Abstract
The influence of cell dose and human leukocyte antigen (HLA) match on double-unit cord blood (CB) engraftment is not established. Therefore, we analyzed the impact of cell dose and high-resolution HLA match on neutrophil engraftment in 84 double-unit CB transplant recipients. The 94% sustained engraftment rate was accounted for by 1 unit in nearly all patients. Higher CD3+ cell doses (P = .04) and percentage of CD34+ cell viability (P = .008) were associated with unit dominance. After myeloablative conditioning, higher dominant unit total nucleated cell (TNC), CD34+ cell, and colony-forming unit doses were associated with higher sustained engraftment and faster neutrophil recovery (P = .07, P = .0008, and P < .0001, respectively). Total infused TNC (P = .0007) and CD3+ cell doses (P = .001) also significantly influenced engraftment. At high-resolution extensive donor-recipient HLA disparity was frequent, but had no influence on engraftment (P = .66), or unit dominance (P = .13). Although the unit-unit HLA match also did not affect sustained engraftment (P = 1.0), recipients of units closely (7-10 to 10-10) HLA-matched to each other were more likely to demonstrate initial engraftment of both units (P < .0001). Our findings have important implications for unit selection and provide further insight into double-unit biology.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3478.
Disclosures
The authors; the Associate Editor Robert S. Negrin; and the CME questions author Charles P. Vega, University of California, Irvine, CA, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Identify the concept of unit dominance after double-unit CBT
Identify cell dose variables associated with determination of the dominant unit of double-unit CBT
Identify cell dose variables associated with engraftment success after double-unit CBT
Evaluate the effect of the unit-unit HLA match in double-unit CBT
Release date: March 24, 2011; Expiration date: March 24, 2012
Introduction
Unrelated donor cord blood (CB) has the advantages of rapid availability1,2 and less stringent requirement for human leukocyte antigen (HLA) match, but single-unit CB transplantation (CBT) is limited by low total nucleated cell (TNC) dose. Double-unit CBT as a strategy to augment the cell dose of the graft has been successful with improved sustained donor engraftment and posttransplantation survival compared with historic single-unit controls.3-7 Therefore, double-unit CBT has been widely adopted. Interestingly, in almost all double-unit CB transplant recipients, sustained hematopoiesis is accounted for by only 1 of the 2 units, and single-unit dominance is frequently detected as early as day 21 after transplantation.4,5,7,8 However, the determinants of engraftment after double-unit CBT remain poorly understood making optimal selection of double-unit grafts challenging. Although the importance of the TNC and CD34+ cell dose, and from 4/6 to 6/6 HLA-A, HLA-B antigen, HLA-DRB1 allele matching in determining single-unit CB engraftment is well documented,9-15 the effect of the same variables on the kinetics of engraftment after double-unit CBT has not been adequately demonstrated.4-6,8,16 In addition, because donor-recipient matching at 6 HLA loci (HLA-A, HLA-B antigen, and HLA-DRB1 allele) is the current standard for CB unit selection, relatively little is known about the influence on engraftment of matching at 10 HLA alleles (HLA-A, HLA-B, HLA-C, HLA-DRB1, HLA-DQ), with no13,17 or minimal18 influence detected after single-unit CBT, and limited investigation reported in double-unit CBT to date. Moreover, the requirement for HLA-matching between the 2 units of a double-unit graft is based on empiric considerations.
We, therefore, investigated how the infused cell dose and HLA match (at 6 HLA loci and at 10 HLA alleles) between unit and recipient, as well as between the 2 units of the graft, influence the likelihood of sustained donor engraftment, speed of engraftment, and unit dominance in 84 double-unit CB transplant recipients at our institution. Elucidating the mechanisms of engraftment and unit dominance after double-unit CBT is of great scientific interest, especially given the recent reports of a reduced incidence of relapse in double-unit compared with single-unit CB transplant recipients.19,20 Improved understanding of the determinants of engraftment after double-unit CBT also has practical implications for the selection of each unit of a double-unit graft.
Methods
Patient and graft characteristics
A total of 84 consecutive recipients of first allograft with high-risk hematologic malignancies underwent transplantation with double-unit CB grafts at our center between October 2005 and October 2009 (Table 1). Double-unit grafts are used exclusively at our institution to augment engraftment after CBT, regardless of recipient age or body weight. The median patient age was 36.5 years (range, 0.9-66 years) and median patient weight was 68 kg (range, 7-117 kg). Sixteen patients were younger than 18 years of age at the time of transplantation, and 68 patients were of adult age. Patients were enrolled on protocols NCT00388102, NCT00387959, NCT00423514, NCT00514579, NCT00574496, and NCT00739141 as trials registered at www.clinicaltrials.gov.
Double-unit CBT patient and graft characteristics
| Characteristic (n = 84) . | Value . |
|---|---|
| Median age (range), y | 36.5 (0.9-66) |
| Median weight (range), kg | 68 (7-117) |
| Diagnosis, n = 84 (%) | |
| Acute myeloid leukemia | 25 (30) |
| Acute lymphoblastic leukemia | 17 (20) |
| Other acute leukemia, MDS, or CML | 6 (7) |
| Lymphoma or chronic lymphocytic leukemia | 36 (43) |
| Preparative regimen, n = 84 (%) | |
| Myeloablative | 61 (73) |
| TBI-based | 49 (58) |
| Chemotherapy-based | 12 (14) |
| Nonmyeloablative | 23 (27) |
| Unit-recipient HLA match, n = 168 (%)* | |
| 6/6 (range 4/10 to 9/10) | 6 (4) |
| 5/6 (range 4/10 to 9/10) | 94 (56) |
| 4/6 (range 2/10 to 7/10) | 68 (40) |
| Median cell and CFU doses after thaw, n = 168 units (range) | |
| Infused TNC × 107/kg | |
| Larger unit, n = 84 | 2.5 (1.4-11.3) |
| Smaller unit, n = 84 | 1.9 (0.9-7.1) |
| Infused CD34+ × 105/kg | |
| Larger unit, n = 84 | 1.14 (0.26-6.42) |
| Smaller unit, n = 84 | 0.61 (0.08-1.52) |
| Infused CD3+ × 106/kg | |
| Larger unit, n = 84 | 4.3 (1.3-12.0) |
| Smaller unit, n = 84 | 3.0 (0.32-10.6) |
| Infused CFU × 104/kg | |
| Larger unit, n = 84 | 4.49 (0.59-15.76) |
| Smaller unit, n = 84 | 2.15 (0.01-10.01) |
| Percentage of CD34+ cell viability after thaw, n = 168 (range) | 92 (34-99) |
| Characteristic (n = 84) . | Value . |
|---|---|
| Median age (range), y | 36.5 (0.9-66) |
| Median weight (range), kg | 68 (7-117) |
| Diagnosis, n = 84 (%) | |
| Acute myeloid leukemia | 25 (30) |
| Acute lymphoblastic leukemia | 17 (20) |
| Other acute leukemia, MDS, or CML | 6 (7) |
| Lymphoma or chronic lymphocytic leukemia | 36 (43) |
| Preparative regimen, n = 84 (%) | |
| Myeloablative | 61 (73) |
| TBI-based | 49 (58) |
| Chemotherapy-based | 12 (14) |
| Nonmyeloablative | 23 (27) |
| Unit-recipient HLA match, n = 168 (%)* | |
| 6/6 (range 4/10 to 9/10) | 6 (4) |
| 5/6 (range 4/10 to 9/10) | 94 (56) |
| 4/6 (range 2/10 to 7/10) | 68 (40) |
| Median cell and CFU doses after thaw, n = 168 units (range) | |
| Infused TNC × 107/kg | |
| Larger unit, n = 84 | 2.5 (1.4-11.3) |
| Smaller unit, n = 84 | 1.9 (0.9-7.1) |
| Infused CD34+ × 105/kg | |
| Larger unit, n = 84 | 1.14 (0.26-6.42) |
| Smaller unit, n = 84 | 0.61 (0.08-1.52) |
| Infused CD3+ × 106/kg | |
| Larger unit, n = 84 | 4.3 (1.3-12.0) |
| Smaller unit, n = 84 | 3.0 (0.32-10.6) |
| Infused CFU × 104/kg | |
| Larger unit, n = 84 | 4.49 (0.59-15.76) |
| Smaller unit, n = 84 | 2.15 (0.01-10.01) |
| Percentage of CD34+ cell viability after thaw, n = 168 (range) | 92 (34-99) |
CML indicates chronic myelogenous leukemia; MDS, myelodysplasia; and TBI, total body irradiation.
The number of units (%) with a unit-recipient HLA match of 6/6, 5/6, and 4/6 are shown with the range of HLA match of these units to the recipient at high resolution.
Patients ineligible for protocol were consented to equivalent treatment plans. All patients received fludarabine-based conditioning except 4 in whom this drug was substituted with clofarabine. Conditioning was myeloablative (n = 61), or nonmyeloablative (n = 23), according to patient age, extent of prior therapy, comorbidities, and diagnosis as previously described.7 No patient received antithymocyte globulin, and all received immune suppression with a calcineurin inhibitor (predominantly cyclosporin A) and mycophenolate mofetil. Mycophenolate mofetil was continued until day 45, and then either stopped or tapered according to protocol. The calcineurin inhibitor was continued until at least day 100, and then tapered in the absence of graft-versus-host disease (GVHD). All patients received granulocyte colony-stimulating factor (usually 5 μg/kg/d to a maximum of 480 μg either intravenously or subcutaneously) starting day 1 after transplantation (in the setting of high-dose total body irradiation) or day 7 after transplantation (all other patients), and continued until neutrophil recovery to ≥ 2.5 × 109/L for at least 2 consecutive days. Patients with markedly delayed engraftment were increased to 12-hourly dosing at the discretion of the treating physician. Patients or their guardians provided written informed consent before transplantation and also consented to analysis of transplantation outcomes for research purposes in accordance with the Declaration of Helsinki, with approval from the Memorial Sloan-Kettering Cancer Center Institutional Review Board. Data were obtained from the prospectively maintained Memorial Sloan-Kettering Cancer Center bone marrow transplant database. The median follow-up of survivors was 23 months (range, 4-53 months).
All CB units had a minimum cryopreserved TNC dose of 1.5 × 107/kg recipient body weight. Above this cell dose threshold, priority was given to the unit-recipient HLA match at HLA-A and HLA-B antigens at intermediate- level resolution (including the serologic equivalent of “splits”), and at HLA-DRB1 alleles. Units with attached segments for identity testing were given preference, and the bank of origin was also taken into account because in our experience this factor can influence the speed of unit acquisition, reliability of product information, and unit quality.8 High-resolution typing of HLA-A, HLA-B, HLA-C, HLA-DRB1 and HLA-DQ was performed on all patients and units, but HLA allele match at loci other than HLA-DRB1 usually did not affect unit selection unless multiple units of similar HLA-A, HLA-B antigen, and HLA-DRB1 allele match grade and TNC dose were available. The majority (n = 163) of units were at least partially red blood cell–depleted before cryopreservation, whereas 5 units were not (only plasma depleted). Only 1 non–red blood cell–depleted unit was permitted per double-unit graft. Unit characteristics are summarized in Table 1.
CB unit thaw and unit assessment
The majority of units (n = 153, 91%) underwent albumin-dextran reconstitution after thawing,7 whereas 15 (9%) were thawed and washed with centrifugation.21 Units were infused sequentially within approximately 2 hours of thaw with an interval of usually less than 45 minutes between units. A small aliquot was removed from the final product of each unit for TNC measurement and flow cytometric assessment of CD34+ and CD3+ cell counts and viability by 7-aminoactinomycin D staining.8 The infused viable CD34+ cell dose per kilogram was calculated using the equation (TNC × percentage of CD45+ cells that are viable × percentage of CD34+ cells in the sample) divided by weight of the patient. A similar equation was used to calculate the infused viable CD3+ cell dose. Colony-forming unit (CFU) assays (burst-forming units erythroid, granulocyte-macrophage progenitors, and CFU granulocyte-erythrocyte-macrophage-megakaryocyte progenitors combined) were performed on all CB units using a total of 1 × 105 cells plated in duplicate. Colony growth was evaluated by light microscopy at 14 days. The original cryobag was sent to the Diagnostic Molecular Pathology Laboratory for DNA extraction and assessment of donor polymorphisms.
Assessment of donor engraftment
All patients were alive on day 14 after transplantation and therefore subject to evaluation for donor-derived neutrophil engraftment. Time to neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count of at least 0.5 × 109/L after the posttransplantation nadir. Donor chimerism was determined serially on bone marrow samples at approximately day 21, 100, 180, and 1 year after CBT, and on whole blood samples on day 28, 60, 100, 180, and 1 year after transplantation (with additional time points as clinically indicated). Donor chimerism using quantitative polymerase chain reaction assays of informative polymorphic short tandem repeat regions of DNA distinguished each donor unit and recipient.4 Sustained engraftment was defined as sustained donor-derived neutrophil recovery with donor chimerism of at least 90% (both units combined). The dominant unit was defined either as the only unit detected, or, in the case of the presence of 2 units, the unit contributing more than 50% of the total donor chimerism in serial testing. Graft failure was defined as the lack of sustained neutrophil recovery of 0.5 × 109/L or more regardless of donor chimerism with cause of death because of graft failure defined according to the algorithm by Copelan et al22 In addition, autologous recovery was also defined as failure of donor engraftment. Secondary graft failure was defined as a drop in neutrophil count to 0.5 × 109/L or less for at least 14 consecutive days after initial donor-derived neutrophil recovery.
Statistical analyses
The influence of infused cell dose, percentage CD34+ cell viability, and HLA match level on the likelihood of sustained donor engraftment, the speed of donor-derived neutrophil recovery, and unit dominance were analyzed. Only recipients of myeloablative conditioning were included in analyses assessing the speed of neutrophil recovery given the majority of nonmyeloablative CB transplant recipients have early autologous neutrophil recovery after transplantation before transitioning to donor-derived hematopoiesis. All patients were included in all other analyses.
The probability of donor-derived neutrophil engraftment was estimated using the cumulative incidence function with early death as the competing risk. Kaplan-Meier methodology was used to investigate engraftment kinetics in the subanalysis of recipients of myeloablative conditioning who engrafted (excluding graft failures). Wilcoxon rank-sum test was used in analyses of cell dose and HLA match and time to neutrophil recovery in addition to analyses of continuous or ordinal level factors associated with engraftment. Fisher exact test was used to examine the relationship between cell dose parameters and HLA match on sustained donor engraftment, and the association between HLA match and the persistence of the nondominant unit. To study variables associated with unit dominance, a Wilcoxon signed-rank test was used for continuous factors, and a McNemar test was used for categorical factors. A P value of .05 or less was considered to be statistically significant.
Results
Neutrophil engraftment and donor chimerism
Double-unit CB transplant recipients have a high incidence of sustained donor engraftment, which was almost always accounted for by 1 unit.
Overall, 79 of 84 patients had sustained donor engraftment. The cumulative incidence of sustained donor engraftment was 93% (95% confidence interval [CI], 87%-100%) in myeloablative transplant recipients with a median time to neutrophil recovery of 23 days (range, 12-43 days). There were 4 recipients of myeloablative conditioning who had graft failure (3 primary, 1 early secondary), all of whom died. The majority of nonmyeloablative transplant recipients had early and transient autologous recovery contributing to an earlier median time to neutrophil recovery of 9.5 days (range, 7-36 days). Sustained donor-derived neutrophil engraftment was subsequently achieved in 96% (95% CI, 85%-100%) of nonmyeloablative CB transplant recipients, with only 1 patient having primary graft failure and autologous recovery. Although a dominant unit was evident by chimerism studies in all 5 of the graft failure patients in the study, donor hematopoiesis from that unit did not result in sustained donor-derived count recovery.
Donor hematopoiesis was detected in the bone marrow of all of 83 patients analyzed on day 21 after transplantation, and 1 additional nonmyeloablative CB transplant recipient was 100% donor when evaluated for the first time at day 28. Donor hematopoiesis in the day-21 bone marrow was derived from a single unit in the majority of patients regardless of conditioning intensity: 53/61 (87%) recipients of myeloablative CB transplants and 14/22 (64%) recipients of nonmyeloablative CB transplants including 4 of the 5 patients who had clinical graft failure. Both units were present in the day-21 bone marrow of the remaining 8 myeloablative and 8 nonmyeloablative CB transplant recipients (including 1 patient who had graft failure). However, in the patients with engraftment of both units, 1 unit dominated, and in nearly all engraftment of the dominant unit progressed such that sustained engraftment of both units was observed in only 1/43 patients at 1 year and beyond.
Analyses of cell doses
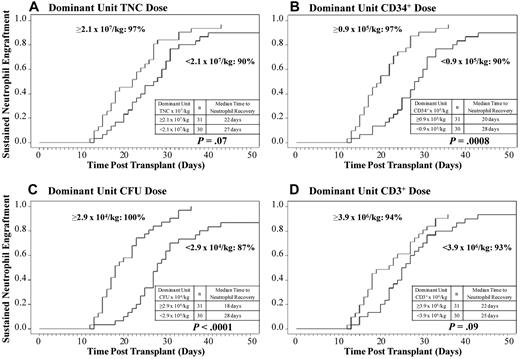
Higher infused TNC, CD34+cell, and CFU dose of the dominant unit is associated with higher likelihood of sustained donor engraftment and faster neutrophil recovery after myeloablative conditioning.
Given that hematopoiesis was derived from a single (dominant) unit in the majority of patients, the relationship between the infused TNC, CD34+ cell, CFU, and CD3+ cell doses of the dominant unit and the likelihood and speed of neutrophil engraftment was examined by univariate analysis in the 61 patients who received myeloablative conditioning. A TNC, CD34+ cell, and CFU dose of the dominant unit at or above the median were each associated (Figure 1A-C) with enhanced neutrophil engraftment (P = .07, P = .0008, and P < .0001, respectively). Thus, in recipients of myeloablative CB transplants, the cumulative incidence of sustained donor engraftment for recipients with dominant unit cell doses equal to or above the median was 97% (95% CI, 87%-100%) for TNC, 97% (95% CI, 87%-100%) for CD34+ cells, and 100% for CFU. The influence of the CD3+ cell dose of the dominant unit on engraftment (Figure 1D) was not statistically significant (P = .09).
The relationship between the infused cell doses of the dominant unit and neutrophil engraftment. The cumulative incidences of sustained donor neutrophil engraftment after myeloablative conditioning (n = 61) according to the infused (A) TNC dose, (B) CD34+ cell dose, (C) CFU dose, and (D) CD3+ cell dose of the dominant unit are shown. An infused CD34+ cell or CFU dose of the dominant unit above or equal to the median is associated with a higher likelihood of sustained engraftment and faster neutrophil recovery.
The relationship between the infused cell doses of the dominant unit and neutrophil engraftment. The cumulative incidences of sustained donor neutrophil engraftment after myeloablative conditioning (n = 61) according to the infused (A) TNC dose, (B) CD34+ cell dose, (C) CFU dose, and (D) CD3+ cell dose of the dominant unit are shown. An infused CD34+ cell or CFU dose of the dominant unit above or equal to the median is associated with a higher likelihood of sustained engraftment and faster neutrophil recovery.
When only engrafting myeloablated CB transplant recipients were analyzed, the significant relationships between TNC, CD34+ cell, and CFU dose of the dominant unit and speed of neutrophil recovery were maintained (P = .01, P = .0004, and P = .001, respectively). Specifically, the median time to neutrophil recovery with a dominant unit cell dose at or above the median was 4 days faster for TNC dose (22 days [range, 12-43 days] compared with 26 days [range, 13-40 days]); 9 days for CD34+ cells (19 days [range, 12-36 days] compared with 28 days [range, 13-43 days]); and 8 days for CFU dose (19 days [range, 12-36 days] compared with 27 days [range, 13-43 days]).
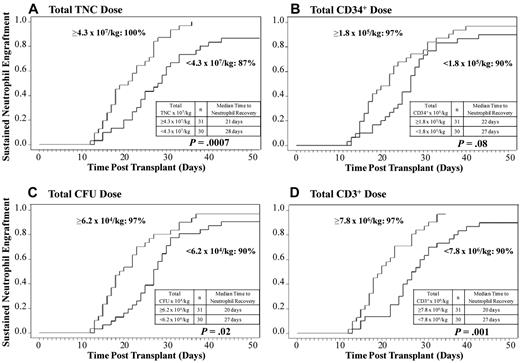
Higher infused total graft TNC, CD34+ cell, CFU, and CD3+ cell dose is associated with higher likelihood of sustained donor engraftment and faster neutrophil recovery after myeloablative conditioning.
To investigate the influence of total graft cell dose on the probability of and time to neutrophil engraftment, the cell doses of both CB units combined (dominant + nondominant) were analyzed in the 61 recipients of myeloablative conditioning. Interestingly, although the influence of the total graft CD34+ cell and CFU doses had a lesser impact on engraftment (P = .08 and P = .02, respectively) than those doses of the dominant unit, the effects of the total TNC (P = .0007) and CD3+ cell dose (P = .001) on the likelihood and speed of engraftment were highly significant (Figure 2A-D).
The relationship between the total infused cell doses (both units combined) and neutrophil engraftment. The cumulative incidences of sustained donor neutrophil engraftment after myeloablative conditioning (n = 61) according to the total infused (A) TNC dose, (B) CD34+ cell dose, (C) CFU dose, and (D) CD3+ cell dose are shown. An infused total TNC or CD3+ cell dose above or equal to the median is associated with a higher likelihood of sustained engraftment and faster neutrophil recovery.
The relationship between the total infused cell doses (both units combined) and neutrophil engraftment. The cumulative incidences of sustained donor neutrophil engraftment after myeloablative conditioning (n = 61) according to the total infused (A) TNC dose, (B) CD34+ cell dose, (C) CFU dose, and (D) CD3+ cell dose are shown. An infused total TNC or CD3+ cell dose above or equal to the median is associated with a higher likelihood of sustained engraftment and faster neutrophil recovery.
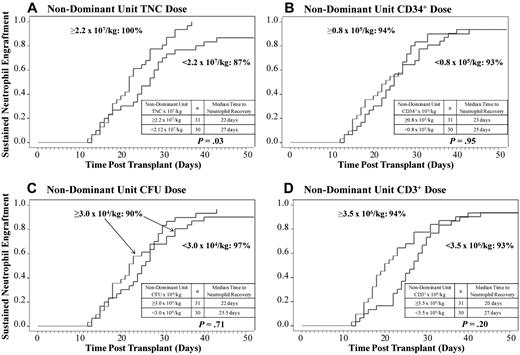
Based on the observation that total graft cell doses influenced engraftment, the effect of the cell doses of the nondominant (nonengrafting) unit was then examined. A higher TNC dose of the nondominant unit was also associated with enhanced neutrophil engraftment in recipients of myeloablative conditioning (P = .03), but the CD34+ cell (P = .95), CFU (P = .71), and CD3+ cell (P = .20) doses had no influence (Figure 3A-D).
The relationship between the infused cell doses of the nondominant unit and neutrophil engraftment. The cumulative incidences of sustained donor neutrophil engraftment after myeloablative conditioning (n = 61) according to the infused (A) TNC dose, (B) CD34+ cell dose, (C) CFU dose, and (D) CD3+ cell dose of the nondominant unit are shown. Although an infused TNC dose of the nondominant unit at or above the median is associated with enhanced neutrophil engraftment, CD34+ cell, CFU, and CD3+ cell doses of the nondominant unit had no influence.
The relationship between the infused cell doses of the nondominant unit and neutrophil engraftment. The cumulative incidences of sustained donor neutrophil engraftment after myeloablative conditioning (n = 61) according to the infused (A) TNC dose, (B) CD34+ cell dose, (C) CFU dose, and (D) CD3+ cell dose of the nondominant unit are shown. Although an infused TNC dose of the nondominant unit at or above the median is associated with enhanced neutrophil engraftment, CD34+ cell, CFU, and CD3+ cell doses of the nondominant unit had no influence.
A higher percentage of viable CD34+ cells in the dominant unit is associated with an increased likelihood of sustained donor engraftment.
We next investigated the influence of the percentage of viable CD34+ cells after thawing on the likelihood of sustained donor engraftment (vs graft failure) in all CB transplant recipients regardless of conditioning intensity. As we have previously shown that units with a post-thaw CD34+ cell viability of < 75% are very unlikely to engraft,8 we used this threshold in our analysis. comparing the CD34+ cell viability of the dominant unit in the 79 patients with sustained donor engraftment with the dominant units in the 5 patients with clinical graft failure, a CD34+ cell viability at 75% or higher (P = .03) in the dominant unit was associated with an increased likelihood of sustained donor engraftment (Table 2).
Association between viability of CD34+ cells in the dominant unit and the risk of graft failure
| CD34+ cell viability of dominant unit . | Patients with sustained donor engraftment, n = 79 (%) . | Patients with graft failure, n = 5 (%)* . | P . |
|---|---|---|---|
| ≥ 75% | 76 (96) | 3 (60) | .03 |
| < 75% | 3 (4) | 2 (40) |
| CD34+ cell viability of dominant unit . | Patients with sustained donor engraftment, n = 79 (%) . | Patients with graft failure, n = 5 (%)* . | P . |
|---|---|---|---|
| ≥ 75% | 76 (96) | 3 (60) | .03 |
| < 75% | 3 (4) | 2 (40) |
Engrafting patients were more likely to have dominant units with a high CD34+ cell viability (≥ 75% after thaw).
Patients with clinical graft failure had a dominant unit detected by chimerism studies, but the donor hematopoiesis did not result in sustained count recovery.
Neither the likelihood of engraftment nor the speed of neutrophil recovery is influenced by the presence of 2 CB units early after transplantation.
Because some investigators have suggested that initial engraftment of both units may be advantageous, we next examined whether the probability or the speed of neutrophil engraftment was enhanced if both units engrafted. When the analysis included all CB transplant recipients, we found the likelihood of donor engraftment was not influenced by the presence of 1 unit compared with 2 CB units in day-21 bone marrow (data not shown). Moreover, in myeloablative CB transplant recipients, there was no difference in the speed of neutrophil recovery among the patients who had 2 CB units detected in the day-21 bone marrow and those patients who engrafted with a single unit (29 days [range, 12-43 days] vs 23 days [range, 13-37 days], P = .71). In addition, myeloablative CB transplant recipients who engrafted before day 21 were no more likely to have both units detected at day 21 than those who engrafted at or beyond day 21 (3/21 [14%] vs 4/36 [11%], P = .70).
Higher CD3+ cell dose and CD34+ cell viability ≥ 75% are associated with unit dominance.
We next analyzed the relationship between infused cell doses and percentage CD34+ cell viability and which unit dominated in engraftment in the 79 patients with sustained donor engraftment. Although there was no difference in the infused TNC, CD34+ cell, or CFU doses of the dominant and nondominant units in engrafting patients, dominant units had a higher CD3+ cell dose (median 3.9 vs 3.3 × 106, P = .04; Table 3). Dominant units were also more likely to have a CD34+ cell viability of 75% or higher (P = .008).
Infused cell doses and unit dominance
| Infused cell dose . | Median cell dose (range) . | P . | |
|---|---|---|---|
| Dominant unit, n = 79 . | Nondominant unit, n = 79 . | ||
| TNC × 107/kg | 2.1 (1.2-11.3) | 2.3 (0.9-7.3) | .64 |
| CD34+ × 105/kg | 0.9 (0.1-4.1) | 0.8 (0.2-6.4) | .50 |
| CD3+ × 106/kg | 3.9 (0.8-10.6) | 3.3 (0.3-12.0) | .04 |
| CFU × 104/kg | 3.3 (0.2-14.7) | 2.8 (0.2-15.8) | .57 |
| Infused cell dose . | Median cell dose (range) . | P . | |
|---|---|---|---|
| Dominant unit, n = 79 . | Nondominant unit, n = 79 . | ||
| TNC × 107/kg | 2.1 (1.2-11.3) | 2.3 (0.9-7.3) | .64 |
| CD34+ × 105/kg | 0.9 (0.1-4.1) | 0.8 (0.2-6.4) | .50 |
| CD3+ × 106/kg | 3.9 (0.8-10.6) | 3.3 (0.3-12.0) | .04 |
| CFU × 104/kg | 3.3 (0.2-14.7) | 2.8 (0.2-15.8) | .57 |
Infused cell doses and unit dominance in 79 double-unit CB transplant recipients with engraftment are shown. Dominant units had a higher CD3+ cell dose.
Analyses of HLA match
Unit-recipient HLA match does not influence the likelihood of sustained donor engraftment, time to neutrophil recovery, or unit dominance.
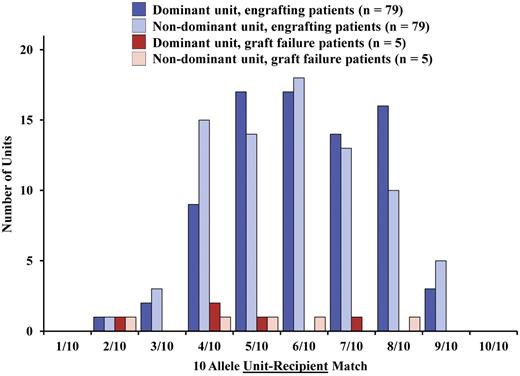
CB units were often markedly HLA-mismatched to the patient when analyzed at high resolution (Table 1). The median unit-recipient HLA-allele match of the 168 CB units was 6/10 (range, 2/10-9/10). When all patients were analyzed, no relationship was found between unit-recipient HLA match and the likelihood of sustained donor engraftment at HLA-A, HLA-B antigen, HLA-DRB1 allele match, or at high-resolution HLA match (Figure 4). Furthermore, in recipients of myeloablative conditioning with sustained donor engraftment (n = 57), unit-recipient HLA match of the dominant unit was not associated with the speed of neutrophil recovery when evaluated at either 6 HLA loci (HLA-A, HLA-B antigen, HLA-DRB1 allele, P = .81) or 10 HLA alleles (P = .34). Finally, there was no relationship between unit-recipient HLA match and unit dominance (Figure 4) when analyzed at HLA-A, HLA-B antigen, HLA-DRB1 allele level (P = .31) or at high-resolution level (P = .13).
Influence of high-resolution unit-recipient HLA match on sustained donor engraftment (n = 84). The degree of HLA-matching of each unit to the recipient does not influence the likelihood of sustained donor engraftment, or the dominant unit in engrafting patients. The distribution of HLA-matching of the dominant units is shown for the 79 patients with sustained donor engraftment and the 5 recipients with graft failure; there was no difference at 6 HLA loci (P = .65) or 10 HLA alleles (P = .66) between the 2 groups of patients.
Influence of high-resolution unit-recipient HLA match on sustained donor engraftment (n = 84). The degree of HLA-matching of each unit to the recipient does not influence the likelihood of sustained donor engraftment, or the dominant unit in engrafting patients. The distribution of HLA-matching of the dominant units is shown for the 79 patients with sustained donor engraftment and the 5 recipients with graft failure; there was no difference at 6 HLA loci (P = .65) or 10 HLA alleles (P = .66) between the 2 groups of patients.
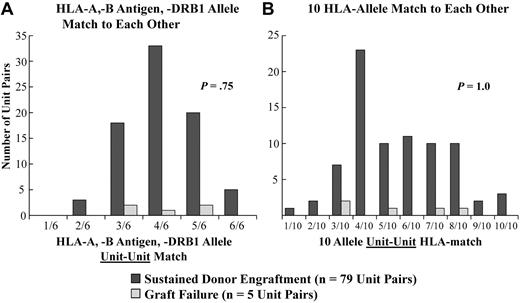
Unit-unit HLA match does not affect sustained donor engraftment or speed of neutrophil recovery.
CB units also had a high degree of HLA-mismatch to each other. Although the majority of double-unit pairs (n = 84) were 4/6 (range, 2/6-6/6) HLA-A, HLA-B antigen, HLA-DRB1 allele matched to each other, they were a median of only 5/10 (range, 1/10-10/10) HLA-matched to each other at high resolution. Notably, when all patients were analyzed, there was no difference between the degree of unit-unit HLA match in patients with sustained donor engraftment and those patients with graft failure at either level of resolution (Figure 5). Furthermore, unit-unit HLA match at either match grade did not influence speed of neutrophil recovery in recipients of myeloablative CBT (data not shown).
Relationship between the unit-unit HLA-match and sustained donor engraftment. A comparison between the 79 patients with sustained donor engraftment and the 5 with graft failure is shown: (A) HLA-match at HLA-A, -B antigens and -DRB1 alleles and at (B) HLA-match at high-resolution 10 HLA-alleles. There was no statistical difference detected in the distributions of the 6 HLA-A, HLA-B antigen, HLA-DRB1 allele unit versus unit HLA match (2/6 vs 3/6 vs 4/6 vs 5/6 vs 6/6, P = .75), or distributions of the 10 HLA allele unit versus unit HLA match (1/10-2/10 vs 3-4/10 vs 5/10-6/10 vs 7/10-8/10 vs 9/10-10/10, P = 1.0) in the 79 patients with sustained engraftment and the 5 patients with graft failure.
Relationship between the unit-unit HLA-match and sustained donor engraftment. A comparison between the 79 patients with sustained donor engraftment and the 5 with graft failure is shown: (A) HLA-match at HLA-A, -B antigens and -DRB1 alleles and at (B) HLA-match at high-resolution 10 HLA-alleles. There was no statistical difference detected in the distributions of the 6 HLA-A, HLA-B antigen, HLA-DRB1 allele unit versus unit HLA match (2/6 vs 3/6 vs 4/6 vs 5/6 vs 6/6, P = .75), or distributions of the 10 HLA allele unit versus unit HLA match (1/10-2/10 vs 3-4/10 vs 5/10-6/10 vs 7/10-8/10 vs 9/10-10/10, P = 1.0) in the 79 patients with sustained engraftment and the 5 patients with graft failure.
High level of unit-unit HLA match is associated with an increased likelihood of engraftment of both units.
We then examined the relationship between the unit-unit HLA allele match and the length of time that the nondominant unit can be detected after transplantation. As shown in Table 4, a closer HLA match, at either 5/6 to 6/6 loci (compared with 1/6-4/6) or 7/10 to 10/10 alleles (compared with 1/10-6/10), between the units was strongly associated with the initial detection and transient persistence of the nondominant unit. This relationship was observed for recipients of both myeloablative and nonmyeloablative conditioning. In addition, to ensure a CD34+ cell viability difference between the unit pairs did not explain this finding, a subgroup analysis was performed in those patients in whom both units had CD34+ cell viability of 75% or more. This analysis also demonstrated a highly significant association between closer unit-unit HLA match and transient persistence of the nondominant unit (data not shown). Notably, thus far, sustained engraftment of both units has only been seen in 1 patient beyond 6 months after transplantaion. This 1 patient received 2 CB units each with a high CD34+ cell viability that were both 9/10 HLA-matched to the recipient and to each other.
Serial detection of both CB units according to unit-unit HLA match
| Time post-CBT . | No. of patients with both units detected in blood or bone marrow (%) . | |||||
|---|---|---|---|---|---|---|
| Unit-unit match at HLA-A, HLA-B antigen, and HLA-DRB1 allele level . | Unit-unit HLA match at 10 allele level . | |||||
| 1/6-4/6 . | 5/6-6/6 . | P . | 1/10-6/10 . | 7/10-10/10 . | P . | |
| Day 21, (n = 83) | 3/56 (5) | 13/27 (48) | < .0001 | 2/56 (4) | 14/27 (52) | < .0001 |
| Day 28, (n = 79) | 2/54 (4) | 12/25 (48) | < .0001 | 0/54 (0) | 14/25 (56) | < .0001 |
| Day 60, (n = 72) | 2/47 (4) | 6/25 (24) | .02 | 0/47 (0) | 8/25 (32) | < .0001 |
| Day 100, (n = 68) | 0/46 (0) | 3/22 (14) | .03 | 0/45 (0) | 3/23 (13) | .04 |
| Day 180, (n = 56) | 0/40 (0) | 2/16 (12) | .08 | 0/38 (0) | 2/18 (11) | .10 |
| Day 365, (n = 43) | 0/30 (0) | 1/13 (8) | .30 | 0/30 (0) | 1/13 (8) | .30 |
| Time post-CBT . | No. of patients with both units detected in blood or bone marrow (%) . | |||||
|---|---|---|---|---|---|---|
| Unit-unit match at HLA-A, HLA-B antigen, and HLA-DRB1 allele level . | Unit-unit HLA match at 10 allele level . | |||||
| 1/6-4/6 . | 5/6-6/6 . | P . | 1/10-6/10 . | 7/10-10/10 . | P . | |
| Day 21, (n = 83) | 3/56 (5) | 13/27 (48) | < .0001 | 2/56 (4) | 14/27 (52) | < .0001 |
| Day 28, (n = 79) | 2/54 (4) | 12/25 (48) | < .0001 | 0/54 (0) | 14/25 (56) | < .0001 |
| Day 60, (n = 72) | 2/47 (4) | 6/25 (24) | .02 | 0/47 (0) | 8/25 (32) | < .0001 |
| Day 100, (n = 68) | 0/46 (0) | 3/22 (14) | .03 | 0/45 (0) | 3/23 (13) | .04 |
| Day 180, (n = 56) | 0/40 (0) | 2/16 (12) | .08 | 0/38 (0) | 2/18 (11) | .10 |
| Day 365, (n = 43) | 0/30 (0) | 1/13 (8) | .30 | 0/30 (0) | 1/13 (8) | .30 |
A higher level of HLA match between the units was associated with engraftment of both units, and this finding was detected at both levels of resolution of HLA match. Incomplete data are a result of time point not reached, lack of assessment during critical illness, or patient death.
Discussion
This is the first detailed analysis evaluating the effect of cell dose and high-resolution HLA match on the probability and speed of engraftment after double-unit CBT in 84 patients transplanted at a single institution. We demonstrate that the infused TNC, CD34+ cell, CFU and CD3+ cell doses, the post-thaw percentage of CD34+ cell viability, and HLA match each influence different aspects of double-unit engraftment. The high incidence of sustained donor engraftment we observed after double-unit CBT is similar to previous reports.3-7 Notably, consistent with the role of CD34+ cell and CFU dose in single-unit CBT,9-11,13-15,23,24 we found that a higher CD34+ cell and CFU dose of the dominant unit were strongly associated with enhanced engraftment after myeloablative conditioning. Interestingly, the impact of the total graft CD34+ cell and CFU doses on engraftment were much less significant, and the corresponding cell doses of the nondominant unit had no influence at all. Haspel et al16 have also reported that a higher CD34+ cell dose of the dominant unit is associated with faster neutrophil recovery.
It is intriguing that we also found a strong association between the total TNC and CD3+ cell doses and engraftment, especially given this relationship had a greater significance than the TNC and CD3+ cell doses of the dominant unit. We speculate that T cells, even if not contributing directly to sustained engraftment (as in the case of the nondominant unit), may afford a graft-facilitating effect in a dose-dependent manner. This hypothesis, and the degree to which variations in the nondominant unit cell dose could influence the engraftment of the dominant unit, will need to be further analyzed in a larger series that would permit multivariate analyses. In the meantime, our demonstration of the critical importance of the dominant unit CD34+ cell and CFU doses suggests that, as with single-unit CBT, cell dose remains a critical attribute of the graft. This is a further argument to standardize progenitor cell measurements between CB banks that could be used in unit selection. Furthermore, we show that the percentage of CD34+ cell viability, which is a reflection of unit quality, is important not only as a determinant of unit dominance,8 but also has significant association with the risk of graft failure.
Although yet to be proven in randomized studies, it has been suggested that the association of double-unit CBT with more rapid neutrophil recovery,4 compared with single-unit historical controls,11 may be because of transient engraftment and a temporary contribution to neutrophil recovery of the nondominant unit.25,26 This suggestion, however, is not supported by our analysis. We found no difference in the likelihood or speed of neutrophil recovery in engrafting myeloablative CB tranplant recipients regardless of whether day-21 hematopoiesis was accounted for by one or both units. Furthermore, only 3/21 patients with neutrophil recovery before day 21 had both units detected in the day-21 bone marrow evaluation. Although we did not assess chimerism at earlier time points, it is very unlikely that the nonengrafting unit could make a meaningful contribution to hematopoiesis and count recovery because it cannot be detected in the majority of patients on day 21 before the median day of neutrophil recovery, and patients with early neutrophil recovery are not more likely to have engrafted with both units. Hence, any enhancement of engraftment after double-unit CBT may be because of improved conditioning and immunosuppression, increasing the chance of infusing at least one good quality unit8 and graft-versus-graft interactions.
In regard to HLA match, our analysis reveals extensive donor-recipient HLA disparity with as little as 2/10 to 3/10 HLA match level by high-resolution typing as previously reported,13,18 and yet sustained engraftment of such units is possible. We were not able to demonstrate an influence of unit-recipient HLA match at either 6 HLA loci or at 10 HLA alleles on neutrophil engraftment after double-unit CBT. Although our results differ from those reported by Delaney et al27 who showed an association between better class I allele match and improved time to neutrophil recovery, their data were not analyzed for the dominant unit. The highly immunosuppressive regimens used for transplantation, in addition to the potential attributes of double-unit grafts, may be able to partially overcome the adverse impact of HLA-mismatch on engraftment. Moreover, analysis of a very large number of patients may be required to demonstrate such a relationship, as has been seen with single-unit CBT.15 Therefore, the influence of HLA match on engraftment as well as other transplantation outcomes after double-unit CBT should be readdressed in the future. In addition, as previously reported for 4/6 to 6/6 HLA-matching,4,8 a better HLA-matched unit at high resolution was not more likely to become the dominant unit.
A new finding of practical significance, however, is our observation that unit-unit HLA match at HLA-A, HLA-B antigens, HLA-DRB1 alleles, or at 10 HLA alleles has no bearing on the likelihood of sustained donor engraftment. Because of early concerns about crossed immunologic rejection, a unit-unit HLA match requirement has traditionally been incorporated into double-unit selection algorithms.4,6,25,28,29 However, obeying this arbitrary requirement may dictate the selection of a second unit with a lower TNC dose to fulfill the requirement of HLA match to both the patient and the first unit. Such a strategy could be disadvantageous to transplantation outcome if the second unit is responsible for sustained donor engraftment. Analyses of a larger number of double-unit CB transplant recipients will be required to determine whether unit-unit HLA match could influence other transplantation outcomes such as GVHD, relapse, or survival. Although these studies will be of great interest, our data show that from the perspective of engraftment, the requirement of a certain degree of HLA-matching between the units of a double-unit graft is not necessary.
A further novel observation that has implications for our understanding of double-unit biology is the highly significant relationship between the degree of unit-unit HLA match and the likelihood of both initial and sustained engraftment of both CB units. There is mounting evidence that unit dominance is related to immune interactions between the dominant and nondominant unit.30-32 Our findings further contribute to the concept that unit dominance is immune-mediated. The present study confirms earlier findings that a higher infused CD3+ cell dose is associated with unit dominance.4,8 More notably, in the setting of less closely HLA-matched units, unit dominance is rapidly established. We hypothesize that this situation results in an enhanced unit-versus-unit immune response, whereas closely HLA-matched units are more likely to be relatively tolerant of each other, and thus at least transient coengraftment is possible.
In summary, we demonstrate that the CD34+ cell and CFU dose of the dominant unit and the total TNC dose are critical determinants of engraftment after double-unit CBT, whereas initial engraftment of both units has no effect. Moreover, the percentage of CD34+ cell viability is associated with engraftment success as well as unit dominance. We found no engraftment benefit of a better HLA match at high resolution, although few patients received units with a donor-recipient HLA match equal to or higher than 8/10. Interestingly, a higher unit-unit HLA match is associated with initial engraftment of both units, but has no relationship with the risk of graft failure.
These findings have practical implications for double-unit graft selection, but also highlight the challenges associated with this process. Firstly, the cell dose analyses in this study are based on infused viable doses, and yet unit selection is necessarily based on the cryopreserved dose without consideration of post-thaw yield and viability. In addition, given the importance of the post-thaw dominant unit CD34+ cell and CFU dose, it would be optimal to select units based on these measurements. However, this selection is not possible as prefreeze CD34+ cell and CFU measurements are not standardized between banks, may not reflect post-thaw results, and CD34+ cell and CFU dose estimates from test vials or segments are not usually available.33,34 Therefore, currently, TNC dose remains the only practical surrogate measurement for progenitor cell dose. Although we have demonstrated that the total TNC of the graft is strongly associated with engraftment success, our data show that the cell dose of each unit is also equally important because a dominant unit with a low TNC, CD34+ cell, and CFU dose poses the risk of delayed or failed engraftment. However, the dominant unit cannot be predicted at the time of unit selection. Unit quality as measured by the percentage of CD34+ viability is also important as units with poor viability are unlikely to engraft.8
Based on this study, it is not yet possible to determine a concrete threshold TNC dose for each unit of the graft. Moreover, it is highly likely that the donor-recipient HLA match of the engrafting unit will be a significant determinant of survival after double-unit CBT as has been demonstrated with single-unit CBT.15 Therefore, although we did not demonstrate that HLA match is important in engraftment in this study, in double-unit selection it is appropriate to balance the importance of cell dose (dominant unit and total graft cell doses) with that of donor-recipient HLA match (clearly established in single-unit CBT outcome). Therefore, we have now increased our threshold for cryopreserved TNC dose from 1.5 to approximately 2.0 × 107/kg for each unit of a double-unit graft. Above this dose, we prioritize 4/6 to 6/6 HLA-A, HLA-B antigen, HLA-DRB1 allele match, taking into consideration the bank of origin (as this may influence unit quality8 ), but not the unit-unit HLA match. This strategy should augment the chance of engraftment by infusing 2 units with a dose in each unit that should be adequate, but also recognizes the importance of HLA match as shown in single-unit CBT. Higher TNC doses for each unit would be ideal, but this could preclude some patients from receiving a transplant, and could increase the use of units with a greater degree of HLA-mismatch and the consequent risk of GVHD, and possibly the risk of opportunistic infection. Larger studies that will permit multivariate analyses of the effect of graft characteristics on engraftment as well as GVHD, transplantation-related mortality, relapse, and survival are required to further refine unit selection criteria for double-unit CBT.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Cladd E. Stevens for thoughtful review of the manuscript.
This work was supported in part by the Gabrielle's Angel Foundation for Cancer Research, the Memorial Sloan-Kettering Cancer Center Society, the Translational and Integrative Medicine Research Grant, and the National Cancer Institute, National Institutes of Health grant P01 CA23766.
National Institutes of Health
Authorship
Contribution: S.A., M.L., A.M.G., W.S., and G.H. analyzed the data and wrote the manuscript; H.C.-M., S.G., N.A.K., and A.S. wrote the manuscript; and J.N.B. directed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juliet N. Barker, MBBS (Hons), FRACP, Associate Professor, Adult Bone Marrow Transplantation Service, Memorial Sloan-Kettering Cancer Center, Box 259, 1275 York Ave, New York, NY 10065; e-mail: barkerj@mskcc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal