Abstract
The low frequency of hematopoietic stem and progenitor cells (HSPCs) in human BM has precluded analysis of the direct biochemical effects elicited by cytokines in these populations, and their functional consequences. Here, single-cell phospho-specific flow cytometry was used to define the signaling networks active in 5 previously defined human HSPC subsets. This analysis revealed that the currently defined HSC compartment is composed of biochemically distinct subsets with the ability to respond rapidly and directly in vitro to a broader array of cytokines than previously appreciated, including G-CSF. The G-CSF response was physiologically relevant—driving cell-cycle entry and increased proliferation in a subset of single cells within the HSC compartment. The heterogeneity in the single-cell signaling and proliferation responses prompted subfractionation of the adult BM HSC compartment by expression of CD114 (G-CSF receptor). Xenotransplantation assays revealed that HSC activity is significantly enriched in the CD114neg/lo compartment, and almost completely absent in the CD114pos subfraction. The single-cell analyses used here can be adapted for further refinement of HSPC surface immunophenotypes, and for examining the direct regulatory effects of other factors on the homeostasis of stem and progenitor populations in normal or diseased states.
Introduction
Adult tissue stem cells are the rare reservoirs of cells responsible for the maintenance and regeneration of diverse tissues including blood, breast, muscle, and colon,1 and are characterized by directed self-renewal and differentiation that are tightly regulated by environmental niches.2 Hematopoiesis is organized as a cellular hierarchy in which HSCs give rise to successively lineage-restricted progenitors that eventually produce all the terminally differentiated cells of the blood.3 Current understanding of the mechanisms regulating HSCs and downstream progeny (collectively termed hematopoietic stem and progenitor cells, or HSPCs), including the cell-intrinsic and extrinsic pathways necessary for their maintenance and functional heterogeneity, draws primarily from manipulation of mouse model systems or long-term analysis of surrogate assays for stem cell function in unseparated human progenitor populations.1,4-7 While valuable information has been gained from these studies, the direct biochemical regulation of the most primitive, currently defined human HSPC populations has not been characterized.
Cytokines play key roles in the homeostasis of the later stages of the hematopoietic system by regulating cell growth, proliferation, survival, and differentiation.8 Several cytokines have been adopted as clinical therapeutics for their effects on hematopoietic lineage cells.9 In particular, G-CSF is used to treat neutropenia of diverse etiologies and to mobilize HSPCs before harvest for clinical hematopoietic cell transplantation.10 The mobilization effects of G-CSF are believed to be indirect, with G-CSF acting on mature resident BM cells which release mediators that cleave the adhesion factors responsible for HSC retention in BM niches.11-13 These and other previous reports have suggested that HSCs do not respond directly to G-CSF.14-16 In contrast, a recent study showed a rapid response to G-CSF in the heterogeneous early mouse KLS (c-kit+Lineage−Sca-l+) progenitor compartment, containing both HSCs and downstream multipotent progenitors (MPPs).17 However, the many differences between mouse and human HSCs—including the disparate markers used to identify them18,19 —necessitate direct investigation of human HSCs before the application of these findings to humans.
In addition to better understanding the biochemical regulatory networks active in human HSCs, there is a greater need to understand the well-described heterogeneity within the HSC compartment (currently best defined as Lin−CD34+CD38− CD90+CD45RA−).20,21 The reasons for this heterogeneity remain unclear.7,22 Possibilities include stochasticity, epigenetic or biochemical diversity of the single-cells composing the HSC pool, technical limitations, or some combination of the above. Full clinical use of HSCs requires a greater understanding of the mechanisms governing their behavior.
Here, single-cell phospho-specific analysis was used to establish the response profile of human HSCs/HSPCs to a broad range of hematopoietic cytokines. This analysis demonstrated that the currently defined HSC compartment is composed of biochemically distinct subsets, and that cellular proliferation can be directly regulated by G-CSF. Importantly, the heterogeneous nature of the G-CSF response led to xenotransplantation assays that ultimately revealed enrichment of human HSC activity in the CD114neg/lo subfraction of the currently defined HSC compartment.
Methods
Cells
Normal adult BM mononuclear cells (BMMCs) and G-CSF–mobilized peripheral blood were purchased from All Cells Inc. Cord blood was collected from human placenta/umbilical cords at the Stanford Medical Center (IRB 5637), separated over Ficoll-Paque Plus (Amersham Biosciences) to remove red blood cells, and either frozen or used immediately for analysis.
Antibodies
The following antibodies (and clones) were used in these studies. BD Biosciences: CD34 (8G12), CD38 (HB7), CD90 (5E10), Stat3 (pY705; 4/P-STAT3), Stat5 (pY694; 47), CD33 (WM53), CD19 (HIB19), CD123 (9F5 in signaling studies; 7G3 other studies), CD114 (LMM741), CD116 (hGMCSFR-M1), CD117 (104D2), CD126 (M5); Biolegend: CD45RA (HI100), CD3 (UCHT1), CD14 (M5E2), CD16 (3G8), CD45 (HI30); Ebiosciences: mouse CD45.1 (A20), Ter119 (Ter-119); Cell Signaling Technologies: Erk (pT202/Y204; E10).
Stimulation of HSPCs, resolution of HSCs, and phospho-signaling analysis
Normal adult BM, G-CSF–mobilized peripheral blood (MPB), or CB progenitors were depleted of cells expressing mature lineage markers (CD2, CD3, CD11b, CD11c, CD14, CD16, CD19, CD24, CD41, CD56, CD66b, glycophorin A) using the Robosep System from StemCell Technologies. In some cases, HSCs were FACS purified as previously described.20 Cells were rested in defined, serum-free media (StemSpan H3000; StemCell Technologies) for 1 hour in an incubator at 37°C with 5% CO2. Cytokines were then added to the cells and cells were returned to the incubator for 15 minutes. Stimuli were recombinant human G-CSF, GM-CSF, IL-3, IL-6 (final concentrations 20 ng/mL each); Tpo (50 ng/mL); Epo (3 units/mL); and FL and SCF (100 ng/mL each). Cytokines were obtained from Peprotech or R&D Systems. To halt signal transduction, cells were fixed with a final concentration of 1.6% paraformaldehyde (PFA; Electron Microscopy Sciences) for 10 minutes at room temperature. Cells were then centrifuged, washed once with staining media to remove residual PFA, and stained for surface markers. Cells were permeabilized with ice-cold 90% EtOH (diluted 200-proof EtOH [Rossville Gold Shield]) with Ultrapure DNAse-, RNAse-free water from Invitrogen for 10 minutes at 4°C. Cells were then washed twice to remove remaining EtOH and were stained with phospho-specific antibodies for 30 minutes. Cells were then analyzed using a BD LSRII (BD Biosciences). A minimum of 50 cell events had to be present in each hematopoietic compartment to be included in analysis. The median number of events analyzed in each compartment was 466.
Cell-cycle analysis
Prospectively sorted HSCs (Lineage-CD34+CD38−CD90+CD45RA−) were cultured in Stemspan H3000 on 96-well plates containing flat hydrogels in media containing only FL and SCF (FS; 100 ng/mL) or FS and G-CSF (FS+G; 40 ng/mL). Cells were washed off the plate 60-68 hours after being plated and were fixed for 10 minutes in PFA to a final concentration of 1.6%. Cells were then permeabilized in 100% ice-cold methanol for 30 minutes at 4°C, washed twice in staining media and stained for 30 minutes at 4°C with Hoechst 33342 (final concentration 2 μg/mL; Invitrogen), pyronin Y (4 μg/mL; Polysciences), and Ki67-Alx488 (BD Biosciences, clone B56).
Hydrogel fabrication
Polyethylene glycol (PEG) hydrogels were produced as previously described.23 Briefly, linear PEG-(SH)2 polymer was purchased from Laysan Bio Inc and 8-arm PEG (40 Kda)–vinyl sulfone (VS) polymer was synthesized as previously described.24 Precursor solutions were generated by dissolving polymers in ultra pure water (PEG-SH) or 0.3M triethanolamine (PEG-VS). Microwell masters were created by casting polydimethylsiloxane (PDMS) on silicon wafers containing the desired topography (generated using standard photolithography techniques). To fabricate micro-patterned hydrogel surfaces (ie, hydrogel arrays), hydrogel precursor solution containing PEG-VS and PEG-SH was polymerized at 37°C in contact with a PDMS replica containing micropillars (the complementary array topography) for 1 hour. Hydrogels were hydrated in PBS containing antibiotics for at least 12 hours. Before cell culture, PEG hydrogels were fixed to the bottom of sterile tissue culture dishes and equilibrated in Stemspan H3000 defined media for at least 12 hours.
Time-lapse microscopy and image analysis
HSPCs were placed directly into wells containing the above-described hydrogel microwell arrays. The plate was then placed in the environmental chamber of an inverted microscope (Zeiss Axiovert 200) equipped with a motorized stage. After cells were randomly distributed and spatially segregated in microwells, the XYZ stage was programmed to repeatedly raster across the microwell array surface, acquiring phase contrast images at 5× magnification of multiple locations in defined time intervals for a period of up to 4 days. The entire surface of the microwell arrays were scanned and the resulting images of the time-lapse experiment were then automatically compiled into a stack (library) using the Volocity software Version 5.0.3 (Improvision). Cells were scored in a blinded fashion and single events were only scored as “division” if there was clear cytokinesis (ie, 1 cell became 2 independently moving cells).
Xenotransplantation and analysis
Transplantations and analyses were carried out as previously described.20,25 Briefly, prospectively sorted HSCs (Lin−CD34+CD38−CD90+CD45RA−) from 3 normal adult BM samples were sorted to high purity based on expression of CD114/G-CSF receptor. Cell-equivalent doses (1000-1500) of cells from each compartment (CD114neg/lo or CD114pos) were then transplanted into the facial veins of sublethally irradiated 2-day-old NOD/SCID/IL-2Rγnull (NSG) pups or into the femurs of adult female NSG mice. BM aspirates were taken 6 and 14 weeks after transplantation and analyzed for human engraftment by flow cytometry. Percentage of human engraftment was calculated as percent human CD45+ cells/(percent human CD45+ cells + percent mouse CD45.1+ cells). Percentages of B cells (human CD19+) and myeloid cells (human CD33+) within the human compartment were determined.
Statistical methods
Synergy was established by comparing the median fluorescence intensity of combination stimulus responses to individual stimulus responses via paired Student t test. Bimodality was established by a likelihood ratio (LR) test comparing the likelihood of the null hypothesis and the alternative hypothesis, a unimodal and 2-component mixture model, respectively. Multimodalities with more than 2 components were not considered. Likelihoods were estimated using a simple Gaussian and a 2-component mixture of Gaussians, with parameters estimated using an Expectation Maximization (EM) procedure as implemented by gmdistribution.fit in the MATLAB statistics toolbox (Mathworks Inc). In the case of mixture models, the likelihood ratio statistic is not distributed as a standard χ2, so an alternate approach was used. We generated a null distribution of LRs and used it to assess P values, similar to the approach presented in.26 To avoid unnecessary parameterization and retain properties typical of flow cytometric data, a reference unimodal flow dataset was used to generate the null LR distribution. The reference distribution was transformed to reflect the data variance for each individual dataset, and the mean of the main peak, assumed to be the mean of the data found after outliers more than 3 SDs from the mean were removed iteratively. Each null LR distribution was generated by sampling 100 000 times from the reference distribution and computing the LR for each sample. LRs for each sample were calculated by fitting a unimodal Gaussian to all data within 3 SDs of the mean and a mixture model for data within 4 SDs of the mean. Because of the stochastic nature of the EM procedure, the LR was derived 10 times for each dataset and the median value was used.
Results
Biochemically distinct subsets within the HSC compartment respond directly to multiple cytokine stimuli
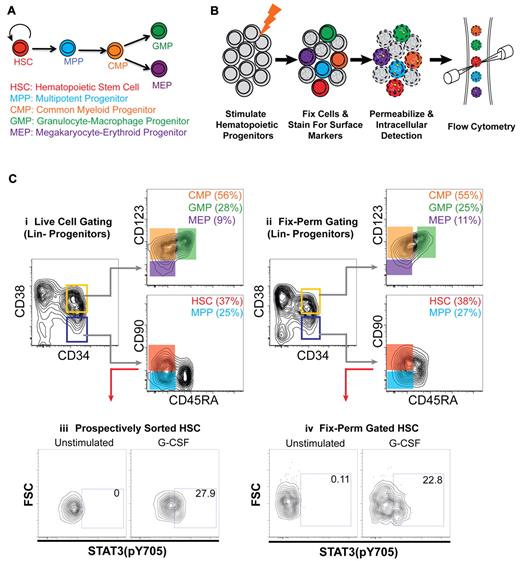
Staining protocols were developed to allow for maintenance of surface marker expression and simultaneous intracellular phospho-protein detection in 5 distinct, well-characterized human HSPC populations without prospective cell sorting. These subsets were HSCs, MPPs, common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), and megakaryocyte-erythroid progenitors (MEPs).19 These methods permitted resolution of HSPC populations in fixed and permeabilized cells from unfractionated BM and/or CB (Figure 1C, compare i and ii).
Intracellular analysis of human HSPCs reveals a cellular subset within the HSC compartment that directly responds to G-CSF. (A) Simplified hematopoietic hierarchy. Self-renewing HSCs give rise to nonself-renewing multipotent progenitors (MPPs). MPPs give rise to common lymphoid (not pictured) or common myeloid progenitors (CMPs), which give rise to either granulocyte-macrophage restricted or megakaryocyte-erythroid restricted progenitors (GMPs and MEPs, respectively). These cells give rise to all mature myeloid lineages. Phenotypic definitions of subsets (all negative for mature lineage markers) are as follows: HSCs are CD34+CD38−CD90+CD45RA−, MPPs are CD34+CD38−CD90−CD45RA−, CMPs are CD34+CD38+CD123+CD45RA−, GMPs are CD34+CD38+CD123+CD45RA+, and MEPs are CD34+CD38+CD123lo/−CD45RA−. (B) Experimental scheme for detection of intracellular signaling events in multiple hematopoietic stem and progenitor populations. See “Stimulation of HSPCs, resolution of HSCs, and phospho-signaling analysis” for details. (C) Flow cytometric analysis of lineage depleted CB progenitors stained for surface markers that identify hematopoietic subsets. Analysis of (i) live cells or (ii) cells after fixation-permeabilization. (iii) Flow cytometric analysis of Stat3(pY705) levels of prospectively sorted, CB HSCs stimulated for 15 minutes with G-CSF. (iv) Flow cytometric analysis of Stat3(pY705) levels in CB HSCs (same samples as panel iii) retrospectively gated after 15-minute stimulation of total lineage-negative progenitors with G-CSF.
Intracellular analysis of human HSPCs reveals a cellular subset within the HSC compartment that directly responds to G-CSF. (A) Simplified hematopoietic hierarchy. Self-renewing HSCs give rise to nonself-renewing multipotent progenitors (MPPs). MPPs give rise to common lymphoid (not pictured) or common myeloid progenitors (CMPs), which give rise to either granulocyte-macrophage restricted or megakaryocyte-erythroid restricted progenitors (GMPs and MEPs, respectively). These cells give rise to all mature myeloid lineages. Phenotypic definitions of subsets (all negative for mature lineage markers) are as follows: HSCs are CD34+CD38−CD90+CD45RA−, MPPs are CD34+CD38−CD90−CD45RA−, CMPs are CD34+CD38+CD123+CD45RA−, GMPs are CD34+CD38+CD123+CD45RA+, and MEPs are CD34+CD38+CD123lo/−CD45RA−. (B) Experimental scheme for detection of intracellular signaling events in multiple hematopoietic stem and progenitor populations. See “Stimulation of HSPCs, resolution of HSCs, and phospho-signaling analysis” for details. (C) Flow cytometric analysis of lineage depleted CB progenitors stained for surface markers that identify hematopoietic subsets. Analysis of (i) live cells or (ii) cells after fixation-permeabilization. (iii) Flow cytometric analysis of Stat3(pY705) levels of prospectively sorted, CB HSCs stimulated for 15 minutes with G-CSF. (iv) Flow cytometric analysis of Stat3(pY705) levels in CB HSCs (same samples as panel iii) retrospectively gated after 15-minute stimulation of total lineage-negative progenitors with G-CSF.
To assess the direct effects of cytokines on the various HSPC populations, cells were treated with a diverse panel of cytokines known to regulate hematopoietic cell survival, proliferation, and differentiation. After 15 minutes of cytokine stimulation, cells were fixed with paraformaldehyde to preserve the signaling state and analyzed by flow cytometry. This analysis revealed that a subset of cells within the CB HSC compartment responded directly to G-CSF with Stat3 phosphorylation (Figure 1Civ). To identify potential artifacts introduced by these methods, CB HSCs were also first prospectively isolated by FACS, stimulated with G-CSF, and subjected to analysis of Stat3 phosphorylation by phospho-specific flow cytometry. Importantly, the response profile remained consistent regardless of whether HSCs were prospectively sorted or retrospectively resolved using the optimized staining protocol (Figure 1Ciii). Therefore, retrospective analysis was used to determine the signaling networks active in the various HSPC populations from normal BM, G-CSF MPB, and CB.
Bone marrow HSCs in adults are considered quiescent27,28 and studies of HSCs in model systems have suggested that only a few “early-acting” cytokines, such as stem cell factor (SCF) and thrombopoietin (Tpo), act directly on this compartment.9,29 In contrast, direct assessment of phosphorylation responses via phospho-specific flow cytometry revealed the adult human BM HSC compartment responded directly and rapidly in vitro to numerous cytokine stimuli through multiple nodes including G-CSF (Stat3, Stat5), GM-CSF (Stat5), FL (Erk), SCF (Erk), Tpo (Stat5, Erk), IL-3 (Stat5), and IL-6 (Stat3). A representative response profile to the full panel of cytokine stimuli is shown in Figure 2A for the BM HSC compartment, while the average response profile of all BM HSPC compartments is shown in Figure 2B (CB and MPB progenitor data are reported in supplemental Figures 1-2; averages for all subsets are shown in supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Adult HSC compartment responds to multiple cytokine stimuli and expresses cytokine receptors. (A) Representative flow cytometric plot of phosphorylation responses [Stat3(pY705), Stat5(pY694)] after 15-minute stimulation with the indicated cytokines in healthy adult BM HSCs. HSCs are CD34+CD38−CD90+CD45RA−. The percentage of cells responding via each node is indicated in each quadrant and is calculated relative to the unstimulated control for each sample. Stimuli used were G-CSF (20 ng/mL), GM-CSF (20 ng/mL), FLT3 ligand (FL; 100 ng/mL), SCF (100 ng/mL), thrombopoietin (Tpo; 50 ng/mL), IL-3 (20 ng/mL), IL-6 (20 ng/mL), and a combination of FL, SCF, Tpo, IL-3, and IL-6 (referred to as FST36). Gray arrows represent directionality of response, but in the case where multiple nodes are activated, not necessarily the sequence of activation. (B) Heatmap of the average percentage of cells responding in each stem and progenitor compartment in normal adult BM after 15-minute cytokine stimulation. MPPs are CD34+CD38−CD90−CD45RA−, CMPs are CD34+CD38+CD123+CD45RA−, GMPs are CD34+CD38+CD123+CD45RA+, and MEPs are CD34+CD38+CD123lo/−CD45RA−. Measurements were obtained from 2-4 distinct biologic samples. Epo is erythropoietin, and 3 units/mL was used to stimulate cells. (C) Representative flow cytometric analyses of cytokine receptor expression on adult marrow HSCs. Red histogram is isotype control, blue histogram is cytokine receptor. Median fluorescence intensity (MFI) is indicated with isotype control on top. Measurements were taken on 3 distinct biologic samples.
Adult HSC compartment responds to multiple cytokine stimuli and expresses cytokine receptors. (A) Representative flow cytometric plot of phosphorylation responses [Stat3(pY705), Stat5(pY694)] after 15-minute stimulation with the indicated cytokines in healthy adult BM HSCs. HSCs are CD34+CD38−CD90+CD45RA−. The percentage of cells responding via each node is indicated in each quadrant and is calculated relative to the unstimulated control for each sample. Stimuli used were G-CSF (20 ng/mL), GM-CSF (20 ng/mL), FLT3 ligand (FL; 100 ng/mL), SCF (100 ng/mL), thrombopoietin (Tpo; 50 ng/mL), IL-3 (20 ng/mL), IL-6 (20 ng/mL), and a combination of FL, SCF, Tpo, IL-3, and IL-6 (referred to as FST36). Gray arrows represent directionality of response, but in the case where multiple nodes are activated, not necessarily the sequence of activation. (B) Heatmap of the average percentage of cells responding in each stem and progenitor compartment in normal adult BM after 15-minute cytokine stimulation. MPPs are CD34+CD38−CD90−CD45RA−, CMPs are CD34+CD38+CD123+CD45RA−, GMPs are CD34+CD38+CD123+CD45RA+, and MEPs are CD34+CD38+CD123lo/−CD45RA−. Measurements were obtained from 2-4 distinct biologic samples. Epo is erythropoietin, and 3 units/mL was used to stimulate cells. (C) Representative flow cytometric analyses of cytokine receptor expression on adult marrow HSCs. Red histogram is isotype control, blue histogram is cytokine receptor. Median fluorescence intensity (MFI) is indicated with isotype control on top. Measurements were taken on 3 distinct biologic samples.
The percentage of cells within the HSC compartment activated via JAK/Stat signaling in CB was not significantly different from BM with the exception of the G-CSF response, which activated a significantly higher percentage of cells from the BM HSC compartment via Stat3 and Stat 5 than HSCs from other sources (supplemental Figure 3A-B). Interestingly, cells within the HSC compartment derived from MPB had a lower percentage of cells responding to G-CSF (Stat3 and Stat5), IL-3 (Stat5), and GM-CSF (Stat5) compared with BM (supplemental Figure 3C-D). The responsiveness of the HSC compartment to Tpo and IL-6 was unchanged in all cell sources tested (supplemental Figure 3E-F). In accordance with their ability to respond to cytokines, cells within the HSC compartment also expressed commensurate cytokine receptors, including those for G-CSF, GM-CSF, IL-3, and IL-6, (Figure 2C).
Cytokine responses in mature lymphocytes and monocytes are often unimodal.30 In contrast, only subsets of cells with the HSC compartment responded to most stimuli (Figure 2A; eg, Stat5 response to IL-3 and GM-CSF). To investigate if the heterogeneous nature of the direct responses indicated the presence of physiologically distinct cell subsets, mixture modeling was applied to the response profiles to determine the statistical likelihood that multiple subpopulations were present.31 This analysis showed that nearly all the cytokine responses within the BM HSC compartment were consistent with the presence of multiple populations (supplemental Table 2). The use of a combination stimulus of FL, SCF, Tpo, IL-3, and IL-6 (referred to as FST36) revealed further heterogeneity within the HSC compartment. BM HSCs consistently showed a greater percentage of cells responding via Stat5 and Erk phosphorylation and a higher per-cell response (> 1.8 fold increase in median fluorescence intensity, P < .001) with combination FST36 versus any individual cytokine (Figure 2B and supplemental Table 3). These data suggest that biochemically distinct cell subsets with unique signaling physiology exist within the currently defined HSC pool and may underlie the functional heterogeneity of HSCs.7,22
Cytokine responsivity of other early hematopoietic progenitors
The intracellular methods used here allowed for the simultaneous analysis of multiple downstream progenitor compartments (Figure 2A). The immediate downstream progenitor of HSCs, the nonlong-term self-renewing MPPs, had a response profile that largely mirrored that of the HSC compartment, suggesting that although cytokine responsiveness is important for homeostasis, additional cell-intrinsic mechanisms govern self-renewal (Figure 2B, supplemental Figures 1-2, and supplemental Table 1). In normal marrow, G-CSF, Tpo, and IL-6 most potently induced phosphorylation in the early HSC/MPP populations (ie, stimulating more cells within these populations than within other subsets) while the common β-chain cytokines GM-CSF and IL-3 potently induced JAK/Stat signaling throughout the entire early hematopoietic hierarchy (Figure 2B). Although G-CSF is known to instruct lineage commitment in myeloid progenitors,32 G-CSF did not induce a significant Stat phosphorylation response in the GMP. This is likely because of the fact that GMP have much higher basal activation compared with the HSC compartment (supplemental Figure 4, compare Ai and Bi), and thus are not significantly further induced by cytokine stimulation. In contrast to the clinically utilized G-CSF and GM-CSF, which induced phosphorylation in the earliest (HSC/MPP) compartments, clinically utilized Epo directly stimulated signaling in the downstream MEPs, but not the HSC/MPP compartment, and only in adult derived samples (BM and MPB), not in CB (supplemental Figure 5). As was seen with HSCs, mixture modeling revealed distinct biochemical subpopulations within these myeloid progenitor compartments, highlighted by the IL-6/Stat3 response (supplemental Table 2).
G-CSF drives proliferation and active cell-cycle entry in the HSC compartment
To determine the functional relevance of these responses, we further characterized the response of the HSC compartment to G-CSF because of its widespread clinical use in treating neutropenia and mobilizing HSCs/HSPCs for clinical hematopoietic cell transplantation.12 The mobilization effects of G-CSF are believed to be indirect, and reports have suggested that HSCs do not respond directly to G-CSF.12,14-16 To investigate the direct effect of G-CSF on proliferation of cells within the HSC compartment, these cells were FACS-purified and deposited into hydrogel microwell arrays to spatially segregate individual cells and give numerous single-cell replicates for each defined culture condition. Individual wells within each array (all exposed to the same defined culture condition) were monitored with time-lapse photography for several days, permitting visualization of proliferation at the single-cell level.23 As was the case with the phospho-signaling responses, there was heterogeneity in proliferation within a given culture monitored over 80 hours, as individual cells underwent no division, a single division, or multiple divisions (Figure 3A). The functional identity of the progeny cells could not be determined; therefore, proliferation in direct response to G-CSF was scored as the percentage of single plated cells from the HSC compartment undergoing at least 1 cell division during the image acquisition period.
G-CSF drives proliferation and cell-cycle entry within the HSC compartment. (A) Representative time-lapse images of prospectively sorted HSCs on hydrogel microwells at the beginning of acquisition (t = 24 hour) and final time point (t = 80 hour). Proliferation of single HSCs was tracked over time with some cells undergoing no divisions (red) and others dividing (blue). (B) Frequency of proliferation (≥ 1 cell division) for prospectively sorted, single HSCs plated on hydrogel microwells in serum-free, defined media supplemented with FL and SCF (FS) alone or in combination with 40 ng/mL G-CSF. Differences between proliferation frequency for CB (black) and normal BM (gray) at 80 and 92 hours, respectively, were statistically significant (P = .0004, paired, 1-tailed t test). (C) Representative flow cytometric detection of DNA (Hoechst 33342) and RNA (Pyronin Y) in prospectively sorted HSCs cultured on hydrogel substrate for 68 hours with gates corresponding to cell-cycle phase. (D) Frequency of cells in active cycle (ie, G1-S-G2-M) after 60-68 hours of culture with FS or FS+G. Difference in frequency of cells in active cycle is statistically significant (P = .0085, paired, 1-tailed t test).
G-CSF drives proliferation and cell-cycle entry within the HSC compartment. (A) Representative time-lapse images of prospectively sorted HSCs on hydrogel microwells at the beginning of acquisition (t = 24 hour) and final time point (t = 80 hour). Proliferation of single HSCs was tracked over time with some cells undergoing no divisions (red) and others dividing (blue). (B) Frequency of proliferation (≥ 1 cell division) for prospectively sorted, single HSCs plated on hydrogel microwells in serum-free, defined media supplemented with FL and SCF (FS) alone or in combination with 40 ng/mL G-CSF. Differences between proliferation frequency for CB (black) and normal BM (gray) at 80 and 92 hours, respectively, were statistically significant (P = .0004, paired, 1-tailed t test). (C) Representative flow cytometric detection of DNA (Hoechst 33342) and RNA (Pyronin Y) in prospectively sorted HSCs cultured on hydrogel substrate for 68 hours with gates corresponding to cell-cycle phase. (D) Frequency of cells in active cycle (ie, G1-S-G2-M) after 60-68 hours of culture with FS or FS+G. Difference in frequency of cells in active cycle is statistically significant (P = .0085, paired, 1-tailed t test).
HSCs reside in environmental niches subjected to a complex cytokine milieu; thus a physiologic assessment of the effect of G-CSF on HSC behavior requires the presence of other factors known to regulate HSCs. Addition of G-CSF to basal cultures containing FL and SCF, factors that contribute to HSC maintenance in vivo,33 significantly increased the proliferation of single cells from the HSC compartment of both CB and BM (P = .0004; Figure 3B) without altering the kinetics of first division (data not shown). Similarly, addition of G-CSF to a cocktail of cytokines including FL, SCF and members of the Wnt/Notch families—cytokines also known for their role in HSC maintenance34 —also significantly increased the proliferation of single-plated CB HSCs (supplemental Figure 6). Furthermore, addition of G-CSF to HSC cultures containing FL and SCF significantly increased the percentage of cells in active cycle (ie, G1-S-G2/M) by day 3 (P = .0085; Figure 3C-D), and increased the frequency of cells expressing high levels of the proliferation antigen Ki67 (data not shown). These results demonstrate that the direct action of G-CSF regulates the behavior of the cells within the HSC compartment, driving them into the cell cycle and increasing their proliferation.
Lack of CD114 expression distinguishes bona fide HSCs from transiently reconstituting multilineage progenitors
While G-CSF elicited both molecular (Stat3 phosphorylation) and cellular (increased proliferation, cell cycle) responses within the HSC compartment (Lin−CD34+CD38−CD90+CD45RA−), it remained unclear whether these responsive cells represented true HSCs. The mixture modeling results indicated that the Stat3-response profile to G-CSF in the HSC compartment was consistent with the presence of multiple, biochemically distinct subsets (supplemental Table 2). We hypothesized that these previously unidentified subsets within the HSC compartment would be informative for further refining the phenotypic identity of true HSCs. The methods that allow intracellular detection of phosphorylation responses downstream of cytokine stimulation render the cells nonviable. Consequently, the traditional assay that would be used to confirm that the Stat3-responsive cells within the HSC compartment are bona fide HSCs, transplanting them into irradiated immunocompromised mice and assessing their ability to contribute to both myeloid and lymphoid lineages after > 12 weeks, was not available.
Therefore, to determine whether the G-CSF responsive cells within the HSC compartment possessed HSC activity, cells were sorted based on G-CSF receptor (CD114) expression (supplemental Figure 7), and equivalent numbers of CD114neg/lo and CD114pos cells within the HSC compartment were then transplanted into NOD/SCID/IL-2Rγnull (NSG) mice. BM aspirates were taken after 6 and 14 weeks to assess short- and long-term human engraftment (ie, > 0.1% human chimerism in BMMCs). Figure 4A and Table 1 show that at 6 weeks after transplantation, both CD114neg/lo and CD114pos cells give equivalent levels of overall human engraftment. Progeny cells from both populations consisted of myeloid (CD33+) and lymphoid (CD19+) components (supplemental Table 4), demonstrating that both populations contain multilineage potential. However, at 14 weeks after transplantation, significantly fewer mice that received a transplantation of CD114pos cells (2 of 10) exhibited human engraftment compared with those that received a transplantation of CD114neg/lo cells (12 of 14; Table 1, P = .002), and the overall level of human chimerism was significantly lower (Figure 4B, P = .001). These results indicate that long-term human HSC activity is significantly enriched in the CD114neg/lo population within the previously identified HSC compartment.
Lack of CD114 expression distinguishes bona fide HSCs from transiently reconstituting multilineage progenitors. The percentage of human engraftment (determined by expression of human CD45) in the BM of NOD/SCID/IL-2Rγnull (NSG) mice that received a transplantation of either CD114neg/lo or CD114pos subfractions of the human HSC compartment is indicated at (A) 6 weeks, or (B) 14 weeks after transplantation. Engrafted mice are defined as greater than 0.1% human chimerism of BMMCs. Black line represents the average percent human chimerism in each group of mice. The difference in the level of engraftment at week 14 is statistically significant (P = .001, 2-tailed Mann-Whitney test).
Lack of CD114 expression distinguishes bona fide HSCs from transiently reconstituting multilineage progenitors. The percentage of human engraftment (determined by expression of human CD45) in the BM of NOD/SCID/IL-2Rγnull (NSG) mice that received a transplantation of either CD114neg/lo or CD114pos subfractions of the human HSC compartment is indicated at (A) 6 weeks, or (B) 14 weeks after transplantation. Engrafted mice are defined as greater than 0.1% human chimerism of BMMCs. Black line represents the average percent human chimerism in each group of mice. The difference in the level of engraftment at week 14 is statistically significant (P = .001, 2-tailed Mann-Whitney test).
Lack of CD114 expression distinguishes bona fide HSC from transiently reconstituting multilineage progenitors
| Time . | Subfraction . | Engraft . | No Engraft . | Statistic . |
|---|---|---|---|---|
| Week 6 | CD114neg/lo | 10 | 4 | 0.21 |
| CD114pos | 4 | 6 | ||
| Week 14 | CD114neg/lo | 12 | 2 | 0.002 |
| CD114hi | 2 | 8 |
| Time . | Subfraction . | Engraft . | No Engraft . | Statistic . |
|---|---|---|---|---|
| Week 6 | CD114neg/lo | 10 | 4 | 0.21 |
| CD114pos | 4 | 6 | ||
| Week 14 | CD114neg/lo | 12 | 2 | 0.002 |
| CD114hi | 2 | 8 |
The presence of human engraftment in the bone marrow of NOD/SCID/IL-2Rγnull (NSG) mice transplanted with either CD114neg/lo or CD114pos subfractions of the human HSC compartment was determined 6 and 14 weeks after transplantation. No statistically significant difference in engraftment was observed at 6 weeks; however, by 14 weeks, engraftment was markedly enriched in the CD114neg/lo subfraction. The Fisher exact, 2-tailed test was used for statistic calculation.
Discussion
The integration of cytokine stimuli by cells of the hematopoietic system is crucial to their survival, differentiation, and homeostasis. However, a comprehensive evaluation of the ability of G-CSF and other cytokines to directly act on the best-defined primitive human HSC/HSPC populations, and the resulting functional consequences, has not been reported. In this study, single-cell response-specific flow cytometry was used to define the signaling networks directly activated in 5 previously defined human HSPC compartments after cytokine exposure, representing direct biochemical analysis of these critical populations. While data from studies using model systems have depicted the HSC compartment as responding to a limited set of “early-acting” cytokines such as SCF and Tpo,9,29 these single-cell intracellular analyses demonstrated that the currently defined human HSC compartment consists of biochemically distinct subsets with the ability to respond rapidly and directly to numerous cytokine stimuli (G-CSF, GM-CSF, FL, SCF, Tpo, IL-3, and IL-6) through multiple signaling nodes (JAK/Stat and Erk) in vitro. While functional cellular heterogeneity of the HSC compartment has been previously described,7,35-37 a debate has remained as to whether these differences are the result of stochastic processes, such as location in niches or clonal origin, or cell-intrinsic properties. The heterogeneous nature of the signaling responses to single stimuli and the ability of combination stimuli to synergize in the cells making up the HSC compartment (Figure 2, and supplemental Tables 2-3) supports the presence of biochemical heterogeneity within currently defined HSC pools. Moreover, this heterogeneity proved informative for better purifying HSC activity within the currently defined HSC compartment (Lin−CD34+CD38−CD90+ CD45RA−). Within this population, cells that lacked expression of CD114, the G-CSF receptor, showed bona fide stem cell activity—long-term, multilineage repopulation—in immune-compromised mice, while this activity was almost completely absent in CD114pos cells. Thus, in addition to providing insight into the biochemical activity in the earliest HSPC compartments, these analyses provided a novel method leading to improved phenotypic purification of stem cell activity.
G-CSF is routinely used clinically to mobilize HSPCs to the periphery before harvesting for hematopoietic cell transplantation. Many of the mechanisms underlying this mobilization act in trans, as G-CSF receptor knockout hematopoietic progenitors can be mobilized by G-CSF,13 and thus G-CSF has not been believed to act directly on HSCs to regulate their activity.11,12,14-16 Recent data from the mouse system has suggested a direct role for G-CSF in HSC regulation. Direct action was hinted at by a recent report that early mouse progenitors can be activated rapidly by G-CSF; however, the known diversity of cells contained within the analyzed KLS compartment, and the differences between the markers used to isolate these mouse cells and human HSCs, made the relevance of this finding to human HSCs uncertain.17-19 Other recent data have suggested that exogenous G-CSF administration can permanently affect the HSC pool. Data from the mouse system showed that G-CSF injection resulted in an increased proliferation of “dormant” HSC clones,38 though the time point used to assay this proliferation (70 days) allows for secondary mechanisms mediated by other cells acted on by G-CSF. The data presented in this report demonstrate a direct action of G-CSF within the HSC compartment (active in under 15 minutes). However, they also show that cells expressing the CD114 (G-CSF receptor) are depleted for long-term repopulating activity. This strongly suggests that true HSCs—cells able to give rise to long-term, multilineage repopulation—do not respond directly to G-CSF. Other cytokine receptors are expressed within the HSC compartment (Figure 2C). Future work will detail whether these markers can also be used to enrich for long-term repopulation activity within the HSC compartment, or if these and other cytokines directly regulate true HSCs.
Signal transduction dysregulation characterizes a number of hematopoietic malignancies including myeloproliferative neoplasms and acute myeloid leukemia (AML).39 There is growing evidence that this dysregulation can originate in early progenitor compartments, including possibly the stem cell compartment.40 Dysregulation of Stat3 and Stat5 responses downstream of G-CSF are effective in diagnosing, and identifying/predicting relapses and AML.39,41 Many of the cells exhibiting signaling dysregulation also show cell surface attributes of a stem/progenitor cell phenotype. Future work that combines additional surface markers that uniquely demarcate diseased versus normal hematopoietic cells, such as CD47 in AML,42 can indicate where in the hematopoietic hierarchy signaling dysregulation first manifests, as well as facilitate screening for targets that specifically impair leukemic stem cells while leaving normal cells unperturbed. The single-cell analyses presented here provide a framework for better elucidating the role of direct cytokine action on the stem cell pool, which is a crucial step for better elucidating their phenotype and illuminating mechanisms of signaling dysregulation in disease states.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sean Bendall, Erin Simonds, Ken Schulz, and Erika O'Donnell for critical manuscript review and helpful conversation. We thank Astraea Jager for animal assistance.
This research is supported by National Institutes of Health (NIH) grant U01HL099999 to I.L.W. and R.M., and by NIH grants 1 P50 CA114747, P01 CA034233-24, 0158 G KB065, NCI grant 1R01CA130826-01, NHLBI grant N01-HV-28 183 and Leukemia & Lymphoma Society grant 7017-6 to G.P.N. K.G. is funded by a National Science Foundation Graduate Research Fellowship and a Stanford DARE Fellowship. R.M. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund.
National Institutes of Health
Authorship
Contribution: K.D.G. and R.M. designed and performed experiments, analyzed results, and wrote the manuscript; P.M.G. and H.M.B. synthesized hydrogels and wrote the manuscript; K.S. performed statistical analysis and wrote the manuscript; F.Z. performed experiments; I.L.W. designed experiments; and G.P.N. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: G.P.N. is a paid consultant of Becton Dickinson, a provider of some reagents for this study. The remaining authors declare no competing financial interests.
Correspondence: Garry P. Nolan, PhD, 269 Campus Dr, CSSR 3205, Stanford, CA 94305; e-mail: gnolan@stanford.edu; or Ravindra Majeti, MD, PhD, 265 Campus Dr, G3021, MC 5461, Stanford, CA 94305; e-mail: rmajeti@stanford.edu.
References
Author notes
G.P.N. and R.M. contributed equally to this study.

![Figure 2. Adult HSC compartment responds to multiple cytokine stimuli and expresses cytokine receptors. (A) Representative flow cytometric plot of phosphorylation responses [Stat3(pY705), Stat5(pY694)] after 15-minute stimulation with the indicated cytokines in healthy adult BM HSCs. HSCs are CD34+CD38−CD90+CD45RA−. The percentage of cells responding via each node is indicated in each quadrant and is calculated relative to the unstimulated control for each sample. Stimuli used were G-CSF (20 ng/mL), GM-CSF (20 ng/mL), FLT3 ligand (FL; 100 ng/mL), SCF (100 ng/mL), thrombopoietin (Tpo; 50 ng/mL), IL-3 (20 ng/mL), IL-6 (20 ng/mL), and a combination of FL, SCF, Tpo, IL-3, and IL-6 (referred to as FST36). Gray arrows represent directionality of response, but in the case where multiple nodes are activated, not necessarily the sequence of activation. (B) Heatmap of the average percentage of cells responding in each stem and progenitor compartment in normal adult BM after 15-minute cytokine stimulation. MPPs are CD34+CD38−CD90−CD45RA−, CMPs are CD34+CD38+CD123+CD45RA−, GMPs are CD34+CD38+CD123+CD45RA+, and MEPs are CD34+CD38+CD123lo/−CD45RA−. Measurements were obtained from 2-4 distinct biologic samples. Epo is erythropoietin, and 3 units/mL was used to stimulate cells. (C) Representative flow cytometric analyses of cytokine receptor expression on adult marrow HSCs. Red histogram is isotype control, blue histogram is cytokine receptor. Median fluorescence intensity (MFI) is indicated with isotype control on top. Measurements were taken on 3 distinct biologic samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/16/10.1182_blood-2010-07-298232/4/m_zh89991169820002.jpeg?Expires=1765897644&Signature=UO1HeUcUVNzvfhTcTsKk4MnrFpNgo~5gm8I3Lt1HWkh5GwPWsUiPemfTEOFZx4JhBHDmJYlalKfBuZ5HITPtvZwKyC87aHOSSGgMupIthacqzqGczaCTiuM09zX4TQj6cxATuna2uCYDME-V-arqeHcOzM76Vy~MtXHrRzPh21nJAF~L78ykGRVN~kMSafZpZXrX9KEMlvye1rRzXg8w0HIUfWPJskN8y~tEngzP6p8Y0YrpL7hr2cujEYG3T2iH2dVP2SUe-A5-69-2Wev3uuRYxoUTSjUYcdl6Dh5dHvhQGFyh0t4~1kapnafRiilSk7hQt4ga0qSKliL-qssG-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

