Abstract
The retrovirus, human T-cell–lymphotrophic virus-1 (HTLV-I) is the etiologic agent of adult T-cell leukemia (ATL) and the neurological disorder HTLV-I–associated myelopathy/tropical spastic paraparesis (HAM/TSP). The HTLV-I–encoded protein tax constitutively activates interleukin-2 (IL-2), IL-9, and IL-15 autocrine/paracrine systems that in turn activate the Jak3 (Janus kinase 3)/STAT5 (signal transducers and activators of transcription 5) pathway, suggesting a therapeutic strategy that involves targeting Jak3. We evaluated the action of the Jak3 inhibitor CP-690,550 on cytokine dependent ex vivo proliferation that is characteristic of peripheral blood mononuclear cells (PBMCs) from select patients with smoldering or chronic subtypes of ATL, or from those with HAM/TSP whose PBMCs are associated with autocrine/paracrine pathways that involve the production of IL-2, IL-9, IL-15, and their receptors. CP-690,550 at 50nM inhibited the 6-day ex vivo spontaneous proliferation of PBMCs from ATL and HAM/TSP patients by 67.1% and 86.4%, respectively. Furthermore, CP-690,550 inhibited STAT5 phosphorylation in isolated ATL T cells ex vivo. Finally, in an in vivo test of biological activity, CP-690,550 treatment of mice with a CD8 T-cell IL-15–transgenic leukemia that manifests an autocrine IL-15/IL-15Rα pathway prolonged the survival duration of these tumor-bearing mice. These studies support further evaluation of the Jak3 inhibitor CP-690,550 in the treatment of select patients with HTLV-I–associated ATL and HAM/TSP.
Introduction
Human T-cell–lymphotropic virus 1 (HTLV-I) is the etiological agent of adult T-cell leukemia/lymphoma (ATL), an aggressive malignancy of CD4+ CD25+ T lymphocytes and of the neurodegenerative disease HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP).1-5 The HTLV-I–encoded 40-kDa Tax protein is crucial for viral replication and malignant transformation.6,7 Activation of nuclear factor-κB (NF-κB) by Tax up-regulates the expression of a number of cytokines and their receptor genes, actions that are thought to play important roles in promoting the proliferation and survival of tumor cells in the early phases of ATL, and the proliferation of CD4 and CD8 T cells in patients with HAM/TSP.8-10 One such cytokine/cytokine receptor pair is interleukin-2 (IL-2) and its specific receptor subunit, IL-2Rα (CD25). IL-2Rα, along with IL-2/IL-15Rβ and the common γ chain (γc) receptor, form the heterotrimeric high-affinity IL-2 receptor.11-13
The autocrine IL-2/IL-2R loop is activated in infected T cells in early stages of ATL and in HAM/TSP.11 These observations provided the scientific basis for our use of anti-Tac, an antibody directed toward IL-2Rα that blocks its interaction with IL-2 in the treatment of patients with ATL.14,15 Responses were observed in 6 of 19 patients with ATL treated with anti-Tac.15 Subsequently, we demonstrated another Tax-induced autocrine stimulation loop that involves IL-15 and its private IL-15 receptor, IL-15Rα, and a paracrine stimulation loop that involves IL-9 expression by ATL cells and IL-9Rα expression on monocytes.8,9 The presence of two autocrine loops and one paracrine loop highlights the limitations for a therapeutic strategy that involves the use of a single monoclonal antibody directed toward the IL-2Rα receptor. Indeed, to inhibit ex vivo proliferation of ATL cells, we had to simultaneously add antibodies to IL-2, IL-9, and IL-15 or to their receptors.13 A potential alternative to this strategy was suggested by the observation that these 3 cytokines share the common γc cytokine receptor subunit and its associated signal transduction element Jak3 (Janus kinase 3).16-18 A corollary of sharing of this signal transduction element is that targeting of Jak3 might yield greater efficacy than could be achieved by antibody-mediated inhibition of a single cytokine system.
Jak3 is inducible in T, B, and myeloid cells but not in nonhematopoietic cells.16-18 In addition, Jak3 is activated by each of the cytokines that interact with the common γc except IL-4, but is not essential for signaling through other pathways. Furthermore, in humans and mice, a defect of Jak3 is associated with an immunodeficiency characterized by the natural killer− (NK−), B+, T− form of severe combined immune deficiency (SCID), but is not associated with disorders of nonimmune systems.19-22
Rational drug design at Pfizer Laboratories contributed to development of CP-690,550, an immunosuppressant that disrupts signaling by inhibiting Jak3 in nanomolar concentrations.23-27 CP-690,550 was first reported to have an approximately 20- and 100-fold lower potency, respectively, in inhibiting Jak2 and Jak1 relative to Jak3; however, subsequent reports suggested that the inhibitory potential of this drug for these 3 receptors is nearly equipotent. In 2 separate studies, CP-690,550 was effective at reducing the rejection of organ allografts in mice and in nonhuman primates.26,27 This Jak3 inhibitor is being evaluated in phase 2 clinical trials in patients undergoing renal allografts and phase 3 clinical trials in patients with autoimmune diseases. In the present study, CP-690,550 was utilized as an inhibitor of ex vivo proliferation of peripheral blood mononuclear cells (PBMCs) from patients with HTLV-I–associated ATL and HAM/TSP, in whom proliferation was inhibited by the addition of nanomolar concentrations of CP-690,550. These in vitro studies support the evaluation of CP-690,550 in an in vivo syngeneic CD8 T-cell autocrine IL-15–transgenic mouse leukemia model. CP-690,550 continuously administered by pumps to mice receiving the IL-15–transgenic CD8-leukemic cell line B1 intravenously prevented subsequent development of leukemia and significantly prolonged the survival of tumor-bearing animals. The encouraging results of these ex vivo and in vivo studies support a clinical trial involving CP-690,550 in the treatment of patients with HAM/TSP and those with smoldering and chronic forms of ATL that manifest constitutive Jak3/STAT5 (signal transducers and activators of transcription 5) activation and whose PBMC proliferation is inhibited by ex vivo addition of this Jak3 inhibitor.
Methods
Compound and patient materials
The small-molecule Jak3 inhibitor CP-690,550 was synthesized by Jian-kang Jiang and Craig Thomas.28 Stock solutions of 10mM CP-690,550 were made in dimethylsulfoxide and subsequently diluted in RPMI 1640 medium before use.
ATL patient samples were obtained from patients under the care of the Clinical Trials Team, Metabolism Branch, National Cancer Institute (NCI), and all samples from patients with HAM/TSP were obtained from the Neuroimmunology Branch, National Institute of Neurological Disorders and Stroke, the National Institutes of Health (NIH). This study protocol was approved by the institutional review boards of the NCI and the National Institute of Neurological Disorders and Stroke. Informed consent was obtained in accordance with the Declaration of Helsinki.
Proliferation assays
NK92 or 32Dβ cells were washed and cultured in media free of cytokines for 24 hours. Cells were seeded into 96-well plates at 100 μL/well of RPMI 1640 medium with and without cytokines or CP-690,550 and incubated at 37°C for 1 hour. Aliquots of 1.5 × 104 cells/100 μL/well of NK92 or of 3.5 × 104 cells/100 μL/well of 32Dβ were plated and incubated at 37°C, 5% CO2 in an incubator for 48 hours. After 42 hours of culture, cells were pulsed for 6 hours with 1 μCi of 3H-thymidine, harvested, and counted with a β-plate counter (Wallac-Perkin Elmer). The assay was performed in triplicate on 3 occasions.
Ex vivo cultures of PBMCs from ATL and HAM/TSP patients
PBMCs from patients with ATL and HAM/TSP were cultured ex vivo in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) for ATL patients or 10% human AB serum (Gemini Bio-Products) for HAM/TSP patients as described in Chen et al.13 On day 6 of the culture, supernatants were collected and stored at −80°C. IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kits according to manufacturer's instructions.
NK92-stimulation assays
NK92 cells were washed, cultured in RPMI 1640 with 10% FBS free of cytokines for 24 hours, resuspended at a concentration of 1.5 × 105/mL, seeded into 96-well plates at 100 μL/well with 6-day ex vivo culture supernatants of PBMCs from patients with ATL and HAM/TSP with and without antibodies against the cytokine IL-2, IL-9, or IL-15 or with CP-690,550, and incubated at 37°C for 1 hour. Aliquots of 1.5 × 104/100 μL/well cells were plated and incubated at 37°C, 5% CO2 for 48 hours. The cells were pulsed for 6 hours with 1 μCi of 3H-thymidine after 42 hours, and then cells were harvested and counted with a β-plate counter. Assays were performed in triplicate.
Isolation of T cells
PBMCs were separated by Ficoll-Hypaque density gradient centrifugation from heparinized blood of patients with ATL and from healthy donors. T cells were isolated using the Human Pan T-Cell–Isolation Kit II (Miltenyi Biotec) according to manufacturer's instructions.
Cell proliferation
Immunoblotting
Immunoblotting was performed on isolated T cells as described in Azimi et al.8 The blots were incubated with a rabbit monoclonal antibody to phosphor-STAT5, or a rabbit polyclonal antibody to STAT5 and β-actin (Cell Signaling Technology).
Therapeutic study of CP-690,550 in IL-15–transgenic CD8 T-cell leukemia–bearing mice
A human IL-15–transgenic mouse was generated in our laboratory.31 These mice develop a CD8 T-cell leukemia that manifests an autocrine IL-15/IL-15Rα stimulation loop that is required for leukemic cell viability and proliferation. An IL-15–transgenic CD8 T-cell leukemic cell line called B1 that produces a fatal leukemia when injected into syngenic mice was developed. The leukemia model was established by intravenous injection of 0.5 × 106 B1 cells into B6.SJL mice (Jackson ImmunoResearch Laboratories). For the therapeutic study, CP-690,550 was dissolved in polyethylene glycol 300 (PEG300; VWR Scientific Products) at 50 mg/mL, and continuously administered via a subcutaneous mini-osmotic pump (ALZET). Mini-osmotic pumps were implanted 2 days prior to or 1 day after tumor inoculation. The mini-osmotic pumps delivered vehicle or therapeutic levels of drug at a flow rate of 0.5μL/h for 14 days. The antibody M111, directed toward human IL-15, was used at 100 μg per injection by intraperitoneal injection once per week for 2 weeks. All animal experiments were approved by the animal care and use committee of the NCI.
Therapeutic study of CP-690,550 in MET-1 human ATL–bearing mice
The human ATL cell population MET-1 was established from the peripheral blood of a patient with acute ATL and was maintained by serial transfer in nonobese diabetic (NOD)/SCID mice (Jackson ImmunoResearch Laboratories) as described in Phillips et al32 (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Measurement of tumor growth
Measurements of serum concentrations of human IL-15 or mouse IL-15Rα (B1 leukemia) and sIL-2Rα or β2-microglobulin (β2μ; MET-l leukemia) were performed using an ELISA assay as indicated in manufacturer's kit inserts (R&D Systems).
Statistical analysis
Data were analyzed using the Student t test. P values < .05 were considered significant.
Results
CP-690,550 inhibited proliferation of a cytokine-dependent NK92 cell line mediated by cytokines that utilized the common γc Jak3 signaling pathway
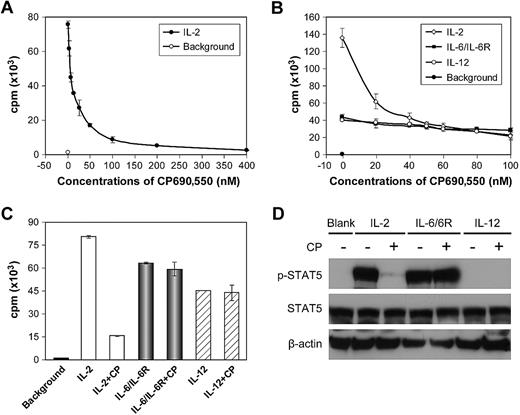
We evaluated the Jak3 inhibitor CP-690,550 in terms of its capacity to decrease IL-2–, IL-9–, and IL-15–mediated ex vivo proliferation of T cells from patients with ATL and those with HAM/TSP. Prior to these studies, to evaluate the specificity of inhibition mediated by CP-690,550, we performed a proliferation assay using a cytokine-dependent human NK cell line, NK92,33 which responded to cytokines whose signaling pathways involve the common γc and Jak3 (eg, IL-2, IL-9, and IL-15), and also to cytokines whose signaling pathways do not involve Jak3 (eg, IL-6/IL-6R and IL-12). After cytokine withdrawal for 24 hours, NK92 cells were stimulated with human IL-2 with and without increasing concentrations of CP-690,550 in culture for 48 hours, with 3H-thymidine added during the final 6 hours. At concentrations of 50 and 100nM, CP-690,550 effectively inhibited IL-2–stimulated NK92-cell proliferation by 78% and 89%, respectively (Figure 1A), but did not inhibit proliferation of NK92 cells that were stimulated with IL-6/IL-6R (500 ng/mL each) or IL-12 (500 pg/mL; Figure 1B-C). STAT5 was phosphorylated in NK92 cells and thus was activated in response to stimulation by IL-2 or IL-6/IL-6R, but not by IL-12. CP-690,550 inhibited IL-2–stimulated phosphorylation of STAT5 in NK92 cells, but did not inhibit STAT5 phosphorylation mediated by IL-6/IL-6R (Figure 1D).
CP-690,550 inhibited the proliferation of the cytokine-dependent NK92 cell line mediated by cytokines that signal through Jak3/STAT5 but not by cytokines that use other pathways. (A) After cytokine starvation for 24 hours, NK92 cells were stimulated by the addition of human IL-2 for 48 hours with and without the addition of serially increasing concentrations of CP-690,550. 3H-thymidine was added during the last 6 hours of the cultures. Cells were then harvested and analyzed for 3H-thymidine incorporation. (B) Cytokine-starved NK92 cells were stimulated with human IL-2, combined IL-6/IL-6R, or IL-12 for 48 hours with and without serially increasing concentrations of CP-690,550. (C) The NK92 cells were treated as those in panel B with and without a single 50nM dose of CP-690,550 (CP). Data are presented as means ± SD (A-C) and are representative of 3 independent experiments. (D) After cytokine starvation for 24 hours, NK92 cells were stimulated with 30 ng/mL of IL-2, 100 ng/mL of combined IL-6/IL-6R, or 100 ng/mL of IL-12 for 1 hour with and without the addition of CP-690,550 (CP). The cell lysates were immunoblotted with an anti–phospho-STAT5 monoclonal antibody and an anti-STAT5 antibody. β-Actin was used as an input control. Data are representative of 3 independent experiments.
CP-690,550 inhibited the proliferation of the cytokine-dependent NK92 cell line mediated by cytokines that signal through Jak3/STAT5 but not by cytokines that use other pathways. (A) After cytokine starvation for 24 hours, NK92 cells were stimulated by the addition of human IL-2 for 48 hours with and without the addition of serially increasing concentrations of CP-690,550. 3H-thymidine was added during the last 6 hours of the cultures. Cells were then harvested and analyzed for 3H-thymidine incorporation. (B) Cytokine-starved NK92 cells were stimulated with human IL-2, combined IL-6/IL-6R, or IL-12 for 48 hours with and without serially increasing concentrations of CP-690,550. (C) The NK92 cells were treated as those in panel B with and without a single 50nM dose of CP-690,550 (CP). Data are presented as means ± SD (A-C) and are representative of 3 independent experiments. (D) After cytokine starvation for 24 hours, NK92 cells were stimulated with 30 ng/mL of IL-2, 100 ng/mL of combined IL-6/IL-6R, or 100 ng/mL of IL-12 for 1 hour with and without the addition of CP-690,550 (CP). The cell lysates were immunoblotted with an anti–phospho-STAT5 monoclonal antibody and an anti-STAT5 antibody. β-Actin was used as an input control. Data are representative of 3 independent experiments.
CP-690,550 demonstrated a selective effect on cytokine-mediated proliferation of 32Dβ cells
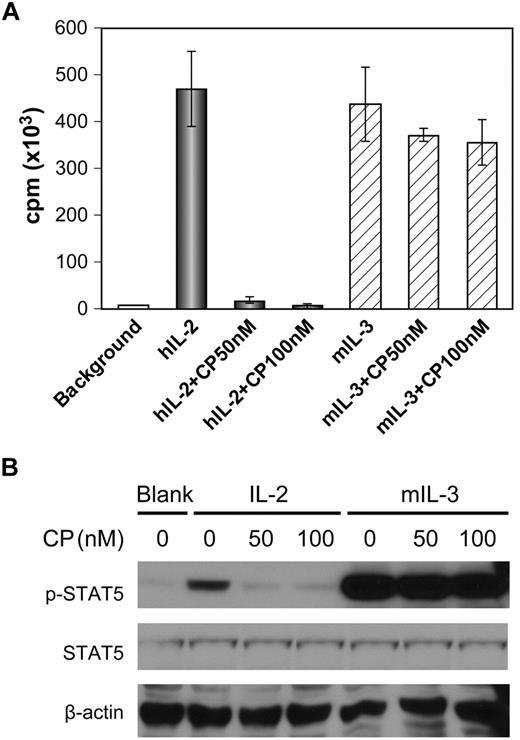
The 32Dβ myeloid precursor cell line generated by transfection of the 32D cell line with IL-2/IL-15Rβ (CD122) proliferates in response to both murine IL-3, which signals through Jak2, and to IL-2, which signals through Jak3.34 After cytokine starvation for 24 hours, 32Dβ cells were stimulated with either human IL-2 (10 ng/mL) or murine IL-3 (125 pg/mL) for 48 hours with and without the addition of CP-690,550. CP-690,550 at 50 and 100nM inhibited IL-2–mediated proliferation of 32Dβ cells by 96% and 98%, respectively, but had a lesser effect on IL-3–mediated proliferation, with inhibitions of 15% and 19%, respectively (Figure 2A). The addition of CP-690,550 inhibited IL-2–stimulated phosphorylation of STAT5, but did not inhibit IL-3–stimulated phosphorylation of STAT5 (Figure 2B).
Inhibition of cytokine-mediated proliferation of 32Dβ cells by CP-690,550 showed specificity for the γc/Jak3/STAT5-signaling pathway. (A) After cytokine starvation for 24 hours, 32Dβ cells were stimulated with human IL-2 or murine IL-3 for 48 hours with and without CP-690,550. 3H-thymidine was added during the last 6 hours of the cultures. Cells were then harvested and analyzed for 3H-thymidine incorporation. (B) After cytokine starvation for 24 hours, 32Dβ cells were stimulated with 1 μg/mL of human IL-2 or 10 ng/mL of murine IL-3 for 2 hours with and without the addition of CP-690,550. The cell lysates were immunoblotted with an anti–phospho-STAT5 monoclonal antibody and an anti-STAT5 antibody. β-Actin was used as an input control. Data are presented as means ± SD (A) and are representative of 3 independent experiments.
Inhibition of cytokine-mediated proliferation of 32Dβ cells by CP-690,550 showed specificity for the γc/Jak3/STAT5-signaling pathway. (A) After cytokine starvation for 24 hours, 32Dβ cells were stimulated with human IL-2 or murine IL-3 for 48 hours with and without CP-690,550. 3H-thymidine was added during the last 6 hours of the cultures. Cells were then harvested and analyzed for 3H-thymidine incorporation. (B) After cytokine starvation for 24 hours, 32Dβ cells were stimulated with 1 μg/mL of human IL-2 or 10 ng/mL of murine IL-3 for 2 hours with and without the addition of CP-690,550. The cell lysates were immunoblotted with an anti–phospho-STAT5 monoclonal antibody and an anti-STAT5 antibody. β-Actin was used as an input control. Data are presented as means ± SD (A) and are representative of 3 independent experiments.
CP-690, 500 inhibited 6-day ex vivo spontaneous proliferation of PBMCs from patients with smoldering and chronic ATL
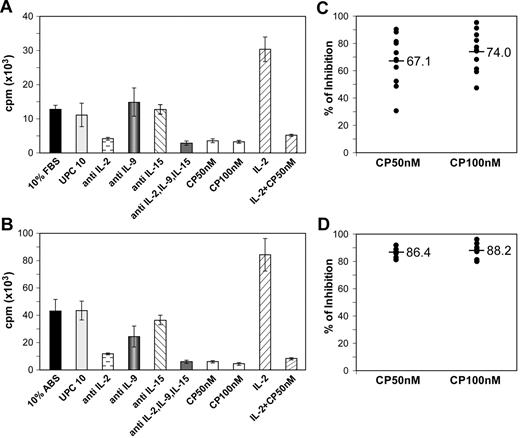
It has been noted that PBMCs from patients in the smoldering/chronic phases of ATL, who are in the IL-2–dependent autocrine phase of their disease, spontaneously proliferate ex vivo.9,13,35 The PBMCs from patients with HAM/TSP manifest similar spontaneous proliferation ex vivo.8,36,37 The spontaneous proliferation of ex vivo 6-day cultures assessed by 3H-thymidine uptake was determined for PBMCs from 12 patients with smoldering/chronic ATL who were in the IL-2–dependent phase of their disease, 11 patients with acute ATL who were in the IL-2–independent phase, and 16 healthy donors (normal controls). The ex vivo–cultured PBMCs from normal controls and from 9 of the 11 patients with acute ATL had a 3H-thymidine uptake of less than 5000 cpm/105 cells in culture. In contrast, mean 3H-thymidine uptake observed in 12 patients with smoldering/chronic ATL ranged from 10 × 103 cpm/105 cells to 46 × 103 cpm/105 cells (see supplemental Figure 1). Using CFSE labeling, we demonstrated that CD4+, CD3Low, CD25bright ATL leukemic cells were among the cells proliferating. Spontaneous proliferation of PBMCs from 12 patients with smoldering/chronic ATL, but not from 2 patients with acute ATL whose cells proliferated ex vivo, was partially inhibited by the addition of 10 μg/mL of individual antibodies that were specifically directed toward IL-2, IL-9, or, to a lesser extent, IL-15, whereas only a modest inhibition was observed upon addition of the nonspecific control antibody UPC10 (10 μg/mL). More profound inhibition of ex vivo proliferation was achieved by the simultaneous addition of antibodies directed against all 3 cytokines (5 μg/mL each; Figure 3A). The addition of CP-690,550 at both 50 and 100nM also inhibited 6-day ex vivo spontaneous proliferation of PBMCs of patients with smoldering/chronic ATL by 67.1% and 74%, respectively (Figure 3C). CP-690,550 also inhibited proliferation of the positive control for this study, ATL PBMCs, whose proliferation was significantly enhanced by the addition of exogenous IL-2 (Figure 3A).
CP-690,550 inhibited the 6-dayex vivospontaneous proliferation of PBMCs from patients with ATL and with HAM/TSP. The PBMCs from a patient with ATL (A) or a patient with HAM/TSP (B) were cultured ex vivo for 6 days with and without CP-690,550 or with 10 μg/mL of antibody when a single antibody directed against the cytokine IL-2, IL-9, or IL-15 was used or with their combination (5 μg/mL each). 3H-thymidine was added during the last 6 hours of the cultures. Cells were then harvested and analyzed for 3H-thymidine incorporation. The percentage of inhibition of proliferation with CP-690,550 addition was determined for 12 patients with ATL (C) or 9 patients with HAM/TSP (D). The value was calculated as: % inhibition = (cpm of proliferation without CP − cpm of proliferation with CP)/(cpm of proliferation without CP) × 100. Black bars indicate the mean inhibition percentage.
CP-690,550 inhibited the 6-dayex vivospontaneous proliferation of PBMCs from patients with ATL and with HAM/TSP. The PBMCs from a patient with ATL (A) or a patient with HAM/TSP (B) were cultured ex vivo for 6 days with and without CP-690,550 or with 10 μg/mL of antibody when a single antibody directed against the cytokine IL-2, IL-9, or IL-15 was used or with their combination (5 μg/mL each). 3H-thymidine was added during the last 6 hours of the cultures. Cells were then harvested and analyzed for 3H-thymidine incorporation. The percentage of inhibition of proliferation with CP-690,550 addition was determined for 12 patients with ATL (C) or 9 patients with HAM/TSP (D). The value was calculated as: % inhibition = (cpm of proliferation without CP − cpm of proliferation with CP)/(cpm of proliferation without CP) × 100. Black bars indicate the mean inhibition percentage.
CP-690,550 inhibited 6-day ex vivo spontaneous proliferation of PBMCs from patients with HAM/TSP
The spontaneous proliferation observed with PBMCs from 9 patients with HAM/TSP was partially inhibited by the addition of single antibodies to IL-2, IL-9, or IL-15, and was more extensively inhibited by the addition of a combination of these 3 antibodies (Figure 3B). Furthermore, CP-690,550 at both 50 and 100nM inhibited the 6-day ex vivo spontaneous proliferation of HAM/TSP PBMCs by 86.4% and 88.2%, respectively (Figure 3D).
CP-690,550 inhibited 6-day ATL culture supernatant–mediated NK92-cell proliferation
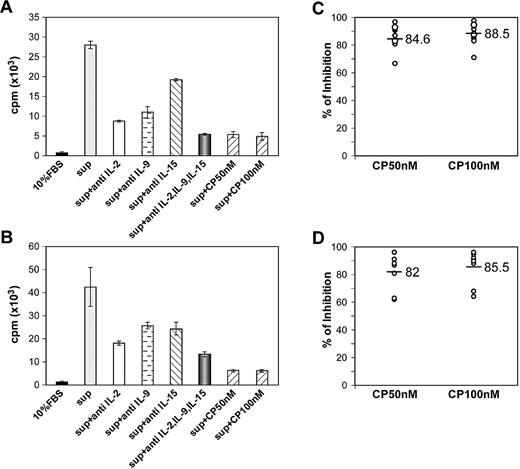
The results of assays on the supernatants from 6-day ex vivo cultures of PBMCs for levels of IL-2, IL-9, and IL-15 using ELISA kits are shown in supplemental Figure 2.
The 6-day ex vivo spontaneous proliferation of ATL and HAM/TSP PBMCs was associated with secretion into culture media of IL-2, IL-9, and modest quantities of IL-15 (supplemental Figure 2). To further characterize this finding, we performed proliferation assays using the NK92 cell line. After 24 hours of cytokine starvation of NK92 cells, 6-day ex vivo culture supernatants of ATL PBMCs were added to NK92 cells and their proliferation was assessed by 3H-thymidine incorporation. The supernatants from cells of patients with smoldering/chronic ATL stimulated the proliferation of NK92 cells, whereas supernatants from the PBMCs of normal controls and patients with acute ATL did not. The 6-day ATL culture supernatant stimulated NK92-cell proliferation that was partially inhibited by the addition of antibodies to IL-2, IL-9, or IL-15 individually and to a greater extent by the addition of a combination of all 3 antibodies (Figure 4A). CP-690,550 at 50 and 100nM inhibited the ATL culture supernatant–mediated proliferation of NK92 cells by a mean of 84.6% and 88.5%, respectively (Figure 4C).
CP-690,550 inhibited ATL and HAM/TSP PBMC 6-day culture supernatant–mediated NK92-cell proliferation. After cytokine starvation for 24 hours, NK92 cells were stimulated with a 6-day ex vivo culture supernatant of PBMCs from an ATL patient (A) or a HAM/TSP patient (B) for 48 hours. Antibodies directed against the cytokines IL-2, IL-9, or IL-15 or CP-690,550 were added to the plates 1 hour prior to cell seeding. 3H-thymidine was added during the last 6 hours of the cultures. Cells were then harvested and analyzed for 3H-thymidine incorporation. The percentage of inhibition of proliferation with CP-690,550 was determined for 11 ATL culture supernatants (C) or 8 HAM/TSP culture supernatants (D). Black bars indicate the mean percentage of inhibition.
CP-690,550 inhibited ATL and HAM/TSP PBMC 6-day culture supernatant–mediated NK92-cell proliferation. After cytokine starvation for 24 hours, NK92 cells were stimulated with a 6-day ex vivo culture supernatant of PBMCs from an ATL patient (A) or a HAM/TSP patient (B) for 48 hours. Antibodies directed against the cytokines IL-2, IL-9, or IL-15 or CP-690,550 were added to the plates 1 hour prior to cell seeding. 3H-thymidine was added during the last 6 hours of the cultures. Cells were then harvested and analyzed for 3H-thymidine incorporation. The percentage of inhibition of proliferation with CP-690,550 was determined for 11 ATL culture supernatants (C) or 8 HAM/TSP culture supernatants (D). Black bars indicate the mean percentage of inhibition.
CP-690,550 inhibited HAM/TSP culture supernatant–mediated NK92-cell proliferation
The 6-day HAM/TSP PBMC culture supernatant–stimulated proliferation of NK92 cells was also partially inhibited by the addition of individual antibodies to IL-2, IL-9, or IL-15, but was more extensively inhibited by the simultaneous addition of all 3 antibodies (Figure 4B). The addition of CP-690,550 at 50 and 100nM inhibited culture supernatant–mediated proliferation of NK92 cells by 82% and 85.5%, respectively (Figure 4D).
CP-690,550 inhibited activation of the Jak3/STAT5-signaling pathway in T cells from patients with ATL
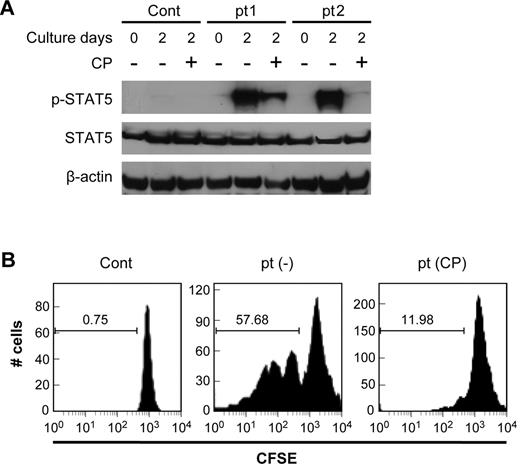
To determine the impact of the addition of CP-690,550 on the activation status of the Jak3/STAT5-signaling pathway in patient's cells, PBMCs were obtained from 10 patients with ATL, including 7 with smoldering/chronic ATL and 3 with acute ATL. T cells were immediately isolated from one aliquot of the PBMCs, and a second aliquot of PBMCs was cultured in RPMI 1640 supplemented with 10% FBS ex vivo for 2 days with and without the addition of CP-690,550. T cells were also isolated from these PBMCs (including those that had been cultured for 2 days), and cell lysates were analyzed for STAT5 phosphorylation by Western blotting. No phosphorylation of STAT5 was detected in T cells from normal controls or from patients with acute ATL at either day 0 or day 2. This lack of phosphorylation of STAT5 was also observed with T cells from patients with smoldering/chronic ATL when assessed at day 0. However, STAT5 was phosphorylated in T cells that had been cultured as part of PBMCs for 2 days ex vivo prior to T-cell isolation. Such phosphorylation was partially or completely inhibited by CP-690,550 addition at the initiation of culture (Figure 5A).
CP-690,550 inhibited the activation of the Jak3/STAT5 pathway present in patients ATL cells. (A) CP-690,550 inhibited the phosphorylation status of STAT5 in isolated T cells from patients with ATL. The T cells from a patient with ATL and those from a normal control were immediately isolated from the freshly separated PBMCs and lysed with cell lysis buffer; alternatively, the T cells were isolated from PBMCs cultured for 2 days with and without 50nM CP-690,550. Cell lysates were immunoblotted with anti–phospho-STAT5 monoclonal antibody and anti–STAT5 antibody. β-actin was used as an input control. (B) CFSE staining of CD3LowCD25+ lymphocytes was used to monitor cell division. ATL PBMCs were labeled with CFSE at day 0, and then cultured with (CP) or without (−) CP-690,550 for 6 days. FACS analysis of CD3LowCD25+CFSE triple-positive ATL cells were performed on day 6. Normal donor PBMCs (Cont) were used as a control. Because normal PBMCs proliferated under stimulation of anti-CD3/CD28 and ATL PBMCs spontaneously proliferated regardless of such stimulation, the nonstimulated normal and ATL PBMCs were chosen for analysis.
CP-690,550 inhibited the activation of the Jak3/STAT5 pathway present in patients ATL cells. (A) CP-690,550 inhibited the phosphorylation status of STAT5 in isolated T cells from patients with ATL. The T cells from a patient with ATL and those from a normal control were immediately isolated from the freshly separated PBMCs and lysed with cell lysis buffer; alternatively, the T cells were isolated from PBMCs cultured for 2 days with and without 50nM CP-690,550. Cell lysates were immunoblotted with anti–phospho-STAT5 monoclonal antibody and anti–STAT5 antibody. β-actin was used as an input control. (B) CFSE staining of CD3LowCD25+ lymphocytes was used to monitor cell division. ATL PBMCs were labeled with CFSE at day 0, and then cultured with (CP) or without (−) CP-690,550 for 6 days. FACS analysis of CD3LowCD25+CFSE triple-positive ATL cells were performed on day 6. Normal donor PBMCs (Cont) were used as a control. Because normal PBMCs proliferated under stimulation of anti-CD3/CD28 and ATL PBMCs spontaneously proliferated regardless of such stimulation, the nonstimulated normal and ATL PBMCs were chosen for analysis.
CP-690,550 inhibited proliferation of ATL cells
PBMCs were isolated from the blood of patients with ATL and from normal controls, stained with CFSE dye, resuspended in RPMI 1640 medium, and cultured with and without precoated anti-CD3 (10 μg/mL) and anti-CD28 (10 μg/mL) for 6 days in the presence or absence of CP-690,550. Upon anti-CD3/CD28 stimulation, normal PBMCs became activated and proliferated, whereas ATL PBMCs, in particular the leukemic population of CD3LowCD25+ cells, were spontaneously activated and proliferated regardless of the stimulation mediated by anti-CD3 and anti-CD28. Upon addition of CP-690,550, the proliferation of the leukemic cells was inhibited (Figure 5B).
CP-690,550 prolonged the survival of mice bearing CD8 IL-15/IL-15Rα autocrine leukemia derived from IL-15–transgenic mice in an in vivo therapeutic study
The studies discussed in “Results” involved analysis of the effects of CP-690,550 on ex vivo proliferation of PBMCs from patients with ATL or HAM/TSP. An additional evaluation of this agent was carried out to define its in vivo effect in an IL-15 autocrine CD8 T cell–leukemia model. Human IL-15–transgenic mice that were established by our group31 consistently manifested a late onset of a CD8 T-cell leukemia that expressed an autocrine IL-15/IL-15R loop that was required for leukemic cell survival. B1, a cell line derived by ex vivo cell culture of leukemic cells of these IL-15–transgenic mice, also manifested the autocrine IL-15/IL-15Rα loop with an associated constitutive activation of the Jak3/STAT5 pathway. This B1-leukemic cell line, when injected intravenously into syngeneic mice, yielded a leukemia that was lethal in approximately 2 to 3 weeks. The B1-leukemic cells in vitro and in vivo showed constitutive activation of Jak3/STAT5, as demonstrated by immunoblotting. The ex vivo proliferation of IL-15–transgenic CD8 T-cell–leukemic B1 cells could be inhibited by the addition of M111, an antibody directed to human IL-15. The addition of CP-690,550 at 50nM also inhibited the in vitro proliferation of cultured B1-leukemic T cells.
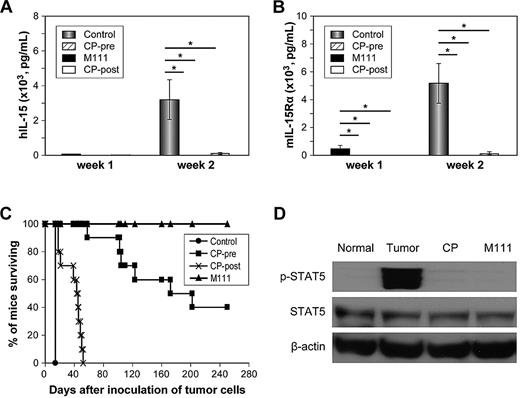
The therapeutic efficacy of CP-690,550 was then evaluated in vivo in B6.SJL mice that had received B1 T cell–leukemic cells intravenously. There were 5 groups of 10 mice each in the therapeutic trial: groups 1 through 4 received an intravenous inoculation of 5 × 105 B1 T-leukemic cells. Group 1 (CP-690,550-pre group) received implanted 14-day mini-osmotic pumps containing CP-690,550 2 days prior to tumor inoculation. The CP-690,550 in PEG300 was administered at a continuous rate of 30 mg/kg/d, yielding therapeutic levels of drug at the time of tumor inoculation. There was an 80% decline in the number of circulating NK cells, confirming that effective inhibition of in vivo IL-15 signaling had been achieved (data not shown). Group 2 (CP-690,550-post group) were implanted with mini-pumps containing CP-690,550 1 day after tumor inoculation. In this group, therapeutic levels of drug were only obtained at 3 days following tumor inoculation. Group 3 (M111 group) received M111 at 100 μg intraperitoneally beginning on day 3 following tumor inoculation and then once per week for 2 weeks. Group 4 (control group) received the vehicle PEG300 by mini-osmotic pump infusion for 14 days. Group 5 received no tumor and no therapy, and served as a control. Serum concentrations of human IL-15 and mouse IL-15Rα were assessed weekly after inoculation of leukemic cells as surrogate markers of tumor load.
Two weeks following inoculation of leukemic cells, the serum concentration of human IL-15 of the control group had increased significantly to a mean of 3196 ± 1137 pg/mL, whereas the IL-15 concentrations of the CP-690,550-pre group and CP-690,550-post group were 5 ± 10.3 pg/mL (P < .0001) and 71.4 ± 41.5 pg/mL (P < .0001), respectively (Figure 6A). In parallel, mean levels of murine IL-15Rα at 2 weeks following inoculation of tumor cells in the control group had risen to 5181 ± 1439 pg/mL, whereas those of the CP-690,550-post group increased to 93.2 ± 168 pg/mL (P < .0001), and that of the CP-690,550-pre group remained below 78 pg/mL, the minimum level of detection (Figure 6B).
CP-690,550 showed therapeutic efficacy in a mouse model of B1 IL-15–transgenic CD8 T-cell leukemia that manifested an autocrine IL-15/IL-15Rα loop that acts through Jak3/STAT5. (A) Serum levels of human IL-15 from the human IL-15–transgenic CD8 T-cell leukemia–bearing mice after inoculation of tumor cells. (B) Serum levels of mouse IL-15Rα from the IL-15–transgenic CD8 T-cell leukemia–bearing mice after inoculation of tumor cells. (C) Kaplan-Meier analysis demonstrating CP-690,550 prolongation of the survival of mice bearing the IL-15–transgenic CD8 T-cell leukemia. Mice in the control group received PEG300 by subcutaneous pump for 14 days. Mice in the CP-690,550-pre group (CP-pre) received a pump infusion with CP-690,550 at 30 mg/kg/d for 14 days that was placed into the mice 2 days before tumor cell inoculation. Mice in the CP-690,550-post group (CP-post) received a pump infusion with CP-690,550 at 30 mg/kg/d for 14 days that was placed into the mice 1 day after inoculation of the leukemic cells. Mice in the anti–human IL-15 monoclonal antibody M111 group received M111 at 100 μg intraperitoneally weekly for 2 weeks starting at 3 days after inoculation of the leukemic cells. (D) CP-690,550 inhibited the phosphorylation status of Jak3/STAT5 in splenocytes from IL-15–transgenic CD8 T-cell leukemia–bearing mice. Mice in the tumor-only group received PEG300 by a subcutaneous pump for 14 days. Mice in the CP-690,550 group (CP) received a pump infusion with CP-690,550 at 30 mg/kg/d for 14 days that was placed into the mice 1 day after inoculation of the leukemic cells. Mice in the M111 group received M111 at 100 μg intraperitoneally weekly for 2 weeks starting at 3 days after inoculation of the leukemic cells. Mice in the normal group (no tumor, no treatment) were used as a control. The splenic cells were separated 13 days after tumor cell inoculation and checked for the phosphorylation status of STAT5. *P < .00001.
CP-690,550 showed therapeutic efficacy in a mouse model of B1 IL-15–transgenic CD8 T-cell leukemia that manifested an autocrine IL-15/IL-15Rα loop that acts through Jak3/STAT5. (A) Serum levels of human IL-15 from the human IL-15–transgenic CD8 T-cell leukemia–bearing mice after inoculation of tumor cells. (B) Serum levels of mouse IL-15Rα from the IL-15–transgenic CD8 T-cell leukemia–bearing mice after inoculation of tumor cells. (C) Kaplan-Meier analysis demonstrating CP-690,550 prolongation of the survival of mice bearing the IL-15–transgenic CD8 T-cell leukemia. Mice in the control group received PEG300 by subcutaneous pump for 14 days. Mice in the CP-690,550-pre group (CP-pre) received a pump infusion with CP-690,550 at 30 mg/kg/d for 14 days that was placed into the mice 2 days before tumor cell inoculation. Mice in the CP-690,550-post group (CP-post) received a pump infusion with CP-690,550 at 30 mg/kg/d for 14 days that was placed into the mice 1 day after inoculation of the leukemic cells. Mice in the anti–human IL-15 monoclonal antibody M111 group received M111 at 100 μg intraperitoneally weekly for 2 weeks starting at 3 days after inoculation of the leukemic cells. (D) CP-690,550 inhibited the phosphorylation status of Jak3/STAT5 in splenocytes from IL-15–transgenic CD8 T-cell leukemia–bearing mice. Mice in the tumor-only group received PEG300 by a subcutaneous pump for 14 days. Mice in the CP-690,550 group (CP) received a pump infusion with CP-690,550 at 30 mg/kg/d for 14 days that was placed into the mice 1 day after inoculation of the leukemic cells. Mice in the M111 group received M111 at 100 μg intraperitoneally weekly for 2 weeks starting at 3 days after inoculation of the leukemic cells. Mice in the normal group (no tumor, no treatment) were used as a control. The splenic cells were separated 13 days after tumor cell inoculation and checked for the phosphorylation status of STAT5. *P < .00001.
All mice in the control group died by day 14 after inoculation of B1 cells, whereas all mice of the CP-690,550-post group died by day 52 and had a median survival duration of 44 days (P < .0001). Among the mice in the pre-transplant CP-690,550 implantation group, 6 mice died between days 58 and 202, whereas the remaining 4 mice survived for greater than 250 days after tumor inoculation, at which time we terminated the experiment (P < .00001). All mice that received M111 survived until the termination of the experiment at 250 days (P < .0001; Figure 6C). These results support the view that treatment of mice receiving the B1 IL-15–transgenic CD8 T-cell leukemia with CP-690,550 that was initiated after tumor inoculation prolonged the survival duration but did not cure leukemia-bearing mice. Treatment of B1 leukemia–bearing mice with CP-690,550 starting just prior to tumor inoculation yielded partial or complete remissions and a prolongation of life.
The activation status of Jak3/STAT5 was determined in the B1 leukemia–bearing mice. Compared with the spleens of normal controls, spleens from B1 leukemia–bearing mice had a marked activation/phosphorylation of STAT5. This STAT5 phosphorylation status was inhibited by the administration of CP-690,550 (Figure 6D).
CP-690,550 did not prolong the survival of MET-1 acute human ATL–bearing mice
To assess the specificity of the therapeutic effects of in vivo CP-690,550, we employed this agent in a therapeutic trial in NOD/SCID mice bearing the MET-1 acute human ATL.32 Human MET-1 cells do not exhibit autocrine γc cytokine stimulation or Jak3/STAT5 phosphorylation. In the MET-1 model of human acute ATL, a 14-day treatment course with CP-690,550 did not demonstrate any therapeutic efficacy, as assessed either by its effects on serum levels of surrogate markers of tumor persistence or by defining the survival of tumor-bearing mice (supplemental Figure 3).
The results of these studies support the view that the efficacy manifested by treatment with CP-690,550 in the IL-15 autocrine leukemia mouse model is not a nonspecific antitumor effect, but rather reflects specific inhibition by CP-690,550 of the Jak3/STAT5 pathway.
Discussion
Currently, there is no uniformly effective therapy for patients with HAM/TSP or HTLV-I–associated ATL. A number of agents, such as corticosteroids, immunosuppressive drugs, cytotoxic agents, and antiretroviral agents, have been evaluated for the treatment of patients with HAM/TSP without clear benefit. In our studies directed at targeting the IL-2Rα subunit, the humanized anti-CD25 antibody daclizumab (Zenapax; Roche) was found to inhibit ex vivo spontaneous lymphoproliferation, reduce the number of circulating activated IL-2Rα–expressing CD4+ lymphocytes, and reduce HTLV-I proviral loads, but did not result in reduction of CD8+-cytotoxic lymphocytes in patients with HAM/TSP.38
There is also no standard therapy for the majority of patients with ATL. Because of these generally poor therapeutic results, there have been several experimental studies of interferons combined with zidovudine (AZT) with and without arsenic.39,40 Partial and complete remissions were observed, largely in smoldering and chronic subtypes of ATL. Little long-term benefit was seen in a recent phase 3 trial comparing an intensive 9-drug combination chemotherapy regimen with dose-intensive CHOP (cyclophosphamide, hydroxydaunorubicin [doxorubicin], oncovin [vincristine], and prednisone/prednisolone) chemotherapy.41 Other studies have suggested that allogeneic hematopoietic stem cell transplantation may be of value in selected patients.42
In a clinical trial, we evaluated treatment with daclizumab, a humanized monoclonal antibody directed against IL-2Rα, in 20 patients with ATL and observed 4 partial responses in 12 patients with the smoldering or the chronic subtypes of ATL.15 A major impediment to the success of this approach has been that daclizumab only blocks IL-2/IL-2 receptor Jak3–pathway activation, but has no effect on the IL-9 and IL-15 paracrine and autocrine pathways that activate Jak3.
Given the limitations in treatment for patients with HAM/TSP and ATL, newer therapeutic strategies are required. A number of studies have suggested Jak3 as an important molecular target in these diseases because it is a final common pathway in signaling for a number of growth-stimulating cytokines for T cells. Constitutive activation of Jak3 was demonstrated in the HTLV-I–transformed cell lines Hut102B2 and MT-2.21,43 In addition, we demonstrated that the proliferation of peripheral blood ATL cells was associated with activation of Jak3/STAT proteins.35 Cell extracts of uncultured leukemic cells from 8 of 12 ATL patients displayed constitutive DNA-binding activity with one or more STAT proteins. Furthermore, immunoblotting with antibodies directed against Jak3 demonstrated the presence of phosphorylated Jak3 in extracts of each of 3 patients with ATL studied.
We focused on the Jak3 inhibitor CP-690,550, 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile, a potent and selective Jak3 inhibitor reported by Paul S. Changelian et al as an orally active immuno-suppressant.23 The original report provided evidence that CP-690,550 inhibited Jak3 at a half-maximal inhibitory concentration (IC50) of 1nM, while inhibiting Jak2, Jak1, Rock-II, and Lck with IC50 values of 20, 112, 400, and 3870nM, respectively. When this Jak3 inhibitor was administered to cynomolgus monkeys by chronic oral dosing, there was a selective reduction of NK and CD8 T-cell numbers—a pattern reminiscent of that observed with a genetic depletion of IL-15.26,27 CP-690,550 has also shown efficacy in treating allograft rejection in a murine model of heterotopic heart transplantation and for rejection prophylaxis of kidney allografts in nonhuman primates.26,27
In the present study, CP-690,550 at 50nM inhibited the abnormal ex vivo proliferation of PBMCs of patients with HAM/TSP to a comparable extent (mean 86.4%) as that achieved by the simultaneous combined addition of antibodies to IL-2, IL-9, and IL-15 or to their corresponding receptors, and inhibited the stimulation of NK92 cells from culture supernatants from the PBMCs of these patients. Previously we demonstrated that ex vivo proliferation of T cells cultured with PBMCs of patients with HAM/TSP was inhibited by the addition of antibodies to IL-2Rα and IL-15 and the shared IL-2/IL-15Rβ subunit (CD122).9,13 Furthermore, we demonstrated that interruption of IL-15 action by the addition of antibodies to IL-15 or to IL-2/IL-15Rβ in HLA-A201-positive patients led to a reduction in the number of antigen-specific (amino acids 11-19 Tax peptide) CD8 T cells that may play a pathogenic role in this disease.44
Clinically, ATL can be divided into smoldering and chronic lymphoma and acute subtypes that have different molecular signatures and outcomes.45 We demonstrated that smoldering/chronic ATL PBMCs spontaneously proliferated ex vivo in a cytokine (IL-2/IL-9/IL-15)–dependent manner.13 In contrast, PBMCs from patients with acute ATL either did not proliferate or proliferated independently of any cytokine stimulation. The simultaneous addition of antibodies to IL-2, IL-9, and IL-15 inhibited the spontaneous proliferation manifested by smoldering/chronic ATL PBMCs. Proliferation of the PBMCs from these patients was also inhibited by CP-690,550. Furthermore, proliferation of the cytokine-dependent NK92 cell line stimulated by 6-day culture supernatants of PBMCs from patients with ATL was markedly inhibited. In contrast to these observations with ex vivo PBMCs from patients with chronic/smoldering ATL, in whom leukemic cells were still in the IL-2–, IL-9–, IL-15–dependent phase, CP-690,550 did not inhibit ex vivo proliferation of the HTLV-I–associated acute ATL cell lines Hut102B2 and MT-2. Although these cell lines manifest STAT5 activation, they proliferate without the addition of cytokines and are not inhibited by the addition of anti-cytokine antibodies. These leukemic cell lines represent cells from the acute IL-2–independent phase of ATL. Similarly, Kirken et al46 demonstrated that the Jak3 inhibitor AG490 did not inhibit proliferation of ATL Hut120B2 and MT-2 cell lines. Our data, together with those of Kirken, suggest that the Jak3/STAT5 pathway in HTLV-I–transformed T cells in the late IL-2–independent phase of ATL has become functionally redundant for proliferation, and that the inhibition of Jak3 may not provide effective therapy in the cytokine-independent phase of acute ATL.
In addition, we evaluated CP-690,550 in a syngeneic autocrine IL-15–dependent mouse CD8 T-cell–leukemia model. IL-15–transgenic mice developed an NK or CD8 T-cell leukemia late in life.47 These mice manifested murine IL-15Rα expression on their leukemic cells, yielding an autocrine IL-15/IL-15R growth loop that was associated with constitutive activation of the Jak3/STAT5 pathway. B1, a spontaneously arising CD8 IL-15 leukemic T-cell line derived from these mice, resulted in a rapidly lethal leukemia when injected into syngeneic mice. Therapy of mice that received the B1 cell line with CP-690,550 prevented the development of leukemia in many of recipient mice and reduced the activation status of the Jak3/STAT5 pathway. In contrast to this efficacy in mice receiving the B1 IL-15 autocrine leukemia model, CP-690,550 did not prolong the survival of mice bearing HTLV-I–associated human MET-1 ATL—an acute type of ATL that does not produce IL-2 and does not exhibit activation of Jak3/STAT5. Thus, our in vitro and in vivo studies suggest that CP-690,550 will likely be active in T-cell malignancies and autoimmune diseases that have T-cell autocrine/paracrine γc cytokine stimulation associated with activation of the Jak3/STAT5 signaling pathway.
Our results support the clinical development of the Jak3 inhibitor CP-690,550 for patients with HTLV-I–associated HAM/TSP. In addition, we suggest that this Jak3 inhibitor might serve as an effective therapeutic agent in patients with chronic and smoldering ATL whose cells produce and require stimulation by γc cytokines for ex vivo spontaneous proliferation and whose T cells manifest constitutive Jak3/STAT5 activation. Furthermore, patients with ATL that may benefit from this therapy could be selected for inclusion into clinical trials if proliferation of their ex vivo PBMCs were inhibited by the addition of nanomolar quantities of CP-690,550.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Sigrid Dubois for technical help with the CFSE staining.
This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, NIH.
National Institutes of Health
Authorship
Contribution: W.J. designed and performed the research, analyzed the data, and wrote the manuscript; M.Z. participated in the design of the research and performed experiments; J.-k.J. and C.J.T. synthesized and provided CP-690,550; U.O. and S.J. provided PBMCs of HAM/TSP patients under their care; B.R.B. and J.C. performed experiments; N.S. and Y.T. provided the IL-15–transgenic B1 cells and performed experiments; J.C.M. and J.E.J. provided blood samples of ATL patients under their care; and T.A.W. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas A. Waldmann, Metabolism Branch, NCI, NIH, Bldg 10, Rm 4N115, 10 Center Dr, Bethesda, MD 20892-1374; e-mail: tawald@helix.nih.gov.