Abstract
All-trans-retinoic acid (ATRA) has greatly modified the prognosis of acute promyelocytic leukemia; however, the role of maintenance in patients in molecular complete remission after consolidation treatment is still debated. From July 1993 to May 2000, 807 genetically proven newly diagnosed acute promyelocytic leukemia patients received ATRA plus idarubicin as induction, followed by 3 intensive consolidation courses. Thereafter, patients reverse-transcribed polymerase chain reaction–negative for the PML-RARA fusion gene were randomized into 4 arms: oral 6-mercaptopurine and intramuscular methotrexate (arm 1); ATRA alone (arm 2); 3 months of arm1 alternating to 15 days of arm 2 (arm 3); and no further therapy (arm 4). Starting from February 1997, randomization was limited to ATRA-containing arms only (arms 2 and 3). Complete remission was achieved in 761 of 807 (94.3%) patients, and 681 completed the consolidation program. Of these, 664 (97.5%) were evaluated for the PML-RARA fusion gene, and 586 of 646 (90.7%) who tested reverse-transcribed polymerase chain reaction–negative were randomized to maintenance. The event-free survival estimate at 12 years was 68.9% (95% confidence interval, 66.4%-71.4%), and no differences in disease-free survival at 12 years were observed among the maintenance arms.
Introduction
The advent of all-trans-retinoic acid (ATRA) and its inclusion in the treatment of acute promyelocytic leukemia (APL) has greatly modified the prognosis of this peculiar subtype of acute myelogenous leukemia.1 However, when given alone, ATRA does not cure APL, and all patients eventually relapse.2 Therefore, a modern approach to the treatment of APL requires that ATRA be combined with standard chemotherapeutic protocols to achieve a high cure rate, as demonstrated by several cooperative groups.3-7
Despite these results, concerns still exist about the antileukemic drugs that must be associated with ATRA for treating APL. Early reports, pioneered by Bernard et al,8 had indicated a high sensitivity of APL to the anthracycline drug daunorubicin (DNR) when used as a single agent during the induction phase. This high sensitivity of APL to high-dose DNR was retrospectively confirmed by the Southwest Oncology Group.9 However, one of the main limitations to the use of high-dose DNR is its acute and chronic cardiotoxicity.10,11 Therefore, to overcome or reduce this problem, the anthracycline idarubicin (IDA) has been proposed as a therapeutic option to the use of DNR.12-14 Moreover, the role of cytosine arabinoside in addition to ATRA and anthracyclines for induction and/or consolidation therapy of newly diagnosed patients is still unclear.15-19 Finally, an open debate remains as to whether maintenance therapy is needed in this disease. Before the introduction of ATRA, 2 retrospective studies suggested a statistically significant better disease-free survival (DFS) in APL patients receiving maintenance with low-dose chemotherapy.20,21 These results were not confirmed in a prospective randomized Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) study using 3 consolidation courses and IDA as anthracycline drug 13 . However, after the advent of ATRA, the American intergroup as well as the European APL Group demonstrated in randomized studies that APL patients who achieved complete remission (CR) had a better DFS22 and overall survival (OS)23 when given ATRA-based maintenance treatment. The results of these studies were confirmed in the long-term outcome analysis.24,25
We report here the mature results of the original AIDA study in which newly diagnosed APL patients induced into CR after ATRA plus IDA (AIDA) induction treatment and consolidated with the same 3 consolidation courses used in the previous GIMEMA study13 were randomized, if reverse-transcribed polymerase chain reaction (RT-PCR)–negative for the promyelocytic leukemia/retinoic acid receptor-α (PML-RARA) hybrid gene at recovery from the third consolidation course, to the same 4 maintenance strategies used by the European APL Group.23 However, this strategy has been different from that used by the European APL group and the American intergroup. Indeed, in both these studies, patients who completed the consolidation phase were not tested for minimal residual disease by RT-PCR analysis before randomization to maintenance arms.
Methods
Between July 1993 and May 2000, 995 consecutive patients from 56 GIMEMA, 19 Associazione Italiana di Ematologia ed Oncologia Pediatrica (AIEOP), and 6 European Organization for Treatment of Cancer (EORTC) centers were registered into the study. The study was approved by the institutional review boards of all participating institutions. A complete list of participating GIMEMA, AIEOP, and EORTC centers appears in the supplemental Appendix (available on the Blood web site; see the Supplemental Materials link at the top of the online article).
Eligibility criteria were as follows: (1) a diagnosis of APL cytogenetically or molecularly confirmed by the demonstration of the t(15;17) or the PML-RARA hybrid gene, respectively; (2) age less than 75 years; and (3) informed consent in accordance with the Declaration of Helsinki.
Exclusion criteria were as follows: (1) lack of any of the eligibility criteria; (2) presence of severe cardiac disease; (3) a left ventricular ejection fraction measured by bidimensional echocardiography less than 50%; (4) a history of previous neoplastic disease, including myelodysplasia; (5) serum creatinine levels > 3.0 mg/dL; (6) serum bilirubin levels > 3.0 mg/dL; (7) serum glutamic oxaloacetic transaminase levels > 5 times the normal levels; and (8) a previous antineoplastic treatment.
Study design
The study design is summarized in Figure 1.
Induction treatment.
Patients received oral ATRA 45 mg/m2 daily, starting on day 1 and continuing until CR or for a maximum of 90 days; 12 mg/m2 of intravenous IDA was added to ATRA on days 2, 4, 6, and 8. In patients younger than 20 years, the dosage of ATRA was reduced to 25 mg/m2 daily, whereas the dosage of IDA remained the same.
Consolidation therapy.
All patients who achieved hematologic CR (hCR) were treated with 3 consolidation courses as previously indicated.13
Amended consolidation therapy for patients 60 years of age or older.
In February 1997, an age-adjusted AIDA consolidation regimen was adopted for patients 60 years of age or older. In particular, after an identical induction, these patents received only the first consolidation course; and if in molecular CR (mCR; ie, testing PML-RARA–negative in the marrow) after this cycle received 2 years of maintenance treatment with intermittent ATRA alone (45 mg/m2/d for 15 days every 3 months). As a consequence, since March 1997, patients 60 years of age or older were no longer included in this study.26 However, because some GIMEMA centers did not follow the amendment, some patients 60 years of age or older continued to receive the original AIDA protocol.
Maintenance therapy.
Patients in mCR after 3 consolidation courses and with serum bilirubin < 3.0 mg/dL, serum creatinine < 3.0 mg/dL and serum glutamic oxaloacetic transaminase levels < 5 times the normal values were randomized until January 1997, into the following 4 arms: oral 6-mercaptopurine (6-MP, 90 mg/m2/d) and intramuscular methotrexate (MTX, 15/m2/wk; arm 1); ATRA alone (45 mg/m2 for 15 days every 3 months; arm 2); alternating chemotherapy and ATRA (3 months of arm 1, then alternating to 15 days of arm 2; arm 3); and no further therapy (arm 4).
Amended maintenance therapy.
In January 1997, after randomization of 318 patients to the programmed 4 arms, the protocol was amended; and starting from February 1997, patients in mCR at recovery from the third consolidation course were randomized only to the 2 ATRA-containing options (arms 2 and 3). The total duration of maintenance treatment before and after the amendment was 2 years. The schedules of maintenance arms were identical to that proposed by the European cooperative group.23
Supportive therapy
Management of fever and infections.
During the induction phase, all patients received oral antifungal (oral fluconazole or itraconazole) and antimicrobial prophylaxis (generally ciprofloxacin) until polymorphonuclear cells were greater than 1000/μL. All febrile episodes were treated with empirical broad-spectrum antibiotics according to the protocol in use in each center. However, whenever a pathogen was isolated, antibiotics were given as prescribed by sensitivity studies.
Prevention and treatment of APL coagulopathy.
As for the prophylaxis of the coagulopathy during induction, the use of tranexamic acid (100 mg/kg in continuous infusion) was recommended. However, because there was no general agreement about the best approach for treating the coagulopathy in APL, this complication was treated according to the protocol in use in each center.
Use of blood products.
Supportive platelet transfusions were administered only in the presence of overt hemorrhage or if the platelet count was less than 30 000/μL with or without laboratory signs of severe coagulopathy (fibrinogen < 150 mg/dL and fibrin degradation products > 40 μg/mL or cross-linked fibrin > 400 μg/mL). When needed, it was common practice to transfuse 1 U/10 kg of body weight. In the case of single-donor apheresis, the transfusions provided the equivalent of 8 platelet units. Moreover, less than 15% of patients (n = 112) received more than 10 units of fresh frozen plasma as supportive treatment because of severe coagulopathy. Packed red blood cell units were transfused to maintain hemoglobin levels more than or equal to 8 g/dL.
BM samples for morphology and molecular biology, laboratory monitoring, and follow-up
Besides when clinically required, bone marrow (BM) samples for morphology and molecular biology were mandatory at diagnosis, before the initiation of the first consolidation course, at recovery from the third consolidation course, and every 3 to 4 months during the first 3 years from the end of consolidation. Thereafter, during the fourth and fifth year from the end of consolidation, BM samples were performed yearly. Moreover, BM examinations only for morphology were also mandatory before each consolidation course. Finally, whenever BM samples were collected, full evaluations of clinical chemistry and peripheral blood cell count as well as physical examination were also performed.
Molecular studies
BM samples obtained for molecular studies were processed for RNA extraction and subsequently analyzed with reverse transcriptase-polymerase chain reaction (RT-PCR) for PML-RARA as described elsewhere.27 In case of a positive result after the third consolidation course or during subsequent follow-up, a new sample, collected at least 2 weeks apart, was obtained. If positive amplification for the fusion gene PML-RARA was confirmed in this repeated sample, the patient was defined as having molecular relapse.
Outcome definitions
hCR and hematologic relapse were defined according to the National Cancer Institute criteria.28 mCR and molecular relapse were defined as the disappearance and reappearance of RT-PCR positivity for the PML-RARA fusion transcript.27 All patients who failed to achieve hCR and did not die during the induction treatment were classified as resistant disease.
The OS was calculated from the date of diagnosis until death from any cause; event-free survival (EFS) was calculated from the date of diagnosis until death, resistant disease, and hematologic or molecular relapse, considering as the event that occurred first. The molecular DFS was calculated on all patients in mCR and randomized to maintenance, starting from the day of randomization to maintenance until death in hCR and molecular or hematologic relapse, considered as the event that occurred first.
RAS
Retinoic acid syndrome (RAS)29-32 was defined as “definitely present” in the presence of the following 5 signs and symptoms: fever, dyspnea, pleural and/or pericardial effusion, pulmonary infiltrates on chest radiograph, and weight gain, whereas RAS was “indeterminate” when a combination of 2 to 4 of these signs and symptoms, associated or not with lower extremity edema and/or hypotension, were present. Considering that the diagnosis can be subtle and elusive, a high index of suspicion is required to recognize RAS early. In case of suspicion of RAS, discontinuation of ATRA and the administration of dexamethasone (10 mg total dose, intravenous every 12 hours for a minimum of 3 days) and furosemide were recommended. From December 1996, the protocol was amended, and it became mandatory since the start of induction treatment to use methylprednisolone (0.3 mg/kg/d intravenously) to prevent this syndrome.
Pseudotumor cerebri
This syndrome is characterized by one or more of the following symptoms and signs: severe headache, nausea and vomiting, papilloedema, retinal hemorrhage, and visual changes. It is particularly frequent in pediatric patients treated with a standard dose of ATRA33 and may or may not be associated with RAS. Taking into account that in pediatric patients the maximum tolerated dose34 of ATRA is 60 mg/m2, to reduce this complication it seemed appropriate to treat APL pediatric patients in our series with a reduced dosage of ATRA at 25 mg/m2, which had proven effective in a previously reported dose reduction trial for adult APL.35 In case of the appearance of pseudotumor cerebri syndrome, the use of major analgesic drugs, such as codeine or morphine sulfate, associated with discontinuation of ATRA, and the administration of dexamethasone (10 mg total dose intravenously every 12 hours for a minimum of 3 days) and furosemide were recommended.
General toxicities
Acute and subacute toxicities were graded according to the World Health Organization recommendations.
Randomization
Central randomization was performed stratifying the patients by participating Institutions. Approximate balance among the 4 randomization arms was maintained within and across participating centers.
Statistical methods
The primary objective of the study was to assess the role of maintenance therapy with ATRA, chemotherapy, or both in patients achieving mCR at the end of the consolidation phase. To this purpose, an experimental 2 × 2 factorial design was performed; under the assumption of no interactions among drugs (ie the effect of ATRA as maintenance therapy is the same, with or without 6-MP + MTX) this design allowed assessment of the effect of ATRA (comparing arms 2 and 3 vs arms 1 and 4) and the effect of 6-MP + MTX (comparing arms 1 and 3 vs arms 2 and 4). The endpoint was the molecular DFS. The total number of patients in mCR to be randomized at the end of consolidation was calculated to be 300, with 141 events, considering an accrual period of 3 years and a minimal follow-up of 1 year. Because hCR rate of 90% and a 10% of ineligible patients for randomization were expected, a total of 370 APL patients needed to be studied since diagnosis. All patients were evaluated according to the treatment assigned at randomization after an “intention-to-treat” approach. Differences in distribution between subgroups were tested applying the Fisher exact test or the χ2 test for categorical variables and applying the Wilcoxon or the Kruskal-Wallis test for continuous variables. Molecular DFS as well as EFS and OS probabilities were estimated by the Kaplan-Meier method, and differences were tested by the log-rank test. The analysis of Schoenfeld residuals from the Cox model for molecular DFS allowed detection of a violation of the proportionality assumption, thus indicating the invalidity of the log-rank test and of the Cox model for the assessment of differences. In case of nonproportionality, a Cox model with a time-varying effect was applied. Differences between the stop arm and the other maintenance arms before and after 1.6 years after randomization were also assessed, respectively, by considering only the events occurring within 1.6 years and by considering a left-truncation at 1.6 (landmark analysis). The time threshold was fixed at 1.6 on the basis of the analysis of residuals and considering that 1.6 is also the median time to end of therapy after randomization for arms 1 to 3. Median follow-up time was estimated by reversing the codes for the censoring indicator in a Kaplan-Meier analysis. All tests were 2-sided, accepting P < .05 as indicating a statistically significant difference. The analysis was performed in SAS Release, Version 9.1.3; the tests on Schoenfeld residuals were performed using the SAS macro Schoen (written by E. Bergstralh and T. Therneau, Section of Biostatistics, Mayo Clinic, Rochester, MN).
Results
Accrual and patient characteristics
Of the 995 consecutive patients registered in the study, 85 were not eligible because of patient refusal (n = 7), lack of genetic confirmation (n = 75), concurrent hematologic or oncologic disease (n = 1), low performance status (n = 1), or concomitant infection not responding to antibiotic treatment (n = 1). Moreover, 82 patients 60 years of age or older diagnosed after February 1997 who received only the first cycle of consolidation were not evaluable for this study.26 Therefore, a total of 828 patients were eligible to receive the original AIDA protocol; the main clinical and laboratory characteristics of these patients are summarized in Table 1. The median follow-up of this cohort at the time of the analysis was 7.8 years.
Clinical and laboratory characteristics of patients eligible for the AIDA protocol
| Characteristic . | Value . |
|---|---|
| No. of patients | 828 |
| Sex, male/female | 438/390 |
| Median age, y (range), no. (%) | 37.2 (1.4-74.7) |
| Patients ≤ 20 | 146 (17.65) |
| Patients > 20 and ≤ 60 | 606 (73.28) |
| Patients > 60 | 75 (9.07) |
| Median WBC count (range), /μL | 2900 (300-570 000) |
| Median platelet count (range), /μL | 24 000 (1000-480 000) |
| Patients with hemorrhagic symptoms at diagnosis, no. (%) | 521 (63.9) |
| Risk category according to Sanz et al,36 no. (%) | |
| High risk | 231 (28.17) |
| Intermediate risk | 432 (52.68) |
| Low risk | 157 (19.15) |
| Not evaluable | 8 |
| Characteristic . | Value . |
|---|---|
| No. of patients | 828 |
| Sex, male/female | 438/390 |
| Median age, y (range), no. (%) | 37.2 (1.4-74.7) |
| Patients ≤ 20 | 146 (17.65) |
| Patients > 20 and ≤ 60 | 606 (73.28) |
| Patients > 60 | 75 (9.07) |
| Median WBC count (range), /μL | 2900 (300-570 000) |
| Median platelet count (range), /μL | 24 000 (1000-480 000) |
| Patients with hemorrhagic symptoms at diagnosis, no. (%) | 521 (63.9) |
| Risk category according to Sanz et al,36 no. (%) | |
| High risk | 231 (28.17) |
| Intermediate risk | 432 (52.68) |
| Low risk | 157 (19.15) |
| Not evaluable | 8 |
Induction therapy
Of 828 patients eligible for the study, data were missing for 10 patients. Another 4 were not evaluable, 3 died before starting the treatment, in 3 there were major violations of the protocol, and 1 was lost to follow-up. Therefore, a total of 807 patients were fully evaluable for induction; of these, 761 (94.30%) achieved hCR, 44 (5.45%) died during induction, and 2 (0.25%) were considered resistant to induction. Causes of induction deaths are reported in Table 2. Median time to death during induction was 8 days (range, 0-35 days). Data for the evaluation of the RAS and pseudotumor cerebri were available for a total of 797 of 807 (98.76%) and 799 of 807 (99%) patients, respectively.
Causes of induction deaths
| Cause . | No. (%) . |
|---|---|
| Hemorrhage | 24 (54.5) |
| Early hemorrhage (before day 7) | 17/24 (70.8) |
| Infections | 6 (13.63) |
| Thromboembolism | 3 (6.8) |
| Retinoic acid syndrome | 3 (6.8) |
| Renal failure | 2 (4.5) |
| Cardiovascular disease | 2 (4.5) |
| Myocardial infarction | 1 (2.3) |
| Hepatorenal syndrome | 1 (2.3) |
| Unknown | 1 (2.3) |
| Not specified | 1 (2.3) |
| Cause . | No. (%) . |
|---|---|
| Hemorrhage | 24 (54.5) |
| Early hemorrhage (before day 7) | 17/24 (70.8) |
| Infections | 6 (13.63) |
| Thromboembolism | 3 (6.8) |
| Retinoic acid syndrome | 3 (6.8) |
| Renal failure | 2 (4.5) |
| Cardiovascular disease | 2 (4.5) |
| Myocardial infarction | 1 (2.3) |
| Hepatorenal syndrome | 1 (2.3) |
| Unknown | 1 (2.3) |
| Not specified | 1 (2.3) |
Overall, RAS was definitely present in 6 of 797 (0.75%) patients and indeterminate in 105 of 797 (13.2%) patients. In particular, as of December 1996, the RAS was definitely present in 5 of 263 patients and indeterminate in 45 of 263 patients. Thereafter, RAS was definitely present in 1 of 534 patients and indeterminate in 60 of 534 patients. The difference in the incidence of RAS definitely present and indeterminate before and after December 1996 was statistically significant (P = .0036; Table 3).
RAS definitely present or indeterminate before and after December 1996
| . | No. of patients evaluable for RAS . | RAS definitely present or indeterminate, no. (%) . | No RAS, no. (%) . | P . |
|---|---|---|---|---|
| Before | 263 | 50 (19) | 213 (81) | |
| After | 534 | 61 (11.4) | 473 (88.6) | |
| .0036 |
| . | No. of patients evaluable for RAS . | RAS definitely present or indeterminate, no. (%) . | No RAS, no. (%) . | P . |
|---|---|---|---|---|
| Before | 263 | 50 (19) | 213 (81) | |
| After | 534 | 61 (11.4) | 473 (88.6) | |
| .0036 |
In December 1996 the protocol was amended and methylprednisolone was introduced as prophylaxis of RAS.
Definitely present indicates the presence of the following 5 signs and symptoms: fever, dyspnea, pleural and/or pericardial effusion, pulmonary infiltrates on chest radiograph, and weight gain; and indeterminate, the presence of a combination of 2 to 4 of these signs and symptoms associated or not with lower extremity edema and/or hypotension.
During induction, 16 of 799 (2%) patients had pseudotumor cerebri; no difference in the incidence of this complication was observed before and after December 1996 (P = .8861; Table 4). Median times to white blood cell count (WBCs) more than 1500/μL and platelets more than 100 000/μL were 26 days (range, 1-58 days) and 27 days (range, 2-50 days), respectively.
Incidence of pseudotumor cerebri before and after December 1996
| . | No. of patients evaluable for pseudotumor cerebri . | Yes,* no. (%) . | No, no. (%) . | P . |
|---|---|---|---|---|
| Before | 263 | 5 (1.9) | 258 (98.1) | |
| After | 536 | 11 (2.0) | 525 (98.0) | |
| .8861 |
| . | No. of patients evaluable for pseudotumor cerebri . | Yes,* no. (%) . | No, no. (%) . | P . |
|---|---|---|---|---|
| Before | 263 | 5 (1.9) | 258 (98.1) | |
| After | 536 | 11 (2.0) | 525 (98.0) | |
| .8861 |
In December 1996, the protocol was amended and methylprednisolone was introduced as prophylaxis for RAS.
Among the 16 patients who experienced pseudotumor cerebri, 10 (62.5%) had less than 18 years.
Consolidation therapy
Of 761 patients who achieved hCR, 749 (98.4%) received the first consolidation cycle whereas 12 patients did not receive it because of toxicity (n = 6), protocol violation (n = 2), lost to follow-up (n = 2), refusal (n = 1), or other causes (n = 1). After the first cycle of consolidation, 19 (2.5%) patients did not proceed to the second cycle for the following reasons: severe toxicity (n = 12), lost to follow-up (n = 4), death during the consolidation course (n = 2), or protocol violation (n = 1). Therefore, 730 of 749 (97.46%) patients in hCR received the second consolidation cycle. After this cycle, 47 (6.4%) patients did not proceed to the third consolidation cycle for the following reasons: death during the consolidation course (n = 4), severe toxicity (n = 26), medical decision (n = 2), relapse (n = 3), protocol violation (n = 4), patient refusal (n = 2), or lost to follow-up (n = 6). As a consequence, the third consolidation cycle was administered to 683 of 730 (93.6%) patients who had received the second consolidation course. Of 683 patients, 2 died during the third consolidation and 17 (2.5%) were withdrawn from the protocol because of protocol violation (n = 9), medical decision (n = 6), or relapse (n = 2). Therefore, at the end of 3 consolidation cycles, a total of 664 of 683 (97.22%) patients who had initiated the third consolidation course were evaluable for RT-PCR of the PML-RARA hybrid gene. Detailed grade 3 and 4 toxicities, according to World Health Organization grading system and observed during consolidation, are listed in Table 5 where it is evident that the second consolidation course was significantly more toxic than the first and the third and that this increased toxicity is exclusively the result of a greater incidence of oral mucositis.
Grade 3 and 4 toxicities, according to WHO grading system, observed during consolidation
| Toxicity . | First cycle (n = 749 patients), no. (%) . | Second cycle (n = 730 patients), no. (%) . | Third cycle (n = 683 patients), no. (%) . | P . |
|---|---|---|---|---|
| Hemorrhage | 3 (0.40) | 5 (0.69) | 4 (0.59) | .7693 |
| Liver | 4 (0.53) | 7 (0.96) | 4 (0.59) | .6156 |
| Renal | 0 | 0 | 2 (0.29) | .0997 |
| Cardiac rhythm | 0 | 2 (0.27) | 0 | .2136 |
| Cardiac function | 1 (0.13) | 3 (0.41) | 0 | .2776 |
| Oral mucositis | 2 (0.27) | 27 (3.70) | 4 (0.59) | < .0001* |
| Nausea/vomiting | 5 (0.67) | 8 (1.10) | 7 (1.02) | .6542 |
| Diarrhea | 1 (0.13) | 2 (0.27) | 2 (0.29) | .7485 |
| Total | 16 | 54 | 23 | < .0001† |
| Toxicity . | First cycle (n = 749 patients), no. (%) . | Second cycle (n = 730 patients), no. (%) . | Third cycle (n = 683 patients), no. (%) . | P . |
|---|---|---|---|---|
| Hemorrhage | 3 (0.40) | 5 (0.69) | 4 (0.59) | .7693 |
| Liver | 4 (0.53) | 7 (0.96) | 4 (0.59) | .6156 |
| Renal | 0 | 0 | 2 (0.29) | .0997 |
| Cardiac rhythm | 0 | 2 (0.27) | 0 | .2136 |
| Cardiac function | 1 (0.13) | 3 (0.41) | 0 | .2776 |
| Oral mucositis | 2 (0.27) | 27 (3.70) | 4 (0.59) | < .0001* |
| Nausea/vomiting | 5 (0.67) | 8 (1.10) | 7 (1.02) | .6542 |
| Diarrhea | 1 (0.13) | 2 (0.27) | 2 (0.29) | .7485 |
| Total | 16 | 54 | 23 | < .0001† |
First cycle vs second cycle (P < .0001), second cycle vs third cycle (P < .0001), and first cycle vs third cycle (P = .4332).
First cycle vs second cycle (P < .0001), second cycle vs third cycle (P = .0009), and first cycle vs third cycle (P = .1568).
Supportive care during consolidation
As for transfusions, there was a statistically significant reduction in packed red cell transfusions from the first to the third consolidation cycle (86.24%, 83.24%, and 79.41%, respectively, P = .0034; data not shown). On the contrary, the rate of patients who received platelet transfusions remained stable during the 3 consolidation courses (52.33%, 56.00%, and 56.74%, respectively; P = .2562; data not shown).
As far as intravenous antibiotic treatment is concerned, during the second consolidation course, the patients received intravenous antibiotic treatment for a higher median number of days and for a longer period of time compared with first and third courses, and this difference was statistically significant (7, 10, and 7 days, respectively; P < .0001; data not shown). No significant difference was observed for intravenous antifungal treatment (9, 10, and 9 days, respectively; P = .4161; data not shown).
RT-PCR evaluation of the PML-RARA hybrid gene at the end of the 3 consolidation cycles and randomization to maintenance therapy
Of 664 cases evaluated by RT-PCR of PML-RARA at the end of consolidation, 646 (97.3%) achieved mCR and 18 (2.7%) showed persistent residual disease. Of the 646 patients in mCR, 60 were not randomized for these reasons: protocol violation (n = 27; in particular of these 27, 18 received a different maintenance treatment, 6 were allotransplanted, and 3 autotransplanted with hematopoietic stem cells), randomization refusal (n = 12), toxicity (n = 9), medical decision (n = 7), lost to follow up (n = 4), or relapse before randomization (1 case). As a consequence, 586 of 646 (90.71%) RT-PCR–negative patients were finally randomized to maintenance. Of these 586 patients, 318 observed until January 1997 were randomized to arms 1, 2, 3, and 4, whereas from February 1997, the remaining 268 patients were randomized only to arms 2 and 3. Clinical characteristics at diagnosis of all randomized patients are reported in Table 6.
Characteristics at diagnosis of patients randomized to maintenance treatment before and after amendment
| . | Median WBCs × 103/μL (range) . | Median platelets × 103/μL, (range) . | Median age, y (range) . | Male/female . |
|---|---|---|---|---|
| Before | ||||
| A | 2.5 (0.6-140.0) | 20.0 (2.0-167.0) | 32.9 (7.5-68.8) | 53/30 |
| A + C | 3.0 (0.4-125.4) | 29.0 (3.0-241.0) | 41.6 (2.8-71.6) | 38/43 |
| C | 2.2 (0.3-108.0) | 21.0 (4.0-178.0) | 36.3 (5.1-73.9) | 46/32 |
| O | 2.5 (0.4-43.9) | 21.5 (1.0-480.0) | 35.8 (2.2-67.6) | 41/35 |
| P | .7570 | .1117 | .4736 | .1576 |
| After | ||||
| A | 4.3 (0.3-570.0) | 22.0 (2.0-173.0) | 33.4 (1.9-70.3) | 70/65 |
| A + C | 2.9 (0.3-180.0) | 23.0 (3.0-227.0) | 33.7 (2.0-71.8) | 67/66 |
| P | .1223 | .5419 | .1390 | .8090 |
| . | Median WBCs × 103/μL (range) . | Median platelets × 103/μL, (range) . | Median age, y (range) . | Male/female . |
|---|---|---|---|---|
| Before | ||||
| A | 2.5 (0.6-140.0) | 20.0 (2.0-167.0) | 32.9 (7.5-68.8) | 53/30 |
| A + C | 3.0 (0.4-125.4) | 29.0 (3.0-241.0) | 41.6 (2.8-71.6) | 38/43 |
| C | 2.2 (0.3-108.0) | 21.0 (4.0-178.0) | 36.3 (5.1-73.9) | 46/32 |
| O | 2.5 (0.4-43.9) | 21.5 (1.0-480.0) | 35.8 (2.2-67.6) | 41/35 |
| P | .7570 | .1117 | .4736 | .1576 |
| After | ||||
| A | 4.3 (0.3-570.0) | 22.0 (2.0-173.0) | 33.4 (1.9-70.3) | 70/65 |
| A + C | 2.9 (0.3-180.0) | 23.0 (3.0-227.0) | 33.7 (2.0-71.8) | 67/66 |
| P | .1223 | .5419 | .1390 | .8090 |
A indicates ATRA; A + C = ATRA + chemotherapy; C = chemotherapy; and O = observation.
EFS, OS, and molecular DFS
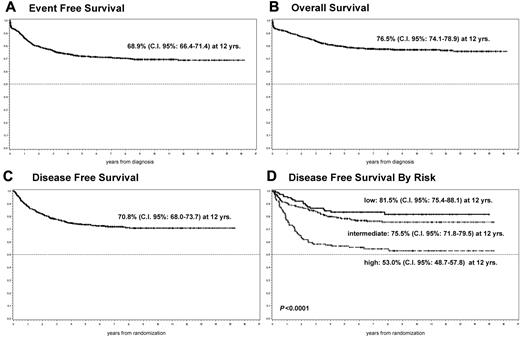
Considering the 828 eligible patients, the EFS and OS estimates at 12 years were 68.9% (95% confidence interval [CI], 66.4%-71.4%) and 76.5% (95% CI, 74.1%-78.9%) respectively (Figure 2A-B). As for the 586 patients in mCR randomized to maintenance, the 12-year estimation was 70.8% (95% CI, 68.0%-73.7%; Figure 2C).
EFS, OS, and DFS. EFS (A) and OS (B) probability for the whole cohort of patients. DFS probability (C) and DFS probability by risk group (D) for patients randomized to maintenance.
EFS, OS, and DFS. EFS (A) and OS (B) probability for the whole cohort of patients. DFS probability (C) and DFS probability by risk group (D) for patients randomized to maintenance.
Molecular DFS by risk group and age
A statistically significant difference (P < .0001) was observed in molecular DFS comparing the entire population of patients randomized to maintenance according to the risk factors described by Sanz et al.36 In particular, the molecular DFS at 12 years was 81.5% (95% CI, 75.4%-88.1%) in the low-risk group (WBCs ≤ 10 000/μL and platelets > 40 000/μL), 75.5% (95% CI, 71.8%-79.5%) in the intermediate-risk group (WBCs ≤ 10 000/μL and platelets ≤ 40 000/μL), and 53.0% (95% CI, 48.7%-57.8%) in the high-risk group (WBCs ≥ 10 000/μL; Figure 2D).
After stratification according to age (≤ 20, > 20 and < 60, and ≥ 60 years), no difference in terms of DFS was found between patients ≤ 20 and > 20 and < 60 years, but the comparison between patients ≥ 60 years and the first 2 groups was significantly different (P = .0468), in favor of patients younger than 60 years (data not shown).
Effect of maintenance treatments on molecular DFS
Before February 1997, 318 patients PML-RARA–negative at the end of consolidation were randomized to receive 6-MP + MTX (arm 1; 78 randomized); ATRA alone (arm 2; 83 randomized); alternating chemotherapy and ATRA (arm 3; 81 randomized); and no further therapy (arm 4; 76 randomized).
Effect of chemotherapy (6-MP + MTX) maintenance on DFS before maintenance amendment.
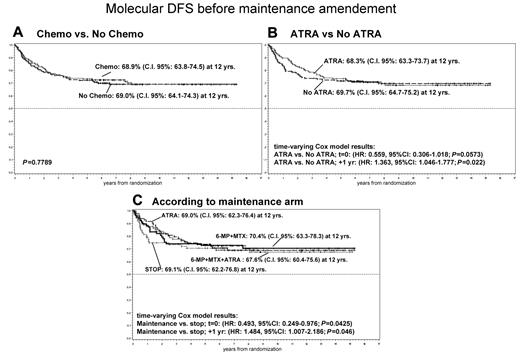
It was evaluated comparing arms 1 and 3 versus arms 2 and 4. The DFS estimations at 12 years were 68.9% (95% CI, 63.8%-74.5%) and 69.0% (95% CI, 64.1%-74.3%), respectively (P = .7789; Figure 3A).
DFS probability before maintenance amendment by randomization. (A) Chemotherapy vs no chemotherapy. (B) ATRA vs no ATRA. (C) According to maintenance arm.
DFS probability before maintenance amendment by randomization. (A) Chemotherapy vs no chemotherapy. (B) ATRA vs no ATRA. (C) According to maintenance arm.
Effect of ATRA maintenance on DFS before maintenance amendment.
It was evaluated comparing arms 2 and 3 versus arms 1 and 4. The DFS at 12 years was quite similar in the 2 groups: 68.3% (95% CI, 63.3%-73.7%) versus 69.7% (95% CI, 64.7%-75.2%), respectively. Patients who did not receive ATRA during maintenance presented more events in the first period (25 vs 17; P = not significant); however, approximately 2 years after randomization, the number of events was the same in the 4 arms (Figure 3B).
Role of the 4 different maintenance strategies on DFS before maintenance amendment.
In the first period after randomization, there was a significant difference between arm 4 (no further therapy) and the other 3 arms, with worse DFS for arm 4 (considering failures within 1.6 years, P = .035; data not shown). Evidence of worse outcomes for arm 4 indeed suggested amending the protocol and randomizing only between arms 2 and 3. Yet, with longer follow-up, it was possible to observe that initial differences disappeared, and at the time of the analysis the 12-year DFS probabilities were very similar in the 4 groups, estimates were: arm 1, 70.4% (95% CI, 63.3%-78.3%); arm 2, 69.0% (95% CI, 62.3%-76.4%); arm 3, 67.6% (95% CI, 60.4%-75.6%); and arm 4, 69.1% (95% CI, 62.2%-76.8%). A Cox model for DFS with time-varying effect estimated that the hazard of failure for arms 1 to 3 compared with arm 4 was initially halved and subsequently increased by 48% each year, therefore returning equivalence approximately 2 years after randomization (Figure 3C).
The initial difference between the 2 groups, treated versus not treated, seems to be explained by relapses because the overall survival of these 2 groups is similar (P = .3317; data not shown).
Role of maintenance on DFS, after maintenance amendment.
From February 1997, an additional 268 patients PML-RARA–negative at the end of consolidation were randomized only to receive ATRA alone (arm 2; 137 randomized) or alternating chemotherapy and ATRA (arm 3; 131 randomized). No statistically significant difference in molecular DFS was observed between these additional 2 groups (Figure 4A).
DFS probability after maintenance amendment by randomization. (A) ATRA vs ATRA plus chemotherapy. (B) DFS probability before and after maintenance amendment by randomized ATRA vs ATRA plus chemotherapy. (C) DFS before vs after maintenance amendment.
DFS probability after maintenance amendment by randomization. (A) ATRA vs ATRA plus chemotherapy. (B) DFS probability before and after maintenance amendment by randomized ATRA vs ATRA plus chemotherapy. (C) DFS before vs after maintenance amendment.
Role of maintenance strategies on DFS before and after maintenance amendment.
Combining those patients who had received ATRA or alternating chemotherapy and ATRA before and after February 1997, no statistically significant difference for molecular DFS between these 2 groups was observed (Figure 4B). Finally, even comparing the 2 molecular DFS obtained before and after the maintenance amendment, no difference in the outcome of patients in mCR was observed (Figure 4C).
Toxicity during maintenance
Of the 290 patients who were randomized to receive chemotherapy with or without ATRA as maintenance, the dosage of MTX and 6-MP was reduced at least once because of WBCs less than or equal to 3000 μL or more than or equal to grade 1 hepatic toxicity in 102 and 75 patients, respectively; definitive discontinuation of the maintenance was never registered. Of the 586 patients randomized to maintenance, 9 (1.5%) died because of infection (n = 3), myocardial infarction (n = 2), solid tumor (n = 2), hemorrhage (n = 1), or suicide (n = 1). Of these 9 patients, 4 were randomized to ATRA plus chemotherapy, 2 to ATRA alone, 2 to chemotherapy alone, and the last to observation. There was only 1 patient who had a sepsis after the first and the third maintenance course. This patient recovered completely and continued to receive maintenance at reduced dosage.
Discussion
This study indicates that, in newly diagnosed APL induced in CR by combining ATRA and IDA and consolidated with the same 3 intensive courses used in the previous GIMEMA protocol,13 the addition of a maintenance treatment does not provide any survival advantage in patients who tested RT-PCR–negative for the PML-RARA hybrid gene at recovery from the third consolidation course. This result, in contrast with previous published experience demonstrating that an ATRA-based maintenance is needed after consolidation to ameliorate DFS or OS survival,22-25 confirms the previous GIMEMA experience conducted in the pre-ATRA era.13
Since the late 1980s, the policy of using IDA in the induction therapy for APL has been a peculiarity of the Italian cooperative group GIMEMA.12,13 In a previous randomized study, the GIMEMA compared IDA versus IDA and cytarabine for induction and maintenance with low-dose chemotherapy versus observation.13 In that study, carried out before the advent of either ATRA or genetic diagnosis, we reported that induction therapy with IDA was the only variable significantly influencing the EFS duration in patients with newly diagnosed hypergranular APL. Moreover, the addition of a maintenance treatment with continuous low-dose 6-MP and weekly intermittent MTX, as previously suggested by 2 retrospective studies,20,21 did not influence the outcome of these patients.13 In the present study, the addition of ATRA to IDA greatly increased, as expected, the CR rate (94.3%). However, despite patients being randomized for maintenance to the identical 4 arms used by the European APL Group, we were unable to demonstrate a benefit on DFS from any of the maintenance options.
The reasons why our results on maintenance are in contrast with the previously published experiences of the North American Intergroup and the European APL Group may be related to several differences in the mentioned studies, which can be summarized as follows. In the AIDA protocol: (1) all patients receiving induction treatment had molecular or cytogenetic evidence of the t(15;17) in the leukemic cells; (2) the used anthracycline in induction as well as in consolidation was IDA instead of DNR; (3) patients induced in hCR in the AIDA received 3 consolidation courses (of which the first and the third were IDA based and the second contained the drugs mitoxantrone and etoposide), whereas only 2 consolidation courses (DNR-based) were used in the other studies; and (4) only patients in molecular remission (ie, those who tested RT-PCR–negative for the hybrid gene PML-RARA at the end of consolidation) were randomized to maintenance in our study.
Recently, a randomized study from the Japan Adult Leukemia Study Group demonstrated that maintenance chemotherapy did not improve DFS in newly diagnosed APL patients who were in molecular remission after consolidation therapy.37 In this Japan Adult Leukemia Study Group study, after an induction phase with ATRA alone or in combination with IDA and cytosine arabinoside, patients were given 3 consolidation courses with IDA, DNR, mitoxantrone, etoposide, and cytosine arabinoside (ie, the same drugs, with the exclusion of DNR, used in the present study).
The primary objective of this study was to assess the role of maintenance therapy in APL patients achieving mCR at the end of the consolidation phase. As soon as we have reached the programmed number of randomized patients (n = 300) to assess this objective and that these patients have been followed for an adequate period of time, a first analysis revealed that patients who did not receive ATRA presented more events than those who received ATRA (25 vs 17; P = not significant) during the first period from randomization. As a consequence, considering the 2 × 2 factorial design, both maintenance arms without ATRA were closed. One can argue: if only arm 4 was closed, would we have received a more clear answer about the need of maintenance in general? Unfortunately, an answer to this question cannot be given. However, in the previous GIMEMA study LAP 0389 in which responding patients were consolidated with 3 consolidation courses identical, for drugs and dosage, to the consolidation courses of the present study, patients randomized to maintenance with 6-MP and MTX did not have a better outcome than those randomized to observation.13
Moreover, to verify the possibility that the amendment made in 1997 could have introduced a bias (ie, those patients in arm 4 who continued on the study without maintenance could have been by chance and despite the random, a selected subgroup who did not need maintenance), we have analyzed the population randomized to arm 4 (observation) before the amendment and have found that, of 76 patients randomized in this arm, 13 (17.1%) were high-risk, 52 (68.4%) were intermediate-risk, and only 11 (14.5%) were low-risk. This result reduced the possibility of a bias because the great majority of patients (65 of 76; 85.5%) randomized to observation belonged to high- and intermediate-risk groups, whereas only 11 of 76 (14.5%) of these patients were in the low-risk category.
As concerns the toxicity observed during consolidation, oral mucositis grades III and IV according to the World Health Organization was significantly more frequent after the second consolidation. Moreover, during this course, patients received intravenous antibiotic treatment for a higher median number of days and for a longer period of time compared with first and third courses, and this difference was statistically significant (P < .0001); severe toxicity during maintenance was very rarely observed.
The analysis of DFS by risk group according to Sanz et al revealed a statistically significant difference (P = .0001) in DFS in favor of the low- and the intermediate-risk group (APL patients with WBCs < 10 000/μL) compared with the high-risk group (APL patients with WBCs > 10 000/μL). Taken together, these results suggest that APL patients should not all receive the same consolidation intensity. The recently reported results of risk-adapted protocols proposed by the PETHEMA and the GIMEMA groups clearly indicate a benefit in terms of both efficacy and safety from the adoption of less or more intensive postremission treatment according to risk group.16,17
The recent published results of AIDA 200017 may raise the question of whether physicians should choose not to give maintenance, based on the present study, or give maintenance based on the AIDA 2000 results. We may not give a solution to this question. However, unlike the AIDA 0493 where the target was the utility of maintenance with or without ATRA after an intensive consolidation, in the AIDA 2000 the target was whether a risk-adapted consolidation without reducing the efficacy of treatment was possible. For this reason, it was decided to maintain all patients with ATRA plus chemotherapy, and this policy was particularly effective in high-risk patients.
As to the incidence of RAS, this was very low (0.075%) if we consider only patients with all the following 5 signs and symptoms: fever, dyspnea, pleural and/or pericardial effusion, pulmonary infiltrates on chest radiograph, and weight gain. However, 105 (13.17%) of the 797 patients evaluable for this syndrome had 2 to 4 of these signs and symptoms, associated (or not) with lower extremity edema and/or hypotension. It is, therefore, possible that the concurrent administration of chemotherapy with ATRA has decreased the incidence of the syndrome compared with other experiences. Moreover, we had the opportunity of monitoring the incidence of this syndrome before and after the addition of steroid prophylaxis to the induction protocol. The results indicate that this amendment to the original induction protocol significantly reduced the incidence of RAS, confirming a previous observation in a very small cohort of patients.38
In conclusion, in this study on a very high number of patients with genetically proven APL, we did not find any long-term beneficial effect of maintenance with low-dose chemotherapy in patients negative for PML-RARA at the end of consolidation. It is, therefore, probable that the usefulness of maintenance therapy in APL may depend on the type of anthracycline (IDA vs DNR), on the intensity of chemotherapy delivered during induction and consolidation, and on the risk group. The results of the present study may, therefore, be useful to design new risk-adapted APL studies in which subgroups of patients, depending on the risk group, may or may not receive maintenance without compromising their outcome, provided they are consolidated with an intensive risk-adapted consolidation.
Presented in part at the 45th American Society of Hematology Annual Meeting, San Diego, CA, December 6-9, 2003. (Abstract 487).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Sandra De Simone (GIMEMA data manager).
This study was supported in part by the Associazione Italiana contro le Leucemie and Associazione Italiana contro il Cancro.
Authorship
Contribution: G.A., F.L.-C., M.C.P., and F. Mandelli designed the study; G.A., F.L.-C., and M.V. analyzed and interpreted the data; F.P.P. and P.F. carried out statistical analysis; G.A. and F.L.-C. wrote the paper; D.D. performed molecular tests; and R.L., G.S., M.B., E.D.B., G.F., F. Marmont, A.R., F.D.R., M.G.K., G.P., E.M.P., G.R., N.C., F.N., A.G., F.F., and S.A. included data of patients treated in their institutions, reviewed the manuscript, and contributed to the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giuseppe Avvisati, Unità Operativa Complessa di Ematologia e Terapia Cellulare, Università Campus Bio-Medico, Via Àlvaro del Portillo, 200, 00128 Roma, Italy; e-mail: g.avvisati@unicampus.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal