Abstract
In BCR-ABL1+ leukemia, drug resistance is often associated with up-regulation of BCR-ABL1 or multidrug transporters as well as BCR-ABL1 mutations. Here we show that the expression level of the transcription factor STAT5 is another parameter that determines the sensitivity of BCR-ABL1+ cells against tyrosine kinase inhibitors (TKIs), such as imatinib, nilotinib, or dasatinib. Abelson-transformed cells, expressing high levels of STAT5, were found to be significantly less sensitive to TKI-induced apoptosis in vitro and in vivo but not to other cytotoxic drugs, such as hydroxyurea, interferon-β, or Aca-dC. The STAT5-mediated protection requires tyrosine phosphorylation of STAT5 independent of JAK2 and transcriptional activity. In support of this concept, under imatinib treatment and with disease progression, STAT5 mRNA and protein levels increased in patients with Ph+ chronic myeloid leukemia. Based on our data, we propose a model in which disease progression in BCR-ABL1+ leukemia leads to up-regulated STAT5 expression. This may be in part the result of clonal selection of cells with high STAT5 levels. STAT5 then accounts for the resistance against TKIs, thereby explaining the dose escalation frequently required in patients reaching accelerated phase. It also suggests that STAT5 may serve as an attractive target to overcome imatinib resistance in BCR-ABL1+ leukemia.
Introduction
More than 99% of all patients with chronic myeloid leukemia (CML) and approximately 30% of acute lymphoid leukemia are characterized by the t(9;22)(q34;q11) translocation and the Philadelphia chromosome. Two chimeric oncogenic tyrosine kinase products, p185BCR-ABL1 or p210BCR-ABL1, may be generated.1,2 Whereas p210BCR-ABL1 is associated with CML, p185BCR-ABL1 is almost exclusively found in acute lymphoid leukemia.3 The BCR-ABL1 oncoprotein promotes leukemia development by activating multiple signal transduction pathways that regulate cell proliferation, transformation, and survival. BCR-ABL1-induced acute lymphoid leukemia is characterized by an excess of lymphoblasts and progresses rapidly, whereas BCR-ABL1+ CML is a stem cell-derived disease with distinct phases: chronic phase (CP), which may last for several years, accelerated phase (AP), and blast crisis (BC).4
Therapy of BCR-ABL1-induced diseases was significantly improved by the development of small molecular weight inhibitors blocking the activity of the ABL1 kinase. Imatinib was the first substance, soon followed by other kinase inhibitors (TKIs), such as dasatinib and nilotinib.5-7 All these TKIs target the enzymatic activity of the ABL1 tyrosine kinases.6,8,9 Imatinib is now the standard first-line therapy for all CML patients. However, not all CML patients respond equally well.10 Moreover, approximately 15% to 25% of the patients who initially responded well acquire resistance against imatinib during therapy. The percentage of nonresponders is even higher in AP.10-12 These patients are treated with increased imatinib dosage (600-800 mg/day), second-generation BCR-ABL1 inhibitors, or stem cell transplantation.13-17 The most frequently reported causes for TKI resistance are mutations in the kinase domain of BCR-ABL1 with a reported frequency of 40% to 90%.13,15,18 Other mechanisms include the up-regulation of BCR-ABL1, increased expression of the drug transporter ABCB1, elevated levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), or TP53 inactivation.14,17,19 Apart from these mechanisms, deregulation of signaling cascades and activation of specific signaling molecules, such as LYN and other SRC family kinases, have also been discussed in contributing to drug resistance in CML.20,21
Signaling pathways activated by BCR-ABL1 include the PI3K-mTOR pathway, the RAS/RAF/MEK/ERK pathway, and the JAK-STAT pathway.22-25 JAK2 and STAT5 are highly activated in CML patient cells. Although JAK-STAT signaling is normally triggered by cytokines, JAK2 and STAT5 activation in BCR-ABL1-transformed cells depends on the ABL kinase activity because imatinib treatment completely abrogates phosphorylation of JAK2 and STAT5.26,27 Treatment of BCR-ABL1+ cells with JAK2 inhibitors or deletion of STAT5 induces apoptosis, even in imatinib-resistant cells.28-30 Furthermore, STAT5 phosphorylation has been shown to be independent of JAK2 in BCR-ABL1+ cells.26 (and authors' observations). STAT5 activates many important antiapoptotic pathways downstream of BCR-ABL1,22,31,32 and we have recently shown that STAT5 is absolutely required for transformation by BCR-ABL1 oncogenes.23,29
In a recent report, Wang et al revealed a correlation between elevated STAT5 phosphorylation and imatinib resistance.19 They attributed the increased phosphorylation of STAT5 to an enhanced GM-CSF synthesis, which activates STAT5 via the tyrosine kinase JAK2.
We delineate here a novel pathway and show that the increased expression of STAT5 itself, independent of JAK2, suffices to mediate imatinib resistance. We also describe that imatinib-resistant patients show an up-regulation of STAT5 in the leukemic cells. Thereby, our study defines the STAT5 protein level as a clinically relevant modulator of imatinib responsiveness.
Methods
Mice
Stat5a/bnull/+, Stat5fl/+, Stat3fl/fl, Mx1-Cre (all mixed C57BL/6J x Sv129), Stat1−/− (C57BL/6J), Cre ERt2 (FVB/NJ), and NOD.CB17-Prkdc∧scid/NCrHsd (NOD/SCID, Harlan Laboratories) mice were maintained at the Biomedical Research Institute (Medical University of Vienna) and genotyped as described previously.33 NOD/SCID mice were used for leukemia engraftment and imatinib in vivo studies. All animal experiments were carried out in accordance with protocols approved by Austrian law.
Tissue culture conditions and infections
Tissue culture conditions, virus preparation, infection of fetal liver cells with viral supernatant from A010 cells or gp+ E86 producer cell lines, and establishment of cell lines was described previously.23
Immunoblotting and electrophoretic mobility shift assay
Immunoblots and electrophoretic mobility shift assay on the β-casein promoter were carried out as described previously.23,34 Membranes were probed with antibodies directed against STAT1 (sc-592X), STAT3 (sc-482X), STAT5A/B (sc-835X), CDK2 (sc-163), CDK4 (sc-260), c-ABL (sc-23, all purchased from Santa Cruz Biotechnology), β-actin (A5441) and α-tubulin (T9026, both Sigma-Aldrich). Antisera against STAT5A and STAT5B have been described previously.35
Plasmids
p210BCR-ABL1, the construct of wild-type Stat5a, the Stat5a mutants cS5F, S5aΔN,36 S5aΔ749,34 S5aEE/AA, S5aY/F,37 and the Stat5b mutant S5b1*638 were expressed in the retroviral vector pMSCv-IRES-eGFP. p185BCR-ABL1 was cloned into a pMSCV backbone. Ecotropic, replication incompetent gp+ E86 producers were generated and selected for high virus titer production by fluorescence-activated cell sorter (FACS) as described previously.36,39
Imatinib treatment of p210BCR-ABL1-IRES-GFP infected bone marrow
For imatinib sensitivity studies of p210BCR-ABL1+ cells, bone marrow cells derived from Stat5+/+ or Stat5null/+ mice were cocultivated on p210BCR-ABL1 retroviral producer cells for 48 hours in the presence of StemPro-34 serum-free medium (Invitrogen) supplemented with interleukin-3 (IL-3; 10 ng/mL), IL-6 (5 ng/mL), GM-CSF (1 ng/mL), Fms-like tyrosine kinase 3 ligand ligand (20 ng/mL), insulin growth factor-1 (40 ng/mL), stem cell factor (100 ng/mL), dexamethasone, and polybrene (7 μg/mL). Three days after infection, StemPro medium was replaced with cytokine-free RPMI supplemented with 10% fetal calf serum, 1% penicillin/streptomycin, and 0.1% β-mercaptoethanol. Five days after StemPro medium deprivation, cells were treated with imatinib for 48 hours and analyzed via FACS for green fluorescent protein (GFP) and MAC-1 expression.
Primary patient samples
Primary cells were obtained from patients treated at the General Hospital, Vienna, Austria. Cells were obtained from patients with CML at routine blood and bone marrow examinations after informed consent was given in compliance with the Declaration of Helsinki. Peripheral blood and bone marrow mononuclear cells were isolated using Ficoll. Samples were analyzed for BCR-ABL1 mutations (complete list of analyzed mutations, supplemental Figure 4A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and BCR-ABL1 mRNA level according to the international standard protocol. Use of human samples was approved by the Ethical Committee of the Medical University of Vienna and is in compliance with Austrian legislation.
Immunocytochemistry was performed as described by Baumgartner et al.40
Statistics
Two-tailed Student t tests were used for statistical analysis. Difference was considered statistically significant when P less than .05. The data are represented as mean ± SD of the number of the determinations and were analyzed by Graph Pad software Version 4.03.
Additional protocols are provided in the supplemental data.
Results
Increased imatinib resistance in Abelson-transformed cells on maintenance in tissue culture
Transformation of hematopoietic cells with p185BCR-ABL1 and v-ABL results in the outgrowth of growth-factor independent CD19+/CD43+/B220+ cells. We noted that different v-ABL-transformed lymphoid pro B-cell clones (v-Abl+ cells) varied significantly in their response to imatinib. We asked whether these differences might be related to the time the cells had been in culture. v-Abl+ cells that had been propagated for less than 3 months after the initial transformation event were defined as short-term cultures (STCs). They were opposed by long-term cultures (LTCs) that had been cultured more than 8 months. First, we compared cell numbers of LTCs and STCs on imatinib treatment. Because LTCs proliferated slightly faster than STCs (supplemental Figure 1A), we set the individual controls to 100% to allow their comparison. As shown in Figure 1A, STCs reacted with a pronounced decrease in cell numbers to 100nM imatinib, whereas LTCs were affected to a significantly minor degree. This difference in imatinib sensitivity also became obvious when the cells were plated in growth-factor free methylcellulose (Figure 1B; summarized in supplemental Figure 1B). Whereas the ability of LTCs to form colonies in the presence of 100nM or 200nM imatinib remained largely unchanged, a significant decrease was observed for STCs. Dose-response curves confirmed the decreased imatinib sensitivity of LTCs (50% inhibitory concentration [IC50] STC = 0.35μM, IC50 LTC = 5.05μM; Figure 1C). Immunoblots revealed that several molecules were expressed at elevated levels in LTCs compared with STCs (Figure 1D). Among the up-regulated factors were the STAT transcription factors. Quantitative analysis by ImageJ software Version 1.42 revealed a highly significant up-regulation of STAT5A/B. STAT1, STAT3, and c-ABL showed a less consistent pattern (Figure 1D; quantitative analysis, supplemental Figure 1C). We asked whether the elevated expression of STAT transcription factors contributes to the reduced imatinib sensitivity in LTCs. To test whether the up-regulation of individual STAT proteins are on their own of functional relevance, Stat1−/− and Stat3Δ/Δ leukemic cell lines were treated with imatinib for 24 hours. No differences on imatinib sensitivity were detectable (supplemental Figure 1D-F).
Prolonged culture times of v-Abl+ lymphoid cells correlate with increased STAT5 protein levels and decreased imatinib sensitivity. (A) Cell counts of LTCs (n = 4) and STCs (n = 6) treated with 100nM imatinib. (B) Representative colony-forming assays of LTCs and STCs in growth-factor free methylcellulose without and supplemented with 200nM imatinib. Pictures of colonies were taken after 14 days (LTCs) and 21 days (STCs). (C) Dose-response curves determined by [3H]-thymidine incorporation of LTCs (n = 6) and STCs (n = 4) treated with imatinib for 24 hours. Control values in the absence of imatinib were set to 100%. (D) Immunoblot analysis of STCs and LTCs for STAT transcription factors and v-ABL. (A,C) Error bars represent mean ± SD. ***P < .001.
Prolonged culture times of v-Abl+ lymphoid cells correlate with increased STAT5 protein levels and decreased imatinib sensitivity. (A) Cell counts of LTCs (n = 4) and STCs (n = 6) treated with 100nM imatinib. (B) Representative colony-forming assays of LTCs and STCs in growth-factor free methylcellulose without and supplemented with 200nM imatinib. Pictures of colonies were taken after 14 days (LTCs) and 21 days (STCs). (C) Dose-response curves determined by [3H]-thymidine incorporation of LTCs (n = 6) and STCs (n = 4) treated with imatinib for 24 hours. Control values in the absence of imatinib were set to 100%. (D) Immunoblot analysis of STCs and LTCs for STAT transcription factors and v-ABL. (A,C) Error bars represent mean ± SD. ***P < .001.
Low STAT5 protein levels are related to increased imatinib sensitivity in p210BCR-ABL1- and v-ABL-dependent cells
Stat5null/null cells cannot be transformed by BCR-ABL1 and v-ABL.23 To evaluate the impact of STAT5 on imatinib sensitivity, we prepared single-cell suspensions of bone marrow (BM) cells from Stat5+/+ and Stat5null/+ mice. Differences in the STAT5 protein levels of the BM were verified via immunoblot (supplemental Figure 2A). These cells were infected with pMSCv-p210BCR-ABL1-IRES-GFP. The initial infection rate was comparable between Stat5+/+ and Stat5null/+ cells and varied between 10% and 20% in individual experiments. After 3 days, the cells were kept under cytokine-free conditions, which increased the percentage of GFP+ cells to 30% to 50% after 5 days. The majority of the GFP+ and hence BCR-ABL1+ cells expressed the myeloid surface marker MAC-1 (supplemental Figure 2B). The cells were then treated with increasing concentrations of imatinib for 48 hours. Imatinib reduced the viability and cell numbers of the BCR-ABL1+/GFP+ cells without affecting the BCR-ABL1-negative population. As additional control, naive BMs were treated with imatinib, which did not exert any effect irrespective of the genotype. In contrast and as shown in Figure 2A-B, Stat5null/+ cells showed a significantly stronger reduction of viable BCR-ABL1+/GFP+ cells compared with BCR-ABL1+/GFP+Stat5+/+ cells. Similar results were obtained with 2 individual p210BCR-ABL1 transformed Stat5fl/+ Cre-ERT2 cell lines that express the progenitor markers c-KIT and SCA-1 (supplemental Figure 2C-D). Deletion of one Stat5a/b allele by tamoxifen treatment enhanced cell death to imatinib exposure.
Stat5null/+ cells depending on p210BCR-ABL1 or v-ABL are highly susceptible to imatinib-induced apoptosis. (A) BM derived from Stat5null/+ (n = 4) and Stat5+/+ (n = 6) mice were infected with a retrovirus encoding for p210BCR-ABL1-IRES-GFP and subsequently treated for 48 hours with increasing dosages of imatinib as indicated. Percentages of BCR-ABL1+/GFP+ cells were analyzed via FACS to evaluate differences in imatinib response. Stat5null/+ cells reacted significantly more sensitive on imatinib treatment compared with Stat5+/+ cells. Shown is one representative FACS profile. (B) Summary of all experiments described in panel A. (C) Immunoblot analysis of STAT5A/B and v-ABL protein expression in Stat5null/+ and Stat5+/+ cells. (D) Dose-response curves determined by [3H]-thymidine incorporation of v-ABL transformed Stat5null/+ and Stat5+/+ cells treated with imatinib for 24 hours (n = 4/genotype). (E) Summary of colony-forming assays of v-Abl+Stat5null/+ and Stat5+/+ cells grown for 21 days in growth-factor free methylcellulose enriched with 10nM or 100nM imatinib (n = 3/genotype). (F-G) Annexin V stained v-ABL transformed Stat5null/+ and Stat5+/+ cells challenged with 100nM imatinib for 24 hours (right, n = 3/genotype). One representative FACS profile is shown in panel G. Data are mean ± SD. *P < .05. **P < .001.
Stat5null/+ cells depending on p210BCR-ABL1 or v-ABL are highly susceptible to imatinib-induced apoptosis. (A) BM derived from Stat5null/+ (n = 4) and Stat5+/+ (n = 6) mice were infected with a retrovirus encoding for p210BCR-ABL1-IRES-GFP and subsequently treated for 48 hours with increasing dosages of imatinib as indicated. Percentages of BCR-ABL1+/GFP+ cells were analyzed via FACS to evaluate differences in imatinib response. Stat5null/+ cells reacted significantly more sensitive on imatinib treatment compared with Stat5+/+ cells. Shown is one representative FACS profile. (B) Summary of all experiments described in panel A. (C) Immunoblot analysis of STAT5A/B and v-ABL protein expression in Stat5null/+ and Stat5+/+ cells. (D) Dose-response curves determined by [3H]-thymidine incorporation of v-ABL transformed Stat5null/+ and Stat5+/+ cells treated with imatinib for 24 hours (n = 4/genotype). (E) Summary of colony-forming assays of v-Abl+Stat5null/+ and Stat5+/+ cells grown for 21 days in growth-factor free methylcellulose enriched with 10nM or 100nM imatinib (n = 3/genotype). (F-G) Annexin V stained v-ABL transformed Stat5null/+ and Stat5+/+ cells challenged with 100nM imatinib for 24 hours (right, n = 3/genotype). One representative FACS profile is shown in panel G. Data are mean ± SD. *P < .05. **P < .001.
Our observations were confirmed in v-ABL-transformed lymphoid cells. Cell lines derived from Stat5null/+ fetal livers display an approximate 50% decreased STAT5 protein expression compared with Stat5+/+ cells as quantified by ImageJ software Version 1.42 (Figure 2C). In contrast, no differences in v-ABL expression were detected. Determination of IC50 values on imatinib treatment in the Stat5null/+ STC revealed a more than 10-fold difference (IC50Stat5null/+ = 31nM, IC50 wild-type = 353nM; Figure 2D). The increased imatinib sensitivity of Stat5null/+ cells was also prominent when cells were seeded in growth-factor free methylcellulose in the presence of 10nM or 100nM imatinib (Figure 2E). Annexin V stainings suggest that the underlying mechanism relies on increased apoptosis in Stat5null/+ cells. Significantly more cells underwent apoptosis in Stat5null/+ STCs compared with wild-type controls on treatment with 100nM imatinib for 24 hours (Figure 2F-G). These experiments implicate STAT5 as a regulator of imatinib responsiveness.
Enhanced expression of STAT5A reduces TKI -induced cytotoxicity
Stat5null/+ cells may harbor additional developmental alterations that account for the differences in imatinib sensitivity. To exclude this possibility, we infected p185BCR-ABL1+ or v-Abl+ STCs with a pMSCv-Stat5a-eGFP based retrovirus to generate transformed B cells that differ from vector controls solely by an elevated STAT5A expression. One representative FACS experiment is shown in Figure 3A. We obtained an infection rate of approximately 20% irrespective of whether we used the empty vector or the vector encoding STAT5A (resulting in “S5ahigh cells”). Subsequently, we challenged the resulting mixed cell population of nontransduced and transduced cells to increasing concentrations of imatinib. Of the viable cells, the percentage of GFP+ cells was analyzed using FACS 24 hours thereafter. The treatment of cells with imatinib did not change the percentage of GFP+ cells infected with empty vector (Figure 3A bottom panel). In contrast, cells expressing elevated levels of STAT5A were selected in the presence of imatinib. This was indicated by the increase in percentage of GFP+-S5ahigh cells (Figure 3A top panel). The effect was most evident at a concentration of 1000nM imatinib, where the percentage of S5ahigh cells increased from 20% to 56% within 24 hours. The experiment was repeated with comparable results using individually derived v-Abl+ cell lines (n = 10) or p185BCR-ABL1+ cell lines (n = 5) (summarized in Figure 3B-C). Similar results were obtained when JAK2-deficient p185BCR-ABL1+ cells were used. The lack of JAK2 did not alter the protective effect of elevated STAT5A levels, indicating the independence of JAK2 mediated signaling (supplemental Figure 3C-D; and data not shown). The effects of our wild-type cells were reinforced when imatinib incubation time of v-Abl+ cells, at a concentration of 100nM, was extended to 48 and 72 hours (supplemental Figure 3A). Similarly, long-term incubations using low concentrations of imatinib (10nM) were capable of selecting for S5ahigh cells over a period of 2 weeks (supplemental Figure 3B).
Enhanced STAT5A expression renders cells less susceptible to imatinib-induced apoptosis. (A) FACS profiles of v-Abl+ cells ectopically expressing STAT5A and GFP (top profile) and vector control cells expressing only GFP (bottom profile) treated 24 hours with imatinib. The percentage of GFP+ cells and the dosage of imatinib administered are indicated. (B-C) Summary of the experiment shown in panel A repeated with 10 individual v-Abl+ (B) and 5 individual p185BCR-ABL1+ (C) cell lines. (D) FACS analysis for propidium iodide (PI) uptake of v-Abl+ cell lines sorted for Stat5a-IRES-eGFP-expressing (S5ahigh) and control (S5alow) cell-treated imatinib for 48 hours (n = 3/group). STAT5A/B expression levels were determined by intracellular FACS staining and are shown in the inset. (E) Representative cytospins of S5ahigh and S5alow cells 48 hours after imatinib treatment. (F) v-ABL-transformed STCs were infected with pMSCv-Stat5a-IRES-eGFP and treated with imatinib, dasatinib, and nilotinib. The experiment was performed as outlined in panel A. The summary of 3 individually performed experiments is shown. (G) [3H]-Thymidine incorporation assay of Ba/F3 p210BCR-ABL1 cells harboring a doxycycline-inducible dominant negative STAT5 (S5aΔ749). [3H]-Thymidine uptake was measured 48 hours after induction of S5aΔ749 expression and 24 hours after TKI treatment. The relative percentage of [3H]-thymidine uptake compared with untreated control cells (black bar) is shown (n ≥ 3/group). (B-E) Error bars represent mean ± SD. *P < .05. **P < .01. ***P < .001.
Enhanced STAT5A expression renders cells less susceptible to imatinib-induced apoptosis. (A) FACS profiles of v-Abl+ cells ectopically expressing STAT5A and GFP (top profile) and vector control cells expressing only GFP (bottom profile) treated 24 hours with imatinib. The percentage of GFP+ cells and the dosage of imatinib administered are indicated. (B-C) Summary of the experiment shown in panel A repeated with 10 individual v-Abl+ (B) and 5 individual p185BCR-ABL1+ (C) cell lines. (D) FACS analysis for propidium iodide (PI) uptake of v-Abl+ cell lines sorted for Stat5a-IRES-eGFP-expressing (S5ahigh) and control (S5alow) cell-treated imatinib for 48 hours (n = 3/group). STAT5A/B expression levels were determined by intracellular FACS staining and are shown in the inset. (E) Representative cytospins of S5ahigh and S5alow cells 48 hours after imatinib treatment. (F) v-ABL-transformed STCs were infected with pMSCv-Stat5a-IRES-eGFP and treated with imatinib, dasatinib, and nilotinib. The experiment was performed as outlined in panel A. The summary of 3 individually performed experiments is shown. (G) [3H]-Thymidine incorporation assay of Ba/F3 p210BCR-ABL1 cells harboring a doxycycline-inducible dominant negative STAT5 (S5aΔ749). [3H]-Thymidine uptake was measured 48 hours after induction of S5aΔ749 expression and 24 hours after TKI treatment. The relative percentage of [3H]-thymidine uptake compared with untreated control cells (black bar) is shown (n ≥ 3/group). (B-E) Error bars represent mean ± SD. *P < .05. **P < .01. ***P < .001.
To exclude that interaction of S5ahigh cells with noninfected cells in the mixed populations interferes with the imatinib response, v-Abl+ cells were sorted for GFP-expressing (S5ahigh) and nonexpressing (S5alow) cells. FACS analysis for intracellular STAT5A/B and immunoblots confirmed higher STAT5 protein levels in S5ahigh cells compared with S5alow cells (Figure 3D inserted histograms; supplemental Figure 3C). The differences in imatinib sensitivity were even more pronounced than in the mixed cell populations. Starting at concentrations of 300nM imatinib, S5ahigh cell lines had a significantly higher viability. All S5alow cells underwent apoptosis when exposed to 1000nM imatinib, whereas still approximately 60% of the S5ahigh cells remained viable after 48 hours (Figure 3D). Cytospins confirmed these results (Figure 3E). These experiments clearly link enforced STAT5A expression to decreased imatinib sensitivity. The effects cannot be attributed to an increase in BCR-ABL1 or v-ABL expression as confirmed by immunoblots (supplemental Figure 4A-B). We also excluded that the imatinib exposure selected for cells expressing high levels of BCR-ABL1 or v-ABL (supplemental Figure 4B). Moreover, we analyzed v-ABL and BCR-ABL1 activity using pCRKL as a surrogate marker. Ba/F3p185BCR-ABL1, Ba/F3p210BCR-ABL1 cells, one v-ABL, and 2 p185BCR-ABL1 transformed primary mouse cell lines were treated for 3 hours with increasing concentrations of imatinib. pCRKL was determined using intracellular FACS and, as expected, decreased in a concentration-dependent manner under imatinib treatment. No differences between S5ahigh or S5alow cells were detectable. This indicates that BCR-ABL1 in both cell types reacts in a comparable manner to imatinib treatment (supplemental Figure 4C-D). These findings make it highly unlikely that the decreased imatinib sensitivity in S5ahigh cells is the consequence of additional mutations or the up-regulation of a multidrug transporter.
We next tested whether the protective effect of elevated STAT5A protein levels is specific for imatinib or extends to other BCR-ABL1 kinase inhibitors. As shown in Figure 3F, the protective effect of STAT5A was also observed in experiments using dasatinib and nilotinib. Most importantly, the protective effect restricted neither to one BCR-ABL1 kinase inhibitor nor to our cellular system. We took advantage of p210BCR-ABL1 transformed Ba/F3 cells that additionally express a doxycycline-inducible dominant negative variant of STAT5A (S5aΔ749).34 The expression of S5aΔ749 was induced for 48 hours by doxycycline treatment. A dosage of doxycycline was defined in preliminary experiments that induced S5aΔ749 expression but had only a minor impact on the viability of the cells (data not shown). To delineate the combined effects of BCR-ABL1 kinase inhibition and STAT5 inhibition, concentrations of the TKIs only slightly affecting the cells were used. The inhibitors imatinib, nilotonib, or dasatinib were added 24 hours after doxycycline. As shown in Figure 3G, the combined inhibition of STAT5 and p210BCR-ABL1 resulted in a significantly decreased viability compared with the inhibition of p210BCR-ABL1 alone. These experiments suggested that transcriptionally competent STAT5 regulates the sensitivity of v-ABL and BCR-ABL1 transformed cells toward BCR-ABL1 kinase inhibition.
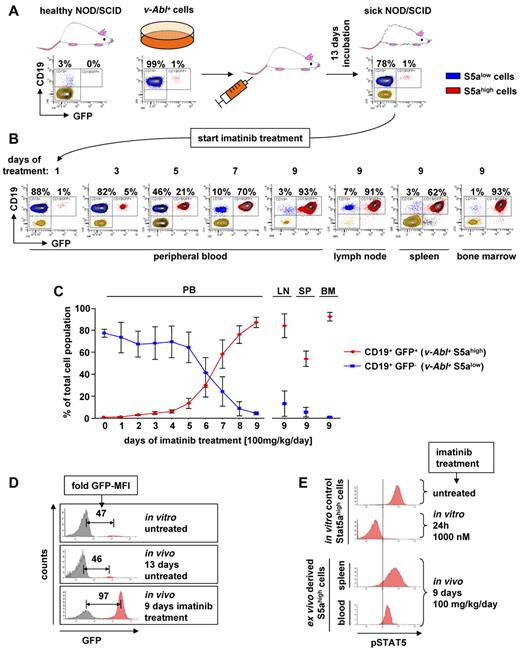
Ectopic expression of STAT5A renders v-ABL transformed cells resistant to in vivo treatment with imatinib
The data obtained so far encouraged us to challenge our concept in an in vivo experimental setting. To mimic the situation of leukemic patients undergoing imatinib treatment, we injected NOD/SCID mice with v-Abl+ cells. We prepared wild-type STCs that contained 99% of uninfected cells (S5alow) enriched by 1% of cells infected with pMSCv-Stat5a-IRES-GFP (S5ahigh). Thus, S5ahigh cells are indicated by GFP expression (Figure 4A top middle panel). A total of 2 × 106 cells/mouse were injected intravenously, and the animals showed first signs of disease, such as reduced mobility and scrubby fur, 13 days thereafter. Blood samples were taken, which revealed the presence of significant amounts of leukemic cells. Approximately 80% of the cells in the PB expressed CD19 and were therefore classified as leukemic cells. No change in the composition of S5ahigh versus S5alow cells was detectable. Still, 1% of the leukemic cells were GFP+, indicating high STAT5A expression levels (Figure 4A top right panel). Hence, under conditions where the leukemic cells spread and proliferate in the animal, the enforced expression of STAT5A did not confer any selective advantage. Treatment with imatinib was initiated with 100 mg/kg once per day by gavage. During therapy, peripheral blood (PB) samples were checked daily by FACS for the presence of leukemic cells. Importantly, the “regular” S5alow leukemic cells steadily declined under imatinib therapy. In contrast, the S5ahigh cells remained unaffected and steadily increased (Figure 4B). Nine days after treatment initiation, the animals had to be killed because they displayed signs of sickness with pronounced weight loss and hind leg ataxia. Pathologic analysis revealed that the mice had a leukemia densely infiltrating spleen, bone marrow (BM), and lymph nodes. FACS analysis showed that the leukemic cells consisted nearly exclusively of GFP+ S5ahigh cells (Figure 4B right panel). This was observed in all mice analyzed (n = 6; Figure 4C). In contrast, no selection of S5ahigh cells was detected in untreated animals (supplemental Figure 5A). Again, determination of the expression level of v-ABL as well as the determination of pCRKL via intracellular FACS confirmed that the effects cannot be attributed to an increased expression or reduced sensitivity of the oncogenic kinase itself (supplemental Figure 5B-C).
Treatment of leukemic mice with imatinib selects for STAT5A overexpressing cells. (A) Top panel: FACS analysis of CD19 and GFP from PB-derived cells of a healthy NOD/SCID mouse (left panel), freshly established stable v-Abl+ cells infected with pMSCv-eGFP based retrovirus encoding for murine STAT5A showing 1% infection efficiency (middle panel) and blood-derived cells from a NOD/SCID mouse 13 days after injection of the v-Abl+ cells (right panel). (B) FACS analysis of PB, lymph nodes (LN), spleen (SP), and BM after imatinib treatment by gavage (100 mg/kg per day). Percentage of CD19+/GFP− (blue) and CD19+/GFP+ (red) cells is shown. (C) Summary of the in vivo experiment shown in panels A and B for a total of 6 mice. Error bars represent mean ± SD. (D) Representative FACS profile of v-Abl+ cells before injection (top panel), 13 days after injection (middle panel), and 9 days after in vivo imatinib treatment (bottom panel). Fold difference of GFP-mean fluorescence intensity of GFP+ (S5ahigh) to GFP− (S5alow) cells are indicated. (E) pSTAT5 level of ex vivo derived S5ahigh cells 9 days after in vivo imatinib (100 mg/kg per day) treatment (bottom histograms) and in vitro control S5ahigh cells before and after 24 hours after imatinib (1000nM) addition (top histograms).
Treatment of leukemic mice with imatinib selects for STAT5A overexpressing cells. (A) Top panel: FACS analysis of CD19 and GFP from PB-derived cells of a healthy NOD/SCID mouse (left panel), freshly established stable v-Abl+ cells infected with pMSCv-eGFP based retrovirus encoding for murine STAT5A showing 1% infection efficiency (middle panel) and blood-derived cells from a NOD/SCID mouse 13 days after injection of the v-Abl+ cells (right panel). (B) FACS analysis of PB, lymph nodes (LN), spleen (SP), and BM after imatinib treatment by gavage (100 mg/kg per day). Percentage of CD19+/GFP− (blue) and CD19+/GFP+ (red) cells is shown. (C) Summary of the in vivo experiment shown in panels A and B for a total of 6 mice. Error bars represent mean ± SD. (D) Representative FACS profile of v-Abl+ cells before injection (top panel), 13 days after injection (middle panel), and 9 days after in vivo imatinib treatment (bottom panel). Fold difference of GFP-mean fluorescence intensity of GFP+ (S5ahigh) to GFP− (S5alow) cells are indicated. (E) pSTAT5 level of ex vivo derived S5ahigh cells 9 days after in vivo imatinib (100 mg/kg per day) treatment (bottom histograms) and in vitro control S5ahigh cells before and after 24 hours after imatinib (1000nM) addition (top histograms).
Interestingly, we did not only see a selection of S5ahigh cells over S5alow cells, but also a selection process within the S5ahigh population with an enrichment of the brightest GFP+ S5ahigh cells indicating very high levels of STAT5A protein. The GFP fluorescence intensity correlates with the amount of ectopically expressed STAT5A (supplemental Figure 5D). We observed a 46-fold higher GFP-mean fluorescence intensity in the S5ahigh population compared with the GFP-negative cells that remained constant during the progression of the leukemia before imatinib treatment (Figure 4D top and middle panels). However, 9 days after imatinib treatment, the GFP-mean fluorescence intensity value directly indicating STAT5A expression had significantly increased from 46-fold to 97-fold (Figure 4D bottom panel; summarized in supplemental Figure 5E). Next, we questioned whether CML cells derived from the murine transplant do have high pSTAT5 levels despite the continuous presence of imatinib. As shown in Figure 4E, S5ahigh leukemic cells that evolved in the presence of imatinib displayed pSTAT5 levels that were only slightly reduced compared with untreated cells in vitro.
Transcriptional activity of STAT5 is required to confer protection from imatinib-induced apoptosis
For transcriptional regulation, STAT5 is tyrosine phosphorylated, dimerizes, and translocates to the nucleus, where it binds to DNA. To investigate whether enforced expression of STAT5 in Abelson-transformed cells is accompanied by increased tyrosine phosphorylation, we quantified pSTAT5 using intracellular FACS. As shown in Figure 5A (left panel), higher levels of phosphorylated STAT5 were present in sorted S5ahigh cells compared with S5alow cells. Accordingly, S5ahigh cells showed increased amounts of DNA-bound STAT5 verified by electrophoretic mobility shift assay (Figure 5A right panel). To investigate whether the transcriptional function of STAT5A accounts for the STAT5A-mediated resistance against imatinib, we took advantage of different STAT5A mutants (shown in Figure 5B). cS5F is a constitutively active STAT5A mutant resulting from a S711F point mutation. S5aΔN lacks the N-terminal domain required for oligomerization but is still capable of functioning as a transcription factor for some STAT5 target genes.36,39 S5aY/F lacks the critical tyrosine phosphorylation (pY) site on position aa693 and does not efficiently translocate to the nucleus, and S5aEE/AA has DNA-binding domain mutations (EE437/438AA) blocking efficient DNA binding to STAT5 response elements.37 Retroviral infection was used to express these mutants in wild-type STCs. Cells were treated for 24 hours with imatinib, and viable cells were analyzed for GFP expression by FACS. Wild-type STAT5A, cS5F, and S5aΔN mediated growth advantage compared with untransfected cells. In contrast, infection with the empty vector or STAT5A mutants incapable of DNA binding had no effect (Figure 5C). These experiments provided evidence that tyrosine phosphorylation and DNA binding of STAT5 are prerequisites for the protective effect against imatinib.
Survival of imatinib-treated v-Abl+ cells depends on the phosphorylation status of STAT5. (A) FACS profiles of cells infected with pMSCv-Stat5a-IRES-eGFP retrovirus before (left histogram) and after sort for GFP+ cells (middle histograms). Tyrosine phosphorylated STAT5 is shown in the right histograms. Right panel: Electrophoretic mobility shift assay of sorted GFP+ (S5ahigh) and GFP− (S5alow) cells for STAT5. Stat5null/null MEFs were used as negative control, and Ba/F3 stimulated with IL-3 were used as positive control. (B) Scheme of murine wild-type and mutant STAT5A variants. Mutants lacking the tyrosine phosphorylation (pY) site or having impaired DNA binding (DB) are indicated by either (+) or (−). (C) v-Abl+ cell lines expressing STAT5A, STAT5A mutants, or vector control along with GFP 24 hours after imatinib administration. The fold increase of GFP-expressing cells is shown. Error bars represent mean ± SD. ***P < .001 for a dosage of 1000nM imatinib (n ≥ 4/group).
Survival of imatinib-treated v-Abl+ cells depends on the phosphorylation status of STAT5. (A) FACS profiles of cells infected with pMSCv-Stat5a-IRES-eGFP retrovirus before (left histogram) and after sort for GFP+ cells (middle histograms). Tyrosine phosphorylated STAT5 is shown in the right histograms. Right panel: Electrophoretic mobility shift assay of sorted GFP+ (S5ahigh) and GFP− (S5alow) cells for STAT5. Stat5null/null MEFs were used as negative control, and Ba/F3 stimulated with IL-3 were used as positive control. (B) Scheme of murine wild-type and mutant STAT5A variants. Mutants lacking the tyrosine phosphorylation (pY) site or having impaired DNA binding (DB) are indicated by either (+) or (−). (C) v-Abl+ cell lines expressing STAT5A, STAT5A mutants, or vector control along with GFP 24 hours after imatinib administration. The fold increase of GFP-expressing cells is shown. Error bars represent mean ± SD. ***P < .001 for a dosage of 1000nM imatinib (n ≥ 4/group).
The protective effect of high STAT5A levels is restricted to tyrosine kinase inhibitor treatment
We next tested mRNA expression levels of critical STAT5 target genes implicated in survival or proliferation of hematopoietic cells. Real-time RT-PCRs for the STAT5 target genes Osm, Pim-1, Cis, c-Myc, Cyclin D2, Socs2, and Bcl-2 were performed. The mRNA levels of S5alow cells served as baseline. A clear up-regulation of all analyzed mRNAs was detected in S5ahigh cell lines (Figure 6A). The pronounced up-regulation of the antiapoptotic Bcl-2 mRNA attracted our interest because a correlation between Bcl-2 overexpression and increased imatinib resistance has been shown. However, already a short incubation of S5ahigh cells with TKIs (3 hours, 100nM dasatinib or 1000nM imatinib) strongly reduced STAT5 target gene mRNA expression to control levels, including Bcl-2 (Figure 6A; and data not shown). Although it appears reasonable that the high levels of antiapoptotic proteins may account for a short-term advantage of STAT5 overexpressing cells, the rapid decrease after TKI treatment makes it highly unlikely to allow for long-term survival of S5ahigh cells (eg, 9-day in vivo treatment). To analyze the dose-dependent effects of imatinib on STAT5 activity, we performed intracellular FACS analysis for pSTAT5. S5ahigh cells showed only a minor reduction of pSTAT5 levels when treated with imatinib for 24 hours up to concentrations of 300nM (Figure 6B top panel). Thereby, 300nM imatinib reduces, but does not entirely abrogate, kinase activity (supplemental Figure 4C). In contrast, the concentration of 300nM imatinib suffices to significantly reduce STAT5 phosphorylation in S5alow cells and to induce apoptosis after 72 hours, underscoring the close correlation of pSTAT5 level and imatinib sensitivity (Figure 6B bottom panel). The pSTAT5 levels in S5ahigh cells were reduced to the levels observed in S5alow cells, only when we used high concentrations of imatinib (1000nM). Under these conditions, we could initiate apoptosis even in S5ahigh cells, albeit with a certain delay. This prompted us to propose the following concept: A threshold of pSTAT5 enables Abelson-transformed cells to survive under imatinib treatment, which may be maintained with higher probability in cells with high STAT5 protein expression. When we performed dose-response curves in S5ahigh and S5alow cells using interferon-β, hydroxyurea, etoposide, puromycin, hygromycin, and Aza-dC, we failed to observe any significant beneficial effect of high STAT5 levels, ruling out any general effects (Figure 6C). These results verify that the protective effect of high STAT5 levels for BCR-ABL+ cells is only relevant under TKI treatment. Figure 6E shows a scheme explaining the mechanism of imatinib resistance mediated by elevated STAT5 levels and the effect of a dose escalation to overcome this resistance.
STAT5A-mediated survival advantage is restricted to TKI-exposed cells and depends on Abelson kinase activity. (A) RT-PCR of S5ahigh and S5alow cells before and after treatment with 100nM dasatinib for 3 hours. Results were normalized by Gapdh mRNA expression. The fold change compared with untreated S5alow cell mRNA levels is shown (n = 3/group). (B) Top panel: Intracellular pSTAT5 staining of S5alow and S5ahigh cells 24 hours after imatinib treatment as indicated. The dashed line indicates the predicted threshold of pSTAT5 level essential for survival. Bottom: FACS analysis for propidium iodide (PI) uptake 72 hours after imatinib treatment as indicated. Percentages of PI-positive cells are shown. (C) Dose-response curves determined by [3H]-thymidine incorporation of S5ahigh and S5alow cells treated with indicated substances for 24 hours. Control values in the absence of any substances were set to 100%. (D) Scheme showing the mechanism of high STAT5 protein levels decreasing imatinib response and how a dose escalation can help to overcome this resistance.
STAT5A-mediated survival advantage is restricted to TKI-exposed cells and depends on Abelson kinase activity. (A) RT-PCR of S5ahigh and S5alow cells before and after treatment with 100nM dasatinib for 3 hours. Results were normalized by Gapdh mRNA expression. The fold change compared with untreated S5alow cell mRNA levels is shown (n = 3/group). (B) Top panel: Intracellular pSTAT5 staining of S5alow and S5ahigh cells 24 hours after imatinib treatment as indicated. The dashed line indicates the predicted threshold of pSTAT5 level essential for survival. Bottom: FACS analysis for propidium iodide (PI) uptake 72 hours after imatinib treatment as indicated. Percentages of PI-positive cells are shown. (C) Dose-response curves determined by [3H]-thymidine incorporation of S5ahigh and S5alow cells treated with indicated substances for 24 hours. Control values in the absence of any substances were set to 100%. (D) Scheme showing the mechanism of high STAT5 protein levels decreasing imatinib response and how a dose escalation can help to overcome this resistance.
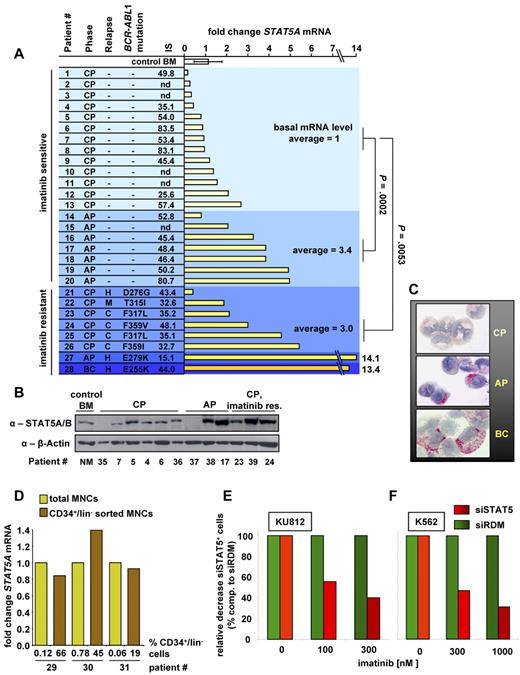
STAT5 expression in leukemic cells of CML patients increases during disease progression and mediates imatinib response of human CML cell lines
We next analyzed STAT5 levels during disease progression to determine the significance of our data for CML patients. To address this, PB- or BM-derived mononuclear leukemic cells of untreated patients at the time points of diagnosis were collected (patient characteristics, supplemental Table 1). STAT5A mRNA levels were determined by real-time polymerase chain reaction (RT-PCR) and BM from 3 healthy patients served as control. A significant increase of STAT5A mRNA was found in samples obtained from patients in AP (n = 7) compared with CP (n = 13) (P = .0002, Figure 7A). In addition, we tested 8 samples from imatinib-treated patients (6 in CP, one in AP, and one in BC) that had developed imatinib resistance and relapsed. A total of 40% to 90% of cases of imatinib resistance are a result of mutations within the kinase domain of BCR-ABL1.18 Although all of our analyzed imatinib-resistant CML patients were tested positive for BCR-ABL1 mutations, a significant up-regulation of STAT5A mRNA compared with patients with imatinib-sensitive CP was registered (P = .0053). The international scale (IS) value for BCR-ABL1 mRNA level and detected BCR-ABL1 mutations are shown (analyzed BCR-ABL1 mutations listed in supplemental Figure 6A). Similar results were obtained for STAT5B mRNA when samples from 3 healthy and 19 CML diseased patients were analyzed (supplemental Figure 6B). The increase in STAT5 mRNA is reflected by changes in protein expression levels: patients with CML in AP or imatinib-resistant CP display higher STAT5 protein levels compared with patients in imatinib-sensitive CP (Figure 7B). To control for the quality of the patient samples additional to β-actin, we analyzed the protein expression of CDK2, CDK4, and α-tubulin, which were comparable between the disease phases (supplemental Figure 6C). Expression of activated STAT5 (pSTAT5) in primary CML cells was further confirmed by immunocytochemistry. The percentage of pSTAT5+ cells varied among patients. In most patients with CP, only a few cells stained positive for pSTAT5, whereas higher counts were recorded in AP and BC (Figure 7C; Table 1) consistent with our previous findings. Furthermore, the pSTAT5 intensity, which reflects the amount of STAT5 proteins in untreated cells, was elevated in AP and BC. Interestingly, even in BC, not all clonal cells (blasts) were found to react with the anti-pSTAT5 antibody.
Elevated STAT5 levels are found in advanced CML phases, and STAT5 down-regulation increases imatinib response of human CML cell lines. (A) RT-PCR of human STAT5A mRNA transcripts of BM derived from healthy control patients (n = 3), PB derived from untreated CML patients in CP (n = 13), AP (n = 7), and relapsed imatinib-resistant patients (n = 6 for CP, one in AP, and one in BC). CML phase, kind of relapse (C indicates cytogenetic; H, hematologic; and M, molecular), BCR-ABL1 mutations, IS value for BCR-ABL1 mRNA level, and fold change STAT5A mRNA levels compared with mean STAT5A mRNA level of imatinib-sensitive CP samples are indicated. nd indicates not determined. Each bar represents data derived from an individual patient. Results were normalized by comparison with their GAPDH mRNA expression. P values for patients in imatinib-sensitive CP compared with imatinib-sensitive AP and imatinib-resistant CP are indicated. (B) STAT5A/B immunoblot of BM derived from a healthy patient (control BM) and PB derived from CML diseased patients in CP (n = 6), AP (n = 3), and imatinib-resistant CP (n = 3). Each lane represents an individual patient. (C) Expression of pSTAT5 in primary CML cells. Mononuclear BM cells from 3 CML patients, one in CP (top image), one in AP (middle image), and one in BC (C, bottom image), were spun on cytospin slides and stained with anti-pSTAT5 antibody AX1. Subsets of CML cells in AP and BC were found to stain positive for pSTAT5, whereas fewer cells expressed pSTAT5 in CP. Representative examples are shown. (D) RT-PCR for STAT5A mRNA from total MNCs and CD34+/lin− sorted MNCs derived from 3 individual CML patient samples. (E) KU812 and (F) K562 cells expressing siSTAT5 or siRDM were treated 48 hours with imatinib. Shown is the relative decrease of siSTAT5+ compared with siRDM+ cells. Percentages of siRDM expressing cells served as baseline and were set to 100%.
Elevated STAT5 levels are found in advanced CML phases, and STAT5 down-regulation increases imatinib response of human CML cell lines. (A) RT-PCR of human STAT5A mRNA transcripts of BM derived from healthy control patients (n = 3), PB derived from untreated CML patients in CP (n = 13), AP (n = 7), and relapsed imatinib-resistant patients (n = 6 for CP, one in AP, and one in BC). CML phase, kind of relapse (C indicates cytogenetic; H, hematologic; and M, molecular), BCR-ABL1 mutations, IS value for BCR-ABL1 mRNA level, and fold change STAT5A mRNA levels compared with mean STAT5A mRNA level of imatinib-sensitive CP samples are indicated. nd indicates not determined. Each bar represents data derived from an individual patient. Results were normalized by comparison with their GAPDH mRNA expression. P values for patients in imatinib-sensitive CP compared with imatinib-sensitive AP and imatinib-resistant CP are indicated. (B) STAT5A/B immunoblot of BM derived from a healthy patient (control BM) and PB derived from CML diseased patients in CP (n = 6), AP (n = 3), and imatinib-resistant CP (n = 3). Each lane represents an individual patient. (C) Expression of pSTAT5 in primary CML cells. Mononuclear BM cells from 3 CML patients, one in CP (top image), one in AP (middle image), and one in BC (C, bottom image), were spun on cytospin slides and stained with anti-pSTAT5 antibody AX1. Subsets of CML cells in AP and BC were found to stain positive for pSTAT5, whereas fewer cells expressed pSTAT5 in CP. Representative examples are shown. (D) RT-PCR for STAT5A mRNA from total MNCs and CD34+/lin− sorted MNCs derived from 3 individual CML patient samples. (E) KU812 and (F) K562 cells expressing siSTAT5 or siRDM were treated 48 hours with imatinib. Shown is the relative decrease of siSTAT5+ compared with siRDM+ cells. Percentages of siRDM expressing cells served as baseline and were set to 100%.
Detection of pSTAT5 in CML cells by immunocytochemistry
| Patient no. . | Source . | Diagnosis . | Treatment . | % pSTAT5+ cells . |
|---|---|---|---|---|
| 22 | BM | CML CP | Nilotinib | < 5 |
| 32 | PB | CML CP | No | < 5 |
| 13 | PB | CML CP | No | 10 |
| 13 | BM | CML CP | No | 20 |
| 20 | BM | CML AP | No | 5 |
| 17 | PB | CML AP | No | 25 |
| 34 | PB | CML BC | No | 25 |
| Patient no. . | Source . | Diagnosis . | Treatment . | % pSTAT5+ cells . |
|---|---|---|---|---|
| 22 | BM | CML CP | Nilotinib | < 5 |
| 32 | PB | CML CP | No | < 5 |
| 13 | PB | CML CP | No | 10 |
| 13 | BM | CML CP | No | 20 |
| 20 | BM | CML AP | No | 5 |
| 17 | PB | CML AP | No | 25 |
| 34 | PB | CML BC | No | 25 |
Mononuclear cells were spun on cytospin slides and stained with the anti-pSTAT5 antibody AX1.
When we blotted the BCR-ABL1 IS values versus STAT5A mRNA levels, no positive linear regression was determined. This excludes a link between STAT5A and BCR-ABL1 expression also for the human CML samples (supplemental Figure 7A). We next sorted CD34+/lin− cells out of human CML mononuclear cells (MNCs) derived from 3 individual patients. This resulted in a more than 300-fold enrichment of CD34+/lin− cells. Comparison of STAT5A mRNA by RT-PCR failed to detect any consistent change between total MNCs and the CD34+/lin− sorted cells (Figure 7D). The up-regulation of STAT5 can therefore not be explained by a shift in cellular populations, such as the presence of increased numbers of immature cells and blasts in the BCR-ABL1+ samples investigated. This statement is further supported by the lack of a positive correlation between STAT5A mRNA level and the percentage of blasts in the PB (supplemental Figure 7B).
To verify that the expression level of STAT5 modulates imatinib sensitivity also in human cells, we used a lentiviral-mediated down-regulation of human STAT5A/B. Cells derived from patient samples as well as the human cell lines K562 and KU812 were transduced with siRNA against STAT5A/B (siSTAT5) and a random control construct (siRDM). Immunoblotting verified the specific knockdown (data not shown). Interestingly, induction of apoptosis mediated by high concentrations of imatinib (3μM) took more than 2 weeks in primary cells derived from patients. Within this time frame, the siRNA-mediated down-regulation of STAT5 had reduced the viability of the primary cells to an extent that precludes obtaining any solid reproducible or trustable information (supplemental Figure 7C-E). This underscores the significance of STAT5 for the viability of human CML cells. To nevertheless obtain information in human cells, the experiments were performed with KU812 and K562 cells. The rapid imatinib-driven induction of apoptosis (24-48 hours) in these cell lines allowed us to determine imatinib sensitivity after siRNA-mediated knockdown. As shown in Figure 7E-F, STAT5 down-regulation enhanced imatinib sensitivity in both KU812 and K562 cells. Within the time frame of the experiment, no decrease in overall viability in siRNA-expressing cells in the absence of imatinib was observed.
Discussion
Response to imatinib treatment and the induction of remission of BCR-ABL1+ CML critically depend on the disease stage at which treatment is initiated.10-12,41 Best responses are achieved in CP. Accordingly, imatinib dosage has to be increased from 400 to 600 or 800 mg in AP and BC, or therapy has to be switched. The underlying reasons for the low responsiveness accompanying advanced CML are only partially understood. Contributing factors include the appearance of point mutations within the kinase domain of BCR-ABL1, increased expression levels of BCR-ABL1, SRC family kinases, or the drug-transporter ABCB1, TP53 deletion, or elevated GM-CSF secretion.13-15,17,19,42 Our studies identify the transcription factor STAT5 as an additional, important regulator that modulates the responsiveness of BCR-ABL1+ cells to ABL1 kinase inhibitors, such as imatinib, nilotinib, and dasatinib for both myeloid and lymphoid cells. In addition, we found a consistent and significant increase in STAT5 expression levels in advanced phase of CML and imatinib-resistant patient samples. This finding is in agreement with a recent report describing a significantly increased mRNA expression of ABCB1, ABCC1, RUNX3, and STAT5A in patients displaying secondary imatinib resistance without BCR-ABL1 mutations compared with newly diagnosed imatinib responders.43
Although constitutive activation of STAT5 in primary CML patient samples has been reported,44 to the best of our knowledge, this is the first report that reveals a causal link between an increased STAT5 mRNA and protein expression and a reduced imatinib response in Abelson-transformed cells. Our findings are supported by a recent report that demonstrates the contribution of GM-CSF, which activates the JAK2-STAT5 pathway, to imatinib resistance.19 This study also reaches the conclusion that pSTAT5 protects BCR-ABL1+ cells from imatinib-induced apoptosis, albeit through a different mechanism. The authors describe increased GM-CSF synthesis and propose that the GM-CSF-induced activation of STAT5 via JAK2 accounts for a decrease in imatinib responsiveness. We here report an additional mechanism; the up-regulation of STAT5 protein itself suffices to confer protection. In our hands, the protective effects were independent of JAK2 but required BCR-ABL1 kinase activity. These differences may be of clinical importance. JAK2 inhibition may be of help in case of GM-CSF-mediated resistance but will have no positive effect when the imatinib resistance is mediated by elevated STAT5 protein levels.
Because the increased resistance depends on the transcriptional activity of STAT5, the most obvious explanation is that STAT5 target genes, such as Bclxl and Bcl-2, build up a barrier against cytotoxicty and apoptosis. Interestingly, we found that the protective effect of STAT5 is restricted to the treatment with TKIs. STAT5 did not prevent cytotoxicity exerted by other agents, such as interferon-β or hydroxyurea. Thereby, our data suggest the following model: a threshold of active STAT5 is a prerequisite for the cells to maintain viability. High levels of STAT5 proteins enhance the probability that even a low remaining kinase activity of BCR-ABL1 will maintain a threshold of tyrosine phosphorylated and thereby transcriptionally active STAT5. Only when the level of pSTAT5 sinks below this critical threshold, apoptosis is induced. This model is also supported by the fact that the application of high imatinib concentrations, which resulted in a complete dephosphorylation of STAT5, was capable of inducing apoptosis irrespective of the STAT5 levels, albeit with a different kinetic and a delayed onset. This different kinetic in cells with high STAT5 levels may be explained by the enhanced expression of antiapoptotic target genes, such as Bclxl and Bcl-2. Our concept is in line with a recent publication that verified the importance of STAT5 for the survival of BCR-ABL1-transformed cells.29 Our model also delivers a possible explanation why dose escalation or switch to more potent BCR-ABL1 inhibitors helps patients who are insensitive to 400-mg imatinib treatment despite harboring no routinely tested BCR-ABL1 mutations or elevated IS values.
The unique and dominating role of STAT5 might also be linked to its capability to interfere with PI3K signaling, a key signaling pathway regulating survival in BCR-ABL1+ cells. Recent publications intertwined pSTAT5 and PI3K activation via the adaptor protein GAB2 and show that pSTAT5 is required to fully activate PI3K.45,46
On progression to BC, the median overall survival decreases to less than 7 months and CML blasts are frequently imatinib-resistant. Recent studies identified the B cell-specific mutator enzyme AID and loss of the transcription factor Ikaros as critical events promoting the development of BC.47,48 Other reports revealed a drastic up-regulation of SOCS2 as well as of Cyclin D2 and BCL-2 during CML progression.49-54 In this regard, it is of interest that SOCS2, Cyclin D2, and BCL-2 are STAT5 target genes, as also evident from our data.27,55,56 Hence, it might be worthwhile to explore STAT5 as a prognostic marker indicating disease progression.
An open question is how expression of STAT5 is regulated on the mRNA and protein level. Our in vivo experiments in mice suggest a “Darwinian” selection process favoring subclones of the leukemic cells with high STAT5 levels. The analysis of human CML cells supports this concept: Imatinib-resistant CP and advanced stages of the disease are accompanied by enhanced STAT5 levels. Even in BC, not all leukemic cells reacted with the pSTAT5 antibody, suggesting subclone formation or expression of pSTAT5 in distinct phases of lineage commitment or differentiation.40 Collectively, our immunocytochemistry data correlated with data obtained in RT-PCR experiments and immunoblots. All together, our data suggest that any therapeutic strategy interfering with the phosphorylation and transcriptional activity of STAT5 may be a promising approach to improve therapy for patients with BCR-ABL1+ leukemia, even in advanced disease stages.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Freissmuth, G. Superti-Furga, and T. Decker for continuous discussion and scientific input.
This work was supported by the Austrian Science Foundation (FWF-SFB 28; V.S., R.M.), the GenAu Program DRAGON (V.S., P.V.), and the Vienna Science and Technology Fund (grant LS07-037; V.S.).
Authorship
Contribution: W.W., K.K., E.E, S.F., K.V.G., A.H., S.C.-R., M.M., G.H., H.H., G.E., and V.S. designed and performed research; W.W., P.V., and V.S. analyzed data; M.D., C.S., P.V., R.M., and V.S. provided vital new reagents and analytic tools; and W.W., P.V., R.M., and V.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Veronika Sexl, Währingerstrasse 13A, A-1090 Vienna, Austria; e-mail: veronika.sexl@meduniwien.ac.at.

![Figure 1. Prolonged culture times of v-Abl+ lymphoid cells correlate with increased STAT5 protein levels and decreased imatinib sensitivity. (A) Cell counts of LTCs (n = 4) and STCs (n = 6) treated with 100nM imatinib. (B) Representative colony-forming assays of LTCs and STCs in growth-factor free methylcellulose without and supplemented with 200nM imatinib. Pictures of colonies were taken after 14 days (LTCs) and 21 days (STCs). (C) Dose-response curves determined by [3H]-thymidine incorporation of LTCs (n = 6) and STCs (n = 4) treated with imatinib for 24 hours. Control values in the absence of imatinib were set to 100%. (D) Immunoblot analysis of STCs and LTCs for STAT transcription factors and v-ABL. (A,C) Error bars represent mean ± SD. ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/12/10.1182_blood-2009-10-248211/5/m_zh89991167160001.jpeg?Expires=1767714158&Signature=1ccek7lbslCe8HG6X5mtjvgXPt4J4G-H6FK55rZ58j17pZkxFQNf57y7bKBRqLpRb6uQs2ZnabUoeI2H5JeXNdDIBE3MZBvXGWf-KOLi6jQOy6F~QW99XwE71zRgQuImDLU66g4DHxIhlkkr6UA-sTOJWrtJoxSckXKbLj3bsOdio8dxbSstNUScplHD3KV8Awp5Hv3s~DQvdPZ8XtIInt77rq~Lpa8LnN66VJOBFUZou6OoFewUqfXw~4DUFgRd8Fnfoisr3aCdTVC8PIqpsG6b~MaDQjlS7WVIa-QwenCTrhDltu~i811X8bDgOKKpL0tJ3WzVmFYi~B-LWWnnow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Stat5null/+ cells depending on p210BCR-ABL1 or v-ABL are highly susceptible to imatinib-induced apoptosis. (A) BM derived from Stat5null/+ (n = 4) and Stat5+/+ (n = 6) mice were infected with a retrovirus encoding for p210BCR-ABL1-IRES-GFP and subsequently treated for 48 hours with increasing dosages of imatinib as indicated. Percentages of BCR-ABL1+/GFP+ cells were analyzed via FACS to evaluate differences in imatinib response. Stat5null/+ cells reacted significantly more sensitive on imatinib treatment compared with Stat5+/+ cells. Shown is one representative FACS profile. (B) Summary of all experiments described in panel A. (C) Immunoblot analysis of STAT5A/B and v-ABL protein expression in Stat5null/+ and Stat5+/+ cells. (D) Dose-response curves determined by [3H]-thymidine incorporation of v-ABL transformed Stat5null/+ and Stat5+/+ cells treated with imatinib for 24 hours (n = 4/genotype). (E) Summary of colony-forming assays of v-Abl+ Stat5null/+ and Stat5+/+ cells grown for 21 days in growth-factor free methylcellulose enriched with 10nM or 100nM imatinib (n = 3/genotype). (F-G) Annexin V stained v-ABL transformed Stat5null/+ and Stat5+/+ cells challenged with 100nM imatinib for 24 hours (right, n = 3/genotype). One representative FACS profile is shown in panel G. Data are mean ± SD. *P < .05. **P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/12/10.1182_blood-2009-10-248211/5/m_zh89991167160002.jpeg?Expires=1767714158&Signature=eRuuORrzCKtsPadhaeKvUIFDkUGLCqG80IvEj80FrcmshLjxxlPnKoK-cNlrwpiN5UsO0awPqHso~sc6Z7c8vA2nR-mS1fyZbYVhAIReza2SJxqMspaMP9qz90q03LHZMvRQSWg8HZ6EQKxEdJxyeQmN2nSc1I9hgRxoPVYrHhjck1uH884z7Mm2gtwdgBLwbxC2kswsyqvhLLeqS5dnDuNVvPjP1ZXxpGaaSJimTPg2YkkHhMKynkpcrnJkwa2MVpRV9S8k64og1kkQ3m20ioWqc6dCkGpQvhc~TpPwK74xTQ1bmjMQdTifiuD~n9fs7-WktUMSCJeoizw06oXf6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Enhanced STAT5A expression renders cells less susceptible to imatinib-induced apoptosis. (A) FACS profiles of v-Abl+ cells ectopically expressing STAT5A and GFP (top profile) and vector control cells expressing only GFP (bottom profile) treated 24 hours with imatinib. The percentage of GFP+ cells and the dosage of imatinib administered are indicated. (B-C) Summary of the experiment shown in panel A repeated with 10 individual v-Abl+ (B) and 5 individual p185BCR-ABL1+ (C) cell lines. (D) FACS analysis for propidium iodide (PI) uptake of v-Abl+ cell lines sorted for Stat5a-IRES-eGFP-expressing (S5ahigh) and control (S5alow) cell-treated imatinib for 48 hours (n = 3/group). STAT5A/B expression levels were determined by intracellular FACS staining and are shown in the inset. (E) Representative cytospins of S5ahigh and S5alow cells 48 hours after imatinib treatment. (F) v-ABL-transformed STCs were infected with pMSCv-Stat5a-IRES-eGFP and treated with imatinib, dasatinib, and nilotinib. The experiment was performed as outlined in panel A. The summary of 3 individually performed experiments is shown. (G) [3H]-Thymidine incorporation assay of Ba/F3 p210BCR-ABL1 cells harboring a doxycycline-inducible dominant negative STAT5 (S5aΔ749). [3H]-Thymidine uptake was measured 48 hours after induction of S5aΔ749 expression and 24 hours after TKI treatment. The relative percentage of [3H]-thymidine uptake compared with untreated control cells (black bar) is shown (n ≥ 3/group). (B-E) Error bars represent mean ± SD. *P < .05. **P < .01. ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/12/10.1182_blood-2009-10-248211/5/m_zh89991167160003.jpeg?Expires=1767714158&Signature=kzwhoA8Q8H-Gb1vnyKgZyf6lMfW77QZVBn2pv6D1yfHL6hbcTtWfw4ZPn4iODbOEd-DU0gN~su~xHexr7912gFD~Y7D9mMB-pV4uZcEHzNOu0fOYCxCScs35JzkarH2wkfvLwCKD3n2pHuEmGinmpVZGSbeGbcVwx8wl-1g6dp9iUEKL8DeTaPm51DRMbB3NYXzrrf8Czx5Tjh8HkobNwoQ4UE~54cjER1Nd1AmSQKD~O~IGE-5kguDP2NN1TktuAamUadFuMuhjy6d5bqbP4agBFpugfTUcqg6eYRRuZWk24XTqGiUaAKnuKRicz~aKqVFgrH8jF~RuKgJFsh7j-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. STAT5A-mediated survival advantage is restricted to TKI-exposed cells and depends on Abelson kinase activity. (A) RT-PCR of S5ahigh and S5alow cells before and after treatment with 100nM dasatinib for 3 hours. Results were normalized by Gapdh mRNA expression. The fold change compared with untreated S5alow cell mRNA levels is shown (n = 3/group). (B) Top panel: Intracellular pSTAT5 staining of S5alow and S5ahigh cells 24 hours after imatinib treatment as indicated. The dashed line indicates the predicted threshold of pSTAT5 level essential for survival. Bottom: FACS analysis for propidium iodide (PI) uptake 72 hours after imatinib treatment as indicated. Percentages of PI-positive cells are shown. (C) Dose-response curves determined by [3H]-thymidine incorporation of S5ahigh and S5alow cells treated with indicated substances for 24 hours. Control values in the absence of any substances were set to 100%. (D) Scheme showing the mechanism of high STAT5 protein levels decreasing imatinib response and how a dose escalation can help to overcome this resistance.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/12/10.1182_blood-2009-10-248211/5/m_zh89991167160006.jpeg?Expires=1767714158&Signature=woPcSXySloWm7edPIXKvDkNXUs6mpK4p8AQkEbM~GJSoC~u0g9Vol7MIEAyDCpykCNQWllU1CSAUByqGsybzSdERM-B7ptzmHPrXBb3lTqjWBdcdAVnZENJdU6XHV4HGXUXJAbmKrRfVIZ4EfCtG6M6Ccuya-oOJ1AWdwWTHEplDfxWC9xWEw9WUgLImXP1LWWXJXhEwSBRW-bK-8TZvjvbRp0Vhapuq1E62EQumvO24sImJA9wxp1RJo8ShZ8LyuNLkicvcKnU8KQijLNgJQiueakFHuaiV0SA0E9PwWP8--Y2iEx9wKZcIBHQAbOJpmU0fvzM7yYjCncCA5yCdbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal