Abstract
The final stages of of megakaryocyte (MK) maturation involve a series of steps, including polyploidization and proplatelet formation. Although these processes are highly dependent on dynamic changes in the microtubule (MT) cytoskeleton, the mechanisms responsible for regulation of MTs in MKs remain poorly defined. Stathmin is a highly conserved MT-regulatory protein that has been suggested to play a role in MK differentiation of human leukemic cell lines. However, previous studies defining this relationship have reached contradictory conclusions. In this study, we addressed this controversy and investigated the role of stathmin in primary human MKs. To explore the importance of stathmin down-regulation during megakaryocytopoiesis, we used a lentiviral-mediated gene delivery system to prevent physiologic down-regulation of stathmin in primary MKs. We demonstrated that sustained expression of constitutively active stathmin delayed cytoplasmic maturation (ie, glycoprotein GPIb and platelet factor 4 expression) and reduced the ability of MKs to achieve high levels of ploidy. Moreover, platelet production was impaired in MKs in which down-regulation of stathmin expression was prevented. These studies indicate that suppression of stathmin is biologically important for MK maturation and platelet production and support the importance of MT regulation during the final stages of thrombopoiesis.
Introduction
Megakaryocytopoiesis is a complex process in which hematopoietic stem cells proliferate, differentiate, undergo terminal maturation, and give rise to circulating platelets.1 During the early stages of megakaryocytopoiesis, megakaryocyte (MK) progenitors are diploid, proliferative and express early markers of the MK lineage. In the later stages, MKs cease to divide but continue to replicate their DNA and become polyploid (ie, DNA content > 2N). This is accompanied by expression of more MK-specific markers and an increase in nuclear complexity and cell size. Lastly, mature MKs develop long cytoplasmic extensions, known as proplatelets, that release large numbers of platelets into the circulation.2
The processes of polyploidization and proplatelet formation are unique to the MK lineage. Both processes are characterized by dramatic changes in the organization of the microtubule (MT) cytoskeleton. After multiple rounds of normal cell divisions, MK progenitors switch from a normal to a form of abortive mitosis, known as endomitosis, in which MKs enter and progress normally through mitosis but fail to undergo cytokinesis and divide.3-5 This process is mediated by an atypical organization of MTs that forms a complex, spherical mitotic spindle.4,6 Recent studies have suggested that the failure of MKs to complete cytokinesis is associated, in part, with defective elongation of spindle MTs.5 On the other hand, studies by Patel et al have demonstrated that the cytoplasm of mature MKs contains bundles of polymerized MTs that extend into the proplatelets and provide their structural scaffold during platelet morphogenesis.7 Although these structural changes are well described at the morphologic level, very little is known about the regulatory mechanisms that are responsible for these unique changes.
Stathmin is a member of a highly conserved family of stathmin-like proteins that have an MT-depolymerizing function.8 Dynamic changes between polymerization and depolymerization of α/β-tubulin heterodimers provide the basis for the MT functions. The MT-depolymerizing activity of stathmin underlies dynamic changes of MT. Alterations of stathmin expression and/or function in different cell types result in morphologic and functional defects of MTs both during interphase and mitosis.9,10 In murine MKs and in human leukemic cell lines with MK-like features, stathmin is down-regulated during differentiation and its level of expression inversely correlates with the level of ploidy.11,12 Furthermore, alterations of stathmin expression in these cell lines are associated with abnormal mitotic spindles and changes in their propensity to become polyploid.12,13 However, these studies reached contradictory conclusions about the effects of stathmin on chemically induced differentiation of human leukemic cells with MK features. This controversy was explained by the different approaches used to interfere with stathmin expression and by the limited ability of transformed MK cell lines to undergo MK differentiation. In this report, we assessed the influence of stathmin on human primary MK development and identify a role for stathmin in MK maturation and platelet formation.
Methods
Primary MK cultures
Human MKs were generated in liquid culture from peripheral blood (PB)-derived CD34+ cells purchased from AllCells and suspended in Iscove modified Dulbecco medium (Invitrogen) supplemented with 1% penicillin/streptomycin, 1% L-glutamine (Invitrogen), 20mM β-mercaptoethanol, 1% bovine serum albumin fraction V (Sigma-Aldrich), 30% serum substitute BIT 9500 (Stem Cell Technologies), 100 ng/mL recombinant human stem cell factor, and 50 ng/mL recombinant human thrombopoietin (R&D Systems). After the initial 6 days, the recombinant human stem cell factor was withdrawn and the cells were cultured for 7 to 10 additional days. CD34+ cells were also plated in semisolid collagen-based cultures in MegaCult media (Stem Cell Technologies) supplemented with recombinant human thrombopoietin, recombinant human interleukin-3, and recombinant human interleukin-6 (R&D Systems) for 16 days.
Generation of FIV-based vectors and lentiviral transductions
Lentiviral vectors were generated by cloning stathmin cDNA into a feline immunodeficiency virus (FIV)–based pCDF1-MCS2-EF1-copGFP plasmid backbone (System Biosciences) using standard molecular cloning techniques. A 1.2-kb BamH I/XbaI fragment of wild-type (STMN-WT) or mutant (STMN-4A) stathmin cDNA was excised from pTRE2-STMN plasmids.13 STMN-4A cDNA was obtained by site-directed mutagenesis replacing serine with alanine at the 4 phosphorylation sites (4A) as described.13 The new plasmids and the empty vector were each cotransfected along with packing pCPR-ΔEnv and envelope pCI-VSV plasmids14 in 293T human embryonic kidney (HEK) cells using Lipofectamine 2000 (Invitrogen). Viral titers were determined by infecting 1 × 105 293HEK cells with 1 mL of viral supernatant. The number of transducing units (TU) were calculated based on the percentage of GFP+ cells detected by flow cytometric analysis. Viral supernatants were concentrated to 109 TU/mL by centrifugation using Peg-It solution (System Biosciences) according to the manufacturer's instructions and stored at −80°C. Primary cells were infected with 108 TU/mL in 12-well tissue culture plates in the presence of 5 μg/mL polybrene (Sigma-Aldrich) and centrifuged at 1450g for 90 minutes at 30°C, followed by incubation at 32°C in a humidified 5% CO2 incubator. After 24 hours, the cells were replated in fresh medium and incubated at 37°C in a humidified 5% CO2 incubator.
RNA quantification
Total RNA was purified using a RNeasy purification kit (QIAGEN). A total of 1 μg of total RNA was used for cDNA synthesis using Omniscript kit (QIAGEN). One-tenth of the cDNA obtained by reverse-transcribed polymerase chain reaction (PCR) was used for quantitative real-time PCR using primers specific for human stathmin, GATA-1, and platelet factor 4 (SA Biosciences). cDNA templates were mixed with 1 times IQ SYBR Green Supermix (Bio-Rad) and 0.2mM of each primer in a total volume of 50 μL in PCR plates (Fisher Scientific) in duplicates. Amplification was performed using a RealPlex MasterCycler (Eppendorf).
Western blotting
Cells were lysed (50mM Tris-Cl, 15mM NaCl, 1% Triton-X, 40 mg/mL protease inhibitor cocktail, Roche Molecular Biochemicals); then 50 μg of protein was separated on 12.5% SDS-PAGE gels and transferred to a polyvinylidene difluoride membrane. Polyclonal antistathmin antibodies (Calbiochem) followed by goat anti–rabbit IgG–horseradish peroxidase–conjugated secondary antibodies (Pierce Chemical) were used to detect stathmin. Monoclonal antiactin antibodies (Oncogene Research Products) followed by goat anti–mouse IgM–horseradish peroxidase secondary antibodies (Calbiochem) were used to detect actin. The proteins were visualized by enhanced chemiluminescence detection (GE Healthcare).
Microscopic analysis
Cells were placed on slides using a Shandon centrifuge (Life Sciences International), fixed in methanol for 5 minutes, and stained with Wright-Giemsa (Sigma-Aldrich) for 20 minutes. MegaCult cultures were fixed and stained using the manufacturer's staining kit (StemCell Technologies). The slides were visualized using an Olympus BX40 microscope and a 40×/0.75 dry objective. Image acquisition and analysis were performed using a Spot Insight 2 camera and Spot software Version 4.5 (Diagnostic Instruments Inc).
Flow cytometric analysis
Cultured cells were incubated with phycoerythrin (PE)-conjugated anti-CD41 antibodies and allophycocyanin (APC)–conjugated anti-CD42b antibodies (BD Biosciences) for 45 minutes and then with 5 μL 7-aminoactinomycin for 15 additional minutes. The data were acquired and analyzed using a FACSCanto II flow cytometer and a FACS Diva software (BD Biosciences). DNA content was evaluated based on propidium iodide staining as described.12
Analysis of culture-derived platelets
MK cultures were harvested and centrifuged at 150g for 5 minutes, and the supernatants were collected and further centrifuged at 2850g for 10 minutes. The pellet containing culture-derived platelets was resuspended in phosphate-buffered saline with 0.1% fetal bovine serum and labeled with CD41-PE and CD42-APC antibodies for 30 minutes at room temperature. Reticulated platelets were identified after incubation with 2 μg/mL thiaozole orange (TO, Sigma-Aldrich; stock solution of 1 mg/mL in methanol) for an additional 15 minutes at room temperature in the dark. The cells were then washed, resuspended in phosphate-buffered saline with 0.1% fetal bovine serum, and analyzed by flow cytometry. PB-derived human platelets were immunolabeled in the same manner and used as control.
FISH
Uninfected and lentivirus-infected MKs were fixed then labeled with 2 different DNA probes, the α-satellite sequence of the centromeric region of chromosome X (Xp11.1-q11.1), and the satellite III sequence of chromosome Y (Yq12) as described.15 These DNA probes were obtained from Abbott Molecular and labeled with Spectrum Orange and Spectrum Green, respectively. Fluorescence in situ hybridization (FISH) probe hybridization signals were visualized using an Axioplan 2 fluorescence microscope (Carl Zeiss) at 100× magnification and imaged with Cyto-Vision software Version 3.6 (Genetix).
Statistical analysis
Data are expressed as mean plus or minus SD and analyzed using Student unpaired t test. P values of less than .05 were considered statistically significant.
Results
Characterization of primary MK cultures
Human MKs were generated in vitro from human CD34+ cells using a 2-step liquid culture differentiation protocol. In the first step, CD34+ cells were plated in the presence of thrombopoietin and stem cell factor for 6 days. In the second step, stem cell factor was removed and MK progenitors were allowed to further differentiate and mature in the presence of thrombopoietin for 7 to 10 additional days. MK differentiation and maturation were monitored by analyzing MK morphology and their ability to produce proplatelets and platelets in culture. MK-specific surface glycoprotein complex GPIIb/IIIa and GPIb expression was monitored by labeling with anti-CD41 and anti-CD42b antibodies, respectively. Immunolabeled cells were analyzed by flow cytometry by acquiring all events on a logarithmic scale to allow the simultaneous identification of MKs and small culture-derived platelets (Figure 1A). MK progenitors were observed as early as day 3 in culture as CD41+/CD42b− cells, whereas mature MKs were observed as early as day 6 as CD41+/CD42b+ cells. By the end of the maturation step (ie, day 14 in liquid culture), the majority of cells (ie, between 80% and 90%) were MKs, out of which 40% to 50% were mature MKs (Figure 1A). Morphologic analysis of cytospin preparations showed a heterogeneous MK population at different stages of differentiation, including easily recognizable large polyploid MKs with multilobulated nuclei (Figure 1B).
Phenotypical characterization of human primary MK cultures. (A) Flow cytometric analysis of MK cultures labeled with PE-conjugated anti-CD41 and APC-conjugated anti-CD42 antibodies. The density plot on the left demonstrates the forward scatter and side scatter properties of the cells that were acquired on a logarithmic scale to identify both MKs and platelet-sized cells (PTL). The density plots on the right show CD41+/CD42b− and CD41+/CD42b+ MKs and culture-derived PTL present in the bottom right and top right quadrants, respectively. (B) MKs derived from CD34+ hematopoietic progenitor cells were visualized by phase-contrast (i) and light microscopy after Wright-Giemsa (ii) and GPIIb/IIIa staining (iii). Mature MKs with polylobulated nuclei and large proplatelet-bearing MKs are present in liquid culture (i-ii, arrows); MKs grown in semisolid culture display cytoplasmic extensions or proplatelets (iii, arrowheads) with nascent platelets at their ends (iii, arrows). (C) Flow cytometric analysis of culture-derived platelets labeled with anti-CD41 antibodies and TO. The dot plots in the upper panels represent human PB PTL labeled with CD41 antibodies (x-axis) and TO (y-axis), which served as a positive control. The plots in the bottom panels represent culture-derived PTL labeled in the same manner.
Phenotypical characterization of human primary MK cultures. (A) Flow cytometric analysis of MK cultures labeled with PE-conjugated anti-CD41 and APC-conjugated anti-CD42 antibodies. The density plot on the left demonstrates the forward scatter and side scatter properties of the cells that were acquired on a logarithmic scale to identify both MKs and platelet-sized cells (PTL). The density plots on the right show CD41+/CD42b− and CD41+/CD42b+ MKs and culture-derived PTL present in the bottom right and top right quadrants, respectively. (B) MKs derived from CD34+ hematopoietic progenitor cells were visualized by phase-contrast (i) and light microscopy after Wright-Giemsa (ii) and GPIIb/IIIa staining (iii). Mature MKs with polylobulated nuclei and large proplatelet-bearing MKs are present in liquid culture (i-ii, arrows); MKs grown in semisolid culture display cytoplasmic extensions or proplatelets (iii, arrowheads) with nascent platelets at their ends (iii, arrows). (C) Flow cytometric analysis of culture-derived platelets labeled with anti-CD41 antibodies and TO. The dot plots in the upper panels represent human PB PTL labeled with CD41 antibodies (x-axis) and TO (y-axis), which served as a positive control. The plots in the bottom panels represent culture-derived PTL labeled in the same manner.
To determine whether the cultured MKs were capable of forming proplatelets and producing platelets in vitro, the cultures were initially examined microscopically to visualize proplatelets and analyzed flow cytometrically to identify and quantify platelets generated in vitro. MKs with cytoplasmic extensions resembling proplatelets were observed as early as day 10 and increased in number progressively until they peaked on day 16. Visualization of proplatelet-bearing MKs was first performed in liquid culture and then recorded in situ in collagen-based semisolid cultures as shown in a representative micrograph in Figure 1B. Culture-derived platelets were first identified by CD41/CD42b immunolabeling and quantified flow cytometrically. An analytic gate was set based on the forward and side scatter light properties of human PB-derived platelets that were used as a positive control for CD41/CD42b expression. Between 11% and 16% of platelet-sized particles were CD41+/CD42b+ (Figure 1A). To ensure that these are platelets that had been generated in vitro rather than contaminating platelets from the primary CD34+ cells preparations, we used TO in parallel with CD41 labeling. TO is an RNA-binding dye that is widely used to distinguish young anucleated blood cells (eg, reticulocytes and newly formed platelets) from their more mature counterparts.16,17 Primary human platelets labeled in a similar manner were used as control. Only 3% to 4% of PB-derived platelets were CD41+/TO+, a value that falls within the normal range reported in the literature.16,17 By contrast, 14% of the cells within the platelet-sized fraction of a typical MK culture were TO+/CD41+, indicating that most of the platelets derived in MK cultures were recently generated (Figure 1C).
Lentivirus-mediated stathmin expression in primary MKs
In the current studies, we assessed the expression of stathmin in human MKs derived from CD34+ hematopoietic progenitor cells. mRNA extracted from MKs harvested at different times in culture was used in quantitative real-time PCR analysis. As illustrated in Figure 2A, the highest levels of stathmin mRNA were found in proliferating MK progenitors on days 3 and 6. A steep decline in stathmin expression coincided with induction of maturation and declined to very low levels in mature MKs. Low levels of stathmin expression in mature MKs were also confirmed at the protein level by immunofluorescence staining for stathmin (Figure 2B). Hypo-lobulated MKs (ie, less mature with low ploidy) were characterized by uniform and intense staining, whereas MKs with multilobulated nuclei (ie, mature with higher ploidy) were characterized by less intense staining for stathmin.
Stathmin expression in human primary MKs. (A) mRNA extracted from MKs generated in liquid culture from CD34+ cells was reverse-transcribed and then amplified by quantitative real-time PCR using human stathmin and GAPDH primers. Relative mRNA amounts obtained at different time points during culture (ie, day 3 [d3] to day 14 [d14]) were plotted after normalization to GAPDH. The results represent the mean ± SD of duplicates in 3 independent experiments. Only statistically significant differences are indicated by the P values. (B) MKs generated in the presence of thrombopoietin for 7 days were immunolabeled with FITC-conjugated antistathmin antibodies to visualize stathmin expression (green fluorescence) and with Hoechst 33342 to visualize nuclear morphology (blue fluorescence). Large mononucleated MKs (ie, less mature with low ploidy) show uniform, intense stathmin expression throughout the cytoplasm (i, arrows). MKs with multilobulated nuclei (ie, mature MKs with higher ploidy) show an overall reduction in stathmin expression which, in some cells, is accompanied by an uneven expression pattern throughout the cytoplasm (ii-iii, arrows). Image acquisition was performed using a Zeiss Axioplan 2 microscope equipped with a 63×/1.4 oil objective and an AxiCam MRm camera (Carl Zeiss). Image analysis was performed using Zeiss AxioVision LE software.
Stathmin expression in human primary MKs. (A) mRNA extracted from MKs generated in liquid culture from CD34+ cells was reverse-transcribed and then amplified by quantitative real-time PCR using human stathmin and GAPDH primers. Relative mRNA amounts obtained at different time points during culture (ie, day 3 [d3] to day 14 [d14]) were plotted after normalization to GAPDH. The results represent the mean ± SD of duplicates in 3 independent experiments. Only statistically significant differences are indicated by the P values. (B) MKs generated in the presence of thrombopoietin for 7 days were immunolabeled with FITC-conjugated antistathmin antibodies to visualize stathmin expression (green fluorescence) and with Hoechst 33342 to visualize nuclear morphology (blue fluorescence). Large mononucleated MKs (ie, less mature with low ploidy) show uniform, intense stathmin expression throughout the cytoplasm (i, arrows). MKs with multilobulated nuclei (ie, mature MKs with higher ploidy) show an overall reduction in stathmin expression which, in some cells, is accompanied by an uneven expression pattern throughout the cytoplasm (ii-iii, arrows). Image acquisition was performed using a Zeiss Axioplan 2 microscope equipped with a 63×/1.4 oil objective and an AxiCam MRm camera (Carl Zeiss). Image analysis was performed using Zeiss AxioVision LE software.
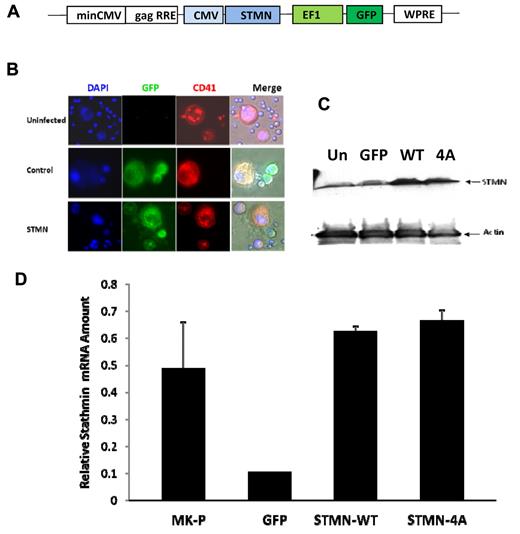
Having confirmed that stathmin is down-regulated during the terminal stages of megakaryocytopoiesis, we designed a strategy to prevent its physiologic down-regulation and investigated the effects of the sustained expression on MK maturation. We selected an FIV-based lentiviral expression system18 to prevent stathmin down-regulation during MK maturation (Figure 3A). We generated 2 different lentiviral vectors, one expressing wild-type stathmin (STMN-WT) and another expressing a phosphorylation-deficient mutant form of stathmin (STMN-4A). The rationale for generating a vector that expresses mutant stathmin was based on previously published data demonstrating that the biologic activity of ectopically expressed STMN-WT is often lost as a result of its phosphorylation by a variety of cellular kinases.19,20 Therefore, expressing a nonphosphorylated protein that is resistant to inactivation allowed us to test the effects of a constitutively active protein that possesses MT-depolymerizing activity. Finally, in addition to stathmin-expressing vectors, we used a vector-expressing GFP only as a control. Each of the 3 transfer vectors was cotransfected separately with the packaging and envelope plasmids into 293T cells to produce lentiviral particles.
Lentivirus-mediated delivery of stathmin in mature MKs. (A) Schematic representation of the transfer vector backbone of the FIV-based lentiviral system in which stathmin (STMN) cDNA is driven by cytomegalovirus (CMV) promoter and GFP gene is driven by elongation factor 1 (EF1) promoter. (B) Immunofluorescence microscopy analysis of MKs infected with stathmin-expressing lentiviruses (green fluorescence) that were labeled with PE-conjugated anti-CD41 antibodies (red fluorescence) and with Hoechst 33342 to visualize DNA and nuclear morphology (blue fluorescence). Image acquisition was performed using a Zeiss Axioplan 2 microscope equipped with a 40×/0.75 dry objective and an AxiCam MRm camera (Carl Zeiss). Image analysis was performed using Zeiss AxioVision LE software. (C) Western blot analysis of protein lysates extracted from 293HEK cells uninfected (Un), infected with control (GFP), and WT or mutant (4A) stathmin-expressing lentiviruses. After blotting, the membranes were incubated with anti–human stathmin polyclonal antibody (top panel) and with antiactin monoclonal antibodies, the latter used as control for protein loading. (D) Quantitative real-time PCR analysis of mRNA extracted from MKs infected with control (MK + GFP), wild-type (MK + STMN-WT), or mutant (MK + STMN-4A) stathmin lentiviruses. mRNA extracted from day 3 MK progenitors (MK-P) was also included in the analysis as reference for basal levels of stathmin expression. Each column represents the mean ± SD of 3 independent experiments performed in duplicates and normalized to GAPDH.
Lentivirus-mediated delivery of stathmin in mature MKs. (A) Schematic representation of the transfer vector backbone of the FIV-based lentiviral system in which stathmin (STMN) cDNA is driven by cytomegalovirus (CMV) promoter and GFP gene is driven by elongation factor 1 (EF1) promoter. (B) Immunofluorescence microscopy analysis of MKs infected with stathmin-expressing lentiviruses (green fluorescence) that were labeled with PE-conjugated anti-CD41 antibodies (red fluorescence) and with Hoechst 33342 to visualize DNA and nuclear morphology (blue fluorescence). Image acquisition was performed using a Zeiss Axioplan 2 microscope equipped with a 40×/0.75 dry objective and an AxiCam MRm camera (Carl Zeiss). Image analysis was performed using Zeiss AxioVision LE software. (C) Western blot analysis of protein lysates extracted from 293HEK cells uninfected (Un), infected with control (GFP), and WT or mutant (4A) stathmin-expressing lentiviruses. After blotting, the membranes were incubated with anti–human stathmin polyclonal antibody (top panel) and with antiactin monoclonal antibodies, the latter used as control for protein loading. (D) Quantitative real-time PCR analysis of mRNA extracted from MKs infected with control (MK + GFP), wild-type (MK + STMN-WT), or mutant (MK + STMN-4A) stathmin lentiviruses. mRNA extracted from day 3 MK progenitors (MK-P) was also included in the analysis as reference for basal levels of stathmin expression. Each column represents the mean ± SD of 3 independent experiments performed in duplicates and normalized to GAPDH.
The ability of the generated lentiviral particles to transduce and express GFP and stathmin in target cells was tested in 293HEK cells and primary MKs. Four days after infection, genomic DNA was extracted from infected cells and assessed for the presence of the virus by PCR and for transgene expression. GFP expression was evaluated by microscopy and flow cytometry, and stathmin expression was evaluated by Western blotting and quantitative RT-PCR. To visualize MKs, the cultures were labeled with PE-conjugated anti-CD41 antibodies and with Hoechst 33342 to visualize nuclear morphology. Representative microphotographs of primary MKs infected with lentiviral particles are shown in Figure 3B. Large CD41+ MKs (red fluorescence) with polylobulated nuclei (blue fluorescence) are present in both uninfected and lentivirus-infected cultures, whereas GFP+ cells (green fluorescence) are seen only in lentivirus-infected cultures. These findings were confirmed by flow cytometric analysis of dual GFP/CD41 expression (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). 7-Aminoactinomycin labeling was used to determine the effects of lentiviral infection on cell viability and to exclude nonviable cells from further analysis. Cell viability was not significantly affected by transduction with control or stathmin-expressing lentiviruses.
Protein lysates from uninfected 293HEK cells and cells infected with control GFP, STMN-WT, or STMN-4A lentiviruses were analyzed by Western blotting to assess lentiviral-mediated stathmin expression at the protein level. As shown in Figure 3C, the stathmin level in cells infected with the control virus was similar to that of the baseline level in uninfected cells. In contrast, cells infected with either STMN-WT or STMN-4A showed a marked increase in the levels of stathmin expression. Because lentiviral transduction efficiency might differ between HEK293 cells and primary MKs, we evaluated the efficiency of transduction of stathmin-expressing lentiviruses in primary MKs. Flow cytometric analysis of CD41+/GFP+ cells had an efficiency of infection ranging from 40% to 53% in MKs and from 23% to 33% in culture-derived platelets (supplemental Figure 1). In several experiments, the efficiencies of transfection (ie, percentage of GFP-expressing MKs) were comparable between control and stathmin-expressing lentiviruses. More importantly, quantitative real-time PCR analysis of mRNA extracted from primary MKs transduced with STMN-WT or STMN-4A lentiviruses showed a 6-fold increase in stathmin expression compared with those infected with control lentiviruses (Figure 3D). These levels of transgene expression in mature MKs were similar to those normally present in MK progenitors before maturation.
Effects of stathmin expression on MK maturation
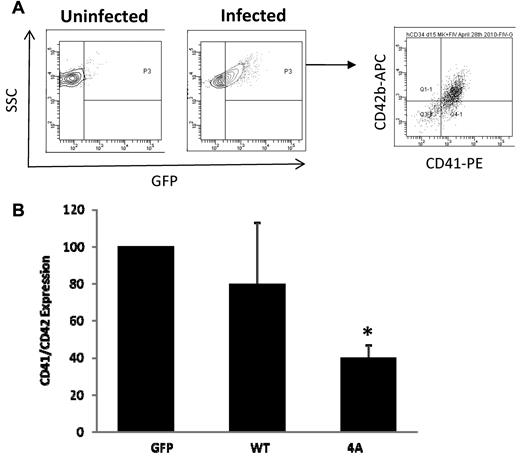
We examined the effects of lentiviral-mediated stathmin expression on cytoplasmic maturation of MKs. Because numerous studies had previously demonstrated that alterations of stathmin expression interfere with cellular proliferation,21 to avoid a potential negative effect of stathmin expression on proliferation of MK progenitors, we used a 2-step liquid culture system that allows for expansion and differentiation of MK progenitors during the first step in the absence of lentiviruses, followed by maturation during the second step in the presence of lentiviruses. Control MKs and MKs infected with stathmin-expressing lentiviruses were analyzed on day 14 to determine the effects of stathmin expression on phenotypic and molecular markers of MK maturation. The presence of GFP in all 3 lentiviruses made it possible to monitor and analyze infected cells exclusively. CD41 expression was used as marker of MK lineage throughout all stages of differentiation, whereas CD42b expression was used to identify more differentiated, mature MKs.22 Uninfected cells were used as negative controls to set a gate for detection of infected cells based on GFP fluorescence. Subsequently, the fraction of CD41+/CD42b+ cells within the GFP+ population was quantified (Figure 4A). Expression of wild-type stathmin did not significantly alter CD41/CD42b expression, whereas expression of the constitutively active form of stathmin resulted in more than 50% reduction in the fraction of mature MKs (Figure 4B).
Flow cytometric analysis of MKs infected with control and stathmin-expressing lentiviruses. (A) The density plot on the left represents uninfected MKs that were used as negative control for GFP expression. The density plot in the middle represents infected MKs showing GFP expression (x-axis). The dot plot on the right represents GFP+ MKs that express CD41 (x-axis) and CD42b (y-axis). (B) Quantification of CD41+/CD42+ MKs infected with control (GFP), WT, or nonphosphorylatable mutant (4A) stathmin-expressing lentiviruses. The results are represented as fold change in the percentage of CD41+/CD42b+ MKs in 3 independent experiments ± SD.
Flow cytometric analysis of MKs infected with control and stathmin-expressing lentiviruses. (A) The density plot on the left represents uninfected MKs that were used as negative control for GFP expression. The density plot in the middle represents infected MKs showing GFP expression (x-axis). The dot plot on the right represents GFP+ MKs that express CD41 (x-axis) and CD42b (y-axis). (B) Quantification of CD41+/CD42+ MKs infected with control (GFP), WT, or nonphosphorylatable mutant (4A) stathmin-expressing lentiviruses. The results are represented as fold change in the percentage of CD41+/CD42b+ MKs in 3 independent experiments ± SD.
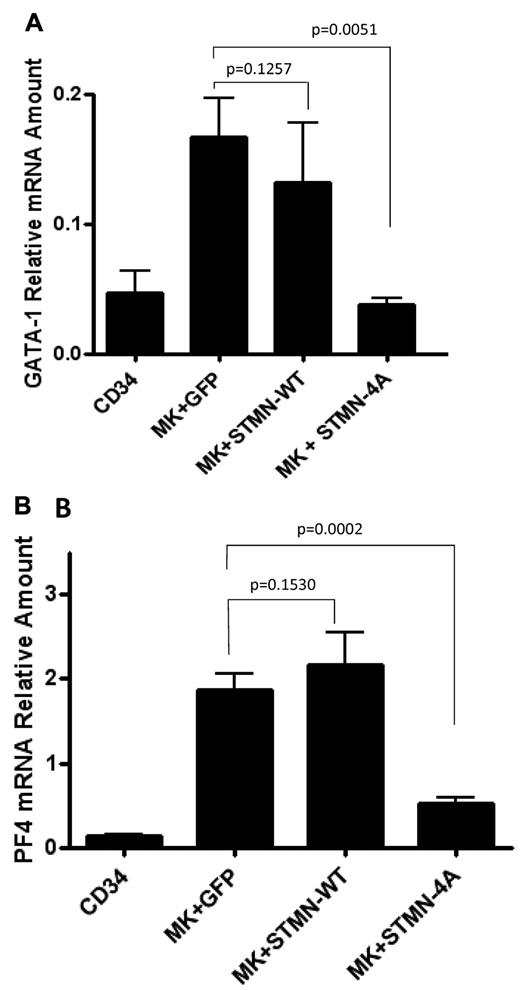
Finally, we evaluated the effects of stathmin expression on markers of MK differentiation by assessing the levels of GATA-1 and platelet factor 4 (PF4) expression. mRNA extracted from MK cultures infected with either control GFP, STMN-WT, or STMN-4A was reverse-transcribed and used for quantitative real-time PCR using primers specific for GATA-1 and PF4. mRNA extracted from undifferentiated CD34+ progenitor cells was used in parallel to determine the baseline expression for both GATA-1 and PF4. The results are expressed as relative mRNA amounts after normalization to GAPDH mRNA and presented in Figure 5. We detected increased GATA-1 levels in mature MKs infected with control lentiviruses compared with primary CD34+ cells. Interestingly, MKs infected with lentiviruses expressing wild-type stathmin were also characterized by up-regulation of GATA-1 expression. In contrast, up-regulation of GATA-1 was not observed in MKs infected with lentiviruses expressing the constitutively active form of stathmin (Figure 5A). PF4 expression was barely detectable in CD34+ cells, whereas it was markedly up-regulated in mature MKs infected with the control lentiviral vector. A small but not statistically significant increase in PF4 expression was observed in MKs that were transduced with the wild-type lentiviral vector. However, up-regulation of PF4 was not observed in MKs transduced with the lentivirus that encodes the constitutively active form of stathmin (Figure 5B). Taken together, these results demonstrate that expression of constitutively active stathmin prevents expression of CD42b, GATA-1, and PF4 expression, supporting the hypothesis that physiologic down-regulation of stathmin is required for terminal MK differentiation.
Quantitative real-time PCR analysis of GATA-1 and PF4 expression. mRNAs extracted from CD34+ cells and from day 14 MKs infected with control (MK + GFP), wild-type (MK + STMN-WT), or nonphosphorylatable mutant (MK + STMN-4A) stathmin-expressing lentiviruses were reverse transcribed and then amplified by quantitative real-time PCR using human GATA-1 (A) and PF4 (B) primers. Each column represents the mean ± SD of 3 independent experiments each performed in duplicate and normalized to GAPDH.
Quantitative real-time PCR analysis of GATA-1 and PF4 expression. mRNAs extracted from CD34+ cells and from day 14 MKs infected with control (MK + GFP), wild-type (MK + STMN-WT), or nonphosphorylatable mutant (MK + STMN-4A) stathmin-expressing lentiviruses were reverse transcribed and then amplified by quantitative real-time PCR using human GATA-1 (A) and PF4 (B) primers. Each column represents the mean ± SD of 3 independent experiments each performed in duplicate and normalized to GAPDH.
Effects of stathmin expression on MK polyploidization
To determine whether sustained stathmin expression interferes with the ability of primary MKs to become polyploid, we analyzed ploidy in MKs after infection with control GFP, STMN-WT, or STMN-4A lentiviruses. Evaluation of ploidy was first performed by flow cytometric analysis of DNA content after propidium iodide staining. Using this method, we were able to obtain an assessment of overall ploidy and found that control and wild-type stathmin-infected cultures had comparable fractions of polyploid MKs (ie, 11.8% and 12.2% MKs with > 4N DNA content, respectively). In contrast, mutant stathmin-infected cultures had a lower fraction of MKs with more than 4N DNA content (ie, 7.1%; Figure 6A). Although we observed a shift from high to low ploidy levels in the presence of sustained stathmin expression, it was difficult to distinguish and quantify different ploidy classes because of the low resolution of the DNA histograms. This was because of a combination of factors, including heterogeneity in cell and nuclear size, mechanical fragility, and the limited number of cells. Thus, we adapted FISH technologies previously used by Raslova et al to evaluate ploidy based on the number of copies of chromosomes.23 MKs transduced with the different stathmin-expressing lentiviruses were placed on slides, labeled with fluorochrome-conjugated probes against X and Y chromosomes, and analyzed by microscopy. Whereas a typical human cell with 2N DNA content (ie, diploid) has one copy of each chromosome X and Y (or 2 copies of an X-chromosome if the cell originated from a female), a cell with 4N DNA content (ie, tetraploid) has 2 copies of each chromosome, a cell with 8N DNA has 4 copies of each chromosome, and so on. Representative examples of MKs with ploidy levels ranging from 2N to 32N are presented in Figure 6B. As shown, the increase in ploidy levels correlates with the nuclear complexity detected by 4,6-diamidino-2-phenylindole staining (blue fluorescence) and with nuclear size. To assess the effects of stathmin on the generation of MKs with high ploidy levels, we analyzed by FISH cells with more than or equal to 8N DNA and categorized them in 3 ploidy classes based on the number of X and Y chromosome copies (Table 1). As shown, we found that sustained stathmin expression was associated with a decrease in the fraction of MKs with 16N and 32N DNA in both wild type- and constitutively active stathmin-expressing cultures. Confirming flow cytometric analysis, this was accompanied by an accumulation of MKs with lower ploidy (ie, 8N DNA content). Taken together, these results indicate that sustained expression of stathmin impaired the ability of MKs to achieve high ploidy levels.
Evaluation of MK ploidy. (A) Representative examples of DNA histograms obtained by flow cytometric analysis of propidium iodide-stained MKs grown in the presence of control and stathmin-expressing lentiviruses (STMN-WT and STMN-4A). The percentages of polyploid cells with more than 4N DNA content are indicated in each condition within the gate P4. (B) FISH analysis of MKs generated in the same conditions. MKs cytospun on slides, fixed, and labeled with Spectrum Orange and Spectrum Green-conjugated probes were visualized by fluorescence microscopy to identify X (red fluorescence) and Y (green fluorescence) chromosomes, respectively. The slides were counterstained with 4,6-diamidino-2-phenylindole to visualize nuclear morphology. One copy of each X and Y chromosome indicates 2N DNA content, 2 copies of each chromosome indicates 4N, 4 copies indicates 8N, and so on. As illustrated, the increase in ploidy is accompanied by an increase in nuclear size and complexity (ie, polylobulation).
Evaluation of MK ploidy. (A) Representative examples of DNA histograms obtained by flow cytometric analysis of propidium iodide-stained MKs grown in the presence of control and stathmin-expressing lentiviruses (STMN-WT and STMN-4A). The percentages of polyploid cells with more than 4N DNA content are indicated in each condition within the gate P4. (B) FISH analysis of MKs generated in the same conditions. MKs cytospun on slides, fixed, and labeled with Spectrum Orange and Spectrum Green-conjugated probes were visualized by fluorescence microscopy to identify X (red fluorescence) and Y (green fluorescence) chromosomes, respectively. The slides were counterstained with 4,6-diamidino-2-phenylindole to visualize nuclear morphology. One copy of each X and Y chromosome indicates 2N DNA content, 2 copies of each chromosome indicates 4N, 4 copies indicates 8N, and so on. As illustrated, the increase in ploidy is accompanied by an increase in nuclear size and complexity (ie, polylobulation).
Analysis of high ploidy MK by FISH
| Ploidy class . | Control . | STMN-WT . | STMN-4A . |
|---|---|---|---|
| 8N | 76.5 | 82.1 | 93.7 |
| 16N | 17.6 | 13.4 | 6.2 |
| 32N | 5.8 | 4.5 | 0 |
| Ploidy class . | Control . | STMN-WT . | STMN-4A . |
|---|---|---|---|
| 8N | 76.5 | 82.1 | 93.7 |
| 16N | 17.6 | 13.4 | 6.2 |
| 32N | 5.8 | 4.5 | 0 |
MKs with ≥ 8N DNA content were categorized in ploidy classes (8N, 16N, and 32N) based on the number of copies of each chromosome as illustrated in Figure 6. The numbers represent percentages of MKs in each culture condition: control, STMN-WT, and STMN-4A. More than 500 cells were analyzed in each condition in 2 independent experiments.
Effects of stathmin expression on platelet formation
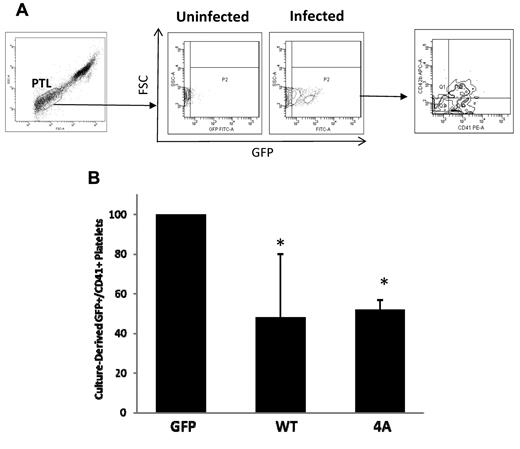
We next examined whether sustained stathmin expression interferes with the process of platelet formation. After infection with control or stathmin-expressing lentiviruses, MK cultures were inspected by microscopy to visualize proplatelet formation. By day 14, control cultures contained large MKs displaying long cytoplasmic extensions resembling proplatelets. The frequency of proplatelet-bearing MKs was much lower in the cultures infected with stathmin-expressing lentiviruses. Because the number of platelets derived in culture is directly proportional with the number of proplatelets formed, we collected and labeled culture-derived platelets from MKs infected with control or stathmin-expressing lentiviruses and quantified them by flow cytometry. Platelets derived from uninfected MK cultures were used as negative control for GFP expression. As illustrated in Figure 7A, platelet-sized particles derived from lentivirus-infected cultures are GFP+, indicating that they were derived from the GFP+ MKs. From the platelet-sized GFP+ events, we quantified only those expressing CD41. The results presented in the bar graph in Figure 7B show a 2-fold reduction in the fraction of platelets derived in vitro from MKs expressing wild-type or phosphorylation-deficient stathmin compared with those produced by control MKs. This finding indicates that sustained stathmin expression has a negative effect on in vitro platelet production, suggesting that alterations of expression of a MT-regulatory protein can interfere with platelet production by primary MKs.
Flow cytometric analysis of platelets derived in culture from MKs infected with control and stathmin-expressing lentiviruses. (A) The dot plot on the left shows the analytic gate containing culture-derived platelets (PTL). This gate was set based on the forward and side scatter light properties of human PB platelets, as described in “Analysis of culture-derived platelets.” The density plots in the middle represent platelets derived from uninfected MKs used as negative control for GFP expression and platelets derived from lentivirus-infected MKs showing GFP expression (x-axis). The density plot on the right represents GFP+ culture-derived platelets showing CD41 expression (x-axis) and CD42b expression (y-axis). (B) Quantification of platelets derived from MKs infected with control (GFP), wild-type (WT), or nonphosphorylatable mutant (4A) stathmin-expressing lentiviruses. The results are represented as fold change in the percentage plus or minus SD of GFP+/CD41+ platelets in 3 independent experiments.
Flow cytometric analysis of platelets derived in culture from MKs infected with control and stathmin-expressing lentiviruses. (A) The dot plot on the left shows the analytic gate containing culture-derived platelets (PTL). This gate was set based on the forward and side scatter light properties of human PB platelets, as described in “Analysis of culture-derived platelets.” The density plots in the middle represent platelets derived from uninfected MKs used as negative control for GFP expression and platelets derived from lentivirus-infected MKs showing GFP expression (x-axis). The density plot on the right represents GFP+ culture-derived platelets showing CD41 expression (x-axis) and CD42b expression (y-axis). (B) Quantification of platelets derived from MKs infected with control (GFP), wild-type (WT), or nonphosphorylatable mutant (4A) stathmin-expressing lentiviruses. The results are represented as fold change in the percentage plus or minus SD of GFP+/CD41+ platelets in 3 independent experiments.
Finally, to evaluate the effects of stathmin expression on microtubule organization, we performed immunofluorescence staining of microtubules in control and stathmin-expressing MKs. A well-organized cytoplasmic network of microtubules was observed in control MKs and MKs infected with wild-type stathmin (supplemental Figure 2). In contrast, MKs infected with the constitutively active form of stathmin were characterized by diffuse tubulin staining and only short bundles of polymerized microtubules. Interestingly, the cultures infected with control lentiviruses had small platelet-like structures lacking nucleus, whereas the cultures infected with wild-type stathmin lentiviruses had mature multi-lobulated MKs bearing cytoplasmic blebs of variable sizes, including platelet-sized structures (supplemental Figure 2). Neither platelets nor MKs with cytoplasmic blebbing were observed in the cultures infected with mutant stathmin lentiviruses. Cytoplasmic blebbing was also confirmed by electron microscopic analysis as illustrated in supplemental Figure 3. Numerous structures reminiscent of microparticles24 were observed in cultures infected with wild-type stathmin-expressing lentiviruses. Moreover, we did not observe demarcation membranes in stathmin-expressing MKs but structures resembling multivesicular bodies (supplemental Figure 3). Of note, the latter structures are an intermediate stage in the formation of platelet α- and dense-granules and precede the demarcation membrane system during MK maturation.25,26
Discussion
MK maturation is characterized by unique structural changes of MT cytoskeleton that are critically important for polyploidization and proplatelet extension. MTs mediate their cellular functions by dynamic changes between polymerization of α/β-tubulin heterodimers and depolymerization of MT polymers.27 These functions are tightly regulated by a balance between MT-stabilizing proteins (ie, promoting polymerization) and MT-destabilizing proteins (ie, promoting depolymerization).8 Despite the recent appreciation of the importance of MT for MK maturation, the mechanisms that regulate MT dynamics during megakaryocytopoiesis remain poorly defined. Stathmin is the prototype of a small family of MT-regulatory proteins that share the functional property of promoting MT depolymerization.8
Although numerous studies have demonstrated the importance of stathmin in cellular proliferation,9,20,28-30 the biologic relevance of its down-regulation as cells undergo terminal differentiation and maturation remains unknown. In this study, we show that stathmin is highly expressed in MK progenitors and is progressively down-regulated as cells differentiate and mature. This is consistent with previous observations that stathmin levels are higher in immature murine MKs than in mature MKs and are undetectable in murine platelets.12,31,32 Because stathmin function is required for cellular proliferation, the observation that it is expressed at high levels in proliferating MK progenitors was not surprising. To test whether down-regulation of stathmin expression is important for MK maturation, we used lentiviral-mediated gene transfer to prevent down-regulation of stathmin in the later stages of megakaryocytopoiesis. We showed that terminal maturation, as reflected by reduced CD42b and PF4 expression, was reduced in the presence of sustained stathmin expression. In addition, the observation that GATA-1 expression was not up-regulated in MKs in which stathmin expression was sustained also supports the negative effects of stathmin on MK maturation. GATA-1 plays a critical role in MK development and its deficiency results in impaired MK differentiation.33 Interestingly, studies by Muntean et al showed that GATA-1-deficient murine MKs express higher levels of stathmin compared with their wild-type counterparts.34 This suggests that stathmin might be regulated by GATA-1 during normal megakaryocytopoiesis. This hypothesis is supported by the presence of several putative GATA-1 binding sites within the stathmin gene promoter.
The first suggestion of a potential involvement of stathmin in polyploidization came from studies showing that alterations of stathmin expression (ie, inhibition or forced expression) in human leukemic cells with MK properties was associated with changes in polyploidization.11,12 On the one hand, Chang et al showed that stathmin overexpression was required for phorbol ester-induced polyploidization during MK differentiation of HEL cells.11 By contrast, we demonstrated that inhibition of stathmin expression increases ploidy, whereas its overexpression decreases ploidy during staurosporine-induced MK differentiation of K562 cells. These contradictory findings might be a reflection of the different experimental models that were used to investigate the potential role of stathmin in megakaryocytopoiesis. In both studies, transformed, aneuploid cell lines were used, which have a limited ability to undergo terminal MK maturation. Moreover, the baseline levels of stathmin in these leukemic cells are unusually high, which raises questions about the relevance of these observations to polyploidization of primary MKs. In the current study, we addressed this issue and showed that sustained levels of stathmin in primary MKs, especially of its functionally inactive form, prevented their ability to achieve high levels of ploidy. Recent studies have suggested that the failure of endomitotic MKs to undergo cytokinesis is associated with defective spindle elongation and is in part the result of defective signaling through the Rho family of proteins.5 Rac1 and cdc42, members of the Rho family, are known to regulate microtubule dynamics by inactivating stathmin.35,36 Furthermore, stathmin can be also inactivated by Aurora B,37 a chromosome passenger protein that is required for proper MK endomitosis.38 This suggests that phosphorylation-mediated inactivation of stathmin may be important for the complex MT spindle reorganization that takes place during endomitosis. It is also possible that the observed effects of sustained stathmin expression on ploidy are not mediated exclusively by its MT-regulatory activities, but they might be a reflection of delayed cytoplasmic maturation. However, although cytoplasmic maturation and polyploidization of MKs take place in parallel, it has been suggested that they are independent processes.39
Unlike cytoplasmic maturation, platelet production was inhibited by both wild-type and phosphorylation-deficient stathmin expression, suggesting that complete suppression of stathmin expression and function may be required for normal platelet production. This is in agreement with earlier studies showing that in vitro platelet formation was impaired by MT-depolymerizing drugs, such as nocodazole and Vinca alkaloids (vincristine, vinblastine).40,41 However, our current studies provide additional evidence that deregulation of a cellular MT-regulatory factor can impair platelet production by primary MKs. The reduction in megakaryocyte maturation and polyploidization that we observed in the presence of sustained levels of constitutively active stathmin was accompanied by decreased platelet production, whereas sustained levels of wild-type stathmin reduced platelet production without affecting megakaryocyte maturation. These observations suggest that wild-type stathmin might interfere with platelet formation independent of its effects on MK maturation. The observation that MKs infected with wild-type stathmin-expressing vectors do not develop a demarcation membrane system suggests that sustained expression of wild-type stathmin may interfere with late events during platelet production. In contrast, sustained expression of the constitutively active form of stathmin may result in more profound interference with microtubule dynamics and interfere with both MK maturation and platelet formation. Finally, MKs that express sustained levels of wild-type stathmin appear to form large numbers of microparticles, suggesting a potential role for microtubules and stathmin in microparticle production by MKs. This intriguing observation requires further investigation in the future. Interestingly, the β1-isoform of tubulin accounts for more than 90% of platelet MT, and its expression is restricted to MK lineage.42 This suggests that the β1-isoform of tubulin may be an excellent therapeutic target for disrupting MT function exclusively in MKs to prevent excessive platelet production in diseases, such as myeloproliferative neoplasms.
In conclusion, we prevented physiologic down-regulation of stathmin in MKs as an experimental tool to investigate its potential role in MT-dependent aspects of MK maturation. We demonstrated that down-regulation of stathmin is required for normal megakaryocytopoiesis by showing that sustained stathmin expression and/or function results in impaired MK maturation and platelet production. Hematologic malignancies, such as leukemia and myelodysplastic syndrome, express very high levels of stathmin.43,44 Deficient platelet production is one of the hallmarks of leukemia and myelodyplastic syndrome, and bleeding from low platelet counts is one of the most common causes of death in these patients.45,46 Therapeutic modalities are being developed to interfere with stathmin expression to inhibit cell proliferation, angiogenesis, and cell migration in several types of cancer.47,48 The present study opens new avenues of investigation into the mechanisms responsible for MK maturation that may prove relevant for hematologic malignancies in which stathmin expression and/or functions are altered.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the National Institutes of Health (DK076796 Career Development Grant; C.I.-R.).
National Institutes of Health
Authorship
Contribution: C.I.-R. designed and performed research, analyzed data, and wrote the paper; D.G. and J.T. performed research; V.N. contributed vital reagents and analytical tools; R.E.G. provided analytical tools; R.H. discussed the results and revised the manuscript; and G.F.A. discussed results and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Camelia Iancu-Rubin, Division of Hematology and Medical Oncology, Department of Medicine and The Tisch Cancer Institute, Mount Sinai School of Medicine, Annenberg Building, Room 24-10, One Gustave Levy Place, Box 1079, New York, NY 10029; e-mail: camelia.iancu-rubin@mssm.edu.


![Figure 2. Stathmin expression in human primary MKs. (A) mRNA extracted from MKs generated in liquid culture from CD34+ cells was reverse-transcribed and then amplified by quantitative real-time PCR using human stathmin and GAPDH primers. Relative mRNA amounts obtained at different time points during culture (ie, day 3 [d3] to day 14 [d14]) were plotted after normalization to GAPDH. The results represent the mean ± SD of duplicates in 3 independent experiments. Only statistically significant differences are indicated by the P values. (B) MKs generated in the presence of thrombopoietin for 7 days were immunolabeled with FITC-conjugated antistathmin antibodies to visualize stathmin expression (green fluorescence) and with Hoechst 33342 to visualize nuclear morphology (blue fluorescence). Large mononucleated MKs (ie, less mature with low ploidy) show uniform, intense stathmin expression throughout the cytoplasm (i, arrows). MKs with multilobulated nuclei (ie, mature MKs with higher ploidy) show an overall reduction in stathmin expression which, in some cells, is accompanied by an uneven expression pattern throughout the cytoplasm (ii-iii, arrows). Image acquisition was performed using a Zeiss Axioplan 2 microscope equipped with a 63×/1.4 oil objective and an AxiCam MRm camera (Carl Zeiss). Image analysis was performed using Zeiss AxioVision LE software.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/17/10.1182_blood-2010-09-305540/4/m_zh89991169840002.jpeg?Expires=1769177718&Signature=dUg6er3IOP8e16okI9rklKpmP2YDsQRS6G8Lf6b89xLXLoG67HRVRtYV5mDe2-r3t5KD5GSI0MqQfawsUfgndAThmdbnlvZF-WJfjNLUEMfBJoknBJZ2499aAaFmAY5LzYAbZXJb7syY-yXHVic9fHQr2RsDtn7jkvAa3jET9ngx0agFx5K~aqf8waP66vE4y887vMolb10l8SWTHZvqSfYGoQOTeeZlc6bVdq~n~00CwisyBCYvg6L0G7FRyKrjtiYDGhpZtqPR0FVJJ2AjIvWqwEjU29vaeIT4qs3vc2lYRq1Bxx-BhMSFIeLceaCRuRQ6KruHzigICSDkc4NaRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal