative of 2 or 3 independent experiments.
A20 controls canonical NF-κB activation in response to B-cell mitogens. (A-C) Western blot of whole cell lysates stimulated for the indicated time points with (A) 10 μg/mL αIgM, (B) 20 μg/mL LPS, and (C) 10 μg/mL αCD40. (D) Western blots on cytoplasmic and nuclear extracts after stimulation with αCD40. Results are representative of 2 or 3 independent experiments.
Loss of A20 in B cells leads to autoimmune pathology in old mice
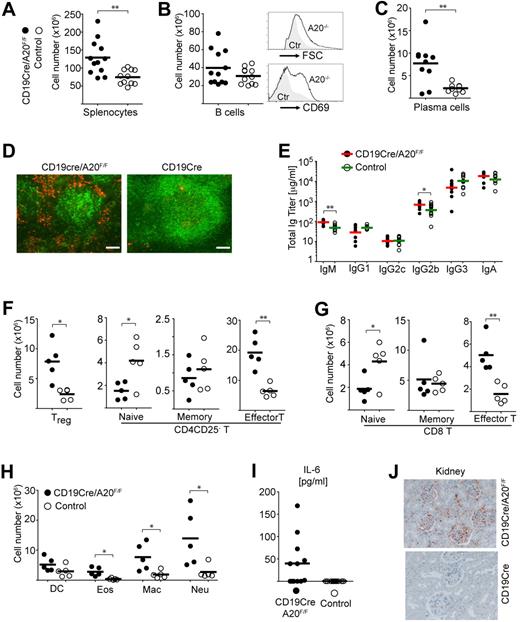
To assess the impact of the chronic proinflammatory state induced by B cell-specific loss of A20 in old age, we aged a cohort of BA20−/− and control mice. Histologic analysis of organs from 20-week-old BA20−/− mice did not yield any signs of inflammation (not shown). BA20−/− mice, more than one year old, on the contrary, were characterized by splenomegaly (Figure 6A; supplemental Figure 6A-B). Although total B-cell numbers were not significantly elevated in old BA20−/− compared with control mice, all A20−/− B cells appeared activated, as judged by the larger cell size and elevated levels of the activation marker CD69 (Figure 6B). In addition, we observed a marked expansion of PCs in the spleen (Figure 6C; supplemental Figure 6C), but not in the bone marrow (data not shown). Immunofluorescence analysis of the enlarged spleens revealed a diffuse pattern of PCs surrounding altered and disrupted B-cell follicles (Figure 6D). Analysis of the serum isotype titers in aged BA20−/− mice revealed higher IgM titers compared with controls, as seen in young mice. However, in contrast to the latter, in old BA20−/− mice the levels of the pathogenic IgG2b isotype29 are elevated (Figure 6E; supplemental Table 2). The increase of effector and regulatory T cells, already apparent in young BA20−/− mice, has further expanded in old mice, at the expense of the respective naive compartments (Figure 6F-G). In addition, the expansion of myeloid cell subsets was much more pronounced (Figure 6H). The progressive nature of the inflammation in the BA20−/− mice is also reflected in elevated levels of serum IL-6 in aged BA20−/− mice (Figure 6I), which could not be detected in the 20-week-old BA20−/− mice (not shown). Histologic analysis of organs from aged mice revealed inflammatory infiltrates in the liver and kidney of most of the old BA20−/− mice (supplemental Figure 6D). Examinations of the renal pathology revealed no extensive glomerular damage but clear IgG immune complex depositions in aged BA20−/− mice (Figure 6J).
Splenomegaly and plasma cell hyperplasia in old BA20−/− mice. Cohort description: age range, 60 to 85 weeks; mean age of experimental group, 68 weeks; mean age of control group, 66 weeks. (A) Absolute cell numbers of splenocytes (n(BA20−/−) = 10, n(control) = 9). (B) Left panel: Absolute cell numbers of B cells (CD19+; n(BA20−/−) = 12, n(control) = 11). Right panel: Representative size and CD69 expression of splenic B220+ B cells. (C) Absolute cell numbers of splenic plasma cells (B220loCD138hi; n(BA20−/−) = 10, n(control) = 9). (D) Representative immunofluorescence analysis of plasma cells in the spleen, plasma cells (red represents αCD138), B cells (green represents αB220). Bar represents 100 μm. (E) Titers of immunoglobulin isotypes in aged mice were determined by ELISA; n(BA20−/−)= 12, n(control) = 11. (F-H) Absolute splenic cell numbers for the following cellular subsets: CD4 T cells. (F) Treg (CD4+CD25+); CD4+ naive (CD25−CD44intCD62Lhi), memory-type (CD25−CD44hiCD62Lhi), and effector T (CD4+CD44hiCD62Llo); CD8-T cells. (G) Naive (CD44intCD62Lhi), memory-type (CD44hiCD62Lhi), and effector T (CD44hiCD62Llo); myeloid cells (H): DC indicates dendritic cell (CD11c+); Eos, eosinophils (Gr1intSiglecF+); Mac, macrophages (Mac1+Gr1lo); and Neu, neutrophils (Gr1hiLy6G+). n(BA20−/−) = 5, n(control) = 5. (I) Serum IL-6 (pg/mL) in aged mice was measured by ELISA; n(BA20−/−) = 12, n(control) = 11. (J) Representative stainings of IgG immune complexes in kidneys of BA20−/− and control mice. Original magnification × 20. *P < .05 (1-way analysis of variance). **P < .001 (1-way analysis of variance).
Splenomegaly and plasma cell hyperplasia in old BA20−/− mice. Cohort description: age range, 60 to 85 weeks; mean age of experimental group, 68 weeks; mean age of control group, 66 weeks. (A) Absolute cell numbers of splenocytes (n(BA20−/−) = 10, n(control) = 9). (B) Left panel: Absolute cell numbers of B cells (CD19+; n(BA20−/−) = 12, n(control) = 11). Right panel: Representative size and CD69 expression of splenic B220+ B cells. (C) Absolute cell numbers of splenic plasma cells (B220loCD138hi; n(BA20−/−) = 10, n(control) = 9). (D) Representative immunofluorescence analysis of plasma cells in the spleen, plasma cells (red represents αCD138), B cells (green represents αB220). Bar represents 100 μm. (E) Titers of immunoglobulin isotypes in aged mice were determined by ELISA; n(BA20−/−)= 12, n(control) = 11. (F-H) Absolute splenic cell numbers for the following cellular subsets: CD4 T cells. (F) Treg (CD4+CD25+); CD4+ naive (CD25−CD44intCD62Lhi), memory-type (CD25−CD44hiCD62Lhi), and effector T (CD4+CD44hiCD62Llo); CD8-T cells. (G) Naive (CD44intCD62Lhi), memory-type (CD44hiCD62Lhi), and effector T (CD44hiCD62Llo); myeloid cells (H): DC indicates dendritic cell (CD11c+); Eos, eosinophils (Gr1intSiglecF+); Mac, macrophages (Mac1+Gr1lo); and Neu, neutrophils (Gr1hiLy6G+). n(BA20−/−) = 5, n(control) = 5. (I) Serum IL-6 (pg/mL) in aged mice was measured by ELISA; n(BA20−/−) = 12, n(control) = 11. (J) Representative stainings of IgG immune complexes in kidneys of BA20−/− and control mice. Original magnification × 20. *P < .05 (1-way analysis of variance). **P < .001 (1-way analysis of variance).
Given this finding, we tested the sera of all old BA20−/− and control mice for indicators of self-recognition. Class-switched antibodies against nuclear self-antigens (ANAs) are the most common autoantibodies observed in autoimmune conditions in mice and humans.30 Sera from BA20−/− mice did not contain significantly higher ANA levels than sera from the controls, and we also did not detect enhanced reactivity to endogenous red blood cells (Figure 7A). In addition, we observed increased levels of rheumatoid factor of the IgM isotype in some BA20−/− mice, but not of the IgG isotype (Figure 7A). However, we detected significantly elevated amounts of anticardiolipin IgG antibodies in aged BA20−/− mice (Figure 7A), demonstrating systemic class-switched autoimmune reactivity. To evaluate whether antiphospholipid antibodies are already detectable in younger mice, we screened the sera of 3-month-old mice. We detected elevated anticardiolipin IgG antibodies in sera from BA20−/− mice (and BA20+/− mice) compared with control sera, but the differences were not statistically significant (Figure 7C).
Autoimmune manifestations in old BA20−/− mice. (A) Analysis of autoantibodies in aged mice. ANA indicates that antinuclear IgG antibodies were detected by ELISA, n(BA20−/−) = 12, n(control) = 21; αRBC-IgM, antierythrocyte IgM was detected by FACS and represented as mean fluorescence intensity, n(BA20−/−) = 8, n(control) = 4. IgG and IgM rheumatoid factor (RF) was measured by ELISA; n(BA20−/−) = 12, n(control) = 11. α-Cardiolipin IgG antibodies were detected by ELISA; n(BA20−/−) = 12, n(control) = 11. *P < .05 (2-tailed unpaired Student t test). **P < .001 (2-tailed unpaired Student t test). (B) Table depicting the self-reactivity of sera from individual BA20−/− mice against the indicated organs from Rag2−/− mice. +, ++, and +++ indicate the severity of autoreactivity; and n.s., not screened. (C) Levels of α-cardiolipin IgG autoantibodies in 3-month-old mice (left panel; n = 8 per genotype) and in 11-month-old mice (right panel; n = 5 per genotype) were detected by ELISA. *P < .05 (1-tailed unpaired Student t test).
Autoimmune manifestations in old BA20−/− mice. (A) Analysis of autoantibodies in aged mice. ANA indicates that antinuclear IgG antibodies were detected by ELISA, n(BA20−/−) = 12, n(control) = 21; αRBC-IgM, antierythrocyte IgM was detected by FACS and represented as mean fluorescence intensity, n(BA20−/−) = 8, n(control) = 4. IgG and IgM rheumatoid factor (RF) was measured by ELISA; n(BA20−/−) = 12, n(control) = 11. α-Cardiolipin IgG antibodies were detected by ELISA; n(BA20−/−) = 12, n(control) = 11. *P < .05 (2-tailed unpaired Student t test). **P < .001 (2-tailed unpaired Student t test). (B) Table depicting the self-reactivity of sera from individual BA20−/− mice against the indicated organs from Rag2−/− mice. +, ++, and +++ indicate the severity of autoreactivity; and n.s., not screened. (C) Levels of α-cardiolipin IgG autoantibodies in 3-month-old mice (left panel; n = 8 per genotype) and in 11-month-old mice (right panel; n = 5 per genotype) were detected by ELISA. *P < .05 (1-tailed unpaired Student t test).
To screen for further autoreactivity against potentially tissue-specific self-antigens, we incubated organ sections of Rag2−/− mice with the sera of the aged mice. We detected autoreactive class-switched IgG antibodies in the sera of BA20−/−, but not of control mice, directed predominantly against kidney, harderian gland, stomach, thyroid gland, eye, liver, and lung (Figure 7B; supplemental Figure 6E). Pancreas, salivary, lacrimal, and adrenal glands were also recognized by sera from few BA20−/− mice but with a lower penetrance compared with the organs mentioned earlier (Figure 7B). Notably, the kidney was recognized by autoantibodies in the sera of the majority of BA20−/− mice. Therefore, we conclude that A20-deficient B cells induce chronic progressive inflammation, which results in significant autoimmune manifestations and pathologic alterations in old mice.
Discussion
The prevalent inactivation of the ubiquitin-editing enzyme A20 in human B-cell lymphoma and the linkage of polymorphisms in the A20/TNFAIP3 gene to human autoimmune diseases raise the question of its function in the B-lineage, especially during B-cell activation.
The major molecular function of A20 uncovered to date is the negative regulation of canonical NF-κB activation.14 We confirm that, also in B cells, A20 limits activation of this signaling pathway by all relevant physiologic inducers. Constitutive activation of canonical NF-κB in B cells induces B-cell hyperplasia, especially pronounced in MZB cells.20 In contrast, we uncovered that A20 activity is required for the correct localization of MZB cells and for MZB cell function. Therefore, we conclude that proper differentiation of A20-deficient MZB cells does not take place. In addition, A20 is needed for the generation or cellular maintenance of peritoneal B1, bone marrow recirculating and thymic B cells, either directly or by mediating their correct localization. Reduced cellular maintenance could be caused by lower sensitivity to survival signals available in naive mice, but also by increased terminal differentiation because of their hyperactivatable state. The elevated IgA serum levels could be a consequence of the enhanced GC reactivity in the GALT of BA20−/− mice. Another possible explanation is that the lower levels of B1a cells could, at least in part, be caused by their enhanced differentiation to IgA-producing plasma cells in response to inducing signals, such as IL-15,31 a cytokine known to induce NF-κB activation. This phenomenon could also contribute to the elevated IgM levels. Because B1 cells are thought to be the main source of naturally occurring autoantibodies in naive mice,32 this could signify that also natural autoantibody IgM titers, which are not pathogenic but rather play a protective role, are enhanced in the BA20−/− mice. The accumulation of A20-deficient precursor populations (transitional B cells and MZB cell precursors) points to blocks in development, although we cannot exclude a role for impaired negative selection.
Taken together, our data on B-cell development and in vitro activation studies suggest the following: A20 activity is required to limit acute B-cell activation induced by stimuli connected to invasion by pathogens. Its absence reduces the activation threshold and enhances survival and proliferation in response to such stimuli. On the other side, the absence of A20 does not render B cells more responsive to maintenance signals required for mature resting cells.
Increased B cell–mediated IL-6 secretion resulting from sporadic local activation and, at least in the case of A20 deficiency, spontaneous production and release of IL-6 is a probable underlying cause of the effector T-cell differentiation and myeloid cell hyperplasia that we observed in BA20−/− and BA20+/− mice. Subsequent cytokine secretion by activated myeloid cells most probably amplifies this response. Indeed, Tsantikos et al recently showed that effector T-cell activation, myeloid cell expansion, and the development of autoimmune disease in Lyn-deficient mice, which strikingly resemble BA20−/− and BA20+/− mice in this regard, are entirely dependent on the secretion of IL-6.33 Interestingly, the authors also observed expansion of CD4+CD25+ T cells but did not investigate whether they represent activated or regulatory T cells.33 The enhanced numbers of Tregs we observe in the BA20−/− and BA20+/− mice appear paradoxical in the context of elevated IL-6 levels.34 We suggest that they expand and/or are induced in reaction to the IL-6-driven inflammation. However, the presence of IL-6 increases the resistance of effector T cells to suppression by regulatory T cells,35 both systemically and locally. Expansion of Tregs has also been observed in the BWF1 mouse model for human SLE.36 Whether, and to what extent, an expanded Treg population serves to keep the sterile inflammation in check is an intriguing question to be tested in future studies.
While this manuscript was in preparation, a study by Tavares et al on the same topic was published.37 The authors also found a convincing gene-dose–dependent effect of A20 ablation on B-cell activation. They detected elevated levels of antibodies in the serum of CD19cre/A20F/F mice that recognize self-antigens on a protein array. However, in their approach, anti–mouse Ig was used to detect autoantibodies, which also recognizes IgM. Indeed, both studies find highly elevated titers of IgM in naive BA20−/− mice (in our case, a 10-fold difference in the geometric mean). Naturally occurring autoantibodies are mostly of the IgM isotype.38,39 Therefore, the increased recognition of self observed by Tavares et al37 could reflect, at least to some degree, this increase in natural autoantibodies. This possibility is substantiated by the fact that only IgM+ dsDNA-recognizing plasma cells were detected and that IgM immune complex deposits were revealed in naive 6-month-old mice, in the absence of any sign of pathology.37 Given that the role of IgM autoantibodies is also discussed as being protective,38,39 it is unclear whether the autoreactivity observed in younger mice contributes to the development of, or helps to prevent, disease.
We observed significant autoreactivity of class-switched antibodies only in old mice, together with autoimmune pathology and inflammation. The chronic inflammation induced by the A20-deficient B cells perhaps contributes to a progressive break in B-cell tolerance resulting in the production of class-switched, autoreactive antibodies. The immune senescence of old age might also contribute to this break in tolerance.32 The inflammatory microenvironment in the spleen, and potentially in other affected organs, together with the elevated presence of the pleiotropic cytokine IL-6, may serve as a niche for the (autoreactive) plasma cells. However, we do not detect significant levels of IgG ANAs, the hallmark of SLE.30 Instead, we observe general IgG autoreactivity to cardiolipin, a common autoantigen in autoimmune disease30,40 in old BA20−/− mice. The presence of tissue-specific class-switched autoantibodies strongly underscores the loss of tolerance. Some pathologic features of the old BA20−/− mice are reminiscent of human Castleman disease, namely, massive infiltrations of plasma cells and the elevated presence of IL-6.28,41 It is indeed possible that the disturbed B-cell development, including the elevated levels of naturally occurring auto-IgM, are preventing a more severe syndrome. Heterozygous BA20+/− mice display inflammation and B-cell hyper-reactivity, but no developmental defects. Therefore, they might represent a good tool for modeling the reduced expression or function of A20 that should underlie the link between mutations and polymorphisms in A20/TNFAIP3 and human autoimmune disease. Indeed, initial analyses revealed elevated class-switched antibodies against cardiolipin in a cohort of 11-month-old BA20+/− mice (Figure 7C).
We demonstrate here that selective loss of A20 in B cells is sufficient to cause an inflammatory syndrome with autoimmune manifestations in old mice. This condition is characterized by a progressive chronic inflammation, elevated levels of IL-6, dramatic plasma cell expansion, and the presence of class-switched systemic and tissue-specifc autoantibodies. The exquisite dose effects of monoallelic loss of A20 make it a prime target for deregulation by proinflammatory miRNAs and oncomirs in disease contexts. Our results demonstrate that B-cell hyper-reactivity caused by reduced A20 function can contribute to the observed link between inherited genetic mutations or polymorphisms in A20/TNFAIP3 and various human autoimmune diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Reinhard Fässler for support; Julia Knogler and Barbara Habermehl for technical assistance; Reinhard Voll and Michael Sixt for advice; Guru Krishnamoorthy for help with thymidine incorporation assays; Ludger Klein for tissues of Rag2−/− mice; Claudia Uthoff-Hachenberg for ELISA reagents; and Michael Sixt, Charo Robles, Vigo Heissmeyer, Stefano Casola, Sergei Koralov, Manu Derudder, and Dinis Calado for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB684) and an Emmy Noether grant (M.S.-S.). J.C.V. and K.H. received PhD stipends from the Ernst Schering Foundation and the Boehringer Ingelheim Fonds, respectively. G.v.L. is a FWO postdoctoral researcher with an Odysseus Grant, K.A. was supported by the IZKF Erlangen (project A31) and D.S. by the Cancer Research Institute.
Authorship
Contribution: Y.C. and M.S.-S. designed and performed research, analyzed data, and wrote the paper; J.C.V., D.K., K.H., A.B., E.W., V.S., B.M., and M.R. performed research; D.S. and K.A. analyzed data; and R.B. and G.v.L. contributed new reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marc Schmidt-Supprian, Max Planck Institute of Biochemistry, Am Klopferspitz 18, D-82152 Martinsried, Germany; e-mail: supprian@biochem.mpg.de.


![Figure 4. A20-deficiency amplifies B-cell responses. (A) Expression levels of the respective B-cell activation marker after overnight stimulation with LPS or αCD40. The dot-plots are representative of 3 to 6 independent experiments. (B) [3H]Thymidine incorporation during a 10-hour pulse 48 hours after stimulation of B-cell cultures with αIgM, αCD40, LPS, or CpG. Data are mean ± SD of triplicate measurements. The experiment was repeated with similar results. (C) Cell cycle profile analysis by propidium iodide staining of B cells 2 days after stimulation with the indicated mitogens. Percentages of dead (sub G0) and live cells are indicated at the top of each histogram. The distribution within the cell cycle was calculated with the FlowJo software Version 8.7.3 using the Watson model (values do not add up to 100%). Data are means of 2 independent experiments. (D) Assessment of proliferation by the carboxyfluorescein succinimidyl ester dilution assay: histograms represent carboxyfluorescein succinimidyl ester intensities 3 days after stimulation. The tables under each histogram show the proliferation index (Prol. Index: average number of divisions of the proliferating cells), the percentage of dividing cells (% Divided: the proportion of cells that initially started to divide), and the division index (Div. Index: average number of divisions of all cells); values were calculated with the FlowJo software. Data represent the means of 5 independent experiments, and bold values are significantly different (P < .05) from wild-type according to 1-way analysis of variance analysis. (E) Evaluation of IL-6 production in overnight activated B cells by ELISA (top panels), intracellular FACS (middle panels), and real-time PCR (bottom panels). For intracellular FACS, B cells were stimulated for 3 days. cDNA was quantified relative to porphobilinogen deaminase. Data are mean ± SD of 3 independent experiments. (F) Quantification of TNF production by overnight-stimulated B cells via ELISA. Data are mean ± SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/7/10.1182_blood-2010-09-306019/5/m_zh89991065340004.jpeg?Expires=1765915029&Signature=OR-MI~JNiqHHMgZw9WuHX76dL7-ZERdadbVn0ArTNEYcCMDY9GVW8vlBDEo7tsMscEVdP-J0BkOVXB3DL2Woxt~ijSNqB0p5yyDW6GVm9Ccuu1~zNxpsghhnzZICFekI9CYZIOlKOD~2ITdaxsemyHonBlgJ5Tzo~IXj4wKuxx36coAUjHmQHS3EKKcxcLgBY8OPGA0pSvjliEaPKQRuD7BkBoVD2E0irIdCa-m8fYCwlk-l~lNMU1LXNGcVvPcx8rCAb-N2QYjfwqzK8yDlt7DTGuNUj~XK5-7PM7H-qxSIfSEftaHJile7pQMOfwWBWSzoyyer~-QN03BUPjckqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal