Abstract
GVHD is a syndrome that results from minor and major histocompatibility complex incompatibilities between the donor and recipient. More than 50 years after its initial description, the pathophysiology of GVHD remains poorly understood. Nonetheless, donor T cells have been shown to be critical to the pathophysiology of acute and chronic GVHD, yet precisely how they function remains unclear. The effector mechanisms by which donor T cells mediate tissue inflammation is even less well understood. Identification of several new lineages of CD4+ T cells made in the past decade and their roles in the pathophysiology of T cell–mediated diseases has shed new light on these effector mechanisms. In this review, we summarize the recent descriptions of these T-cell lineages and the current data supporting their role in acute and to a lesser extent chronic GVHD. Investigations into the activity of these new T-cell lineages may provide more rationale approaches to the treatment or prevention of GVHD.
Introduction
The first descriptions of acute GVHD came after investigations into the capacity of radiation to ablate endogenous bone marrow function in the 1950s. When mice were treated in this manner and given splenocytes from a non–congenic donor strain to facilitate the regeneration of blood cells, an illness characterized by progressive weight loss, hunched posture, decreased activity, and diarrhea was noted.1 This was initially termed “secondary disease” or “runting” to differentiate it from the initial toxicity of the conditioning regimen and its resulting aplasia. Subsequent work indicated that donor T cells that recognize minor or major histocompatibility differences in the host were critical mediators of GVHD.2-4
Thus, a role for donor T cells in the pathogenesis of GVHD has been clear for > 30 years. However, the mechanism by which T cells mediate this process is not completely understood. This review focuses on the multiple different new subsets of effector CD4+ T cells that have been recently described and their role in acute and chronic GVHD. We will not focus on regulatory T cells (eg, Tregs)5,6 or T regulatory type 1 (Tr1) cells7 or provide a comprehensive overview of each lineage both of which have been recently reviewed.8-12
T-helper type 1/T-helper type 2 cells
In 1986, Mosmann et al published seminal work on the clonal behavior of T cells.13 From a large panel of antigen-specific and autoreactive T-cell clones, they were able to divide these cells into 2 distinct groups.13 T-helper type 1 (Th1) clones generated IL-2, IL-3, and IFN-γ, whereas T-helper type 2 (Th2) cells generated IL-3, T-cell growth factor 2, and mast cell growth factor 2. Subsequent work by Cherwinski et al showed that Th1 clones generated IL-2, granulocyte-macrophage colony-stimulating factor (GM-CSF) and IFN-γ, whereas Th2 clones generated IL-4 and IL-5.14 Further, Stevens et al showed that both types of T cells induced the generation of immunoglobulin M (IgM) and IgG3 by B cells.15 However, Th1 cells specifically induced IgG2a, whereas Th2 cells induced the production of IgG1 by B cells. Multiple investigators showed that the Th1 and Th2 lineages were fixed and separate because they were not able to generate Th1 clones from polarized Th2 cells and vice versa. Eventually, this became the accepted paradigm,16 with studies showing that Th1 cells played a critical role in the clearance of intracellular organisms, most typically viral pathogens,17 whereas Th2 responses were critical for the response to parasitic infections. In addition, each of these lineages was associated with specific and distinct autoimmune diseases.
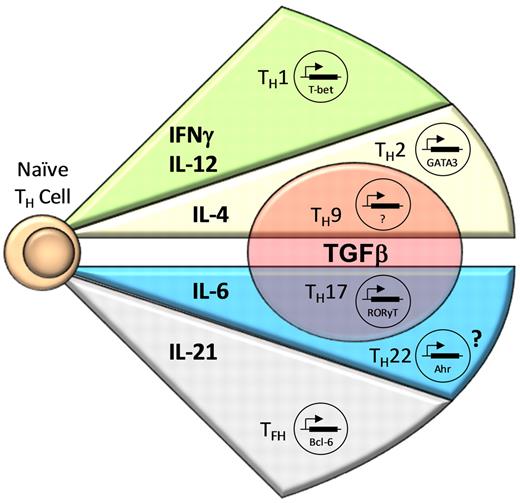
Differentiation of CD4+ T cells by the proteins or mRNA they generated remained inexact, with overlapping activity found for Th1 and Th2 cells. However, critically important work from the laboratories of Zheng and Flavell18 and Szabo et al19 identified lineage-specific transcription factors for Th1 and Th2 cells. T-BET (TBOX21) was found to be the critical transcription factor for the generation of Th1 cells, whereas GATA-3 served that role for Th2 cells (Figure 1).
CD4+ T-cell effector subsets. Antigen-specific stimulation of naive CD4+ T cells in the presence of certain cytokines induces expression of lineage-specific transcription factors, resulting in differentiation into CD4+ T-cell effector subsets. IFN-γ and IL-12 lead to the expression of T-bet, resulting in Th1 cell differentiation. CD4+ T-cell activation in the presence of IL-4 results in Th2 cell development mediated by GATA3; however, the addition of TGF-β causes differentiation into Th9 cells. The combination of IL-6 and TGF-β is necessary for RORγt expression, resulting in Th17 cell development, whereas the presence of IL-6 alone or with other unknown cytokines possibly results in Th22 cell differentiation by expression of AHR. Finally, IL-21 appears to be important for the development of Tfh cells through induction of Bcl-6.
CD4+ T-cell effector subsets. Antigen-specific stimulation of naive CD4+ T cells in the presence of certain cytokines induces expression of lineage-specific transcription factors, resulting in differentiation into CD4+ T-cell effector subsets. IFN-γ and IL-12 lead to the expression of T-bet, resulting in Th1 cell differentiation. CD4+ T-cell activation in the presence of IL-4 results in Th2 cell development mediated by GATA3; however, the addition of TGF-β causes differentiation into Th9 cells. The combination of IL-6 and TGF-β is necessary for RORγt expression, resulting in Th17 cell development, whereas the presence of IL-6 alone or with other unknown cytokines possibly results in Th22 cell differentiation by expression of AHR. Finally, IL-21 appears to be important for the development of Tfh cells through induction of Bcl-6.
Until 10 years ago, Th1 and Th2 T cells were the only truly separate CD4+ lineages. Although groups of T cells such as Tr1 or T-helper type 3 cells were described, it was not clear that these cells were separate lineages.20,21 Thus, much of the work inferring a role for Th1 or Th2 cells in the biology of GVHD has been done with the assumption that Th1 or Th2 cells were the sole T-cell lineages. In this review, we summarize the data suggesting that other CD4+ T-cell lineages also are critical to the pathophysiology of GVHD.
Th17 cells
The initial description of a subset of CD4+ T cells separate from Th1 cells that mediated inflammation came from the interesting finding that mice lacking the IL-12 p40 chain behaved differently than mice lacking the IL-12 p35 chain. This conundrum was explained when work showed that p40 could pair with a second chain, p19, to generate the cytokine IL-23. This cytokine was shown to be important for the generation of T cells producing IL-17A. However, whether T cells producing IL-17A differed from Th1 cells was not clear, until 2 seminal studies clearly established the Th17 lineage as independent and distinct.22,23
Early work suggested a critical role for IL-23 in the development of Th17 cells from naive T cells (Tns; Figure 1). However, the initial polarization of Tns was found to depend critically on the presence of transforming growth factor β (TGF-β), a cytokine that had previously been shown to inhibit both Th124 and Th225 development. At least one other proinflammatory cytokine must be combined with TGF-β to effectively induce Th17 cells.26-32 In mice, IL-6 is the critical second mediator,26,27 and it also appears to be required for their normal development in vivo.33 In humans, the nature of this second signal is less clear, with IL-6, IL-21, IL-23, and IL-1β all being implicated.29-32
In mice, Th17 polarization proceeds on TCR and CD28 engagement in the absence of Th1- and Th2-polarizing cytokines in the presence of both TGF-β and IL-6. The latter has been shown to directly activate the transcription factor signal transducer and activator of transcription 3 (STAT-3), which is required for Th17 differentiation34 (Figure 1). Full expression of the Th17 phenotype depends on the orphan nuclear receptor, retinoid-related orphan receptor γ t (RORγt). RORγt induces the expression of IL-17A and IL-17F,33 contributes to the generation of IL-23R,35,36 and mediates IL-22 production.37 Recently, a second transcription factor, RORα, has been found to respond to STAT-3 and has several overlapping functions with RORγt, including the transcription of IL-17A and to a lesser extent IL-17F.
IFN-γ is the prototypical Th1 cytokine, driving initial Th1 polarization and serving as their principal inflammatory mediator. It is becoming increasingly clear, however, that equating IFN-γ production with Th1 cells is an oversimplification, because other cell types, including Th17 cells, are able to produce abundant IFN-γ under certain conditions. Several groups, including our own, have reported that a substantial fraction of IL-17A–producing Th17 cells coexpress IFN-γ both in vitro and in vivo.38-42 In addition, recent data have shown that the Th17 lineage is not stable. Lee et al43 and Mukasea et al44 found that Th17 cells cultured in the presence of IL-12 with or without exogenous TGF-β rapidly extinguished IL-17F and RORγt expression and up-regulated IFN-γ by signaling through STAT-4 and T-bet, consistent with skewing toward the Th1 lineage. Our group and others45,46 found similarly that almost a pure population of Th17 cells extinguished production of IL-17A and IL-17F if cultured in the presence of IL-12. Especially relevant for GVHD pathophysiology, this process occurs more readily in a lymphopenic environment.47
The previous studies and data showing that inducible Tregs can convert under certain conditions to Th17 cells and vice versa have led investigators to evaluate the mechanism for this finding.10 Studies have shown substantial epigenetic modifications for lineage changes by T cells. Several recent studies have shown that chromatin alterations at critical lineage-specific loci such as FOXP3 or RORC or both are critical in these changes and suggest that the notions about lineage fixation by T cells is overly simplistic.48
T follicular helper cells
Previous investigators demonstrated that antibody generation against pathogens and critically immunoglobulin class switching required the interaction of CD4+ T cells with B cells. Because IL-4 could mediate much of this effect, the presumption had been that this process was mediated by Th2 cells. However, in 2002, a third effector T-cell lineage, T follicular helper (Tfh) cells, was described simultaneously by Breitfield et al49 and Schaerli et al.50 Tfh cells express the chemokine receptor, CXCR5, which is critical for their migration into the germinal center where antibody generation and class switching occur. IgG and IgA generation by B cells was significantly increased in the presence of Tfh cells. Chtanova et al using gene chip analysis demonstrated that Tfh cells express a distinct transcription profile characterized by the differential expression of several genes, including BCL6, IL6R, CD30l, CD27, CD84, CD200, and IL21 in addition to CXCR5 and IL4 compared with Th1, Th2, central, and effector memory cells.51
Tfh cells migrate from the blood into the B-cell follicle where they reach B cell–rich areas. The mechanism by which exposure to antigen program cells to become Tfh cells is not entirely clear, although TCR affinity for class II major histocompatibility complex (MHC)/peptide appears to be important because Tfh cells have higher affinity TCRs.8 The transcriptional repressor, BCL-6, is critical for Tfh activity because forced expression in T cells generates features consistent with Tfh cells, and, perhaps most importantly, its absence leads to an inability to generate Tfh cells and as a consequence complete loss of germinal center B cells and the germinal center reaction.52 Sites for the binding of BCL-6 were found in the promoters of TBOX21 and RORC. Moreover, forced expression of BCL-6 diminished the expression of TBOX21 and GATA3, suggesting that T cells undergoing the program to generate Tfh cells suppress transcription factors critical for Th1 or Th2 polarization.52
Th22 cells
IL-22 is a cytokine that is a member of the IL-10 superfamily and signals by the IL-22 receptor composed of the common IL-10R2 and specific IL-22Rα1.9 The function of IL-22 is complex, because it has been shown to mediate both proinflammatory and anti-inflammatory activity. IL-22 was initially shown to be produced by Th17 cells and the expansion of cells generating this dependent on IL-23.53 IL-22 in conjunction with IL-17A or IL-17F or both is important in the generation of small antimicrobial peptides important for the health of the skin.
Eyerich et al were the first to describe a specific cellular T-cell lineage for the production of IL-22.54 They evaluated human T cells from patients with psoriasis, atopic eczema, and allergic contact dermatitis. A subset of T cells secreting IL-22 alone was found primarily in the blood and skin of patients with atopic eczema. Th22 clones generated IL-22 and in addition IL-10 or TNF or both. Sorting for the expression of the skin homing chemokine receptor, CCR10, enriched for IL-22–expressing memory T cells. Transcriptome analysis indicated that Th22 cells generated CCL7, CCL15 and, unlike Th17 cells, did not up-regulate CCL20. The transcription factors BNC2 and FOXO4 were overexpressed by Th22 cell clones, whereas there was reduced expression of RORC, GATA3, and TBOX21 compared with Th1, Th2, or Th17 cells. A second group used expression of the chemokine receptors CCR4, CCR6, and, in particular, CCR10 to characterize human CD4+ T cells.55 Transcription factors critical for T-cell polarization could be differentiated on the basis of the expression of CCR10. CCR10+ T cells expressed the aryl hydrocarbon receptor (AHR), whereas CCR10− T cells expressed RORC. Knocking down RORC inhibited the production of IL-17A and IL-22 by T cells, whereas targeting AHR specifically inhibited the production of IL-22. This group's Th22 clones generated IL-22, IL-13, and TNF. Subsequent work suggested that Langerhan cells, a specific type of skin dendritic cell, can stimulate Th22 cells independent of Th1 or Th17 activity.56 At this time, a specific Th22 lineage has yet to be described in mice.
Th9 cells
IL-9, a cytokine traditionally associated with the Th2 lineage, has been implicated in immunity to helminths, allergic responses, and the expansion of B cells. More recently, IL-9 expression has been linked to other T-cell subtypes, including Th17 cells and inducible Tregs, and shown to mediate both proinflammatory and immunomodulatory effects in vivo.57,58 Data are now emerging to suggest that IL-9–producing T cells may represent another, distinct Th subset. Th9 cells have been shown by 2 groups to generate abundant IL-9 and IL-10, with minimal expression of IL-4, IL-5, IL-13, IFN-γ, or TNF.59,60 Further, these cells were not found to express any of the known T helper transcription factors that define the Th1, Th2, iTreg, or Th17 lineages (TBOX21, GATA3, FOXP3, or RORC, respectively). Th9 cells could be generated de novo from naive CD4+CD25− cells under unique polarizing conditions that included exogeneous IL-4 in the presence of TGF-β. In addition, committed Th2 cells could be “reprogrammed” to a Th9 phenotype on reactivation in the presence of TGF-β alone, with extinction of GATA3 and IL4 expression.59 These Th2 precursors could not be polarized back to Th1 cells despite repeated stimulation in the presence of IL-12, suggesting that Th9 transition may represent a unique plasticity for the Th2 lineage.
At the present time, the normal tissue distribution and immune function of these cells is poorly understood. In general, and with the exception of IL-9–producing Tregs, Th9 cells appear to be proinflammatory despite their abundant IL-10 production and have been shown to exacerbate intestinal inflammation in T-cell adoptive transfer colitis models.60 Interestingly, these cells may also possess a strong tropism for nervous tissue, because they are able to induce severe peripheral neuritis in murine recipients.60 Th9 cells, unlike Tr1 cells, which also produce abundant IL-10, do not exhibit any apparent immunosuppressive properties in vitro and appear to proliferate normally in response to anti-CD3 stimulation.60
T-cell subsets and GVHD: preclinical evaluations
Th1 cells
In most murine models, CD4+ T cells are critical to the induction of GVHD either by the need for CD4+ T cells to produce IL-2, which mediates robust allospecific CD8+ T-cell proliferation, or by the generation of effector proteins such as TNF or cytolytic activity mediated by FAS/FAS ligand. Early studies have shown significant expression of IFN-γ in GVHD target organs, suggesting that Th1 cells were critical mediators of tissue pathology.61 From these findings, investigators hypothesized that blocking the function of the Th1 effector cytokine, IFN-γ, early after stem cell transplantation (SCT) would diminish or perhaps prevent acute GVHD. Interestingly, this was not found. Our group evaluated whether donor T cells incapable of generating IFN-γ could mediate GVHD in a murine model.62 The median survival for recipient mice given IFN-γ knockout (KO) donor cells was 21 days compared with 38 days for the use of wild-type (WT) T cells. We demonstrated a similar effect with the use of anti–IFN-γ monoclonal antibody. These data suggested that not only was IFN-γ not critical for the generation of acute GVHD, but that its absence also exacerbated GVHD lethality. Subsequent studies have shown that donor-derived IFN-γ production worsened gastrointestinal (GI) GVHD but reduced lung injury.63 Interestingly, our group found that IFN-γ, although is critical for GI tract disorders, also mediated the induction of indolamine 2-3 dioxygenase, which catabolizes the critical amino acid tryptophan to kynurenine and blocks T-cell proliferation in the GI tract.64,65 Thus, IFN-γ may mediate separate functions at the same target organ during GVHD.
A second approach to evaluate the function of different T-cell subsets in GVHD focused on the signaling proteins critical to the generation of IFN-γ and, as a result, Th1 cells.66 Nikolic et al evaluated the induction of acute GVHD with the use of donors unable to generate STAT4 (Th1 impaired) or STAT6 (Th2 impaired).67 They found that STAT4 KO donor T cells mediated acute GVHD with delayed kinetics compared with WT or STAT6 KO T cells. Interestingly, recipients of STAT4 KO cells showed substantial liver disorders with hepatocellular necrosis and extensive cutaneous changes with marked dermal infiltration and lysis along the epidermal-dermal junction, similar to that recently described after the infusion of Th17 cells generated ex vivo.38 This severe skin disorder was not found with the use of STAT6 KO donors. Recipient mice given STAT6 KO donor T cells had earlier and more severe clinical GVHD, and they manifested profound inflammation in the colon with little involvement of the skin or liver. These data have suggested that Th1 cells play a critical role in tissue inflammation in the GI tract (Figure 2).
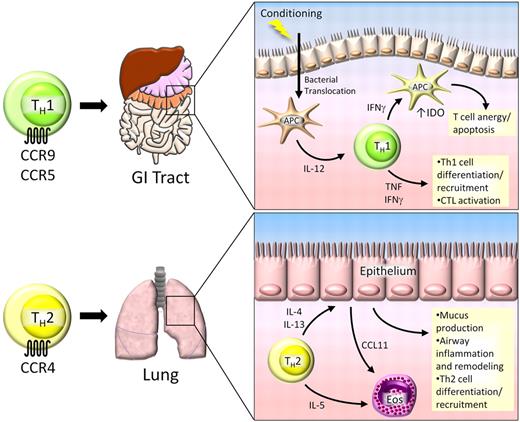
Th1 and Th2 cells in GVHD. Th1 cells play a significant role in GVHD pathogenesis in the gastrointestinal (GI) tract. Donor-specific Th1 cells migrate to the GI tract and liver by the chemokine receptors CCR9 and CCR5. During conditioning before transplantation, the integrity of the epithelial barrier is compromised, resulting in translocation of bacterial products and activation of local antigen presenting cells (APCs). These activated APCs secrete IL-12, which is necessary for Th1 cell development and expansion. Th1 cells secrete IFN-γ, which has dual roles in the GI tract. IFN-γ and TNF facilitate further Th1 cell development and activation of allospecific CTLs, resulting in tissue damage. Conversely, Th1-derived IFN-γ can induce the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) in APCs, causing T-cell anergy and apoptosis. Th2 cells migrate to the lung by CCR4, where they secrete IL-4, IL-5, and IL-13. IL-4 and IL-13 act on lung epithelium, causing inflammation and tissue remodeling that ultimately leads to pulmonary fibrosis. IL-5 facilitates expansion of eosinophils (Eos) that are recruited to the lung by CCL11, which can further exacerbate lung tissue damage.
Th1 and Th2 cells in GVHD. Th1 cells play a significant role in GVHD pathogenesis in the gastrointestinal (GI) tract. Donor-specific Th1 cells migrate to the GI tract and liver by the chemokine receptors CCR9 and CCR5. During conditioning before transplantation, the integrity of the epithelial barrier is compromised, resulting in translocation of bacterial products and activation of local antigen presenting cells (APCs). These activated APCs secrete IL-12, which is necessary for Th1 cell development and expansion. Th1 cells secrete IFN-γ, which has dual roles in the GI tract. IFN-γ and TNF facilitate further Th1 cell development and activation of allospecific CTLs, resulting in tissue damage. Conversely, Th1-derived IFN-γ can induce the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) in APCs, causing T-cell anergy and apoptosis. Th2 cells migrate to the lung by CCR4, where they secrete IL-4, IL-5, and IL-13. IL-4 and IL-13 act on lung epithelium, causing inflammation and tissue remodeling that ultimately leads to pulmonary fibrosis. IL-5 facilitates expansion of eosinophils (Eos) that are recruited to the lung by CCL11, which can further exacerbate lung tissue damage.
Th2 cells
Initial studies have shown that Th2 cells, when added to donor splenocytes, could mitigate acute GVHD with significant attenuation of GVHD disorder in the GI tract and liver.68,69 Follow-up studies have shown that the release of proinflammatory cytokines induced by lipopolysaccharide was reduced when Th2 cells were added to the donor inoculums,70 suggesting that Th2 cells could be therapeutically beneficial. Quite recently, 2 groups have sought to evaluate the role of Th2 cells in acute GVHD with the use of genetic approaches.71,72 Tawara et al used T cells from mice in which the IL4/IL5/IL13 gene cluster was targeted by homologous recombination and crossed to IL9 KO mice.71 In a complete MHC-mismatched model, they found enhanced GVHD that correlated with increased donor T-cell proliferation, production of TNF in vivo, and T-cell production of IL-2, IFN-γ, and IL-17A in vitro. Yi et al used a similar approach by infusing donor T cells unable to generate IFN-γ, IL-17A, or IL-4.72 Absence of IFN-γ and IL-17 led to diminished inflammation in the colon, liver, and skin but modestly increased inflammation in the lung. With the use of an anti–IL-4 antibody, they found reduced lung pathology scores, suggesting a role for IL-4 generation in idiopathic pneumonia syndrome/GVHD in the lung (Figure 2). They did not find GVHD in any other target organs in the absence of IL-17 and IFN-γ, suggesting that Th2 cells may not play a critical role in the pathophysiology of GVHD in the colon, liver, skin, or small bowel in animal models. These and additional studies indicate that Th1 cells are critical for acute GVHD pathology in the GI tract, but that IFN-γ itself has detrimental and beneficial effects that are organ system dependent.64,65 A specific role for Th2 cells themselves could not be found, with the exception of idiopathic pneumonia syndrome after SCT. A role for Th2 cells was inferred from the pathology found with donor mice unable to polarize to a Th1 response (STAT4 KO), which may be complicated by the ability of other T-cell lineages such as Th17 cells to mediate these effects.
Chronic GVHD
Our understanding of the function of T cells in chronic GVHD is less advanced compared with that in acute GVHD partly because of the difficulty with generating animal models that duplicate clinical disease. Organs such as the kidney, which is rarely involved clinically, are often a significant site of disorders in chronic GVHD murine models.73 Nevertheless, studies have suggested in both nonconditioned F1 recipients using donor T cells alone and in minor mismatched models with sublethal irradiation, that Th1 polarization can reduce tissue pathology, whereas Th2 polarization exacerbated this.73,74
Th17 cells
Th17 cells generate the proinflammatory cytokines IL-17A, IL-17F, IL-21, IL-22, and TNF, and their function is mediated by the transcription factors RORγt and RORα (Figure 1). The contribution of Th17 cells to GVHD disorders in mice is controversial. Our group addressed this issue by generating highly pure B6 (H-2b) Th17 cells in vitro and administering them alone or combined with B6 Tns to haploidentical B6D2 F1 (H-2bxd) recipients. Infusion of Th17 cells resulted in aggressive, lethal GVHD, with unusually severe pathology observed in the skin and lungs38 (Figure 3). These effects depended partly on IL-17A, because neutralization with an anti–IL-17A antibody greatly ameliorated skin disease, whereas blocking TNF ameliorated systemic GVHD. Further, these effects were independent of IFN-γ, because Th17 cells generated from IFNG KO B6 animals produced nearly identical results. Hill et al suggested a prominent role for Th17 cells in cutaneous GVHD.75 The investigators demonstrated that donor mice undergoing stem cell mobilization with human granulocyte CSF show an increased proportion of CD4+ and CD8+ IL-17A–producing T cells within their spleens in an IL-21–dependent manner. When granulocyte CSF–mobilized splenocytes were subsequently transferred into irradiated recipients, sclerodermatous skin lesions developed which depended critically on the production of IL-17A (Figure 3).
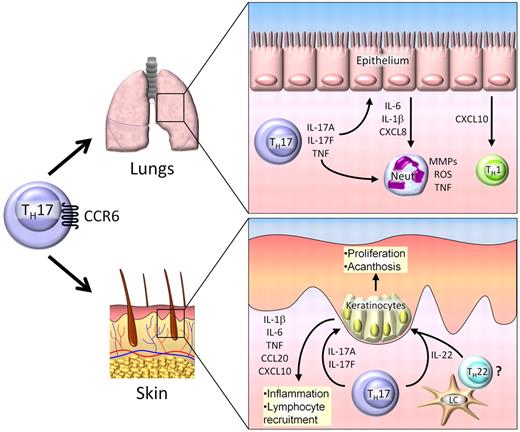
Th17 cells in GVHD. Th17 cells traffic to the lung and skin by CCR6, where they mediate tissue damage. In the lung, Th17 cells secrete IL-17A, IL-17F, and TNF, which induce secretion of proinflammatory cytokines and chemokines by epithelial cells. Th17-derived cytokines act directly on neutrophils (neut), resulting in production of matrix metalloproteinases (MMPs), reactive oxygen species (ROS), and TNF. In the skin, IL-17A and IL-17F produced by infiltrating Th17 cells cause the production of several proinflammatory cytokines and chemokines by keratinocytes, resulting in further leukocyte recruitment and local tissue damage. IL-22 is secreted byTh17 cells and possibly Th22 cells activated by Langerhan cells (LCs). IL-22 induces keratinocyte proliferation, resulting in acanthosis and cutaneous GVHD pathology.
Th17 cells in GVHD. Th17 cells traffic to the lung and skin by CCR6, where they mediate tissue damage. In the lung, Th17 cells secrete IL-17A, IL-17F, and TNF, which induce secretion of proinflammatory cytokines and chemokines by epithelial cells. Th17-derived cytokines act directly on neutrophils (neut), resulting in production of matrix metalloproteinases (MMPs), reactive oxygen species (ROS), and TNF. In the skin, IL-17A and IL-17F produced by infiltrating Th17 cells cause the production of several proinflammatory cytokines and chemokines by keratinocytes, resulting in further leukocyte recruitment and local tissue damage. IL-22 is secreted byTh17 cells and possibly Th22 cells activated by Langerhan cells (LCs). IL-22 induces keratinocyte proliferation, resulting in acanthosis and cutaneous GVHD pathology.
However, it has been harder to show a conclusive role for the cytokine, IL-17A, in GVHD induced by Tns. Kappel et al found that whole T cells from IL17a KO B6 animals generated GVHD responses that were essentially identical to those induced by WT cells, although, when purified CD4+IL17a KO T cells were used, there was improved transplantation outcomes with recipients showing a prolonged median survival time.40 Overall survival was unaffected, however, leading the investigators to conclude that, although IL-17A contributes to the early development of CD4+ T cell–mediated inflammation, it is dispensable for GVHD as a whole.
Substantially different results were obtained by Yi et al with the use of a similar model.76 IL17a KO splenocytes and whole T cells were found to exacerbate GVHD and to accelerate recipient mortality compared with WT cells. The inferior outcomes seen with IL17a KO cells could be prevented by the administration of exogeneous IL-17A or neutralizing anti–IFN-γ, leading the investigators to conclude that, in the absence of IL-17A, Th1 differentiation was enhanced, which has been found in other models.77 Because the only difference in the experimental systems that generated these disparate results was the use of a lower dose of radiation, it is possible that the function of IL-17A after transplantation is timing or conditioning dependent or is affected by the microbiotica in the different investigator's colonies.78
Given the plasticity of the Th17 lineage10 and its numerous inflammatory mediators, the most straightforward approach to addressing the contribution of Th17 cells to GVHD pathogenesis is to use donor immune cells lacking RORγt, which would be unable to generate Th17 cells.33 In work by Iclozan et al, the investigators transferred WT or RORγt KO purified CD4+ B6 T cells to lethally irradiated Balb/c mice and observed nearly identical overall survival percentages, median survival times, and percentage of weight loss between the 2 treatment groups.39 This led the investigators to conclude that “Th17 cells are sufficient but not necessary to induce GVHD.”39p177 Although the investigators transferred between 1 and 2 × 106 CD4+ cells per recipient, uniform lethality was not seen in either group as late as transplantation day 50. Our own group has conducted similar experiments with WT B6 versus RORγ KO (lacking both isoforms RORγ and RORγt) B6 donor animals, using both Balb/c and B6D2 F1 mice as recipients. In both systems, using a lower dose of donor T cells, RORγ KO cells generated greatly attenuated GVHD and prolonged recipient survival, although this was less profound in the complete MHC mismatch model,79 suggesting that targeting both isoforms of RORγ substantially affected acute GVHD. The reasons for the apparent discordant results with Iclozan et al39 are unclear, but they may relate to the dose of T cells used, the difference in the infusion of whole versus CD4+ T cells or whether the knockout targets the entire RORC locus or specifically RORγt.
Recent work in which alloreactive T cells are transferred into recombinase activating gene KO syngeneic hosts to generate a model of chronic GVHD alloreactivity leading to autoreactivity found autoimmune damage was not modified by blocking IL-17A genetically or by using monoclonal antibody therapy.80 Whether other cytokines generated by Th17 cells, such as IL-21, which plays a critical role in antigen-specific B-cell function, are important in chronic GVHD is not yet known.81
T-cell subsets and GVHD: clinical evaluations
Much of the information about the role of T-cell subsets in clinical GVHD is confusing and contradictory and comes from the presence of specific cytokines in the serum of patients with GVHD,82-84 in lesional tissue biopsies,82,85 or is inferred from single nucleotide polymorphisms present in cytokines genes.86,87 Interestingly, despite the perceived skewing of GVHD toward a Th1 process, multiple investigators have not found increased serum levels of IL-12 after allo-SCT compared with healthy donors.88 Consistently, investigators have found increased protein or mRNA for IL-6, TNF, and IL-1β, which in one study correlated with disease severity82-84 and in another with disease onset.89 Interestingly, these cytokines would polarize toward a Th17 response. Th17 cells have been found increased by enzyme-linked immunospot assay and intracellular cytokine staining in the peripheral blood of patients with acute and chronic GVHD, and this correlated in one study with active disease.41 Single nucleotide polymorphisms in the regulatory region of IFN-γ leading to increased IFN-γ expression have been associated with an increased incidence of acute GVHD but only when present in the recipient and not the donor.86,87 Thus, serum evaluations would support indirectly a role for Th17 cells in the pathophysiology of acute GVHD.
At lesional sites, we found increased expression of IL2, IL4, and IFNg mRNA from skin biopsy samples in patients with GVHD, suggesting a mixed T-cell response.82 Several investigators have noted increased expression of TNF in the skin of patients with acute GVHD, with one group correlating this with CCR10-infiltrating T cells.85 This corresponds to the 70% response rate at this site with the use of the TNF-targeted therapy infliximab.90 Dander et al noted increased numbers of Th17 cells in the skin of patients with active chronic GVHD compared with control samples collected from patients with skin cancer, with most of these IL-17A–producing cells coexpressing IFN-γ.41 Somewhat conflicting results were obtained in a larger series from Broady et al.91 Little to no IL-17 or IL-22 was found from ex vivo–stimulated T cells isolated from the skin of patients with GVHD compared with healthy controls or samples obtained from patients with psoriasis. A third series examined Th17 involvement in human cutaneous GVHD.42 Skin biopsies were obtained from patients with either acute or chronic lichenoid cutaneous GVHD and from skin-infiltrating Th17 cells identified on the basis of their expression of IL-17A by immunohistochemistry. At the same time, infiltrating Tregs were quantitated with Foxp3 staining. The investigators demonstrated that the Th17/Treg ratio was significantly lower than that seen in their non-GVHD controls, which argued against a pathogenic role for the Th17 subset.
Thus, at this time there are data to support involvement of Th1 and Th17 cells in skin GVHD with perhaps a greater involvement of Th17 cells in chronic GVHD of the skin. The reasons for the differences between these clinical studies and the preclinical data are not entirely clear. One concern is that calcineurin inhibitors block the production of IL-17A, which may lead to an underestimation of Th17 cells if they are only identified according to their production of IL-17A. A second issue would be the plasticity of Th17 cells, which may preclude longitudinal assessment of these cells ex vivo in the absence of polarizing cytokines. The timing of these evaluations may be critical because Th17 cells may readily convert to Th1 cells after the cytokine storm induced by conditioning has abated.
The overwhelming preponderance of preclinical data suggest that Th1 cells are critical mediators of tissue pathology in the GI tract. The little human data that do exist have essentially yielded similar results to the animal studies, with little evidence for a robust Th17 response within GI biopsies taken from patients with GVHD.42 Tissue inflammation in the liver after SCT has been harder to define. Dander et al found dual IL-17A, IFN-γ–expressing T cells in the liver in patients with active chronic GVHD, suggesting a role for Th17 cells skewed toward a Th1 response.41 Interestingly, the efficacy of infliximab is poorest for treating patients with GVHD involving the liver, suggesting that TNF may not be a critical mediator from Th17 cells at this site.
Other lineages
Although there are no current data about the function of Th22 cells in GVHD, the activity of IL-22 makes it an interesting target for patients with chronic GVHD (Figure 3). Our group has preliminary data, suggesting substantial generation of mRNA for IL22 in the skin of patients with chronic GVHD (J.M.C. and J.S.S., unpublished data, December 2010). Because IL-22 appears to be critical for keratinocyte proliferation and acanthosis in an inflammatory setting and epidermal thickening one of the hallmarks of chronic GVHD is markedly induced byIL-22, IL-22 production by Th22 or Th17 cells may play an important and targetable role in skin pathology during chronic GVHD.9 In addition to alloantibody and autoantibody responses,92-94 a coordinated T-B response to minor histocompatibility antigens has been well described in human chronic GVHD.95 A role for Tfh cells in autoimmune diseases has been described96,97 and suggests that further investigation of these cells in GVHD pathogenesis will define further a role for Tfh cells and B cells in chronic GVHD.
What role, if any, Th9 cells play in the pathogenesis of GVHD remains purely speculative. In solid organ transplantation models, IL-9 expression by inducible Tregs has been shown to promote graft tolerance through the recruitment of immunosuppressive mast cells.58 This would suggest a possible protective role for the cytokine in the hematopoietic SCT setting. Conversely, Th9 cells might worsen acute GVHD by directly exacerbating intestinal injury, or it could potentiate chronic GVHD responses by promoting pathogenic B-cell expansion or autoantibody production. Future work should evaluate for the presence of these cells, especially in the GI tract in animal models and clinical samples.
Summary
Since the initial description of Th1 and Th2 cells, there has been a substantial increase in our understanding of T-cell fate determination. In this review, we have provided a framework for understanding these new lineages as they effect acute or chronic GVHD. Current data would suggest that Th1 cells play a critical role in the pathophysiology of acute GVHD with a substantial amount of data suggesting that GI tract involvement is mediated in part or in total by Th1 cells. There is little preclinical or clinical information to implicate Th2 cells in acute GVHD globally or at a specific site with the possible exception of the lung. Th2 cells may be critical however in chronic GVHD. The effector lymphocytes that mediate GVHD in the skin and liver are less clear, although preclinical and some but not all clinical data suggest a role for Th17 or Th1 cells or both. In addition, T-cell lineage commitment is much more dynamic than previously believed, and it may be more appropriate to evaluate inflammatory nodes such as a combined Th1/Th17/Th22 axis for acute GVHD or a combined Th2/Tfh/Th9 axis for chronic GVHD. Recent work would support this approach.98 Over the past decade much new has been learned. The time has come to get past the Th1/Th2 paradigm and to address the complexity and plasticity of T-cell lineage commitment as a new approach for the treatment/prevention of GVHD.
Acknowledgments
This work was supported in part by research funding from the National Institutes of Health (R01 AI064363 and R56 AI082219; J.S.S. and R01 HL56067, AI34495, and CA72669; B.R.B.) and by the Mary Elizabeth Thomas Endowment fund (J.S.S.). J.M.C. was supported by National Institutes of Health training award K12CA120780.
National Institutes of Health
Authorship
Contribution: J.M.C. and S.S. assisted in writing the manuscript, T.P.M. assisted in writing the manuscript and generated the figures, W.J.M. edited the manuscript, B.R.B. assisted in writing and editing the manuscript, and J.S.S. conceived of the project and assisted in writing and editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan Serody, Lineberger Comprehensive Cancer Center, 450 West Dr, Chapel Hill, NC 27599-7295; e-mail: jonathan_serody@med.unc.edu.