Abstract
We retrospectively analyzed 12 consecutive adult severe aplastic anemia patients who received unrelated umbilical cord blood transplantation after a reduced-intensity conditioning regimen (RI-UCBT). The conditioning regimen consisted of 125 mg/m2 fludarabine, 80 mg/m2 melphalan, and 4 Gy of total body irradiation. The median infused total nucleated cell number and CD34+ cell number were 2.50 × 107/kg and 0.76 × 105/kg, respectively. Eleven of the 12 patients achieved primary neutrophil and platelet engraftment. All patients who achieved engraftment had complete hematologic recovery with complete donor chimerism, except for one patient who developed late graft failure 3 years after RI-UCBT. Two of the 12 patients died of idiopathic pneumonia syndrome, and the remaining 10 patients are alive, having survived for a median of 36 months. Our encouraging results indicate that RI-UCBT may become a viable therapeutic option for adult severe aplastic anemia patients who lack suitable human leukocyte antigen-matched donors and fail immunosuppressive therapy.
Introduction
Bone marrow transplantation from a human leukocyte antigen (HLA)-matched sibling is recommended as first-line therapy for younger patients with severe aplastic anemia (SAA).1,2 However, many patients lack HLA-matched sibling donors. Bone marrow transplantation from an HLA-matched unrelated donor has been an alternative therapeutic option for patients who fail one or more courses of immunosuppressive therapy, but high rates of graft failure (GF), graft-versus-host disease (GVHD), and infection still remain to be solved.3 The number of unrelated umbilical cord blood transplantations (UCBTs) has been increasing.4 However, little information has been available on whether UCBT is feasible for SAA patients. We reported successful urgent UCBT using reduced-intensity (RI) conditioning for a 70-year-old SAA patient in 2003.5 Here we present successful sustained engraftment of 11 consecutive patients with SAA who received RI-UCBT with the same RI conditioning regimen after the first report.
Methods
This study included 12 consecutive adult patients with acquired SAA who underwent RI-UCBT at our institute from September 2002 through January 2009. The patients' characteristics and umbilical cord blood (UCB) units are summarized in Table 1. Their median age was 49 years (range, 20-70 years). Four cases of severe, 6 of very severe, and 2 of fulminant type were included according to criteria as previously reported.2,6 Fulminant type was defined as no neutrophils in the peripheral blood at diagnosis despite administration of granulocyte-colony stimulating factor. Ten patients, except for the 2 patients with fulminant type, had failed at least one course of immunosuppressive therapy. All patients gave their written informed consent in accordance with the Declaration of Helsinki, and the study was approved by the Toramon Hospital Institutional Review Board. UCB units were obtained from the Japanese Cord Blood Bank Network, and single UCB unit was infused in all the studied patients. All UCB units were serologically typed for HLA-A, -B, and -DR antigen before selection and were tested by high-resolution DNA typing before transplantation. The degree of mismatch is expressed using antigen level at HLA-A and -B, and allele level at DRB1. ABO incompatibility was not incorporated as one of the factors used in CB unit selection. The median total nucleated cell number and CD34+ cell number at cryopreservation were 2.50 × 107/kg (range, 1.83-4.39 × 107/kg) and 0.76 × 105/kg (range, 0.27-1.52 × 105/kg), respectively. Anti-HLA antibodies were screened before transplantation in 6 patients using a FlowPRA method (One Lambda), and LAB Screen PRA or Single Antigen (One Lambda) was used to identify HLA antibody specificities.7,8 All patients were conditioned with 25 mg/m2 fludarabine daily for 5 days, 40 mg/m2 melphalan daily for 2 days, and 4 Gy of total body irradiation in 2 fractions in 1 day. GVHD prophylaxis consisted of cyclosporine in 2, tacrolimus in 2, and tacrolimus plus mycophenolate mofetil in 8. Assessment of engraftment, GF, chimerism, GVHD, and supportive care during transplantation were performed as previously reported.9,10 Karnofsky performance status score was assessed as surrogate for quality of life of the survivors. Overall survival was estimated using the Kaplan-Meier method.
Characteristics of patient, grafts, and GVHD prophylaxis
| Case no. . | Age, y . | Previous treatment . | Interval from diagnosis to UCBT, mo . | Previous transfusion times (RBCs/platelet) . | Disease status at UCBT . | HLA match . | HLA Ab (reactive to CB) . | ABO group (R/D) . | TNC × 107/kg . | CD34+, × 105/kg . | GVHD prophylaxis . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | CSA | 3 | 11/14 | SAA | 4/6 | NT | A/A | 4.00 | 1.23 | CSA |
| 2 | 20 | ATG + CSA | 78 | > 20/> 20 | VSAA | 4/6 | NT | B/O | 2.65 | 1.07 | CSA |
| 3 | 22 | ATG + CSA, PSL | 157 | > 20/> 20 | SAA | 4/6 | NT | A/O | 2.26 | 0.27 | Tac |
| 4 | 26 | ATG + CSA | 3 | > 20/> 20 | VSAA | 5/6 | NT | A/A | 2.65 | 0.70 | Tac |
| 5 | 59 | ATG + CSA | 8 | > 20/> 20 | SAA | 5/6 | Positive (no) | O/O | 2.15 | 1.52 | Tac + MMF |
| 6 | 49 | ATG + CSA, PSL | 12 | > 20/> 20 | VSAA | 3/6 | NT | A/A | 2.04 | 0.62 | Tac + MMF |
| 7 | 70 | None | 1 | 5/8 | Fulminant | 4/6 | Positive (yes) | A/O | 4.39 | 1.29 | Tac + MMF |
| 8 | 52 | None | 1 | 4/6 | Fulminant | 4/6 | NT | AB/A | 3.20 | 0.49 | Tac + MMF |
| 9 | 46 | ATG + CSA | 45 | > 20/> 20 | VSAA | 4/6 | Positive (no) | AB/O | 1.83 | 0.42 | Tac + MMF |
| 10 | 49 | ATG + CSA, PSL | 327 | > 20/> 20 | VSAA | 6/6 | Positive (no) | B/O | 2.34 | 0.82 | Tac + MMF |
| 11 | 65 | CSA | 6 | 16/> 20 | VSAA | 6/6 | Positive (no) | A/A | 3.31 | 0.56 | Tac + MMF |
| 12 | 31 | ATG + CSA, PSL | 215 | > 20/> 20 | SAA | 4/6 | Positive (no) | B/O | 2.09 | 1.26 | Tac + MMF |
| Case no. . | Age, y . | Previous treatment . | Interval from diagnosis to UCBT, mo . | Previous transfusion times (RBCs/platelet) . | Disease status at UCBT . | HLA match . | HLA Ab (reactive to CB) . | ABO group (R/D) . | TNC × 107/kg . | CD34+, × 105/kg . | GVHD prophylaxis . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | CSA | 3 | 11/14 | SAA | 4/6 | NT | A/A | 4.00 | 1.23 | CSA |
| 2 | 20 | ATG + CSA | 78 | > 20/> 20 | VSAA | 4/6 | NT | B/O | 2.65 | 1.07 | CSA |
| 3 | 22 | ATG + CSA, PSL | 157 | > 20/> 20 | SAA | 4/6 | NT | A/O | 2.26 | 0.27 | Tac |
| 4 | 26 | ATG + CSA | 3 | > 20/> 20 | VSAA | 5/6 | NT | A/A | 2.65 | 0.70 | Tac |
| 5 | 59 | ATG + CSA | 8 | > 20/> 20 | SAA | 5/6 | Positive (no) | O/O | 2.15 | 1.52 | Tac + MMF |
| 6 | 49 | ATG + CSA, PSL | 12 | > 20/> 20 | VSAA | 3/6 | NT | A/A | 2.04 | 0.62 | Tac + MMF |
| 7 | 70 | None | 1 | 5/8 | Fulminant | 4/6 | Positive (yes) | A/O | 4.39 | 1.29 | Tac + MMF |
| 8 | 52 | None | 1 | 4/6 | Fulminant | 4/6 | NT | AB/A | 3.20 | 0.49 | Tac + MMF |
| 9 | 46 | ATG + CSA | 45 | > 20/> 20 | VSAA | 4/6 | Positive (no) | AB/O | 1.83 | 0.42 | Tac + MMF |
| 10 | 49 | ATG + CSA, PSL | 327 | > 20/> 20 | VSAA | 6/6 | Positive (no) | B/O | 2.34 | 0.82 | Tac + MMF |
| 11 | 65 | CSA | 6 | 16/> 20 | VSAA | 6/6 | Positive (no) | A/A | 3.31 | 0.56 | Tac + MMF |
| 12 | 31 | ATG + CSA, PSL | 215 | > 20/> 20 | SAA | 4/6 | Positive (no) | B/O | 2.09 | 1.26 | Tac + MMF |
RBC indicates red blood cell; CB, cord blood; R, recipient; D, donor; TNC, total nucleated cells; CSA, cyclosporine-A; ATG, antithymocyte globulin; PSL, prednisone; VSAA, very severe aplastic anemia; NT, not tested; Tac, tacrolimus; and MMF, mycophenolate mofetil.
Results and discussion
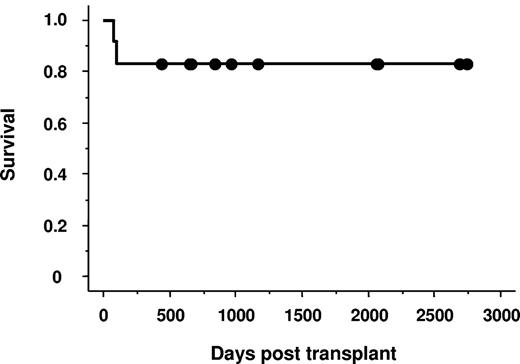
Patients' outcomes are summarized in Table 2. Eleven of the 12 patients achieved primary neutrophil and platelet engraftment. The median times to achieve neutrophil engraftment and platelet count more than 20 × 109/L were 18 days (range, 12-28 days) and 42 days (range, 26-64 days), respectively. All patients who achieved engraftment had complete hematologic recovery and were free from transfusion, and they showed complete donor chimerism at the time of the first chimerism analysis (median, 14 days; range, 11-73 days). One patient developed primary GF and was later found to have antibody against mismatched HLA on donor cells. Another patient developed secondary GF 3 years after UCBT. Both patients underwent a second RI-UCBT and obtained rapid donor engraftment. The negative impact of multiple transfusions before transplantation was not detected (Tables 1–2). Among 11 evaluable patients, 2 developed grade I and 5 developed grade II acute GVHD. Of the 9 patients who survived longer than 100 days after transplantation, 3 developed limited type of chronic GVHD. No patients developed grade III-IV acute GVHD and extensive type of chronic GVHD. Two of the 12 patients died of idiopathic pneumonia syndrome, and the remaining 10 patients are alive, having survived for a median of 36 months (range, 14-91 months). The probability of overall survival at 3 years was 83.3% (Figure 1). The surviving patients had high Karnofsky performance status score with a median of 90% (range, 60%-100%).
Outcomes of 12 patients after reduced-intensity unrelated cord blood transplantation
| Case no. . | Days to ANC > 0.5 × 109/L . | Days to PC > 20 × 109/L . | % Donor chimerism (days tested, methods) . | aGVHD . | cGVHD . | Discontinuation of IS (mo) . | Complications . | Survival (mo) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 52 | 100 (14, FISH) | Grade II (skin) | No | Yes (3) | Possible IPA | Alive (91) |
| 2 | 20 | 64 | > 90 (49, PCR-STR) | Grade II (skin) | Limited | Yes (2) | No | Alive (90) |
| 3 | 26 | 42 | 100 (26, FISH) | No | No | Yes (26) | Yes | Alive (69) |
| 4 | 18 | 53 | 100 (18, FISH) | No | No | Yes (5) | Pneumocystis jirovecii, late GF, rescued by second RI-UCBT | Alive (69) |
| 5 | 16 | 26 | 96.6 (14, FISH) | Grade I (skin) | Limited | Yes (14) | Norwalk virus colitis, EBV-PTLD | Alive (39) |
| 6 | 28 | 64 | 99.6 (11, FISH) | No | NE | No | IPS | Dead; IPS (3) |
| 7 | No | No | 48.8 (10, FISH), 4.3 (15, FISH) | NE | NE | NE | Primary GF, rescued by second RI-UCBT | Alive (32) |
| 8 | 18 | 28 | 99.2 (13, FISH) | Grade II (skin, gut) | No | Yes (7) | CMV colitis, EBV-PTLD | Alive (28) |
| 9 | 28 | 43 | > 90 (14, PCR-STR) | Grade I (skin) | NE | No | HSV pneumonia, IPS | Dead; IPS (3) |
| 10 | 15 | 27 | 99 (73, FISH) | No | Limited | No | No | Alive (22) |
| 11 | 15 | 27 | 100 (20, FISH) | Grade II (skin, gut) | No | No | No | Alive (22) |
| 12 | 13 | 28 | 100 (14, FISH) | Grade II (gut) | No | No | No | Alive (14) |
| Case no. . | Days to ANC > 0.5 × 109/L . | Days to PC > 20 × 109/L . | % Donor chimerism (days tested, methods) . | aGVHD . | cGVHD . | Discontinuation of IS (mo) . | Complications . | Survival (mo) . |
|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 52 | 100 (14, FISH) | Grade II (skin) | No | Yes (3) | Possible IPA | Alive (91) |
| 2 | 20 | 64 | > 90 (49, PCR-STR) | Grade II (skin) | Limited | Yes (2) | No | Alive (90) |
| 3 | 26 | 42 | 100 (26, FISH) | No | No | Yes (26) | Yes | Alive (69) |
| 4 | 18 | 53 | 100 (18, FISH) | No | No | Yes (5) | Pneumocystis jirovecii, late GF, rescued by second RI-UCBT | Alive (69) |
| 5 | 16 | 26 | 96.6 (14, FISH) | Grade I (skin) | Limited | Yes (14) | Norwalk virus colitis, EBV-PTLD | Alive (39) |
| 6 | 28 | 64 | 99.6 (11, FISH) | No | NE | No | IPS | Dead; IPS (3) |
| 7 | No | No | 48.8 (10, FISH), 4.3 (15, FISH) | NE | NE | NE | Primary GF, rescued by second RI-UCBT | Alive (32) |
| 8 | 18 | 28 | 99.2 (13, FISH) | Grade II (skin, gut) | No | Yes (7) | CMV colitis, EBV-PTLD | Alive (28) |
| 9 | 28 | 43 | > 90 (14, PCR-STR) | Grade I (skin) | NE | No | HSV pneumonia, IPS | Dead; IPS (3) |
| 10 | 15 | 27 | 99 (73, FISH) | No | Limited | No | No | Alive (22) |
| 11 | 15 | 27 | 100 (20, FISH) | Grade II (skin, gut) | No | No | No | Alive (22) |
| 12 | 13 | 28 | 100 (14, FISH) | Grade II (gut) | No | No | No | Alive (14) |
ANC indicates absolute neutrophil count; PC, platelet count; aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; IS, immunosuppressant; FISH, fluorescence in situ hybridization; PCR-STR, PCR of short tandem repeat; NE, not evaluable; IPA, invasive pulmonary aspergillosis; EBV-PTLD, Epstein-Barr virus–associated posttransplantation lymphoproliferative disorder; and IPS, idiopathic pneumonia syndrome.
Survival of 12 patients with SAA undergoing unrelated cord blood transplantation.
Survival of 12 patients with SAA undergoing unrelated cord blood transplantation.
The present study demonstrated that our RI conditioning regimen allows a sufficient sustained engraftment of UCB in adult SAA patients. The RI conditioning regimen was originally developed in our institute for UCBT for various hematologic malignancies.9 Eleven of the 12 patients achieved primary engraftment, which compares favorably with previously reported engraftment rates of UCBT for SAA.11-16 Our RI conditioning regimen would be more potent than the others to overcome immunologic barriers for engraftment. Cell dose has been known to significantly influence the rate of engraftment after UCBT.14 In the present study, although the cell dose was not very large, sufficient engraftment was seen. Any significant relationship between cell dose (total nucleated cell, ≥ 2.5 vs < 2.5 × 107/kg; CD34+, ≥ 0.8 vs < 0.8 × 105/kg) and engraftment kinetics were observed (data not shown). Thus, not just cell dose but other factors, such as the intensity of the conditioning regimen and posttransplantation immunosuppression, may be important to achieve better engraftment after UCBT for SAA patients. Interestingly, all 6 patients who were screened for HLA antibodies before transplantation had HLA antibodies, and the one case who had positive HLA antibodies against an HLA on a transplanted UCB unit was the only one who failed primary engraftment. Recently, Takanashi et al reported that, in large number of UCBT for various hematologic malignancies, the patients with anti-HLA antibodies, when the specificity corresponding to mismatched antigen in UCB graft, showed significantly lower neutrophil or platelet recovery than those with antibody-negative or -positive but not corresponding to UCB graft.17 Although the observations may differ from that of diverse populations and warrants further investigation, if possible, the use of a UCB unit with corresponding HLA antibodies in the recipient should be avoided.
Three-year survival in the studied patients was 83.3%. In addition to high rate of engraftment, the low risk of severe GVHD might contribute to high survival rate with good quality of life, and seems to be one of the important advantages of using a UCB unit for SAA patients. The other advantage of the use of UCB units is rapid availability. In the present study, 2 patients with fulminant type could be rescued by urgent hematopoietic stem cell transplantation using UCB units. More than 90% of recipients can find a suitable UCB unit in Japan; thus, UCB expands the chance to receive transplantation for those who need it urgently.
In conclusion, this retrospective study strongly suggests the feasibility and effectiveness of RI-UCBT for adult SAA patients. RI-UCBT may become a viable therapeutic option for those who lack suitable HLA-matched donors and who fail or relapse after immunosuppressive therapy. Although our results should be interpreted with caution because of the small number of patients and still short follow-up duration, we think that RI-UCBT with the conditioning regimen presented here deserves further evaluation in a prospective trial, hopefully in a multicenter setting.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank data coordinators Kaori Kobayashi, Madoka Narita, Rumiko Tsuchihashi, and Naomi Yamada for their invaluable assistance as well as the physicians, nurses, pharmacists, and support personnel for their care of patients in this study.
This work was supported in part by the Japanese Ministry of Health, Labor, and Welfare (Research Grant for Allergic Disease and Immunology H20-015).
Authorship
Contribution: H.Y. and D.K. performed transplantation, analyzed extracted data, and contributed to writing the paper; A.Y. reviewed histopathologic sections; H.Y. and N.M. performed statistical analysis; N.U., K. Izutsu, and S. Taniguchi reviewed study design and methods; and K. Ishiwata, H.A., S. Takagi, M.T., N.N., Y.A.-M., K.M., A.W., and S.M. performed transplantation and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Naoyuki Uchida, 2-2-2 Toranomon, Minato-ku, Tokyo 105-8470, Japan; e-mail: nuchida@toranomon.gr.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal