Abstract
Prolyl-4-hydroxylation is necessary for proper structural assembly of collagens and oxygen-dependent protein stability of hypoxia-inducible transcription factors (HIFs). In vitro function of HIF prolyl-4-hydroxylase domain (PHD) enzymes requires oxygen and 2-oxoglutarate as cosubstrates with iron(II) and vitamin C serving as cofactors. Although vitamin C deficiency is known to cause the collagen-disassembly disease scurvy, it is unclear whether cellular oxygen sensing is similarly affected. Here, we report that vitamin C–deprived Gulo−/− knockout mice show normal HIF-dependent gene expression. The systemic response of Gulo−/− animals to inspiratory hypoxia, as measured by plasma erythropoietin levels, was similar to that of animals supplemented with vitamin C. Hypoxic HIF induction was also essentially normal under serum- and vitamin C–free cell-culture conditions, suggesting that vitamin C is not required for oxygen sensing in vivo. Glutathione was found to fully substitute for vitamin C requirement of all 3 PHD isoforms in vitro. Consistently, glutathione also reduced HIF-1α protein levels, transactivation activity, and endogenous target gene expression in cells exposed to CoCl2. A Cys201Ser mutation in PHD2 increased basal hydroxylation rates and conferred resistance to oxidative damage in vitro, suggesting that this surface-accessible PHD2 cysteine residue is a target of antioxidative protection by vitamin C and glutathione.
Introduction
Oxygen is essential for several physiologic processes, particularly for cellular respiration and energy metabolism. On the molecular level, response to hypoxia is mediated by hypoxia-inducible transcription factors (HIFs). Under continuous oxygen supply, 2 distinct prolyl residues within the oxygen-dependent degradation domain of HIFα subunits are hydroxylated by prolyl-4-hydroxylase domain–containing enzymes (PHDs). Hydroxy-HIFα is recognized by the von Hippel–Lindau tumor suppressor protein (pVHL) and subsequently targeted for proteasomal degradation.1,2 When oxygen is limited, PHD activity ceases, and nonhydroxylated HIFα is stabilized and heterodimerizes with the HIFβ subunit to activate expression of numerous target genes.3 Moreover, an asparaginyl hydroxylase termed factor-inhibiting HIF hydroxylates a C-terminal Asn residue of HIFα subunits in an oxygen-dependent manner, thereby regulating cofactor recruitment and HIFs' transcriptional activity.4
Three PHD isoforms have been characterized so far, termed PHD1, PHD2, and PHD3; they differ in size, subcellular localization, and tissue distribution.5 PHD2 is the most ubiquitously expressed isoform, responsible for the normoxic control of HIFα.6 Accordingly, genetic ablation of PHD2 but not PHD1 or PHD3 results in embryonic lethality in mice.7 Suggesting a fundamental role in the hematopoietic and circulatory systems, somatic inactivation of PHD2 leads to increased erythropoiesis and angiogenesis as a result of HIFα stabilization followed by activation of its target genes, including erythropoietin (EPO) and vascular endothelial growth factor.8 Knockout of either PHD1 or PHD3 had no effect on hematologic parameters. However, combined PHD1/PHD3 knockout animals showed a slight increase in hematocrit, hemoglobin, and red blood cell counts.9 Clinical data on patients with erythrocytosis revealed Pro317Arg or Arg371His mutations in the gene encoding for PHD2, altering the hydroxylation efficiency of the mutant protein.10,11 A third point mutation in PHD2 (His374Arg) was found in a patient suffering from erythrocytosis and paraganglioma.12 These case reports emphasize the critical role of PHD2 in regulating erythropoiesis and maintaining red blood cell homeostasis in humans.
PHDs belong to a larger superfamily of 2-oxoglutarate and Fe(II)-dependent dioxygenases. Similar to collagen prolyl-4-hydroxylase ([C-P4H]; EC 1.14.11.2), PHDs require molecular oxygen and 2-oxoglutarate as cosubstrates, as well as ferrous iron and probably l-ascorbic acid (vitamin C) as cofactors for enzymatic activity.13 Km values of PHDs for oxygen are strikingly higher than those of other prolyl-4-hydroxylases.13 The relatively low oxygen affinity is essential for effective oxygen sensing, because even small changes in oxygen partial pressure can influence hydroxylation activity.13-15
In a previous study, we reported on the dose-dependent regulation of the in vitro activity of all 3 PHD isoforms by their essential cosubstrates and cofactors, including vitamin C.16 Primates, including humans, lost the ability to synthesize vitamin C de novo and thus depend on dietary vitamin C intake. C-P4Hs hydroxylate proline residues to stabilize the collagen triple helix structure. Because ascorbate is an essential cofactor for C-P4Hs, persistent ascorbate deficiency results in disassembly of connective tissue structures, a common symptom of the nowadays rare disease scurvy.17 With Km values ranging from 140 to 180μM, the requirement of PHDs for vitamin C in vitro is only 2-fold lower than for C-P4H, suggesting that HIF hydroxylases also could well be affected by vitamin C malnutrition.13 Mice lacking a functional Gulo gene have been described as a model to study vitamin C deficiency.18 Gulo encodes for l-gulono-1,4-lactone-oxidase (EC 1.1.3.8), a key enzyme involved in the final step of vitamin C biosynthesis. Dietary vitamin C deprivation leads to body weight loss, anemia, aortic wall damage, and internal hemorrhages in these mice.18
Although the interaction between the target prolyl residue, molecular oxygen, 2-oxoglutarate, and iron during the reaction cycle in the active center of PHDs has been described in detail previously, the apparently inevitable presence of vitamin C for the in vitro function of the PHDs remains elusive.19,20 Because of its antioxidative properties, vitamin C might maintain ferrous iron in the reduced state. Given the enzymatic relationship between HIFα and C-P4Hs, we set out to investigate the effect of dietary vitamin C on the regulation of the PHD-HIF oxygen–sensing pathway in Gulo−/− mice under normoxemic and hypoxemic conditions.
Methods
Cell culture
HeLa human cervix carcinoma cells were adapted to Ham nutrient mixture F-12 (Sigma-Aldrich), free of ascorbate and fetal calf serum (FCS), containing the following supplements: epidermal growth factor (50 ng/mL; Sigma-Aldrich), insulin (5 μg/mL; Sigma-Aldrich), apo-transferrin (5 μg/mL; Sigma-Aldrich), hydrocortisone (100nM; Sigma-Aldrich) buffered with 15mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid pH 7.4 as well as 100 IU/mL penicillin and 100 μg/mL streptomycin. HepG2 human hepatoma cells stably transfected with a HIF-dependent firefly luciferase reporter gene termed HRG1 have been described previously.21 If not indicated otherwise, all cells were maintained in DMEM with 10% FCS, 100 IU/mL penicillin, and 100 μg/mL streptomycin. Cell number and viability were determined using a ViCell counter (Beckman Coulter).
HIF transactivation activity
HeLa cells (5 × 105) were cotransfected with 500 ng of the HIF-dependent pH3SVL reporter vector containing 6 HIF binding sites in total derived from regulatory elements of the transferrin gene22 and 40 ng of pRL-CMV Renilla luciferase expression plasmid (Promega) essentially as described previously.14 Twenty-four hours after transfection, cells were split and exposed to graded oxygen concentrations (21%-0.2% oxygen) for 24 hours by using cross-calibrated oxygen-controlled CO2 incubators (CB 150; Binder). Stably transfected HRG1 HIF reporter cells were adapted to 1% FCS overnight and treated with 50μM desferrioxamine mesylate (Dfx; Sigma-Aldrich) or 100μM CoCl2 and 1 to 10mM reduced l-γ-glutamyl-l-cysteinyl-glycine ([GSH] 250mM stock solution adjusted to pH 7.0) or 0.2 to 2mM ascorbate for 24 hours. For hypoxic experiments, cells were grown under 2% O2 for 24 hours and treated with GSH or ascorbate. HRG1 cells were transfected with pRL-SV40 Renilla luciferase to control for non–HIF-mediated effects of ascorbate and GSH on the heterologous simian virus 40 minimal promoter present in both constructs. Cells were lysed using passive lysis buffer, and luciferase activities were determined according to the manufacturer's instructions (Promega) by using a 96-well luminometer (Berthold Technologies). Data are expressed as relative luciferase activities per total cellular protein of experiments performed in triplicates by calculating the ratio of firefly/Renilla activities per well.
Expression and purification of recombinant PHD enzymes
Recombinant PHD proteins were expressed and purified as glutathione transferase (GST)–fusion proteins from baculovirus-infected Sf9 insect cells as described previously.14 Untagged enzyme preparations were obtained by introducing a PreScission protease cleavage site between the GST-tag and the PHD open reading frame. A Cys201Ser point mutation was introduced into the human PHD2 expression plasmid by site-directed mutagenesis (Stratagene). Untagged PHD2 was expressed in Sf9 cells and purified by conventional ion-exchange chromatography (kind gift of Dr Felix Oehme, Bayer Healthcare, Wuppertal, Germany). Purity of the enzyme preparations was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by Coomassie staining or immunoblotting.
Prolyl-4-hydroxylation assay
Activity of recombinant PHD enzymes was measured by a microtiter plate-based peptide hydroxylation assay as described previously.23 In brief, recombinant PHDs were used to hydroxylate a biotinylated peptide derived from HIF-1α (amino acid residues 556-574) coupled to streptavidin-coated 96-well plates. Hydroxylation reaction was performed for 1 hour at room temperature in the presence of 10μM FeSO4, 0.5mM 2-oxoglutarate, and 2mM ascorbate in 20mM Tris-HCl pH 7.5, 5mM KCl, and 1.5mM MgCl2. Hydroxylated peptides were detected by recombinant, thioredoxin-tagged von Hippel–Lindau/elongin B/elongin C (VBC) complex. Reactions were stopped by removing the reaction mix and adding 1mM H2O2. Bound VBC complex was detected by rabbit anti-thioredoxin antibodies and secondary horseradish peroxidase (HRP)–conjugated anti-rabbit antibodies (Sigma-Aldrich) by using the 3,3,5,5-tetramethylbenzidine substrate kit (Pierce). The peroxidase reaction was stopped by adding 2M H2SO4, and absorbance was determined at 450 nm in a microplate reader. Background values as determined by using a mutant HIF-1α (Pro564Ala) peptide were subtracted for each experiment.
Ascorbate determination
Ascorbate content of in vitro hydroxylation assay samples was quantified by high-performance liquid chromatography (HPLC) as described previously.24 In brief, a 10-fold dilution of the enzyme reaction mix containing 2mM ascorbate was analyzed before and after 1 hour of hydroxylation reaction. After dilution in the mobile phase (60mM phosphoric acid, pH 3.1), a 20-μL sample was injected onto a Nucleosil C18 column (Macherey Nagel) and eluted applying an acetonitrile gradient (0%-60%). Ascorbate elution was monitored at 254 nm, corresponding to 96% absorbance of ascorbate and only 4% of dehydroascorbate.25 Chromatograms and standard curve of pure ascorbate ranging from 25 to 200μM were used to calculate the content of ascorbate in study samples (supplemental Figure 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Ascorbate levels in plasma samples of mice were determined by HPLC (Swiss Vitamin Institute).
Immunoblot analyses
Total soluble cellular proteins were extracted with a high salt extraction buffer containing 0.4M NaCl, 0.1% Nonidet P-40, 10mM Tris-HCl pH 8.0, 1mM ethylenediaminetetraacetic acid, 1mM dithiothreitol, 1mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail (Sigma-Aldrich). Protein concentration was measured by the method of Bradford, and 60 to 70 μg of cellular protein was subjected to immunoblot analyses. Membranes were probed using the following dilutions of mouse monoclonal (mAb) or rabbit polyclonal antibodies: mAb anti–HIF-1α (1:1000; BD Transduction Laboratories), mAb anti-CA9 (M75; kindly provided by S. Pastorekova, Bratislava, Slovak Republic), mAb anti–β-actin (1:5000; Sigma-Aldrich), rabbit anti-PHD1 (1:2000; Genway), rabbit anti-PHD2 (1:1000; Novus), or rabbit anti-PHD3 (1:1000; Novus), followed by secondary HRP-conjugated antibodies (1:2000; all Pierce).
mRNA quantification
Total RNA purification and mRNA determination by real-time PCR has been described previously.14 Transcript levels of the HIF-dependent and -independent genes were quantified by reverse transcription (RT) quantitative (q) PCR using SYBR Green qPCR reagent kit (Sigma-Aldrich) in combination with an MX3000P light cycler (Stratagene). Initial copy number of each sample was calculated by comparison with serial dilutions of a calibrated standard. For mouse tissues, ribosomal protein S12 mRNA was used as a housekeeping gene, and ribosomal protein L28 mRNA served as control for samples from human cell lines. Primer sequences are given in supplemental Table 1.
OxyBlot detection of protein oxidation
PHD2 carbonylation was determined with a protein oxidation detection kit (OxyBlot; Millipore). In brief, 50 ng/μL recombinant PHD2 was exposed to either 100μM CoCl2, 10μM FeSO4, 2mM ascorbate, 0.5mM 2-oxoglutarate, or 1mM H2O2 in the presence of 400nM wild-type or Pro564Ala mutant HIF-1α peptide in 20mM Tris–HCl, 5mM KCl, and 1.5mM MgCl2 for 1 hour at room temperature. Then, 5 μL of the reaction mix was mixed with 5 μL of 12% sodium dodecyl sulfate, and the carbonyl groups were derivatized with 10mM 2,4-dinitrophenylhydrazine for 15 minutes. Dinitrophenyl groups were detected by immunoblotting using rabbit anti-DNP antibodies (1:150) followed by secondary goat anti–rabbit HRP-conjugated antibodies (1:300). For loading controls, PHD2 was detected using rabbit anti-PHD2 antibodies (Novus).
Animal studies
Gulo−/− mice were maintained on vitamin C–supplemented water containing 0.33 g/L l-ascorbic acid and 0.01mM EDTA as described previously.18 At 3 months of age, ascorbic acid supplement was withdrawn from 6 Gulo−/− males but continued for the control males. After 5 weeks, mice were killed with an overdose of 2,2,2-tribromoethanol, and tissues were collected and stored in RNAlater (Applied Biosystems) at −20°C until use. For hypoxia studies, 22 Gulo−/− males with an average age of 16 weeks were allocated to 4 groups so that no significant differences were observed in mean body weight and age of the animals at the beginning of the experiment. The body weight was determined every second day. After 36 days of ascorbate withdrawal, mice were exposed to 8% oxygen for 24 hours in a hypoxia tent (Coy Laboratory Products). Control animals were maintained at ambient oxygen concentration. Heparinized whole blood was collected from all mice by cardiac puncture after intraperitoneal injection anesthesia using 4 mg/mL xylazine and 20 mg/mL ketamine at a dosage of 0.1 mL/20 g body weight. Blood samples of hypoxic animals were collected inside the hypoxic tent. Animal experiments were conducted at 2 centers with the appropriate consent by the Institutional Animal Care and Use Committees of the University of North Carolina at Chapel Hill for breeding and normoxic gene expression studies or by the Veterinary Office of the Canton Zürich (119/2010) for hypoxia studies.
Blood parameters and plasma EPO concentrations
Plasma EPO levels were measured by enzyme-linked immunosorbent assay following the procedures recommended by the manufacturer (Quantikine; R&D Systems). EPO concentrations were determined by comparison with a calibrated recombinant mouse EPO standard. Hematologic parameters of mouse whole blood were analyzed by the Division of Hematology (University Hospital, Zürich, Switzerland).
Results
Ascorbate is not required for HIF induction by hypoxia in HeLa cells
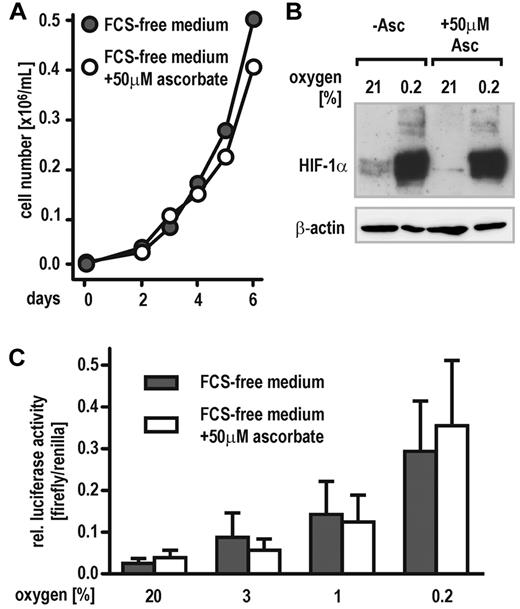
HeLa cells have been described previously to grow in serum-free medium supplemented with hormones and growth factors.26 To achieve cell culture conditions avoiding ascorbate contamination derived from animal sera, HeLa cells were adapted to a chemically defined medium free of ascorbate for at least 2 weeks. Control cells were grown in the same medium supplemented with 50μM ascorbate. Both cell groups proliferated at the same rate with no differences noticed between ascorbate-free and supplemented cells (Figure 1A). Despite its need for PHD activity in vitro, hypoxic HIF-1α protein accumulation was similar in ascorbate-containing and -deficient cells. However, a faint normoxic induction of HIF-1α could be observed in ascorbate-free cells only (Figure 1B). Accordingly, cells transfected with a HIF-responsive reporter gene (pH3SVL) and subsequently exposed to graded oxygen concentrations (0.2%, 1%, 3%, or 21% O2, respectively) revealed similar induction levels of luciferase activity under both culture conditions (Figure 1C).
Cellular oxygen sensing by the PHD-HIF pathway does not require vitamin C. (A) Proliferation of HeLa cells growing in FCS-free, chemically defined medium containing either no or 50μM ascorbate. (B) Stabilization of HIF-1α protein in HeLa cells maintained in FCS-free medium containing either no (−Asc.) or 50μM ascorbate. Cells were exposed to 21% and 0.2% oxygen for 6 hours, and then protein levels were analyzed by immunoblotting. (C) Induction of HIF-dependent luciferase activity (pH3SVL vector) in HeLa cells maintained in FCS-free medium containing either no or 50μM ascorbate and exposed to 0.2% to 21% oxygen for 24 hours.
Cellular oxygen sensing by the PHD-HIF pathway does not require vitamin C. (A) Proliferation of HeLa cells growing in FCS-free, chemically defined medium containing either no or 50μM ascorbate. (B) Stabilization of HIF-1α protein in HeLa cells maintained in FCS-free medium containing either no (−Asc.) or 50μM ascorbate. Cells were exposed to 21% and 0.2% oxygen for 6 hours, and then protein levels were analyzed by immunoblotting. (C) Induction of HIF-dependent luciferase activity (pH3SVL vector) in HeLa cells maintained in FCS-free medium containing either no or 50μM ascorbate and exposed to 0.2% to 21% oxygen for 24 hours.
GSH can substitute for vitamin C in the hydroxylation reaction catalyzed by PHDs
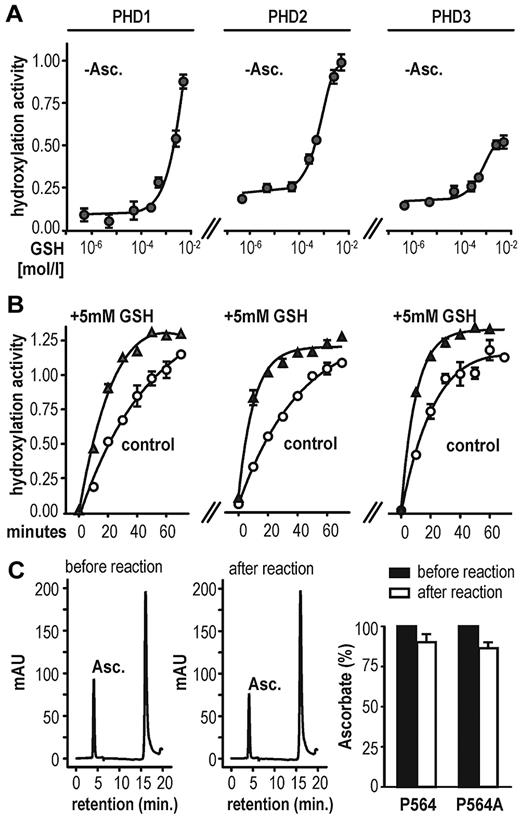
Because HeLa cells grown in a medium containing no ascorbate maintained hypoxic HIF-1α stabilization, we speculated that other antioxidants could compensate for vitamin C loss in these cells. Thus, several compounds with antioxidative properties were tested for their effects on PHD hydroxylation activity by using a previously described in vitro hydroxylation assay.23 Surprisingly, some compounds such as n-propyl gallate16 and the superoxide dismutase mimetic Mn(III) tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride were potent inhibitors of PHD enzymes (supplemental Figure 1A). However, GSH enhanced HIFα hydroxylation by all 3 PHDs. Because the recombinant enzyme preparations in the initial experiments were expressed and purified as GST-fusion proteins, we could not exclude interference of the tested antioxidants, particularly GSH, with the GST-tag. Therefore, a PreScission protease cleavage site was engineered in between the 2 fusion partners. Chimeric GST.PHD and tag-free PHD enzymes showed comparable hydroxylation activity (supplemental Figure 1B). GSH increased the activity of all 3 untagged PHD isoforms, even in the absence of ascorbate (Figure 2A). Addition of GSH (+ 5mM in Figure 2B) to 2mM ascorbate increased the reaction rate compared with ascorbate alone (control in Figure 2B), suggesting 2 independent reaction modes of GSH, one mode replacing vitamin C and an additional mode enhancing the reaction rate. Of note, only minor changes in ascorbate oxidation were found before and after 1 hour of PHD2-mediated substrate hydroxylation (Figure 2C). The minor decrease in reduced ascorbate was probably because of air-dependent oxidation rather than enzymatic consumption because it was independent of the presence of the hydroxyl-acceptor substrate (Figure 2C right panel). In conclusion, as shown previously for C-P4H,27 ascorbate is not consumed during coupled PHD-catalyzed hydroxylation reactions.
GSH substitutes for vitamin C as a cofactor in HIF-1α hydroxylation in vitro. (A) GSH can enhance PHD hydroxylation activity in the absence of ascorbate (−Asc.) in a dose-dependent manner. Hydroxylation activity was determined using a multiwell VBC binding assay. (B) PHD-dependent hydroxylation reaction rate in the presence of 2mM ascorbate (control) or 2mM ascorbate combined with 5mM GSH (+5mM GSH). Shown are mean values ± SEM of triplicates. (C) Ascorbate determination by HPLC before and after 1 hour of PHD2-dependent hydroxylation reaction (left panels). Ascorbate content is only slightly decreased after 1 hour of incubation and independent of target hydroxylation (right panel). Shown are mean values ± SEM of 3 independent experiments normalized to values measured at time point zero.
GSH substitutes for vitamin C as a cofactor in HIF-1α hydroxylation in vitro. (A) GSH can enhance PHD hydroxylation activity in the absence of ascorbate (−Asc.) in a dose-dependent manner. Hydroxylation activity was determined using a multiwell VBC binding assay. (B) PHD-dependent hydroxylation reaction rate in the presence of 2mM ascorbate (control) or 2mM ascorbate combined with 5mM GSH (+5mM GSH). Shown are mean values ± SEM of triplicates. (C) Ascorbate determination by HPLC before and after 1 hour of PHD2-dependent hydroxylation reaction (left panels). Ascorbate content is only slightly decreased after 1 hour of incubation and independent of target hydroxylation (right panel). Shown are mean values ± SEM of 3 independent experiments normalized to values measured at time point zero.
GSH decreases HIF activity in CoCl2-treated hepatoma cells
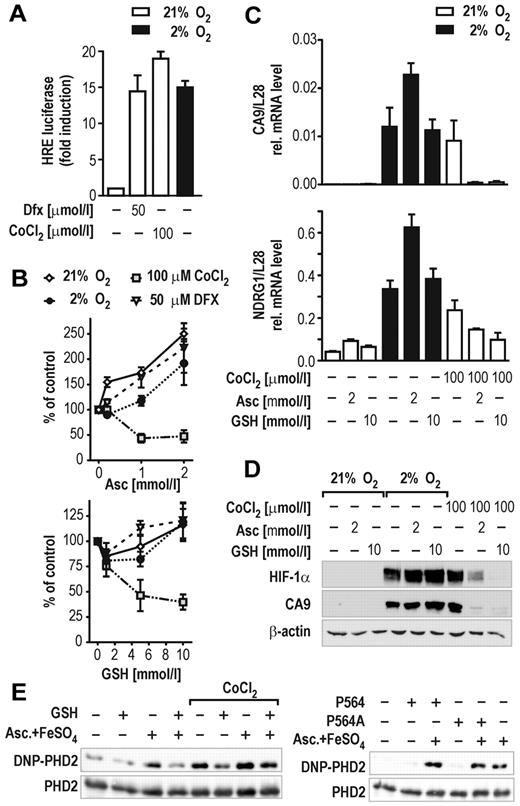
In cell culture models, Co(II) and Ni(II) have been shown to substantially decrease cellular ascorbate content by catalyzing ascorbate oxidation to dehydroascorbate followed by irreversible hydrolysis to diketogulonate.28 Interestingly, exogenous ascorbate administration completely blunted the Co(II)-induced hypoxic response in lung epithelial cells.29 To test whether GSH could similarly compensate for reduced ascorbate levels after Co(II) stimulation, HIF transcriptional activity was further studied in HepG2 hepatoma cells stably transfected with an HIF-dependent luciferase reporter gene (HRG1 cells). We first determined the concentrations of CoCl2 required to activate HIF-dependent reporter gene expression to a similar extent as exposure of the cells to 2% oxygen or the hypoxia mimicking iron chelator Dfx (Figure 3A). Subsequently, HRG1 cells were treated with 100μM CoCl2 or 50μM Dfx under 21% or 2% oxygen. Control cells were kept at ambient oxygen concentrations. All cells were cotreated with 1 to 10mM GSH or 0.2 to 2mM ascorbate. Indeed, ascorbate and GSH reduced HIF activity exclusively in CoCl2-treated HRG1 cells (Figure 3B). A substantial increase of HIF activation was noted particularly when cells were treated with 2mM ascorbate, which might be explained by the pro-oxidative function ascorbate exerts if applied at high concentration (Figure 3B top panel).30 In line with these observations, both ascorbate and GSH reduced the expression levels of the endogenous HIF target genes CA9 and NDRG13 only in cells treated with CoCl2, whereas 2mM ascorbate enhanced hypoxic activation of both genes by almost 2-fold (Figure 3C). To evaluate whether these effects reflected differential activities of cellular PHD enzymes, HIF-1α protein accumulation was analyzed by immunoblotting. As expected, only CoCl2-stabilized HIF-1α was down-regulated by cotreatment with ascorbate or GSH (Figure 3D). Interestingly, 10mM GSH was a more potent inhibitor of CoCl2-induced HIF-1α stabilization than 2mM ascorbate, a concentration that showed saturated inhibition of the HIF-reporter in the same cell line (Figure 3B top panel).
GSH impairs HIF activation in cells. (A) Induction of HIF-dependent luciferase reporter gene activity in stably transfected HRG1 hepatoma cells by 2% O2, 50μM Dfx, or 100μM CoCl2 for 24 hours. (B) Effects of ascorbate (Asc, 0.2-2mM; top panel) or GSH (1-10mM; bottom panel) in combination with hypoxia, Dfx, or CoCl2 treatment on HIF-dependent luciferase activity relative to the protein concentration of the lysates. Shown are mean values ± SEM of 3 independent experiments normalized to the reporter activity in the absence of either ascorbate or GSH (control). (C) CA9 and NDRG1 HIF target gene mRNA levels in HRG1 cells after treatment with 2mM ascorbate or 10mM GSH combined with 2% O2 or 100μM CoCl2 for 24 hours. Shown are mean values ± SEM of 3 independent experiments relative to the mRNA content of ribosomal protein L28. (D) HIF-1α and CA9 protein levels in HRG1 cells after treatment with 2mM ascorbate or 10mM GSH combined with 2% O2 or 100μM CoCl2. (E) OxyBlot analyses of recombinant PHD2 protein carbonylation. GSH (5mM) reduced PHD2 carbonylation by either 2mM ascorbate/10μM FeSO4 or 100μM CoCl2 (left panel). PHD2 oxidation is independent of target hydroxylation as shown by using a wild-type or a Pro564Ala mutant HIF-1α hydroxyl-proline acceptor peptide in in vitro hydroxylation reactions (right panel).
GSH impairs HIF activation in cells. (A) Induction of HIF-dependent luciferase reporter gene activity in stably transfected HRG1 hepatoma cells by 2% O2, 50μM Dfx, or 100μM CoCl2 for 24 hours. (B) Effects of ascorbate (Asc, 0.2-2mM; top panel) or GSH (1-10mM; bottom panel) in combination with hypoxia, Dfx, or CoCl2 treatment on HIF-dependent luciferase activity relative to the protein concentration of the lysates. Shown are mean values ± SEM of 3 independent experiments normalized to the reporter activity in the absence of either ascorbate or GSH (control). (C) CA9 and NDRG1 HIF target gene mRNA levels in HRG1 cells after treatment with 2mM ascorbate or 10mM GSH combined with 2% O2 or 100μM CoCl2 for 24 hours. Shown are mean values ± SEM of 3 independent experiments relative to the mRNA content of ribosomal protein L28. (D) HIF-1α and CA9 protein levels in HRG1 cells after treatment with 2mM ascorbate or 10mM GSH combined with 2% O2 or 100μM CoCl2. (E) OxyBlot analyses of recombinant PHD2 protein carbonylation. GSH (5mM) reduced PHD2 carbonylation by either 2mM ascorbate/10μM FeSO4 or 100μM CoCl2 (left panel). PHD2 oxidation is independent of target hydroxylation as shown by using a wild-type or a Pro564Ala mutant HIF-1α hydroxyl-proline acceptor peptide in in vitro hydroxylation reactions (right panel).
GSH protects PHD2 from metal-catalyzed oxidation
Enzymatic activity of the PHDs is sensitive to reactive oxygen species and transition metal ions.31 However, the mechanism(s) by which reactive oxygen species or metal ions inhibit hydroxylase activity remained speculative. Besides its general antioxidative properties as radical scavenger, vitamin C actively interferes with the oxidation state of metal ions by serving as electron donor in a redox reaction. As such, it largely differs from GSH that is a major cellular antioxidant protecting cysteinyl and methionyl residues in proteins from oxidative modifications. Both CoCl2 and H2O2 inhibited all 3 PHD isoforms in vitro, with PHD2 being slightly more resistant to CoCl2 (supplemental Figure 3). To directly determine protein oxidation by these compounds, carbonyl group formation in PHD2 was estimated by OxyBlot technology. As shown in Figure 3E (left panel), 2mM ascorbate and 10μM FeSO4 (as used in the standard reaction buffer for in vitro hydroxylation) substantially increased carbonylation of recombinant PHD2 during 1 hour of hydroxylation reaction. Surprisingly, CoCl2 increased carbonylation of PHD2 whether ascorbate/FeSO4 was present or not. After addition of 5mM GSH, oxidation of PHD2 by ascorbate/FeSO4, CoCl2, and H2O2 was markedly reduced. To examine whether PHD2 protein oxidation is coupled to its dioxygenase activity, the reaction was performed in the presence of a mutant Pro564Ala HIF-1α peptide substrate. As shown in Figure 3E (right panel), PHD2 protein oxidation was independent of the presence of a hydroxylation acceptor proline, providing evidence that protein oxidation is not caused by the hydroxylation reaction cycle.
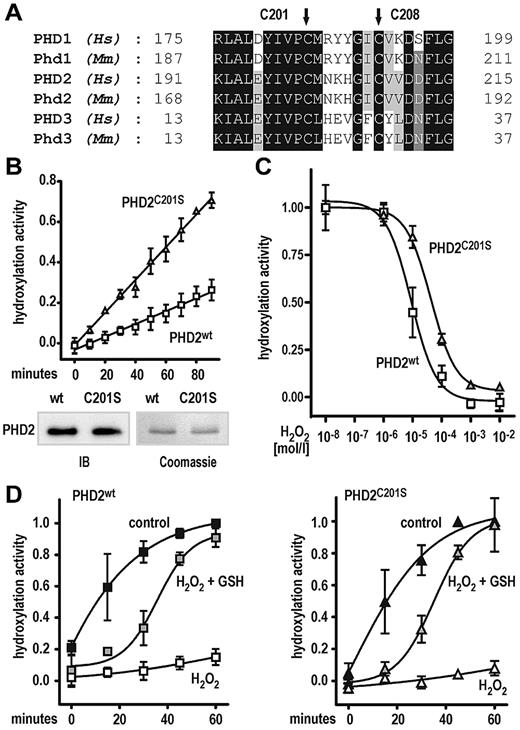
Cysteine 201 affects PHD2 hydroxylation activity
Recently, cysteine residue 201 within the catalytic domain of PHD2 has been identified as a surface-accessible, highly nucleophilic residue predominantly interacting with thiol compounds.32 Moreover, Cys201 and Cys208 were proposed to provide an additional metal binding site in PHD2.33 Both, Cys201 and Cys208 are highly conserved among all 3 human and mouse PHD isoforms (Figure 4A). To investigate the functional relevance of Cys201, recombinant PHD2, wild type, or Cys201Ser mutant was purified from Sf9 cells. Surprisingly, the Cys201Ser mutation significantly (P < .0001) increased the PHD2 reaction rate by 2.5-fold (Figure 4B top panel). Equal concentrations of wild-type and mutant PHD2 proteins were confirmed by Coomassie staining of the undiluted stock solutions and immunoblotting of the diluted assay solutions (Figure 4B bottom panel). To further test the hypothesis that the Cys201Ser mutation might protect the PHD2 enzyme from oxidative damage, the effect of H2O2 on hydroxylation activity was measured. As shown in Figure 4C, the half-maximal inhibitory concentration for H2O2 was roughly 5-fold higher for the Cys201Ser mutant compared with wild-type PHD2 (half-maximal inhibitory concentration of 8.9 × 10−6 and 4.3 × 10−5 M H2O2 for wild-type and Cys201Ser mutant PHD2, respectively). We further tested whether the H2O2-mediated loss of PHD2 activity could be rescued by sequential addition of GSH. For both enzyme preparations, preincubation with 1mM H2O2 for 30 minutes inhibited subsequent substrate hydroxylation reactions, despite 2mM ascorbate being freshly added to start the hydroxylation reaction (Figure 4D). Interestingly, further addition of 5mM GSH could similarly reactivate both enzyme preparations, although reaction kinetics was substantially slower for the reactivated enzymes (Figure 4D).
A Cys201Ser mutation enhances PHD2-dependent hydroxylation reaction rate and protects from protein oxidation. (A) Conservation of cysteine residues (Cys201 and Cys208 in human PHD2) in all 3 human (Hs) and mouse (Mm) PHD isoforms. (B) Increased reaction rate of Cys201Ser mutant PHD2 as measured by the hydroxylation-dependent VBC binding assay. Shown are mean values ± SEM of 3 independent experiments. Linear regression analyses were performed, revealing highly different slopes (P < .0001). (C) The Cys201Ser mutation confers resistance of PHD2 to H2O2-mediated inhibition of hydroxylation activity. Shown are mean values ± SEM of a representative experiment performed in triplicates. (D) GSH can rescue PHD2 wild type and Cys201Ser hydroxylation activities after H2O2-mediated enzyme damage. In brief, enzyme preparations were preincubated with 1mM H2O2 for 30 minutes (H2O2) or left untreated for a similar period (control). For rescue experiments, enzymes after H2O2 treatment were incubated with 5mM GSH (H2O2 + GSH) for 15 minutes. Hydroxylation reactions were carried out at standard assay conditions for 60 minutes. Note that all reactions contained 2mM ascorbate freshly added when hydroxylation reactions were started. Data are given as mean values ± SEM of 3 independent experiments normalized to hydroxylation activities of control reactions obtained after 60 minutes.
A Cys201Ser mutation enhances PHD2-dependent hydroxylation reaction rate and protects from protein oxidation. (A) Conservation of cysteine residues (Cys201 and Cys208 in human PHD2) in all 3 human (Hs) and mouse (Mm) PHD isoforms. (B) Increased reaction rate of Cys201Ser mutant PHD2 as measured by the hydroxylation-dependent VBC binding assay. Shown are mean values ± SEM of 3 independent experiments. Linear regression analyses were performed, revealing highly different slopes (P < .0001). (C) The Cys201Ser mutation confers resistance of PHD2 to H2O2-mediated inhibition of hydroxylation activity. Shown are mean values ± SEM of a representative experiment performed in triplicates. (D) GSH can rescue PHD2 wild type and Cys201Ser hydroxylation activities after H2O2-mediated enzyme damage. In brief, enzyme preparations were preincubated with 1mM H2O2 for 30 minutes (H2O2) or left untreated for a similar period (control). For rescue experiments, enzymes after H2O2 treatment were incubated with 5mM GSH (H2O2 + GSH) for 15 minutes. Hydroxylation reactions were carried out at standard assay conditions for 60 minutes. Note that all reactions contained 2mM ascorbate freshly added when hydroxylation reactions were started. Data are given as mean values ± SEM of 3 independent experiments normalized to hydroxylation activities of control reactions obtained after 60 minutes.
Oxygen sensing is fully functional in ascorbate-deficient Gulo−/− mice
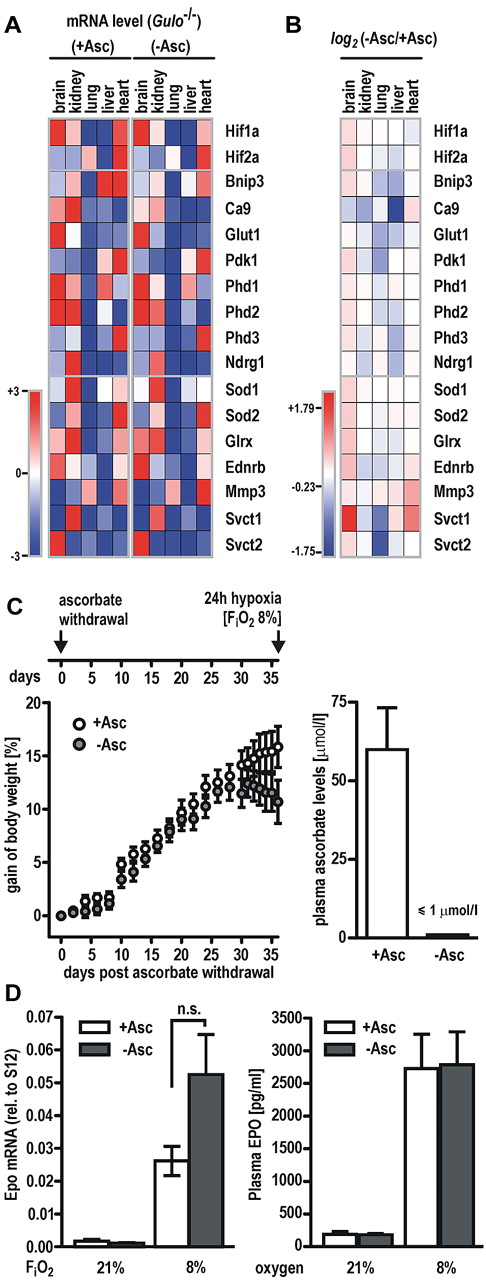
Gulo−/− mice received a diet with or without vitamin C for 5 weeks. Transcript levels of known HIF target genes (Bnip3, Ca9, Glut1, Pdk1, Phd2, Phd3, and Ndrg1) as well as genes involved in antioxidative defense (Sod1, Sod2, and Glrx), the ascorbate transporters Svct1 and Svct2, or oxygen-independent genes (Ednrb, Mmp3, and Phd1) were determined by RT-qPCR in brain, lung, kidney, heart, and liver. Similar tissue-specific expression levels for most genes involved in different pathways were observed in both groups (Figure 5A). Conclusive with our finding from cell culture experiments, expression levels of most of the HIF target genes remained largely unaffected by vitamin C deficiency or even showed reduced levels (blank and blue squares, respectively, in the heatmap shown in Figure 5B). Expression of the ascorbate transporter Svct1, however, was moderately induced in animals fed without ascorbate, possibly reflecting compensatory mechanisms for ascorbate deprivation.
Hypoxic response is fully functional in vitamin C–depleted Gulo−/− mice.Gulo−/− male mice received a diet with (+Asc) or without (−Asc) ascorbate for 5 weeks. (A) Transcript levels of HIF target genes (Bnip3, Ca9, Glut1, Pdk1, Phd2, Phd2, Phd3, and Ndrg1) as well as genes involved in antioxidative defense (Sod1, Sod2, and Glrx), the ascorbate transporters Svct1 and Svct2, or oxygen-independent genes (Ednrb, Mmp3, and Phd1) were quantified by RT-qPCR in brain, kidney, lung, liver, and heart. Values are expressed relative to S12 mRNA levels and visualized in a heatmap (Genepattern; Broad Institute). Lowest and highest mRNA levels of each gene were arbitrarily defined as −3 (dark blue) and +3 (dark red), respectively. (B) Heatmap of gene expression changes following a vitamin C–deficient diet. Log2 (−Asc/+Asc) ratios revealed that the majority of HIF target genes remained either unchanged or showed a slightly reduced expression pattern. Data ranged from −1.75 (dark blue) and +1.79 (dark red), respectively. (C) Scheme depicting the experimental setup for hypoxic experiments with vitamin C–depleted Gulo−/− animals (top panel). Relative gain of body weight of Gulo−/− mice (n = 11 animals per group) after ascorbate withdrawal (−Asc) or ascorbate supplementation (+Asc) for 36 days (left panel). Ascorbate levels in the plasma of Gulo−/− mice (n = 6 animals per group) after 5 weeks of ascorbate withdrawal compared with mice kept on an ascorbate-supplemented diet (right panel). (D) Epo mRNA (left panel) and circulating EPO protein (right panel) levels in Gulo−/− mice maintained on a diet with (+Asc) or without (−Asc) for 5 weeks followed by exposure to 8% or 21% oxygen for 24 hours. Data represent mean values ± SEM derived from at least 5 animals per group; n.s., indicates not significant.
Hypoxic response is fully functional in vitamin C–depleted Gulo−/− mice.Gulo−/− male mice received a diet with (+Asc) or without (−Asc) ascorbate for 5 weeks. (A) Transcript levels of HIF target genes (Bnip3, Ca9, Glut1, Pdk1, Phd2, Phd2, Phd3, and Ndrg1) as well as genes involved in antioxidative defense (Sod1, Sod2, and Glrx), the ascorbate transporters Svct1 and Svct2, or oxygen-independent genes (Ednrb, Mmp3, and Phd1) were quantified by RT-qPCR in brain, kidney, lung, liver, and heart. Values are expressed relative to S12 mRNA levels and visualized in a heatmap (Genepattern; Broad Institute). Lowest and highest mRNA levels of each gene were arbitrarily defined as −3 (dark blue) and +3 (dark red), respectively. (B) Heatmap of gene expression changes following a vitamin C–deficient diet. Log2 (−Asc/+Asc) ratios revealed that the majority of HIF target genes remained either unchanged or showed a slightly reduced expression pattern. Data ranged from −1.75 (dark blue) and +1.79 (dark red), respectively. (C) Scheme depicting the experimental setup for hypoxic experiments with vitamin C–depleted Gulo−/− animals (top panel). Relative gain of body weight of Gulo−/− mice (n = 11 animals per group) after ascorbate withdrawal (−Asc) or ascorbate supplementation (+Asc) for 36 days (left panel). Ascorbate levels in the plasma of Gulo−/− mice (n = 6 animals per group) after 5 weeks of ascorbate withdrawal compared with mice kept on an ascorbate-supplemented diet (right panel). (D) Epo mRNA (left panel) and circulating EPO protein (right panel) levels in Gulo−/− mice maintained on a diet with (+Asc) or without (−Asc) for 5 weeks followed by exposure to 8% or 21% oxygen for 24 hours. Data represent mean values ± SEM derived from at least 5 animals per group; n.s., indicates not significant.
To further test whether the absence of ascorbate limits PHD function under hypoxic conditions, Gulo−/− males were deprived of vitamin C for 5 weeks, whereas control animals were supplemented with vitamin C. As described previously,18 the animals developed a scorbutic phenotype marked by a substantial loss of body weight after 35 days on a ascorbate-free diet, indicating that systemic stores of vitamin C have been exhausted (Figure 5C left panel). In line with this observation, plasma ascorbate levels in Gulo−/− mice fed an ascorbate-free diet were below the detection limit (≤ 1μM), whereas plasma of control animals contained 40.3 to 123.8μM vitamin C (Figure 5C right panel), corresponding to ascorbate plasma levels in healthy humans.34
After 5 weeks of vitamin C deprivation, mice were breathing 8% oxygen for an additional period of 24 hours, whereas control groups were kept under ambient oxygen concentration (see scheme in Figure 5C). Both ascorbate-deficient and supplemented animals responded to the hypoxic treatment with a robust induction of Epo mRNA in the kidney that was higher in Gulo−/− males fed without ascorbate, although differences did not reach the level of significance (P = .07, Student t test; Figure 5D left panel). Circulating EPO protein levels in mouse plasma were induced by hypoxic exposure to a similar extent in both groups (Figure 5C right panel). Of note, no significant changes of the red cell lineage hematologic parameters were observed in Gulo−/− mice after 5 weeks of ascorbate depletion, indicating that the oxygen transport capacity was similar in both treatment groups. Hypoxic increases in hematocrit values are known to be delayed and reach the level of significance not before 72 hours of continuous exposure to hypoxia,35 explaining the lack of an increase of either hematocrit values or red blood cell counts in our experimental setting with a hypoxic period of only 24 hours (supplemental Table 2).
Discussion
Ascorbic acid and ferrous iron have been reported as essential cofactors for PHD-dependent HIFα hydroxylation in vitro.13 Unexpectedly, we found a fully functional cellular oxygen-sensing pathway in HeLa cells maintained under strictly ascorbate-free culture conditions, indicating that ascorbate is dispensable for HIFα hydroxylation in vivo. In a search for the nature of antioxidative compounds substituting for ascorbate during prolyl-4-hydroxylation, we identified GSH as a potent activator of all 3 PHDs in vitro, increasing HIFα peptide hydroxylation in a dose-dependent manner. Notably, ascorbate and GSH are the most abundant reducing compounds within eukaryotic cells.36 Given the distinct antioxidative properties of vitamin C and GSH, the 2 compounds might affect prolyl-4-hydroxylation by different ways. Ascorbate might be required to reduce occasionally oxidized ferric Fe(III) generated in the active center of PHDs by uncoupled reaction cycles as it has been described for C-P4H.27 However, to the best of our knowledge, no experimental evidence has been reported for enzymatic activity of PHDs in the absence of a hydroxyl-acceptor substrate. Strikingly, the major iron form bound to purified PHD2 is ferrous Fe(II) even when purified under oxygenated conditions,37 arguing against an essential role of ascorbate in reducing PHD iron. In support of this notion, iron and 2-oxoglutarate have been reported to copurify with 50% and 5%-10% of PHD2, respectively, whereas ascorbate did not copurify at all.37 Recent work by Flashman et al38 showed that ascorbate does not directly interact with the catalytic domain of PHD2; however, its intrinsic ene-diol–reducing moiety was found to be important to promote hydroxylation by PHD2.
GSH fully stimulated in vitro PHD hydroxylation activity only at rather high concentrations, which is in line with findings reported previously for N-terminally truncated PHD2.38 The millimolar GSH concentrations used in our study reflect physiologically relevant levels of this compound in living cells.39 Moreover, we found that ascorbic acid is not consumed by coupled substrate hydroxylation, suggesting that exogenously added GSH does not simply regenerate potentially copurified oxidized dehydroascorbate. Our data rather favor an alternative function of GSH by preventing oxidative damage to the enzyme itself. Physiologic concentrations of GSH were able to reduce transition metal- or peroxide-induced PHD enzyme carbonylation. Despite being generally referred to as antioxidant, ascorbate, together with oxygen and transition metals such as Fe(III) or Cu(II), also exerts pro-oxidative effects by generating hydroxyl radicals in a Fenton-like reaction.40 Indeed, we found increased PHD2 carbonylation by ascorbate-iron in vitro, suggesting that GSH might protect PHDs from the adverse effects of ascorbate. In line with 2 distinct reaction modes, addition of GSH to hydroxylation reactions containing saturating ascorbate concentrations markedly increased the hydroxylation rate of PHDs in vitro. Of note, Co(II) induced ascorbate depletion, as suggested for cultured cells,28,29 cannot account for PHD inhibition in our cell-free assays, because we showed previously that Co(II) only inefficiently catalyses ascorbate oxidation by air under these assay conditions.16 Direct interference of Co(II) with the enzymes is supported by the observation that Co(II) strongly carbonylated purified PHD2 even in the absence of ascorbate. Moreover, ascorbate and GSH exclusively blunted Co(II)-induced HIF activation in our cellular models, demonstrating a complementary function of GSH and ascorbate in oxygen sensing by living cells. Although simple chelation of Co(II) by ascorbate and GSH cannot be fully excluded in cell culture experiments, it should be mentioned that metal chelators naturally occurring in serum (eg, histidine, glutamic acid, and albumin but also GSH) are essential to facilitate Co(II)-induced ascorbate oxidation, because “free” Co(II) is unable to directly oxidize ascorbate in simple aqueous solutions at neutral pH (for a review, see Salnikow and Kasprzak28 ). Thus, the actual redox potential of the ion in such ternary complexes —rather than the intracellular concentration of “free” metal—determines its efficacy to act as a “hypoxia mimetic.”
One of the specific GSH functions is to prevent or reduce inappropriate disulfide bond formation. Two recent reports identified PHD2 cysteinyl residues Cys201 and Cys208 to be highly nucleophilic and surface accessible.32,33 Moreover, crystallographic analyses predicted that these 2 cysteines might form disulfide bonds.32,33 Cys201Ser mutation protects recombinant PHD2 from oxidative damage in vitro and results in a 2.5-fold higher specific hydroxylation activity. One might speculate that a certain fraction of wild-type PHD2 enzyme constantly undergoes oxidative modification of Cys201, leading to reduced activity. As such, PHD enzymes could combine oxygen- and redox-sensing properties, providing a possible explanation for previous work on redox factors modulating PHD activity.41-43 However, potentiation of PHD activity by GSH clearly involves mechanisms distinct from Cys201 oxidation, because both wild-type and Cys201Ser mutated PHD2 enzymes could be efficiently reactivated from H2O2-induced damage by GSH.
Translating our biochemical and cellular findings into a systemic context, we did not observe marked alterations in HIF target gene expression after dietary vitamin C deprivation in Gulo−/− mice, providing evidence that other antioxidants might substitute for vitamin C in vivo. Accordingly, the hypoxic response of Gulo−/− mice with low or undetectable ascorbate in the plasma was similar to that of mice receiving an ascorbate-supplemented diet. Consistent with antioxidant redundancy in vivo, a study using the same animals backcrossed to a BALB/c genetic background found vitamin C–independent de novo synthesis of collagen in allografted tumors and unchanged levels of hydroxyproline-collagen. Dermal hydroxyproline content of collagen was even increased.44 Of note, treatment of vitamin C–deprived guinea pigs with a cell-permeable GSH monoethyl ester significantly attenuated the severity of scurvy-related symptoms, also suggesting a cooperative function of both antioxidants for P4H function in vivo.45 Moreover, Gulo−/− mice have increased levels of total glutathione in brain and liver, possibly explained by a compensatory mechanism for antioxidative defense in these animals.46
Interestingly, tumor growth and angiogenesis were retarded in a syngenic tumor model using vitamin C–deprived Gulo−/− mice, but no changes were observed for HIF-1α protein levels in the respective tumor tissue.47 The possible value of vitamin C in cancer therapy recently experienced a renaissance, because it has been shown that pharmacologic doses of vitamin C decrease growth of tumor xenografts in mice by increasing peroxide levels in neoplastic tissue.30,48 Such tumoricidal effects of antioxidants might, at least partially, involve destabilization of HIFα after increased hydroxylase activity.49,50 Our data support a model of cooperative function of GSH and vitamin C in regulating the efficiency of PHD oxygen sensors. Translated to chemotherapy of cancers, combined treatment with both clinically approved molecules might even boost their antitumorigenic function.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank F. Oehme and S. Pastorekova for providing materials and B. Blattmann and M. Bordoli for technical advice. We also thank C. Schofield for helpful discussion. The use of the ZIHP Core Facility for Rodent Physiology is gratefully acknowledged.
This work was supported by National Institutes of Health grant HL42630 (to N.M.), Swiss National Science Foundation grant 31003A_129962/1 (to R.H.W. and D.P.S.), and the Forschungs-kredit of the University of Zürich (to D.P.S.).
National Institutes of Health
Authorship
Contribution: K.J.N. designed and performed experiments, analyzed data, and wrote the manuscript; K.J.N., N.M., P. Schläfli, and P. Spielmann performed in vivo experiments; R.H.W. designed experiments and wrote the manuscript; and D.P.S. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel P. Stiehl, Institute of Physiology, University of Zürich, Winterthurerstr 190, CH-8057 Zürich, Switzerland; e-mail: daniel.stiehl@access.uzh.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal