Abstract
The individual risk of infection and requirements for medical treatment after high-dose chemotherapy have been unpredictable. In this prospective, multicenter, open-label study we investigated the potential of granulocyte colony-stimulating factor (G-CSF) responsiveness as a predictor. A total of 168 patients with multiple myeloma or lymphoma received a single dose of subcutaneous G-CSF (lenograstim, 263 μg) after high-dose chemotherapy. Highly variable leukocyte peaks were measured and grouped as low (quartile 1; leukocytes 100-10 100/μL), medium (quartile 2; leukocytes > 10 100-18 300/μL), and high (quartiles 3/4; leukocytes > 18 300-44 800/μL). G-CSF responsiveness (low vs medium vs high) was inversely correlated with febrile neutropenia (77% vs 60% vs 48%; P = .0037); the rate of infection, including fever of unknown origin (91% vs 67% vs 54%; P < .0001); days with intravenous antibiotics (9 vs 6 vs 5; P < .0001); and antifungal therapy (P = .042). In multivariate analysis, G-CSF responsiveness remained the only factor significantly associated with infection (P = .016). In addition, G-CSF responsiveness was inversely correlated with grade 3/4 oral mucositis (67% vs 33% vs 23%; P < .0001). G-CSF responsiveness appears as a signature of the myeloid marrow reserve predicting defense against neutropenic infection after intensive chemotherapy. This study is registered at http://www.clinicaltrials.gov as NCT01085058.
Introduction
High-dose chemotherapy followed by autologous blood stem cell transplantation is a standard treatment for multiple myeloma1-3 and relapsed lymphoma4,5 that can improve patient survival. Major clinical problems occurring with this type of therapy are infection, mucositis, and bleeding during the cytopenic phase, which lasts 1-2 weeks. Solid tumors, lymphoma, leukemia, and multiple myeloma appear to have a similar risk for infectious complications.6,7 However, complications and supportive care requirements can vary extensively from patient to patient. Hitherto, it has not been possible to predict these risks for the individual patient, and prophylaxis of infection and supportive treatment cannot yet be adjusted to the needs of the patients.
Previously, we evaluated the marrow response to a single dose of granulocyte colony-stimulating factor (G-CSF) 1 day after high-dose melphalan and before autologous blood stem cell transplantation in patients with multiple myeloma at our center.8 The magnitude of the G-CSF–induced leukocyte peak in the blood was used as the reading for G-CSF responsiveness (G-Rsp) of the individual patient. G-Rsp was found to be highly variable, and ordered categories on the basis of the observed distribution were inversely correlated with infection during neutropenia and engraftment. Therefore, we performed a prospective multicenter study in patients with multiple myeloma and lymphoma who received different high-dose regimens to confirm the association between G-Rsp and the risk of infection and to analyze the consecutive medical treatment for the different G-Rsp subgroups.
Methods
Patients
Patients with multiple myeloma or lymphoma at the age of 18-70 years with adequate organ functions could be included into the study when they were planned to receive high-dose chemotherapy with autologous blood stem cell transplantation at the respective study center. Patients had to have a blood stem cell transplant with at least 2 × 106 CD34+ cells per kilogram. Exclusion criteria were known intolerance to lenograstim, serious infections such as HIV or hepatitis B/C, a second malignancy, and outpatient treatment after transplantation. Additional exclusion criteria were a serum creatinine level > 2 mg/dL, an elevation of a serum aspartate aminotransferase or alanine aminotransferase, or serum bilirubin level > 3 times the upper limit of the normal range. All patients provided written informed consent, and the study protocol was approved by the ethics committee at each study site.
High-dose chemotherapy
Fifteen centers in Germany participated in this trial. The types of high-dose chemotherapy applicable for the study were predefined as melphalan (MEL) 140, MEL200, BEAM, BEAC, or BUCY. In an amendment of the study protocol, the study was opened for second transplants and the CBV regimen (ie, cyclophosphamide, BCNU, and VP-16), but only 1 patient receiving a second high-dose therapy and 1 lymphoma patient receiving CBV were included because the recruitment of the planned patient number had been reached faster than expected. Most patients received either BEAM or MEL high-dose therapy, whereas only 4 patients received other regimens. The BEAM regimen consisted of cumulative doses of carmustine 300 mg/m2, etoposide 800 or 1200 mg/m2, cytarabine 1600 mg/m2, and melphalan 140 mg/m2 and was administered over the course of 4-5 days. MEL high-dose chemotherapy was administered at a total dose of 140 mg/m2 or 200 mg/m2 melphalan, given over the course of 2 days. Appropriate guidelines for diagnosing and treating of infection in neutropenic patients were applied.9
Study design and medication
The aim of this prospective, open-label, multicenter study was to confirm the results of the single-center study8 and to investigate in greater detail the predictive potential of the magnitude of the leukocyte peak induced by a single dose of G-CSF for the occurrence of infection and other complications after high-dose chemotherapy. As the study medication, the single dose of subcutaneous G-CSF (lenograstim 263 μg) was administered in all patients 1 day (day −1) before autologous blood stem cell transplantation after a blood sample was taken for a baseline leukocyte count in the evening. On the next morning (day 0), 12-14 hours after the single G-CSF dose, another blood sample was drawn to determine the induced leukocyte peak in the blood. This day 0 leukocyte count was taken as the reading for the G-Rsp of the patient. After this, autologous blood stem cell transplantation was performed. The administration of G-CSF was restarted on day +5 from stem cell transplantation with a daily subcutaneous dose of lenograstim 263 μg until postnadir neutrophils had recovered (not considered as study medication). The study was conducted in accordance with the Declaration of Helsinki (revised version from 2000), the International Conference on Harmonization guideline on good clinical practice (ICH-E6; CPMP/135/95), and respective laws in Germany (AMG 10. Amendment from July 11, 2000).
Assessments
Clinical and laboratory assessments were prespecified for different time points, which were inclusion of patients, pre- and post- the single G-CSF dose (days −1 and 0), the posttransplant phase (daily), discharge of patients from hospital, and further clinical controls (2 times weekly) until day +30 from transplantation.
From day −1, daily assessments of patients included documentation of the physical examination, blood count, antimicrobial prophylaxis and treatment, fever and infection, and red blood cell and platelet transfusions. Occurring side effects were classified by the use of National Cancer Institute Common Toxicity Criteria grading. Severe adverse events and unexpected severe adverse events were reported to the principal investigator (C. Straka) and the responsible contract research organization (Winicker Norimed GmbH).
The documentation of infection distinguished microbially and clinically defined infections and fever of unknown origin as proposed by the Immunocompromised Host Society10 and prespecified bacteremia, pneumonia, enterocolitis, urinary tract infection, infectious stomatitis, catheter infection, viral infection, fungal infection, and others, as the type and/or location of infection.
Study centers included patients after a formal prestudy visit of the responsible contract research organization. For documentation, study-specific case report forms were used. Monitoring visits by the contract research organization were performed at regular intervals to ensure that all aspects of the protocol were being followed and the trial was conducted according to good clinical practice.
Statistical analysis
The data management performed by the contract research organization included a check of case report forms for plausibility, completeness, and validity by on-site monitoring in all study centers. Two independent individuals transferred the data to 2 identically structured databases with following validation. Patients who did not fulfill the inclusion criteria were to be excluded from the analyses (ie, noneligible). All other patients were included in the intention-to-treat analyses with respect to the primary end point. A second analysis (ie, according to protocol) restricted the study population to patients who had undergone diagnostics and treatments as specified in the study protocol.
The primary objective of the study was to show a decrease in the infection rate across 3 ordered categories for G-Rsp, which were defined prospectively but determined by the actually observed distribution (“low” group: ≤ 1st quartile; “medium” group: > 1st quartile and ≤ median; and “high” group: > median). To detect the assumed trend (40%, 30%, and 15% of infections in the respective risk groups) and on the basis of the relative group sample size of 1:1:2 according to the definition of the categories, 160 patients were to be enrolled to achieve 80% power with a 2-sided type I error of 5%. This results from the application of a logistic regression model for the trend test on infection rates, according to Nam,11 as implemented in the NQuery Advisor 4.0 software (Statistical Solutions). The assumed infection rates for this trend were chosen with respect to the single-center experience with the use of documented infection.
Although the different categories of infection10 were documented and validated separately, we had planned to use a composite end point for the infection rate. The original primary end point had been defined as the proportion of microbially plus the clinically defined infections as was performed in the single-center study.8 During a plausibility and accuracy check of documentation of infection at the end of the study, before the data were entered into the database, it was noted that there was a highly overproportional rate of cases with a positive blood culture defined microbial infection (44% vs 9%) at one particular center. This one center accounted for 35% in this category of infection but only for 9% of patients and was contrary to the trend of the other 14 centers. Because this suggested a potential unreliability of microbially defined infections in the multicenter setting to us, we amended the protocol at that time point with respect to the primary end point and included fever of unknown origin into a comprehensive composite end point infection. This amended primary end point therefore was defined as the overall rate of infection, which added fever of unknown origin to documented infection. The intention was to compensate the center effect. Variability in identifying microorganisms influences the quantitative relation between documented infection and fever of unknown origin but does not grossly change the overall rate of infection. No interim analyses were planned nor performed.
Proportions, such as infection rates, across the G-Rsp categories were analyzed with the use of an exact version of the Cochrane-Armitage trend test, whereas the association to quantitative parameters was assessed by the Kruskall-Wallis test. Time to neutrophil and platelet engraftment was estimated according to the method of Kaplan-Meier12 and compared with the log-rank test. For multivariate analysis on predictors of infection rates, including all parameters with P < .1 in univariate analysis, a logistic regression model was applied, with stepwise backward variable selection (exclusion in case of P > .1). A Cox model13 was used for multivariate analyses of time to engraftment. All reported P values are 2-sided.
The statistical planning was performed by WISP Research Institute. The analysis of data was performed by the investigators, the WISP Research Institute, and the contract research organization.
Results
High-dose chemotherapy
Of a total of 169 patients, who were recruited from 15 centers, 168 were eligible and were analyzed (intent-to-treat population; Table 1). The majority of patients had multiple myeloma (62%), whereas others were treated for Hodgkin disease (8%), indolent lymphoma (10%), and aggressive lymphoma (20%). The median age of patients was 57 years. Most of the patients (71%) were in complete or partial remission when the high-dose therapy was administered. Patients with multiple myeloma received high-dose MEL, patients with lymphoma BEAM, or in a few cases other regimens. MEL200 was used in 35%, MEL140 in 27%, BEAM in 36%, and other regimens in 2%.
Patient and treatment characteristics
| Characteristic . | n . | % . |
|---|---|---|
| Patients | ||
| Recruited | 169 | |
| Intent to treat | 168 | 100 |
| Per protocol | 162 | 96 |
| Sex | ||
| Male | 91 | 54 |
| Female | 77 | 46 |
| Age, y | ||
| < 40 | 24 | 14 |
| 40-59 | 80 | 48 |
| ≥ 60 | 64 | 38 |
| Diagnosis | ||
| Multiple myeloma | 104 | 62 |
| Hodgkin disease | 14 | 8 |
| Indolent lymphoma | 17 | 10 |
| Aggressive lymphoma | 33 | 20 |
| ECOG performance status | ||
| 0 | 68 | 40 |
| 1 | 93 | 56 |
| 2 | 7 | 4 |
| Previous cycles of chemotherapy | ||
| ≤ 6 | 120 | 71 |
| > 6 | 48 | 29 |
| Previous radiotherapy | ||
| Yes | 59 | 35 |
| No | 109 | 65 |
| Remission status | ||
| Complete remission | 22 | 13 |
| Partial remission | 97 | 58 |
| Less than partial remission | 49 | 29 |
| High-dose regimens | ||
| MEL140 | 45 | 27 |
| MEL200 | 59 | 35 |
| BEAM | 60 | 36 |
| Others | 4 | 2 |
| CD34+ cells infused, ×106/kg | ||
| 2.0-5.0 | 106 | 63 |
| > 5.0 | 62 | 37 |
| Characteristic . | n . | % . |
|---|---|---|
| Patients | ||
| Recruited | 169 | |
| Intent to treat | 168 | 100 |
| Per protocol | 162 | 96 |
| Sex | ||
| Male | 91 | 54 |
| Female | 77 | 46 |
| Age, y | ||
| < 40 | 24 | 14 |
| 40-59 | 80 | 48 |
| ≥ 60 | 64 | 38 |
| Diagnosis | ||
| Multiple myeloma | 104 | 62 |
| Hodgkin disease | 14 | 8 |
| Indolent lymphoma | 17 | 10 |
| Aggressive lymphoma | 33 | 20 |
| ECOG performance status | ||
| 0 | 68 | 40 |
| 1 | 93 | 56 |
| 2 | 7 | 4 |
| Previous cycles of chemotherapy | ||
| ≤ 6 | 120 | 71 |
| > 6 | 48 | 29 |
| Previous radiotherapy | ||
| Yes | 59 | 35 |
| No | 109 | 65 |
| Remission status | ||
| Complete remission | 22 | 13 |
| Partial remission | 97 | 58 |
| Less than partial remission | 49 | 29 |
| High-dose regimens | ||
| MEL140 | 45 | 27 |
| MEL200 | 59 | 35 |
| BEAM | 60 | 36 |
| Others | 4 | 2 |
| CD34+ cells infused, ×106/kg | ||
| 2.0-5.0 | 106 | 63 |
| > 5.0 | 62 | 37 |
ECOG indicates Eastern Cooperative Oncology Group.
G-Rsp
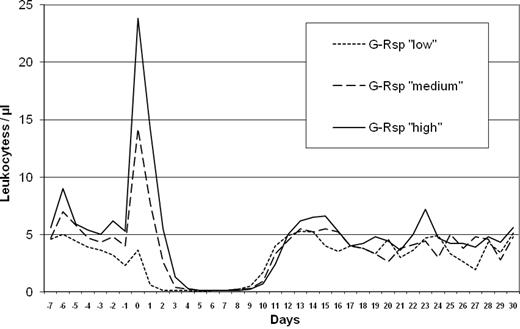
After the end of high-dose chemotherapy, the test dose of G-CSF (lenograstim 263 μg) was given in the evening (day −1). The leukocyte count at this time point was a median of 3800/μL (range, 200-12 700). On the next morning (day 0) 12-14 hours after this single G-CSF dose and before autologous transplantation, a blood cell count was performed to determine the induced leukocyte peak. The median leukocyte peak was 18 300/μL (range, 100-44 800). According to the distribution of leukocyte peaks, groups were defined as having a low (< 10 100/μL leukocytes; quartile 1), a medium (> 10 100-18 300/μL leukocytes; quartile 2), or a high (> 18 300/μL leukocytes; quartiles 3 + 4) G-Rsp for associations with clinical end points. The safety analysis did not point to any unknown risks related to the study medication. The leukocyte course for G-Rsp subgroups until day +30 is shown in Figure 1.
The G-CSF–induced leukocyte peaks were generally lower after the BEAM regimen (median, 8900/μL; range, 100-38 800) than for MEL (median, 21 900/μL; range, 5600-44 800). However, 42% of patients receiving BEAM showed a medium or high G-Rsp.
Stem cell engraftment
For autologous transplantation, a median of 4.2 × 106 CD34+ cells per kilogram (range, 2.0-22.0) was infused. Accordingly, all patients had received at least the standard dose of 2 × 106 CD34+ cells per kilogram. A greater CD34+ cell dose (> 4.0 × 106 per kilogram) was associated with a faster engraftment of neutrophils (P = .0026) and platelets (P = .086). The time to neutrophil engraftment with postnadir neutrophils > 500/μL was a median of 10 days (range, 7-14 days). Platelet engraftment to a count > 20 000/μL took place after a median of 11 days (maximum, 21 days).
Neutropenia and fever
Although the low G-Rsp cases had received the greatest CD34+ cell doses (Table 2), neutropenia was the longest in this group. The median duration of severe neutropenia (< 500 neutrophils/μL) was 8 days (range, 6-15 days) for the low group, 7 days (range, 5-10 days) for the medium group, and 6 days (range, 4-9 days) for the high group (P < .0001). The reason for the longer duration of neutropenia in the low and medium groups was an earlier onset of neutropenia (Figure 1). In 98 of 168 patients (58%) a febrile neutropenia developed (Table 3). For G-Rsp subgroups the incidence and median duration of febrile neutropenia were 77% and 2 days for the low group, 60% and 1 day for the medium group, and 48% and 0 days for the high group (P = .0037 and P = .0010, respectively).
Antimicrobial treatment and supportive care
| Treatment . | Granulocyte colony-stimulating factor responsiveness . | P . | ||
|---|---|---|---|---|
| Low . | Medium . | High . | ||
| Intravenous antibiotics, d | ||||
| Incidence, % | 84 | 69 | 60 | .0093 |
| Mean ± SD | 9.4 ± 6.4 | 6.3 ± 6.1 | 4.1 ± 3.9 | |
| Median (range) | 9 (0-28) | 6 (0-25) | 5 (0-18) | < .0001 |
| > 7 days % | 65 | 33 | 14 | < .0001 |
| Intravenous antimycotics, d | ||||
| Incidence, % | 26 | 10 | 12 | .091 |
| Mean ± SD | 2.3 ± 4.7 | 0.5 ± 1.7 | 0.7 ± 2 | |
| Median (range) | 0 (0-20) | 0 (0-7) | 0 (0-9) | .042 |
| Intravenous virustatics, d | ||||
| Incidence, % | 16 | 21 | 10 | .24 |
| Mean ± SD | 1.4 ± 4.1 | 2.0 ± 5.6 | 0.5 ± 1.8 | |
| Median (range) | 0 (0-18) | 0 (0-33) | 0 (0-11) | .16 |
| Red blood cell transfusions, units | ||||
| Mean ± SD | 2.5 ± 2.2 | 2.1 ± 1.8 | 1.8 ± 1.9 | |
| Median (range) | 2 (0-11) | 2 (0-6) | 2 (0-10) | .27 |
| Platelet transfusions, units | ||||
| Mean ± SD | 3.1 ± 2.1 | 2.1 ± 1.5 | 1.4 ± 1.9 | |
| Median (range) | 3 (0-8) | 2 (0-8) | 1 (0-14) | < .0001 |
| Applications of G-CSF from day +5, d | ||||
| Mean ± SD | 7.1 ± 2.7 | 7.6 ± 2.3 | 7.2 ± 1.7 | |
| Median (range) | 7 (0-13) | 7 (0-13) | 7 (0-12) | .52 |
| ≥ 10 days % | 21 | 14 | 8 | .05 |
| Hospitalization from transplantation, d | ||||
| Mean ± SD | 17.0 ± 4.0 | 15.6 ± 3.6 | 14.5 ± 2.5 | |
| Median (range) | 16 (11-27) | 15 (12-28) | 14 (11-26) | .0017 |
| Transplanted CD34+ cells, ×106/kg | ||||
| Mean ± SD | 5.9 ± 4.1 | 4.2 ± 2.3 | 5.2 ± 2.7 | * |
| Median (range) | 5.0 (2.2-22.0) | 3.4 (2.0-12.3) | 4.2 (2.3-14.2) | |
| Treatment . | Granulocyte colony-stimulating factor responsiveness . | P . | ||
|---|---|---|---|---|
| Low . | Medium . | High . | ||
| Intravenous antibiotics, d | ||||
| Incidence, % | 84 | 69 | 60 | .0093 |
| Mean ± SD | 9.4 ± 6.4 | 6.3 ± 6.1 | 4.1 ± 3.9 | |
| Median (range) | 9 (0-28) | 6 (0-25) | 5 (0-18) | < .0001 |
| > 7 days % | 65 | 33 | 14 | < .0001 |
| Intravenous antimycotics, d | ||||
| Incidence, % | 26 | 10 | 12 | .091 |
| Mean ± SD | 2.3 ± 4.7 | 0.5 ± 1.7 | 0.7 ± 2 | |
| Median (range) | 0 (0-20) | 0 (0-7) | 0 (0-9) | .042 |
| Intravenous virustatics, d | ||||
| Incidence, % | 16 | 21 | 10 | .24 |
| Mean ± SD | 1.4 ± 4.1 | 2.0 ± 5.6 | 0.5 ± 1.8 | |
| Median (range) | 0 (0-18) | 0 (0-33) | 0 (0-11) | .16 |
| Red blood cell transfusions, units | ||||
| Mean ± SD | 2.5 ± 2.2 | 2.1 ± 1.8 | 1.8 ± 1.9 | |
| Median (range) | 2 (0-11) | 2 (0-6) | 2 (0-10) | .27 |
| Platelet transfusions, units | ||||
| Mean ± SD | 3.1 ± 2.1 | 2.1 ± 1.5 | 1.4 ± 1.9 | |
| Median (range) | 3 (0-8) | 2 (0-8) | 1 (0-14) | < .0001 |
| Applications of G-CSF from day +5, d | ||||
| Mean ± SD | 7.1 ± 2.7 | 7.6 ± 2.3 | 7.2 ± 1.7 | |
| Median (range) | 7 (0-13) | 7 (0-13) | 7 (0-12) | .52 |
| ≥ 10 days % | 21 | 14 | 8 | .05 |
| Hospitalization from transplantation, d | ||||
| Mean ± SD | 17.0 ± 4.0 | 15.6 ± 3.6 | 14.5 ± 2.5 | |
| Median (range) | 16 (11-27) | 15 (12-28) | 14 (11-26) | .0017 |
| Transplanted CD34+ cells, ×106/kg | ||||
| Mean ± SD | 5.9 ± 4.1 | 4.2 ± 2.3 | 5.2 ± 2.7 | * |
| Median (range) | 5.0 (2.2-22.0) | 3.4 (2.0-12.3) | 4.2 (2.3-14.2) | |
Not an outcome variable.
Infections
Infections as defined by the Immunocompromised Host Society10 occurred in 112 of 168 patients (67%; Table 3). The definition of infection was microbial in 19%, clinical in 15%, and fever of unknown origin in 42%. The trend for the association with low, medium, and high G-Rsp was significant for clinically defined infection (P = .01), fever of unknown origin (P = .0013), but not for microbially defined infections (P = .73). In this context, the center effect on blood culture testing as detailed in “Statistical analysis” needs to be considered. Specific infections with a greater risk for a serious course were less frequent in patients with a high G-Rsp as observed for pneumonia (1% vs 9%; P = .034) or enterocolitis (1% vs 7%; P = .12). Fungal infections were only found in patients with a low G-Rsp (2%). The mortality rate of high-dose therapy was low (< 1%). One patient with a low G-Rsp died on day +9 after high-dose chemotherapy because of Escherichia coli sepsis. The initial primary end point (microbially plus clinically) documented infection did not reach statistical significance (P = .32). A clearly negative impact of the center effect on the initial primary end point could be demonstrated by testing the exclusion of this one center, resulting in borderline significance (P = .06). A highly significant association of the amended primary end point, including fever of unknown origin with G-Rsp was noted, which was 91% for the low, 67% for the medium, and 54% for the high group (P < .0001). There was no significant correlation of the CD34+ cell dose with infection.
Fever and infection during neutropenia
| . | Granulocyte colony-stimulating factor responsiveness . | P . | ||
|---|---|---|---|---|
| Low, n = 43 . | Medium, n = 42 . | High, = 83 . | ||
| Fever and neutropenia, % | ||||
| Fever > 38°C | 84 | 62 | 51 | .0004 |
| Febrile neutropenia | 77 | 60 | 48 | .0037 |
| Documented infections, % | ||||
| Bacteremia | 12 | 17 | 11 | |
| Pneumonia | 7 | 12 | 1 | |
| Enterocolitis | 7 | 7 | 1 | |
| Fungal infection | 2 | – | – | |
| Viral infection | 5 | 5 | 1 | |
| Urinary tract infection | 2 | – | 2 | |
| Stomatitis | 2 | 2 | 1 | |
| Catheter infection | – | 2 | 1 | |
| Others | 5 | 5 | 6 | |
| Categories of infection, % | ||||
| Microbially defined (type A) | 16 | 29 | 16 | .73 |
| Clinically defined (type B) | 23 | 21 | 8 | .01 |
| Fever of unknown origin (type C) | 65 | 38 | 33 | .0013 |
| Primary composite end points, % | ||||
| Initial (type A + B) | 30 | 43 | 24 | .32 |
| Amended (type A + B + C) | 91 | 67 | 54 | < .0001 |
| . | Granulocyte colony-stimulating factor responsiveness . | P . | ||
|---|---|---|---|---|
| Low, n = 43 . | Medium, n = 42 . | High, = 83 . | ||
| Fever and neutropenia, % | ||||
| Fever > 38°C | 84 | 62 | 51 | .0004 |
| Febrile neutropenia | 77 | 60 | 48 | .0037 |
| Documented infections, % | ||||
| Bacteremia | 12 | 17 | 11 | |
| Pneumonia | 7 | 12 | 1 | |
| Enterocolitis | 7 | 7 | 1 | |
| Fungal infection | 2 | – | – | |
| Viral infection | 5 | 5 | 1 | |
| Urinary tract infection | 2 | – | 2 | |
| Stomatitis | 2 | 2 | 1 | |
| Catheter infection | – | 2 | 1 | |
| Others | 5 | 5 | 6 | |
| Categories of infection, % | ||||
| Microbially defined (type A) | 16 | 29 | 16 | .73 |
| Clinically defined (type B) | 23 | 21 | 8 | .01 |
| Fever of unknown origin (type C) | 65 | 38 | 33 | .0013 |
| Primary composite end points, % | ||||
| Initial (type A + B) | 30 | 43 | 24 | .32 |
| Amended (type A + B + C) | 91 | 67 | 54 | < .0001 |
Mucositis
Muscositis was evaluated during daily clinical routine inspection of the patient by the use of National Cancer Institute Common Toxicity Criteria grading. Severe oral mucositis of grade 3 or 4 was also inversely correlated with G-Rsp, amounting to 67% in the low, 33% in the medium, and 23% in the high subgroup (P < .0001).
Treatment of infection and supportive care
The association of G-Rsp subgroups with medical treatment and supportive care is shown in Table 2. There was a highly significant difference in the duration of intravenous antibiotic therapy, which was a median of 9 days for the low, 6 days for the medium, and 5 days for the high G-Rsp subgroups (P < .0001). Although the use of intravenous antimycotic therapy was rare, significantly more days with antimycotics were noted in the low group (P = .042).
More platelet transfusions (P < .0001), a prolonged G-CSF treatment (≥ 10 days; P = .05), and the duration of hospitalization from time of transplantation (P = .0017) were correlated with a lower G-Rsp. The CD34+ cell dose was associated with the overall use of G-CSF (P = .0001) but not with a prolonged G-CSF use (≥ 10 days) or with other medical treatments.
Prognostic factors for infection in univariate and multivariate analyses
Besides the association of the rate of infections with G-Rsp, other factors of the prospectively defined covariates, such as the baseline leukocyte count (day −1), the number of previous cycles of chemotherapy, and the high-dose regimen used, also were significantly associated with infection (Table 4). The respective impact of the univariately defined (P < .1) prognostic factors was analyzed multivariately by the application of a logistic regression model with a step-by-step strategy for elimination of nonsignificant covariates. In this multivariate analysis, G-Rsp remained the only independent significant prognostic factor (Table 4). Only the cycles of previous chemotherapy seemed to account for some additional independent information. Remarkably, the highly unfavorable association of nonmelphalan high-dose chemotherapy is eliminated when simultaneously taking the G-Rsp test result into account.
Infection rates by prognostic factors in univariate and multivariate analysis
| Parameter/group . | Patients, n . | Patients with infection . | |||
|---|---|---|---|---|---|
| Univariate . | Multivariate . | ||||
| n . | % . | P . | P . | ||
| Baseline leukocytes, /μL | |||||
| < 4000 | 88 | 67 | 76 | .0086 | .61 |
| ≥ 4000 | 80 | 45 | 56 | ||
| Previous radiotherapy | |||||
| No | 109 | 73 | 67 | 1.0 | – |
| Yes | 59 | 39 | 66 | ||
| Previous cycles of chemotherapy | |||||
| ≤ 6 | 120 | 72 | 60 | .0053 | .16 |
| > 6 | 48 | 40 | 83 | ||
| CD34+ cells, ×106/kg | |||||
| 2.0-5.0 | 106 | 73 | 69 | .50 | – |
| > 5.0 | 62 | 39 | 63 | ||
| High-dose chemotherapy | |||||
| Melphalan | 104 | 60 | 58 | .0022 | .80 |
| BEAM and others | 64 | 52 | 81 | ||
| G-CSF responsiveness | |||||
| Low | 43 | 39 | 91 | < .0001 | .016 |
| Medium | 42 | 28 | 67 | ||
| High | 83 | 45 | 54 | ||
| Parameter/group . | Patients, n . | Patients with infection . | |||
|---|---|---|---|---|---|
| Univariate . | Multivariate . | ||||
| n . | % . | P . | P . | ||
| Baseline leukocytes, /μL | |||||
| < 4000 | 88 | 67 | 76 | .0086 | .61 |
| ≥ 4000 | 80 | 45 | 56 | ||
| Previous radiotherapy | |||||
| No | 109 | 73 | 67 | 1.0 | – |
| Yes | 59 | 39 | 66 | ||
| Previous cycles of chemotherapy | |||||
| ≤ 6 | 120 | 72 | 60 | .0053 | .16 |
| > 6 | 48 | 40 | 83 | ||
| CD34+ cells, ×106/kg | |||||
| 2.0-5.0 | 106 | 73 | 69 | .50 | – |
| > 5.0 | 62 | 39 | 63 | ||
| High-dose chemotherapy | |||||
| Melphalan | 104 | 60 | 58 | .0022 | .80 |
| BEAM and others | 64 | 52 | 81 | ||
| G-CSF responsiveness | |||||
| Low | 43 | 39 | 91 | < .0001 | .016 |
| Medium | 42 | 28 | 67 | ||
| High | 83 | 45 | 54 | ||
G-CSF indicates granulocyte colony-stimulating factor.
Fixed G-Rsp risk groups
When we examined the relationship between G-Rsp and the risk of infection beyond the quartile distribution, 3 risk groups also could be distinguished. A G-Rsp < 11 000/μL (low) was associated with a very high risk of infection (ratio infection: no infection above 3:1). For a G-Rsp between 11 000/μL and 20 000/μL (medium), the risk of infection was intermediate (ratio infection: no infection approximately 2:1). For values > 20 000/μL (high), the ratio between infection and no infection was approximately 1:1 and no further risk reduction was seen with further increasing G-Rsp. In comparison with the single-center experience, almost identical thresholds were observed.
Discussion
G-Rsp is associated with several parameters representing the risk of infection. We observed highly significant correlations between G-Rsp and the incidence and duration of febrile neutropenia as well as the duration of severe neutropenia. Our primary objective of this study was to analyze the association between G-Rsp and the rate of infection. For the categorization and quantification of infections, the definitions provided by the Immunocompromised Host Society were used, which distinguish microbially and clinically defined infection from fever of unknown origin.10 A correct categorization of the different types of infections was a major focus of the validation process in this study performed by the responsible contract research organization. All 3 different categories of infection were reduced when G-Rsp was high. Finally, the majority of serious infections, such as pneumonia, enterocolitis, and fungal infection, occurred in patients with a low and medium G-Rsp.
The primary end point infection rate had been planned as a composite end point. Because the original version (microbially plus clinically documented infection) was potentially impaired by a prominent center effect with a highly overproportional rate of positive blood cultures, an alternative version of the primary end point, including fever of unknown origin, was put forth in an amendment of the protocol before the data were entered into the database and before statistical analyses were performed. Because the classification of an infection as fever of unknown origin or documented infection is mutually exclusive, shifts in the relative proportions between fever of unknown origin and documented infection will not grossly change the overall rate of infection. This served as the rationale for trying to compensate this center effect. Unsurprisingly, the initially planned primary end point composition (documented infection) did not reach statistical significance in the association with any potential prognostic factors, including G-Rsp. With the alternative version of the primary end point (overall rate of infection), a meaningful picture emerged with correlations of the infection rate with several patient and treatment characteristics in univariate analyses.
The type of high-dose regimen was one of the prognostic factors for the occurrence of infection in univariate analysis. The intensive BEAM regimen lowered G-Rsp adjunct to chemotherapy compared with MEL-alone regimens. This was accompanied by an increase in the rate of infection after BEAM. Yet, more than 40% of BEAM patients retained a medium or high G-Rsp. This finding demonstrates that the type of high-dose regimen is a significant but not sufficient factor to determine G-Rsp. In multivariate analysis, the unfavorable association of complex high-dose chemotherapy with infection was virtually neutralized. G-Rsp remained the only significant parameter correlating with infection.
The risk of infection in patients receiving intensive chemotherapy is correlated with the duration and severity of neutropenia. This association had been found already some decades ago in patients with leukemia.14 The duration and severity of neutropenia in cancer patients depends on several factors, including the underlying disease, the intensity of chemotherapy, bone marrow reserve, the use of hematopoietic growth factors, and the CD34+ cell dose in case of transplantation. It is not possible to precisely foresee the neutrophil course over time after chemotherapy. Therefore, from a biometric point of view, the duration and severity of neutropenia is an outcome variable rather than a “predictor” of infection. In addition, the duration of neutropenia is not sufficient to describe the risk of infection for a patient because it does not account for the differences in the individual susceptibility. A greater G-Rsp, however, also influences the duration of neutropenia by resulting in a longer decline of leukocytes to nadir values. This could be one obvious reason for the prognostic importance of G-Rsp. In our single-center study,8 G-Rsp was found to be independent from and more informative than the duration of neutropenia in the prediction of infection. In a logistic regression model, the effect of G-Rsp in the multicenter study remained virtually unchanged, whereas the duration of neutropenia showed no independent significant impact. The results from both studies indicate that the role of G-Rsp as a predictor of infection cannot be reduced to the associated differences in neutropenia duration.
Following our single-center study, this multicenter trial prospectively confirmed the potential of testing G-Rsp for the occurrence of infection in the neutropenic patient. When we compared the single center with multicenter experience, 3 similar risk groups could be distinguished, independently from the quartile distribution, which was used as the basis of statistics. In the multicenter setting, variability between centers in identifying microbials in patients with fever can make a composite end point documented infection unreliable. The addition of fever of unknown origin to the composite end point appeared as a means to compensate this. Because the leukocyte threshold values for risk group separation were determined only when the study was over, the investigators, although unblinded to the results of G-Rsp, did not know to which risk group the patient belonged. Fever is the major trigger for many diagnostic and therapeutic measures in the neutropenic patient and is normally not influenced by subjectivity. Nevertheless, it cannot be excluded that subjectivity of individual investigators had some influence on outcome variables.
In contrast to G-Rsp, the CD34+ cell dose of stem cell transplants although correlated with neutrophil engraftment was not significantly associated with the incidence or duration of febrile neutropenia and the infection rate. Favorably, all patients in this study had received at least a standard dose of 2.0 × 106 CD34+ cells15 and differences in neutrophil engraftment were small.
G-CSF is the central mediator of host defense during neutropenia and infection.16,17 Under these “emergency” conditions, endogenous G-CSF levels increase quickly and excessively, demonstrating the physiologic importance of G-CSF in mobilizing cellular reserves. The first dose of G-CSF releases preformed marrow neutrophils into the blood. The resulting blood leukocyte peaks, which are composed of > 90% neutrophils, appear to represent the myeloid marrow reserve. In patients with a low G-Rsp, somewhat lower leukocyte counts were already present in the blood before G-Rsp was tested. This exhaustion already shortly after high-dose chemotherapy suggests a lower myeloid cell reserve in patients with a low G-Rsp. During later severe cytopenia, the variation of leukocyte release in different patients is no longer obvious and the leukocyte counts are uniformly very low. New insights into the factors influencing the migration and release of immature and mature myeloid cells from the bone marrow microenvironment to the blood have been gained. Stem cell mobilization is associated with a decrease in bone marrow stromal cell-derived factor 1 on G-CSF administration.18 A down-regulation of chemokine receptor CXCR-4 on myeloid cells was found in the bone marrow during G-CSF-induced mobilization to the blood.19 Furthermore, suppressor of cytokine signaling proteins can act as negative regulators of G-CSF signaling and influence G-Rsp of the host.20 The capacity of this hematopoietic network, its activation level, and functional disturbance because of disease and chemotherapy should be important for interindividual differences in G-Rsp.
Pharmacologic G-CSF reduced neutropenia and the risk of infection after myelosuppressive chemotherapy21,22 and after high-dose chemotherapy with autologous transplantation.23-25 In our study, G-CSF was restarted on day +5. Greater CD34+ cell doses were correlated with less applications of posttransplant G-CSF and balanced a low G-Rsp. A greater proportion of lymphoma patients was present in the low G-Rsp subgroup. Because there is usually no need to divide stem cell collections for tandem transplantation as in myeloma, lymphoma patients can receive greater CD34+ cell doses for their single transplantation. When we focused on patients with a prolonged use (≥ 10 days) of G-CSF, however, we found that this feature was correlated with a lower G-Rsp (P = .05) but not with the CD34+ cell dose. Current guidelines for G-CSF application favor a broad use after myelosuppressive or high-dose chemotherapy with stem cell transplantation but the individual requirements of a patient cannot be indicated.26,27
G-Rsp subgroups had clearly different requirements for medical treatment during the posttransplant phase. Patients with a lower G-Rsp needed more antimicrobial and antifungal therapy, more platelet transfusions, and stayed longer in the hospital. This finding is a confirmation of the clinical importance of G-Rsp and also has potential implications for the prospective management of patients.28,29 The patients with a high G-Rsp constitute the low-risk group in this trial, which included approximately two-thirds of myeloma patients receiving high-dose melphalan and approximately 15% of lymphoma patients after BEAM high-dose therapy. Strategies developed for low-risk patients in the past include outpatient management after autologous transplantation30,31 or oral antibiotic therapy for neutropenic fever.32,33 Any adaptation of supportive care determined by the concept of low risk must ensure safety for the patients.
The finding that G-Rsp predicted severe oral mucositis is of special interest. Oral mucositis is a major toxicity of high-dose chemotherapy that promotes infection because a disruption of the mucosal barrier leads to a greater penetration of microorganisms. With the recent availability of drugs against mucositis,34,35 a prediction of this complication could become beneficial for the patients.
A patient stratification as determined by G-Rsp could offer a greater chance for meaningful results for authors investigating the value of specific prophylaxis and treatment of infection and qualifies the setting of autologous transplantation for this. That the intensity of the high-dose regimen is accompanied by plausible changes in G-Rsp suggests that G-Rsp also will be a predictor of infection with other high-dose regimens than used in this study. Because G-Rsp appears to be determined by normal bone marrow functions, which could be impaired by the intensity of pretreatment or bone marrow infiltration, solid tumor patients also should be informative regarding G-Rsp testing. However, in diseases with impairment and reduction of normal stem cells and defects in differentiation and maturation such as in leukemia, this cannot be assumed without investigation.
In the setting of myelosuppressive chemotherapy, any first dose of G-CSF represents testing G-Rsp when a leukocyte count is performed on the next day. During cyclical chemotherapy, a test dose of G-CSF could even be applied before each chemotherapy cycle, potentially offering a new basis to decide on treatment delays or dose adjustments or the use of prophylactic G-CSF treatment. If maximum leukocyte responses are required to achieve an optimal prognostic power, then a greater-than-standard dose of G-CSF would be necessary under steady-state conditions before chemotherapy. A first G-CSF dose then could be the last for that cycle in patients with a low risk of infection. Alternatively, regular or intensified G-CSF regimens or special formulations of G-CSF could be used in high-risk patients. The anticipated lasting importance of applying G-CSF in cancer patients, the high costs of overuse, and even underuse when noneffective are arguments in favor of exploring this concept further.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C. Straka designed and performed research, analyzed data, and wrote the paper; M.S. performed research and analyzed data; H.S. performed research and analyzed data; H.W., B.M., K.H., G. Silling, M.H., D.F., R.S., M.F., O.S., A.G., G.E., W.G., A.E., G. Schlimok, and C. Scheid performed research; P.H. and H.H. designed research; H.E. designed research and analyzed data; A.H. designed research and analyzed data; and B.E. designed and performed research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian Straka, MD, PhD, Schön Klinik Starnberger See, Münchner Straβe 23-29, 82335 Berg, Germany; e-mail: cstraka@schoen-kliniken.de.