Abstract
Allogeneic hematopoietic cell transplantation can be curative in patients with leukemia and lymphoma. However, progressive growth of malignant cells, relapse after transplantation, and graft-versus-host disease (GVHD) remain important problems. The goal of the current murine study was to select a freshly isolated donor T-cell subset for infusion that separates antilymphoma activity from GVHD, and to determine whether the selected subset could effectively prevent or treat progressive growth of a naturally occurring B-cell lymphoma (BCL1) without GVHD after recipients were given T cell–depleted bone marrow transplantations from major histocompatibility complex–mismatched donors. Lethal GVHD was observed when total T cells, naive CD4+ T cells, or naive CD8+ T cells were used. Memory CD4+CD44hi and CD8+CD44hi T cells containing both central and effector memory cells did not induce lethal GVHD, but only memory CD8+ T cells had potent antilymphoma activity and promoted complete chimerism. Infusion of CD8+ memory T cells after transplantation was able to eradicate the BCL1 lymphoma even after progressive growth without inducing severe GVHD. In conclusion, the memory CD8+ T-cell subset separated graft antilymphoma activity from GVHD more effectively than naive T cells, memory CD4+ T cells, or memory total T cells.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) can be curative for patients with lymphoma, especially if the recipient is in complete remission at the time of transplantation.1-4 However, the risk of progressive disease or relapse is considerably greater if the recipient is in partial remission at the time of transplantation or if mixed rather than complete chimerism develops when nonmyeloablative conditioning is used.5-8 The goal of the current study was to identify a freshly isolated subset of donor T cells with potent antilymphoma activity that could promote complete chimerism without inducing severe graft-versus-host disease (GVHD) after allogeneic bone marrow transplantation (BMT) in mice.
Several studies have shown that memory T cells, including memory CD4+, CD8+, and total T cells, induce significantly less GVHD than naive T cells (CD62LhiCD44lo) or combinations of naive and memory T cells.9-15 In some of these studies, the memory cells were freshly isolated from normal mice that had not been primed to alloantigens, and the effector memory CD4+ T cells (CD62L−CD44hi) or effector memory total T cells (CD62L−CD44hi) did not induce GVHD in irradiated major histocompatibility complex (MHC)–mismatched or matched hosts.9-11,13,14 The CD4+ effector memory cells were reported to mediate graft antitumor activity against mouse leukemia cells that were induced by retroviral insertion of the human BCR-ABL fusion cDNA into mouse hematopoietic progenitor cells.11 Because the transduced cells can express xenoantigens, it is not clear whether these CD4+ memory T cells can mediate antitumor activity against naturally occurring mouse tumor cells that ordinarily express weak murine tumor antigens. In studies of freshly isolated memory total T cells from unprimed donors, neither the effector memory cells nor central memory T cells (CD62LhiCD44hi) induced GVHD.13,14 However, the ability of the memory phenotype cells from unprimed allogeneic donors to mediate graft antitumor activity in hosts given syngeneic tumors was not tested.13,14
Similarly, combined CD8+ central and effector memory (CD8+CD44hi) T cells freshly isolated from unprimed donors, or effector memory CD8+ T cells generated after culturing donor CD8+ T cells with host-type dendritic cells, failed to induce GVHD in MHC-matched hosts. Although the dendritic cell–activated CD8+ T cells mediated some antitumor activity in vivo against the naturally occurring EL-4 thymic lymphoma, approximately 90% of tumor-bearing hosts died within 100 days.12 In a more recent study, freshly isolated central memory CD8+ T cells were reported to induce GVHD in both MHC-mismatched and MHC-matched strain combinations with similar severity to that of naive CD8+ T cells.15 In addition, both naive and central memory T cells had similar antitumor activity against retroviral transduced leukemia cells expressing the human BCR-ABL gene construct.15
In the current study, freshly isolated naive CD4+, CD8+, or total T cells, and/or memory CD4+CD44hi, CD8+CD44hi, and total T CD44hi cells from unprimed donors were compared for their capacity to induce GVHD, promote complete chimerism, and mediate antitumor activity against a naturally occurring B-cell lymphoma (BCL1) in an MHC-mismatched model. Only the CD8+CD44hi memory T-cell subset containing both central and effector memory cells was capable of eradicating the lymphoma cells without inducing GVHD. In contrast, CD4+and CD8+ naive T cells, memory CD44hi CD4+ T cells, naive total T cells, and memory CD44hi total T cells either induced lethal GVHD or lacked potent antitumor activity. The tumor-bearing recipients of CD8+CD44hi T cells had a clear survival advantage over those given CD8+ naive T cells because of the lethal GVHD induced by the latter. The CD8+CD44hi T cells were also used in a model of treatment of progressive lymphoma growth after BMT, and were able to promote complete chimerism and eradicate the tumor without GVHD.
Methods
Animals
Wild-type Thy1.2 C57BL/6 (H-2b) male mice (8-12 weeks old) and BALB/c (H-2d) Thy 1.2 male mice (8-12 weeks old) were purchased from the breeding facility of the Department of Comparative Medicine, Stanford University, or from The Jackson Laboratory. The luciferase-expressing (luc+) transgenic B6-L2G85 (H-2b, Thy1.1) mice were used as described previously.16 All mice were housed in a specific pathogen-free facility. Care of all experimental animals was in accordance with institutional and National Institutes of Health guidelines.
Antibodies and flow cytometric analysis
Unconjugated anti-CD16/32 (2.4G2), anti-CD8 phycoerythrin (PE; 53-6.7), anti–T-cell receptorβ (anti-TCRβ) allophycocyanin (APC; H57-597), anti-CD62L fluorescein isothiocyanate (FITC; Mel-14), anti-CD44 PE (IM7), anti–LPAM-1 PE (α4β7 integrin complex; DATK32), anti–H-2Kb FITC (AF6-88.5), anti-CD19 APC (1D3), and anti-B220 Pacific Blue (RA36B2) monoclonal antibodies (mAbs) were purchased from BD Pharmingen. Anti-CD8 Alexa 700 (53-6.7) and anti-Thy1.1 Pacific Blue (OX-7) were obtained from BioLegend. Anti-CCR9 PE (242503) and anti-CXCR3 PE (220803) mAbs were purchased from R&D Systems. Anti-idiotype BCL1 antibody was purified from a hybridoma secreting rat IgG2a. The antibody was conjugated with Alexa Fluor 488 for fluorescence-activated cell sorting (FACS). Staining and flow cytometric analysis and sorting have been described in detail previously.10
Cell preparations
Single-spleen cell suspensions were enriched for CD4+, CD8+, or TCRβ+ total T cells with anti-CD4 and anti-CD8 magnetic Microbeads using the MidiMACS system (Miltenyi Biotec). After staining with anti-CD8 PE, anti-CD4, anti-CD62L FITC, and anti-CD44 APC, cells were sorted into CD62LhiCD44lo naive or total CD44hi (memory) populations using a FACSAria flow cytometer (Becton Dickinson). The sorted naive and memory cells were ≥ 98% pure as judged by reanalysis of sorted cells. Preparation of T cell–depleted (TCD) bone marrow (BM) cells has been reported previously.10 To monitor BCL1 tumor cells and donor chimerism in peripheral blood of transplanted mice, red cells were lysed and enriched white blood cells were used for FACS staining.17
MLR, cytokine assay, and cytotoxicity assay
Sorted naive or memory CD8+ T-cell subsets from C57BL/6 donor mice were used as responders and mixed with irradiated (3000 cGy) allogeneic BALB/c splenocytes as stimulators in the mixed leukocyte reaction (MLR), as described previously.16 3H-thymidine incorporation was measured after 5 days, and cytokine secretion in the supernatants was analyzed in a multiplex assay system with microsphere beads after 60 hours.10 Sorted naive or memory phenotype CD8+ T cells were stimulated in a 1:2 ratio with irradiated (3000 rads) BALB/c splenocytes for 96 hours. Cultured cells were used as effector cells, and mixed with luc+ BCL1 luc/gfp lymphoma target cells.18 Cytolysis was assessed by bioluminescence imaging (BLI), as described previously.19
GVHD model, histopathologic scoring for GVHD severity, and BCL1 tumor model
Acute GVHD was induced as described previously.20 In brief, BALB/c hosts were given 800 cGy of total body irradiation from a 200 Kv X-ray source, and injected with donor cells via the tail vein within 24 hours. Histologic assessment of liver, small bowel, and colonic GVHD was performed in a blinded fashion using the histopathologic scoring system described by Kaplan et al.21 BCL1 is a spontaneously arising B-cell leukemia/lymphoma derived from BALB/c mice with an IgMλ surface Ig phenotype.22 This cell line was maintained by serial passage in BALB/c mice, as described previously.17 The use of BCL1 tumor cells with the luc-gfp gene construct has been reported previously.18
In vivo and ex vivo BLI
In vivo BLI was performed according to the method of Edinger et al.18 Briefly, mice were injected intraperitoneally with luciferin (10 μg/g body weight). Ten minutes later, mice were imaged using an IVIS100 charge-coupled device imaging system (Xenogen) for 5 minutes. Imaging data were analyzed and quantified with Living Image software (Xenogen). Ex vivo BLI was performed according to the method described by Beilhack et al.23
In vivo CFSE proliferation assay
For analysis of cell proliferation, sorted naive or memory CD8+ T cells from Thy1.1 C57BL/6 donors were loaded with a Vybrant CDDA SE carboxyfluorescein diacetate succinimidyl ester (CFSE) tracer kit (Invitrogen), as described previously.23 Thy1.1 CFSE–labeled naive and memory cells (0.5 × 106), along with 2 × 106 TCD BM cells (C57BL/6, Thy1.2) were injected into lethally irradiated BALB/c mice. On day 3+ after BMT, CFSE staining of the infused Thy1.1 cells from the spleen was analyzed by FACS, and comparisons of the number of cell divisions were made using proliferation analysis with FlowJo software Version 9.0.2.
Statistical analysis
Kaplan-Meier survival curves were made using Prism Version 5 software (GraphPad). Statistical differences in animal survival were analyzed with the log-rank test. Differences in mean 3H-thymidine incorporation and cytokine production of replicate in vitro assays were analyzed using the 2-tailed Student t test. The Mann-Whitney U test was used for the comparison of GVHD scores. For all tests, P ≤ .05 was considered significant.
Results
GVHD and antilymphoma activity of donor T-cell subsets
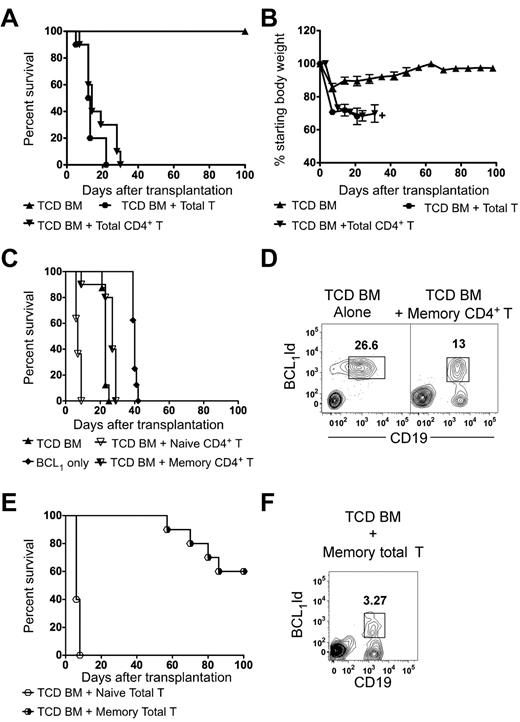
We searched for freshly isolated donor T cells that had graft antilymphoma activity without inducing severe GVHD. Initially, lethally irradiated BALB/c (H-2d) hosts were transplanted with 2 × 106 C57BL/6 (H-2b) donor TCD BM cells along with a constant number (0.5 × 106) of total T cells (naive and memory unseparated) or CD4+ T cells (naive and memory unseparated). Hosts transplanted with TCD BM alone survived more than 100 days and their weights returned to pretransplantation levels (Figure 1A). The CD4+ and total T cells added to TCD BM induced acute GVHD within a week of transplantation, leading to diarrhea, progressive weight loss, and death of all recipients by 3 weeks after transplantation (Figure 1). Body weight and survival were significantly reduced compared with that of recipients given TCD BM alone (P ≤ .0001 and P < .001, respectively; Figure 1A-B).
Survival of BALB/c hosts transplanted with donor C57BL/6 TCD BM cells with or without total T cells or T-cell subsets. (A) Lethally irradiated (800 cGy) hosts were given 2 × 106 TCD BM cells from donors with or without (n = 12) 0.5 × 106 sorted donor total T cells (n = 10) or total CD4+ T cells (n = 10) from the spleen. The data were pooled from 2 independent experiments. (B) Serial measurements of body weights were determined in mice from panel A. +Weight measurements were stopped when no more than 2 mice remained in the group. (C,E) The hosts received 500 BCL1 lymphoma cells 6 hours after irradiation. Lethally irradiated hosts were given 2 × 106 TCD BM cells with or without (n = 8) 0.5 × 106 sorted CD62LhiCD44lo naive (n = 10) or CD44hi memory phenotype CD4+ or total T cells (n = 10). Control untreated hosts were also given tumor cells (n = 8). The data were pooled from 2 independent experiments. (D,F) Representative 2-color flow cytometric analyses of CD19 versus BCL1-idiotype markers in the peripheral blood from recipients with progressive tumor growth in panels C and E, respectively, are shown 28 days after transplantation of TCD BM alone or with memory CD4+ T cells or memory total T cells, respectively.
Survival of BALB/c hosts transplanted with donor C57BL/6 TCD BM cells with or without total T cells or T-cell subsets. (A) Lethally irradiated (800 cGy) hosts were given 2 × 106 TCD BM cells from donors with or without (n = 12) 0.5 × 106 sorted donor total T cells (n = 10) or total CD4+ T cells (n = 10) from the spleen. The data were pooled from 2 independent experiments. (B) Serial measurements of body weights were determined in mice from panel A. +Weight measurements were stopped when no more than 2 mice remained in the group. (C,E) The hosts received 500 BCL1 lymphoma cells 6 hours after irradiation. Lethally irradiated hosts were given 2 × 106 TCD BM cells with or without (n = 8) 0.5 × 106 sorted CD62LhiCD44lo naive (n = 10) or CD44hi memory phenotype CD4+ or total T cells (n = 10). Control untreated hosts were also given tumor cells (n = 8). The data were pooled from 2 independent experiments. (D,F) Representative 2-color flow cytometric analyses of CD19 versus BCL1-idiotype markers in the peripheral blood from recipients with progressive tumor growth in panels C and E, respectively, are shown 28 days after transplantation of TCD BM alone or with memory CD4+ T cells or memory total T cells, respectively.
Several previous studies, including ours, have shown that CD44hi memory CD4+ T cells do not induce lethal GVHD.10 We determined the survival and appearance of tumor cells in the blood of BALB/c transplant recipients given C57BL/6 CD44hi memory CD4+ T cells, TCD BM, and BCL1 lymphoma cells. Other groups received naive CD4+ T cells, naive total T cells, or memory total T cells instead of memory CD4+ T cells at the same dose (Figure 1C-F). All animals that received TCD BM alone died, and had developed lymphoma cells in the blood by day 28 (Figure 1D). There were no signs of GVHD. The animals that received naive CD4+ T cells or naive total T cells along with TCD BM succumbed to lethal GVHD, with severe diarrhea and weight loss within 10 days of transplantation (Figure 1C-E). Although the recipients of memory CD4+ T cells and TCD BM did not show signs of GVHD, they were unable to control the lymphoma growth and all died within 30 days of transplantation with BCL1-idiotype–positive cells in the peripheral blood (Figure 1D). Interestingly, 40% of the hosts that received memory total T cells died with lymphoma cells in the blood (Figure 1F). The remaining 60% of the hosts did not develop lymphoma cells in the blood and survived more than 100 days without clinical signs of GVHD (Figure 1E).
CD8+ naive and memory T cells from unimmunized donors are alloreactive
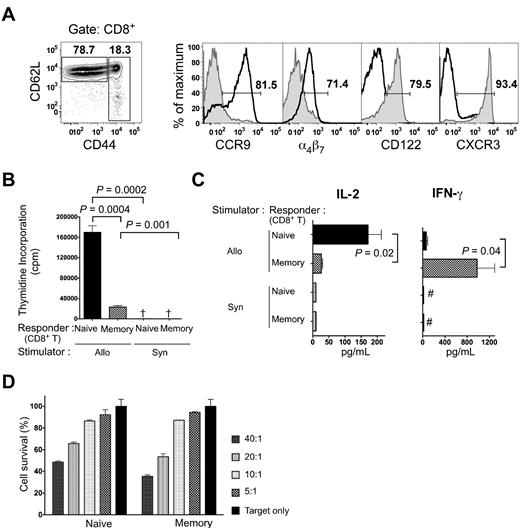
We went on to study separated naive and memory CD8+ T cells for alloreactivity in vitro and for GVHD and GVT activity. Initially, we analyzed the gated naive CD8+ T cells and memory CD44hi CD8+ T cells from untreated C57BL/6 mice for expression of CCR9, α4β7, CXCR3, and CD122. Naive CD8+ T cells showed intense staining for CCR9 and dull staining for α4β7, whereas memory CD8+ T cells showed negative staining for both (Figure 2A). The memory phenotype cells displayed higher levels of CXCR3 and CD122 than naive cells (Figure 2A), confirming that these are memory markers, as has been described previously.24,25
Characterization of naive and memory phenotype CD8+ T cells. (A) C57BL/6 splenocytes were stained with anti-CD8, anti-CD44, and anti-CD62L mAbs. Gated CD62LhiCD44low and CD44hi CD8+ T cells were analyzed for CCR9, α4β7, CD122, and CXCR3 expression. Gates were determined by isotype staining. Histogram plots of the respective markers are overlaid for CD44lo CD8+ T cells (bold line) and CD44hi CD8+ T cells (tinted area). (B) 3H-thymidine incorporation (mean ± SE) of C57BL/6 responder naive or memory phenotype CD8+ T cells (2 × 105) to BALB/c stimulator cells (8 × 105) at day 5 in the MLR in 3 replicate wells for each cell combination. Results are representative of at least 3 MLR experiments. Allo and Syn, BALB/c and C57BL/6 stimulator cells, respectively. †3H-thymidine incorporation < 5000 cpm. (C) Cytokine responses of C57BL/6 donor naive or memory phenotype CD8+ T cells (1 × 105) to irradiated BALB/c stimulator cells (5 × 105) in the MLR are shown at 60 hours. The left panel shows the mean ± SEM concentrations of IL-2 and the right panel shows the mean ± SEM concentrations of IFN-γ. #Cytokine concentration < 10 pg/mL. Results are representative of at least 3 MLR cultures. (D) Sorted naive and memory cells were used in a cytotoxicity assay against luc+ BCL1 cells. Sorted naive or memory phenotype CD8+ T cells were stimulated with irradiated BALB/c splenocytes for 96 hours. BCL1 lymphoma cells expressing luciferase were mixed with stimulated naive or memory phenotype cells at various effector-to-target ratios. Luciferase signal was measured after 16 hours. Percent cytotoxicity was then determined compared with the same target numbers without effector cells at each time point.
Characterization of naive and memory phenotype CD8+ T cells. (A) C57BL/6 splenocytes were stained with anti-CD8, anti-CD44, and anti-CD62L mAbs. Gated CD62LhiCD44low and CD44hi CD8+ T cells were analyzed for CCR9, α4β7, CD122, and CXCR3 expression. Gates were determined by isotype staining. Histogram plots of the respective markers are overlaid for CD44lo CD8+ T cells (bold line) and CD44hi CD8+ T cells (tinted area). (B) 3H-thymidine incorporation (mean ± SE) of C57BL/6 responder naive or memory phenotype CD8+ T cells (2 × 105) to BALB/c stimulator cells (8 × 105) at day 5 in the MLR in 3 replicate wells for each cell combination. Results are representative of at least 3 MLR experiments. Allo and Syn, BALB/c and C57BL/6 stimulator cells, respectively. †3H-thymidine incorporation < 5000 cpm. (C) Cytokine responses of C57BL/6 donor naive or memory phenotype CD8+ T cells (1 × 105) to irradiated BALB/c stimulator cells (5 × 105) in the MLR are shown at 60 hours. The left panel shows the mean ± SEM concentrations of IL-2 and the right panel shows the mean ± SEM concentrations of IFN-γ. #Cytokine concentration < 10 pg/mL. Results are representative of at least 3 MLR cultures. (D) Sorted naive and memory cells were used in a cytotoxicity assay against luc+ BCL1 cells. Sorted naive or memory phenotype CD8+ T cells were stimulated with irradiated BALB/c splenocytes for 96 hours. BCL1 lymphoma cells expressing luciferase were mixed with stimulated naive or memory phenotype cells at various effector-to-target ratios. Luciferase signal was measured after 16 hours. Percent cytotoxicity was then determined compared with the same target numbers without effector cells at each time point.
To assess the alloreactivity of the cell subsets, sorted naive or memory C57BL/6 CD8+ T cells were incubated with irradiated BALB/c spleen cells as allogeneic stimulators. A constant number of sorted responder cells were incubated with a constant number of stimulator cells. The proliferation was measured after 5 days in culture, and interleukin-2 (IL-2) and interferon-γ (IFN-γ) cytokine secretion was measured in the supernatants after 60 hours in culture. Our previous studies using this culture system showed that naive CD4+ T cells were alloreactive but that memory CD4+ T cells were not, as judged by proliferation and cytokine secretion that were no greater than background.10 The naive CD8+ T cells incorporated 7-fold more 3H-thymidine than the memory CD8+ T cells (P = 0.0004; Figure 2B). Both subsets incorporated significantly more 3H-thymidine with allogeneic stimulators compared with syngeneic. Figure 2C shows that sorted naive CD8+ T cells secreted mean concentrations of IL-2 that were 150-200 pg/mL after culture with allogeneic stimulators, and differences between the allogeneic and syngeneic cultures were significant (P < .01). In contrast, the mean concentration of IL-2 secreted by sorted memory CD8+ T cells was below 50 pg/mL in both allogeneic and syngeneic cultures (P = .02 memory vs naive for allogeneic; Figure 2C). Figure 2C shows that allogeneic cultures with sorted memory CD8+ T cells secreted IFN-γ (mean ∼1000 pg/mL) that was significantly increased compared with allogeneic cultures with naive CD8+ T cells (P = .04) or syngeneic cultures (P < .005). All cultures were assayed for IL-4, IL-10, and tumor necrosis factor-α in the supernatants; however, these cytokines were not detected in multiplex cytokine assays (data not shown).
To investigate the cell-mediated cytotoxicity of the memory and naive phenotype CD8+ T cells, we used BCL1 lymphoma target cells transduced with the luciferase gene construct. The naive and memory CD8+ T cells were activated in allogeneic cultures with irradiated BALB/c stimulator cells for 96 hours, and these activated cells were used as effector cells in different ratios against BCL1 target cells. The enzymatic activity of luciferase was used as a measure of BCL1 cell survival. The percentage of BCL1 cell cytolysis was similar with naive and memory CD8+ T cells in all cell ratios tested (Figure 2D). Therefore, the subsets displayed a similar ability to kill tumor cells.
CD8+ naive but not memory T cells induce lethal GVHD
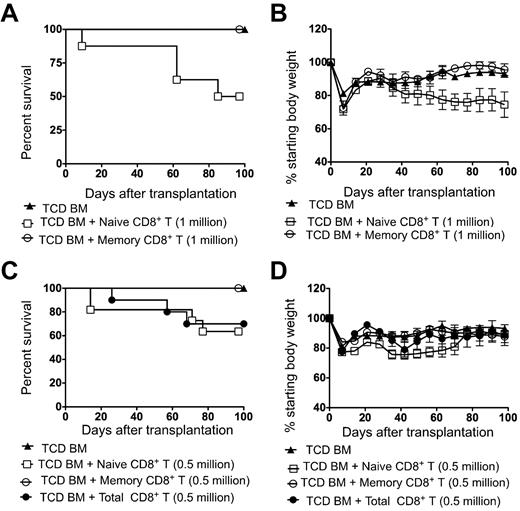
In further experiments, irradiated BALB/c hosts received either 1.0 × 106 or 0.5 × 106 doses of naive or memory C57BL/6 donor CD8+ T cells and/or 2 × 106 TCD BM cells. Figure 3A through C show that all irradiated recipients that received only TCD BM cells survived for 100 days. Although there was a transient weight loss during the first week after irradiation, there was a recovery to baseline during the third week and stabilization thereafter (Figure 3B). These mice did not show typical clinical features of GVHD such as diarrhea, hunched back, ruffled fur, hair loss, and facial swelling. In contrast, approximately 50% of the hosts that received 1 × 106 naive CD8+ T cells developed diarrhea around day 40, and showed progressive weight loss until death between 60 and 100 days after transplantation (Figure 3A-B). In contrast, all recipients survived in the memory CD8+ T-cell group (Figure 3A). There were significant differences in survival (P < .05) and weight loss (P < .01) between the naive and memory CD8+ T-cell groups. When the hosts received a lower cell dose of 0.5 × 106 naive CD8+ T cells, approximately 40% succumbed with clinical features of GVHD (Figure 3C). The survival of recipients given 0.5 × 106 total CD8+ T cells was similar to that of recipients given the same dose of naive CD8+ T cells. Both the total CD8+ T cells and naive CD8+ T cells significantly reduced survival compared with that of memory CD8+ T cells (P = .04; Figure 3C). Autopsies showed histopathologic evidence of GVHD (data not shown). All of the recipients of memory phenotype CD8+ T cells at this cell dose survived more than 100 days and showed similar weight loss as the TCD BM group (Figure 3D). The difference between the survival and weight loss in naive and memory group was significant (P < .05; Figure 3D).
Survival and weight changes of BALB/c recipients transplanted with donor C57BL/6 TCD BM cells with or without sorted, total, naive, or memory phenotype CD8+ T cells. (A,C) Survival of lethally irradiated BALB/c hosts given 2 × 106 TCD BM cells from donors with or without 1.0 × 106 naive T cells, 1.0 × 106 memory T cells, 0.5 × 106 naive T cells, 0.5 × 106 memory T cells, or 0.5 × 106 total CD8+ donor T cells (n = 8-11) is shown. The data were pooled from 2 independent experiments. (B,D) Percentage of starting body weight of host mice given TCD BM with or without sorted naive, memory, or total CD8+ T cells as in panels A and C. Brackets show SEM.
Survival and weight changes of BALB/c recipients transplanted with donor C57BL/6 TCD BM cells with or without sorted, total, naive, or memory phenotype CD8+ T cells. (A,C) Survival of lethally irradiated BALB/c hosts given 2 × 106 TCD BM cells from donors with or without 1.0 × 106 naive T cells, 1.0 × 106 memory T cells, 0.5 × 106 naive T cells, 0.5 × 106 memory T cells, or 0.5 × 106 total CD8+ donor T cells (n = 8-11) is shown. The data were pooled from 2 independent experiments. (B,D) Percentage of starting body weight of host mice given TCD BM with or without sorted naive, memory, or total CD8+ T cells as in panels A and C. Brackets show SEM.
CD8+ memory T cells mediate antilymphoma activity with minimal GVHD
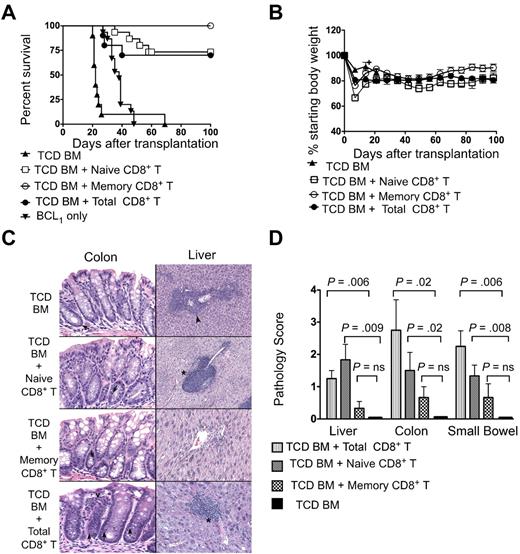
We assessed the antitumor activity of donor cells in lethally irradiated BALB/c mice that were given 500 BCL1 lymphoma cells (nontransduced) followed by 2 × 106 TCD BM with or without 0.5 × 106 total CD8+ T cells or sorted naive or memory CD8+ T cells. Most of the mice that received only TCD BM died by day 30 after transplantation (Figure 4A), and this was associated with the presence of BCL1-idiotype–positive tumor cells in the blood (Figure 5A). Survival of hosts was significantly improved in the groups that received total CD8+ T cells (P < .0001), naive CD8+ T cells (P < .0001), or memory CD8+ T cells (P < .0001) compared with the hosts that received only TCD BM (Figure 4A). Approximately 25% of the hosts died by day 60 in the total CD8+ T-cell and naive CD8+ T-cell groups; none of the hosts in this group had detectable tumor cells in the blood (Figures 4A and 5A). All of the mice that received memory CD8+ T cells survived more than 100 days without any tumor cells in the blood (Figures 4A and 5A). The survival was significantly decreased between groups given total CD8+ T cells or naive CD8+ T cells compared with the group given memory CD8+ T cells (P = .03 and P = .04, respectively). Weight loss was also significantly different (P = .03 and P = .004, respectively). All untreated mice that received BCL1 tumor cells died by day 50.
Survival, weight changes, and organ pathology scores of BALB/c hosts that received 500 BCL1 lymphoma cells followed by transplantation of TCD BM from C57BL/6 donors with or without sorted naive, memory, or total CD8+ T cells. The hosts received 500 BCL1 lymphoma cells 6 hours after irradiation. (A) Survival of irradiated hosts given 2 × 106 TCD BM cells from C57BL/6 donors with or without (n = 10) 0.5 × 106 sorted naive T cells (n = 14), memory T cells (n = 12), or total CD8+ T cells (n = 10). The data were pooled from 2–4 independent experiments. (B) Percentage of starting body weight of host mice given TCD BM with or without sorted naive, memory, or total CD8+ T cells as in panel A. Brackets show SEs of the mean. (C) Histopathologic changes induced with naive, memory phenotype, or total CD8+ T cells and TCD BM only. Representative tissue sections were obtained from the hosts from panel A. Histopathologic specimens from the liver and large intestines of surviving hosts were obtained at 100 days after transplantation and fixed in formalin before embedding into paraffin blocks. Tissue sections of 4- to 5-mm thickness were stained with hematoxylin and eosin. Microscopic images were obtained using an Eclipse E1000M microscope (Nikon) with a SPOT RT digital camera and acquisition software (SPOT advanced 4.6, Diagnostic Instruments) with a final magnification of 300× for all images. Image processing was performed with Adobe Photoshop CS with standard adjustments of brightness, contrast, and color balance to the entire image. Histopathology at day 21 in the TCD BM group is shown (top photos). Except for rare apoptosis (arrow left photo), there is no evidence of GVHD in the colon, but lymphoma is evident (arrowhead right photo) in the liver. In the naive group (second row photos), there was no evidence of GVHD in the colon. Liver portal tracts have prominent lymphocytic infiltrates compatible with grade II GVHD (* right photo). In the memory group (third row of photos), there is no evidence of GVHD or lymphoma in either colon or liver. In TCD BM with total CD8+ T-cell group, increased apoptosis was seen in colonic crypts (arrow), along with increased lamina propria inflammation (open arrowhead left photo fourth row). Similarly, portal inflammation and bile duct injury (*) was seen in liver and was compatible with GVHD. Tissue sections were stained with hematoxylin and eosin. Each photo is representative of 5-10 hosts examined. (D) Mean (± SE) of histopathologic GVHD scores of liver, small bowel, and colon on day 21 or 100 from the 4 groups (n = 6 per group). NS indicates not significant; P > .05.
Survival, weight changes, and organ pathology scores of BALB/c hosts that received 500 BCL1 lymphoma cells followed by transplantation of TCD BM from C57BL/6 donors with or without sorted naive, memory, or total CD8+ T cells. The hosts received 500 BCL1 lymphoma cells 6 hours after irradiation. (A) Survival of irradiated hosts given 2 × 106 TCD BM cells from C57BL/6 donors with or without (n = 10) 0.5 × 106 sorted naive T cells (n = 14), memory T cells (n = 12), or total CD8+ T cells (n = 10). The data were pooled from 2–4 independent experiments. (B) Percentage of starting body weight of host mice given TCD BM with or without sorted naive, memory, or total CD8+ T cells as in panel A. Brackets show SEs of the mean. (C) Histopathologic changes induced with naive, memory phenotype, or total CD8+ T cells and TCD BM only. Representative tissue sections were obtained from the hosts from panel A. Histopathologic specimens from the liver and large intestines of surviving hosts were obtained at 100 days after transplantation and fixed in formalin before embedding into paraffin blocks. Tissue sections of 4- to 5-mm thickness were stained with hematoxylin and eosin. Microscopic images were obtained using an Eclipse E1000M microscope (Nikon) with a SPOT RT digital camera and acquisition software (SPOT advanced 4.6, Diagnostic Instruments) with a final magnification of 300× for all images. Image processing was performed with Adobe Photoshop CS with standard adjustments of brightness, contrast, and color balance to the entire image. Histopathology at day 21 in the TCD BM group is shown (top photos). Except for rare apoptosis (arrow left photo), there is no evidence of GVHD in the colon, but lymphoma is evident (arrowhead right photo) in the liver. In the naive group (second row photos), there was no evidence of GVHD in the colon. Liver portal tracts have prominent lymphocytic infiltrates compatible with grade II GVHD (* right photo). In the memory group (third row of photos), there is no evidence of GVHD or lymphoma in either colon or liver. In TCD BM with total CD8+ T-cell group, increased apoptosis was seen in colonic crypts (arrow), along with increased lamina propria inflammation (open arrowhead left photo fourth row). Similarly, portal inflammation and bile duct injury (*) was seen in liver and was compatible with GVHD. Tissue sections were stained with hematoxylin and eosin. Each photo is representative of 5-10 hosts examined. (D) Mean (± SE) of histopathologic GVHD scores of liver, small bowel, and colon on day 21 or 100 from the 4 groups (n = 6 per group). NS indicates not significant; P > .05.
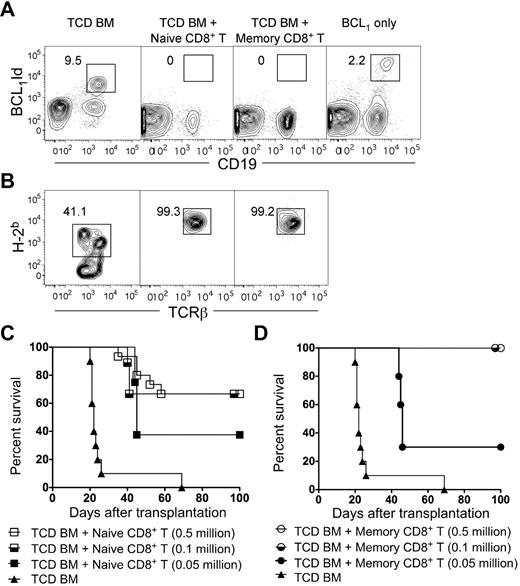
Survival, chimerism, and elimination of BCL1 tumor cells after transplantation of TCD BM with or without sorted naive or memory CD8+ T cells. (A) Representative 2-color flow cytometric analysis of CD19 versus BCL1-idiotype markers in the peripheral blood from recipients 28 days after total body irradiation (800 cGy), BCL1 cells, and a transplantation of 2 × 106 TCD BM cells with or without 0.5 million naive or memory phenotype CD8+ T cells. Untreated control recipients were given BCL1 only. The boxes enclose BCL1-idiotype–positive CD19+ cells, and percentages of cells in boxes are shown. (B) Representative flow cytometric analysis of peripheral blood at day 28 showing percentage of donor (H-2Kb+) cells among gated TCRβ+ cells. (C–D) Survival of lethally irradiated BALB/c hosts given 2 × 106 TCD BM cells from C57BL/6 donors with or without (n = 8) 0. 5 × 106, 0.1 × 106, or 0.05 × 106 sorted naive (n = 15; n = 9; n = 8; C) or memory phenotype phenotype (n = 12; n = 9; n = 8; D) CD8+ T cells. The data were pooled from 2 independent experiments.
Survival, chimerism, and elimination of BCL1 tumor cells after transplantation of TCD BM with or without sorted naive or memory CD8+ T cells. (A) Representative 2-color flow cytometric analysis of CD19 versus BCL1-idiotype markers in the peripheral blood from recipients 28 days after total body irradiation (800 cGy), BCL1 cells, and a transplantation of 2 × 106 TCD BM cells with or without 0.5 million naive or memory phenotype CD8+ T cells. Untreated control recipients were given BCL1 only. The boxes enclose BCL1-idiotype–positive CD19+ cells, and percentages of cells in boxes are shown. (B) Representative flow cytometric analysis of peripheral blood at day 28 showing percentage of donor (H-2Kb+) cells among gated TCRβ+ cells. (C–D) Survival of lethally irradiated BALB/c hosts given 2 × 106 TCD BM cells from C57BL/6 donors with or without (n = 8) 0. 5 × 106, 0.1 × 106, or 0.05 × 106 sorted naive (n = 15; n = 9; n = 8; C) or memory phenotype phenotype (n = 12; n = 9; n = 8; D) CD8+ T cells. The data were pooled from 2 independent experiments.
Figure 4C shows representative tissue sections of the colon and the liver of the BMT recipients. Whereas the colons of recipients given TCD BM with or without memory CD8+ T cells showed preservation of crypts and goblet cells with minimal lymphocytic infiltration, the recipients given naive CD8+ T cells or total CD8+ T cells showed dropout of crypts, loss of goblet cells, and considerable infiltrates. The livers of recipients given TCD BM showed tumor cells surrounding blood vessels, whereas recipients of naive or total CD8+ T cells showed periportal lymphocytic infiltration; recipients of memory CD8 + T cells showed neither of these abnormalities. Figure 4D shows the histopathology scores for GVHD lesions in liver, colon, and small intestine. Whereas there were no significant differences between the scores in recipients given TCD BM with or without memory CD8+ T cells (P > .05), there was a significantly increased score in the groups given TCD BM with naive CD8+ T cells or total CD8+ T cells versus the group with TCD BM alone (P = .02-.008). The GVHD score in the liver was significantly higher (P = .04) in the naive versus the memory group.
We assessed donor-cell chimerism among T cells in the peripheral blood of the 3 groups of recipients at day 28 (Figure 5B). All TCD BM-transplanted hosts were mixed chimeras as shown by representative staining with donor-type (H-2Kb+) cells among TCRβ+ T cells (Figure 5B). All recipients given 0.5 × 106 naive or memory CD8+ T cells had more than 99% donor-type cells and maintained stable donor chimerism through day 100 (data not shown). Thus, the addition of either naive or memory CD8+ T cells resulted in a change from mixed to complete chimerism. Whereas the mixed chimeras developed tumor cells in the blood, the complete chimeras did not (Figure 5A).
We determined the survival of hosts given lower doses of naive or memory CD8+ T cells along with TCD BM and BCL1 cells. Approximately 70% of the hosts that received 0.5 × 106 or 0.1 × 106 naive CD8+ T cells survived more than 100 days (Figure 5C) and were free of the BCL1 lymphoma in blood (data not shown). When the cell dose was 0.05 × 106, approximately 30% of the hosts survived more than 100 days without tumor cells, and approximately 70% died with tumor cells in the blood. In contrast, 100% of the hosts given 0.5 × 106 or 0.1 × 106 memory CD8+ T cells were able to survive more than 100 days without any evidence of lymphoma in the blood, but 80% died with lymphoma when the dose was reduced to 0.05 × 106 (Figure 5D). The survival of the group given 0.1 × 106 memory CD8+ T cells was significantly improved (P < .05) compared with the group given 0.5 × 106 total memory T cells (Figure 1E).
Rapid accumulation of CD8+ naive but not memory T cells in the target organs of GVHD
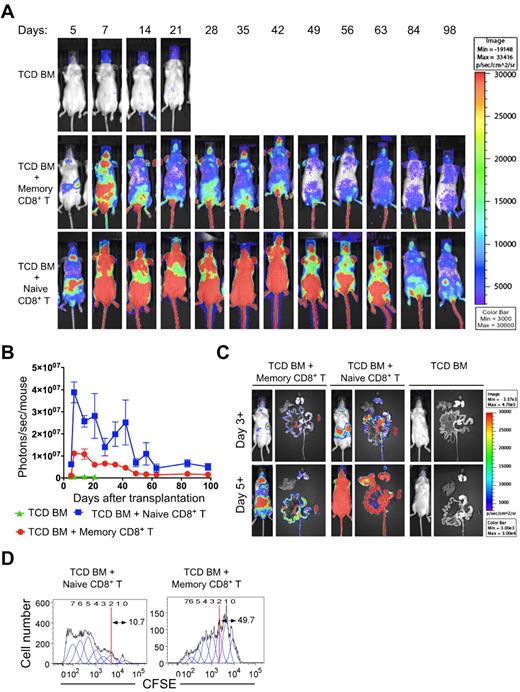
To account for differences in GVHD severity between naive and memory CD8+ T cells, we investigated whether there were any differences in the extent and rapidity of accumulation of these cells in the lymphoid tissues and in target organs of GVHD. The trafficking and survival of naive and memory CD8+ T cells were evaluated by transplantation of 0.5 × 106 naive or memory CD8+ T cells from C57BL/6-L2G85 luc+ mice, along with 2 × 106 TCD BM from wild-type C57BL/6 donors into irradiated BALB/c recipients that received 500 BCL1 tumor cells. Naive CD8+ T cells homed to the spleen and cervical lymph nodes by day 3, and by day 5 intense signals were observed in the gastrointestinal tract and skin (Figure 6A). The signals were much lower in these organs in mice that received memory CD8+ T cells (Figure 6A). TCD BM controls had no signal, and these mice died by day 28 because of lymphoma, as shown previously. The signals from naive CD8+ T cells continued to persist in the gastrointestinal area over the entire observation period of 84 days, whereas the signals were lower from memory CD8+ T cells during this period. Quantification of the photon emission by BLI demonstrated that the signals of naive CD8+ T cells increased rapidly up to day 7 and then declined to approach background by day 70 (Figure 6B). The signal intensity continued to be higher than the memory CD8+ T cells until day 60 (P = .002; Figure 5B). We performed ex vivo BLI on freshly prepared organs such as the liver, spleen, and gastrointestinal tract to analyze the tissue distribution of the signals at days 3+ and 5+ after BMT. Ex vivo images revealed that naive CD8+ T cells homed to secondary lymphoid tissues, including the spleen, mesenteric lymph nodes, and Peyer patches, by day 3+, followed by infiltration of the gastrointestinal tract by day 5+ (Figure 6C). The memory CD8+ T cells displayed a similar pattern; however, the signal from the small and large intestines in recipients of memory CD8+ T cells was much weaker and slower than that in recipients of naive CD8+ T cells (Figure 6C day 5).
Comparison of trafficking and proliferation of luciferase transgenic naive and memory CD8+ T cells after transplantation with nontransgenic TCD BM. BALB/c hosts were lethally irradiated, received 500 BCL1 lymphoma cells, and then were injected with 2 × 106 C57BL/6 (H-2b) wild-type TCD BM cells with 0.5 × 106 naive or memory phenotype CD8+ T cells from B6-L2G85 (H-2b)luc+ mice. (A) BLI images at serial time points after transplantation. (B) Quantitative analysis of photon emission of BLI over time. Recipients in the TCD BM group died by day 28. (C) In vivo imaging of mice and ex vivo imaging of intestinal tract (middle position), liver (top left position), spleen (bottom right position), and lungs (top right position) at day 3+ and day 5+ after transplantation. (D) Lethally irradiated BALB/c recipient mice were injected with 2 × 106 C57BL/6 (H-2b, Thy1.2) TCD BM cells with either CFSE-labeled 0.5 × 106 congenic C57BL/6 (H-2b, Thy1.1) sorted naive or memory phenotype CD8+ T cells. On day 3+, the staining intensity of CFSE from naive and memory phenotype cell Thy1.1+ in the spleen was analyzed.
Comparison of trafficking and proliferation of luciferase transgenic naive and memory CD8+ T cells after transplantation with nontransgenic TCD BM. BALB/c hosts were lethally irradiated, received 500 BCL1 lymphoma cells, and then were injected with 2 × 106 C57BL/6 (H-2b) wild-type TCD BM cells with 0.5 × 106 naive or memory phenotype CD8+ T cells from B6-L2G85 (H-2b)luc+ mice. (A) BLI images at serial time points after transplantation. (B) Quantitative analysis of photon emission of BLI over time. Recipients in the TCD BM group died by day 28. (C) In vivo imaging of mice and ex vivo imaging of intestinal tract (middle position), liver (top left position), spleen (bottom right position), and lungs (top right position) at day 3+ and day 5+ after transplantation. (D) Lethally irradiated BALB/c recipient mice were injected with 2 × 106 C57BL/6 (H-2b, Thy1.2) TCD BM cells with either CFSE-labeled 0.5 × 106 congenic C57BL/6 (H-2b, Thy1.1) sorted naive or memory phenotype CD8+ T cells. On day 3+, the staining intensity of CFSE from naive and memory phenotype cell Thy1.1+ in the spleen was analyzed.
Increased cell division of CD8+ naive versus memory T cells in the spleen
The observations from BLI studies prompted us to investigate differences in the proliferation of naive and memory CD8+ T cells after BMT. To evaluate the proliferation of these 2 cell populations after transplantation, we injected 0.5 × 106 CFSE-labeled sorted naive or memory CD8+ T cells from C57BL/6-L2G85 luc+ Thy1.1 mice, along with wild-type C57BL/6 Thy1.2 TCD BM into irradiated BALB/c mice. We analyzed the rate of cellular division of naive and memory CD8+ T cells in the host spleen on day 3+ after BMT by the change in the intensity of staining for CFSE (Figure 6D). A representative proliferation assay showed that approximately 10% of the naive CD8+ T cells and approximately 5-fold more memory CD8+ T cells had undergone 2 or fewer cell divisions, as determined by proliferation analysis using FlowJo software. These results are consistent with the MLR data (Figure 2B) showing 7-fold less 3H-thymidine incorporation of memory versus naive CD8+ T cells after alloantigenic stimulation.
CD8+ memory T cells are an effective treatment for progressive lymphoma after BMT
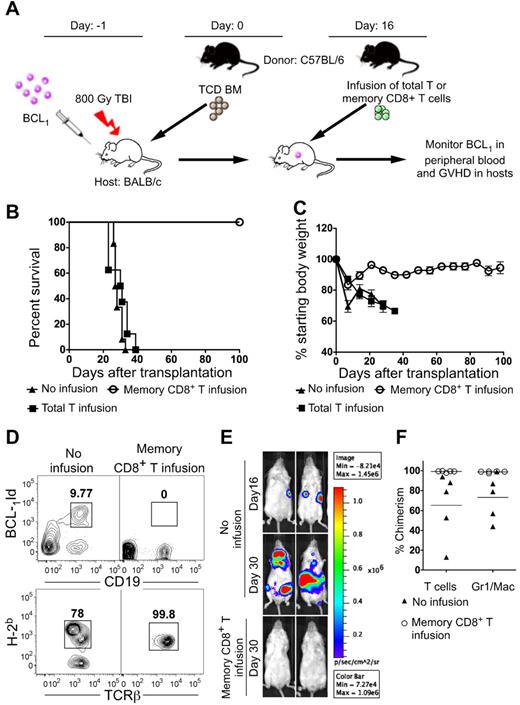
We investigated whether memory CD8+ T cells given as an infusion on day 16 could eradicate BCL1 tumor cells injected 1 day before the transplantation of donor TCD BM cells on day 0 (Figure 7A). Day 16 was chosen for infusion because in preliminary experiments, luc+ tumor cells were already expanding in lymphoid tissues of the transplantation recipients (Figure 7E) at that time, but had not yet been detected in the blood (data not shown). In subsequent experiments, the hosts were serially monitored for the nontransduced BCL1 tumors in the blood and signs of GVHD. All hosts that did not receive the day 16 infusion died by day 35 with tumor cells in the blood (Figure 7B-D). All hosts that received the infusion containing 0.5 × 106 memory CD8+ T cells survived more than 100 days (P < .001) and tumor cells did not appear in the blood (Figure 7B-D). The surviving mice in the infusion-treated group gained at least 90% of their starting body weight at the end of 100 days, and showed no clinical signs of GVHD (Figure 7C). When an infusion containing an equal number of total T cells was given instead of CD8+ memory T cells, then all of the hosts died by day 40 with clinical signs of GVHD. Weight loss was significantly more than in the CD8+ memory T-cell group (P = .004; Figure 7B-C). The group given the CD8+ memory T cells showed > 99% donor T-cell chimerism in all hosts, whereas the control group without infusion therapy showed mixed-donor T-cell chimerism (Figure 7D). Figure 7F shows that 5 of 5 recipients given infusion therapy were complete chimeras in the T-cell lineage and granulocyte/macrophage lineage, whereas recipients without the infusion were all mixed chimeras in these lineages. The yield of B cells was too low for accurate determination of B220 + cells. The group given the total T-cell infusions that developed lethal GVHD also had > 99% T-cell chimerism and BCL1 tumor cells did not appear in the blood (data not shown).
Survival, weight changes, chimerism, and blood-borne BCL1 cells in recipients with progressive tumor growth treated with infusion of total T cells or memory CD8+ T cells. (A) Experimental scheme. Lethally irradiated BALB/c recipient mice were injected with 100 BCL1 cells 6 hours after irradiation. The next day (day 0), they received 2 × 106 C57BL/6 TCD BM cells (n = 10). On day 16 after BMT, some mice received an intravenous infusion of 0.5 × 106 sorted memory phenotype CD8+ T cells (n = 8) or total T cells (n = 8). (B) Survival of recipients with or without infusion. (C) Percentage of starting body weight of host mice given TCD BM with or without infusion as in panel A. (D) Top panels shows 2-color flow cytometric analysis of CD19 versus BCL1-idiotype markers in the peripheral blood from recipients at day 28 after transplantation of 2 × 106 TCD BM cells with (right panel) or without (left panel) infusion. The boxes enclose BCL1-idiotype–positive CD19+ cells. Bottom panels show representative flow cytometric analysis of the peripheral blood at day 28 stained for donor (H-2Kb+) cells versus TCRβ+ cells among gated TCRβ+ cells. (E) Representative examples of BLI of lymphoma growth in mice with or without infusion. After total body irradiation, 100 BCL1-luc+–transduced lymphoma cells were injected into BALB/c hosts, followed by injection of 2 × 106 TCD-BM on the next day (day 0). Sixteen days after BMT, experimental mice received an infusion of 0.5 × 106 memory phenotype CD8+ T cells, and controls received no infusion. Imaging was performed days 16 and 30 after BMT. Two representative mice from each group of 5 mice are shown. (F) Percentage of donor-type cells among TCRβ+ and Mac1/Gr1+ cells in the blood and BMT recipients with and without memory-cell infusion.
Survival, weight changes, chimerism, and blood-borne BCL1 cells in recipients with progressive tumor growth treated with infusion of total T cells or memory CD8+ T cells. (A) Experimental scheme. Lethally irradiated BALB/c recipient mice were injected with 100 BCL1 cells 6 hours after irradiation. The next day (day 0), they received 2 × 106 C57BL/6 TCD BM cells (n = 10). On day 16 after BMT, some mice received an intravenous infusion of 0.5 × 106 sorted memory phenotype CD8+ T cells (n = 8) or total T cells (n = 8). (B) Survival of recipients with or without infusion. (C) Percentage of starting body weight of host mice given TCD BM with or without infusion as in panel A. (D) Top panels shows 2-color flow cytometric analysis of CD19 versus BCL1-idiotype markers in the peripheral blood from recipients at day 28 after transplantation of 2 × 106 TCD BM cells with (right panel) or without (left panel) infusion. The boxes enclose BCL1-idiotype–positive CD19+ cells. Bottom panels show representative flow cytometric analysis of the peripheral blood at day 28 stained for donor (H-2Kb+) cells versus TCRβ+ cells among gated TCRβ+ cells. (E) Representative examples of BLI of lymphoma growth in mice with or without infusion. After total body irradiation, 100 BCL1-luc+–transduced lymphoma cells were injected into BALB/c hosts, followed by injection of 2 × 106 TCD-BM on the next day (day 0). Sixteen days after BMT, experimental mice received an infusion of 0.5 × 106 memory phenotype CD8+ T cells, and controls received no infusion. Imaging was performed days 16 and 30 after BMT. Two representative mice from each group of 5 mice are shown. (F) Percentage of donor-type cells among TCRβ+ and Mac1/Gr1+ cells in the blood and BMT recipients with and without memory-cell infusion.
BCL1 lymphoma progression was also assessed by BLI in an additional group of recipients given BCL1-luc+ cells. Figure 7E shows the lymphoma growth of luc+ BCL1 cells in hosts after TCD BM transplantation with or without memory CD8+ T-cell infusion therapy. There was easily detectable lymphoma accumulation in hosts without infusion at day 16, and the tumor progressed at day 30 with increased intensity and extension to additional tissues. In contrast, there was no detectable BLI signal on day 30 in mice that were infused with memory CD8+ T cells.
Discussion
Previous murine studies showed that memory T cells induce considerably less severe GVHD than naive T cells.9-15 Accordingly, we compared donor total T cells, CD4+ T cells, and CD8+ T cells that had not been separated into purified naive and memory subsets for the induction of lethal GVHD. We also assayed the subsets for anti-BCL1 lymphoma activity in the same MHC-mismatched transplantation model (C57BL/6 → BALB/c) that is most relevant to haplotype-mismatched transplantation. Total T cells, naive total T cells, CD4+ T cells, and CD8+ T cells induced a significant increase in lethal GVHD compared with TCD BM alone. Memory total T cells lacked potent antitumor activity compared with memory CD8+ T cells, because 5-fold fewer memory CD8+ T cells induced complete tumor remissions in a higher percentage of recipients than memory total T cells. Accordingly, memory CD8+ T cells became the focus of the study.
In the C57BL/6 → BALB/c strain combination, CD4+ total and naive T cells induced considerably more severe GVHD than CD8+ total and CD8+ naive T cells. Whereas 0.5 × 106 CD4+ total T cells induced uniformly lethal GVHD by approximately 30 days, an equal number of CD8+ total T cells induced death in approximately 40% of the recipients by 75 days. We did not study the graft antilymphoma activity of the CD4+ total or naive T cells, because death due to GVHD was considerably more rapid than the appearance of tumor cells in the blood and subsequent tumor-associated deaths.
Although memory CD4+ T cells had no in vitro or in vivo alloreactivity,10 memory CD8+ T cells responded to allogeneic stimulator cells in the current study as judged by proliferation and cytokine secretion. However, the proliferation and IL-2 secretion of the memory cells was significantly reduced (∼ 7-fold) compared with the naive cells but IFN-γ secretion was significantly increased. Both subsets showed potent killing of allogeneic target cells after initial alloantigenic stimulation. It is unclear why the memory CD8+ T cells were alloreactive, whereas in our previous study the memory CD4+ T cells were not.10 The large majority of memory CD44hiCD8+ T cells from untreated mice were central memory T cells, whereas the large majority of memory CD4+ CD44hi T cells were effector memory T cells.
CD8+CD44hi memory T cells did not induce lethal GVHD in irradiated hosts, whereas an equal number of 1 × 106 or 0.5 × 106 naive CD8+ T cells or total CD8+ T cells induced lethal GVHD in approximately 40%–50% of recipients without tumor cells. Control recipients given TCD BM and BCL1 tumor cells all died of progressive tumor growth. When the latter recipients were given 0.5 × 106 naive or total CD8+ T cells, none died from tumor growth. However, approximately 25% died from GVHD, and the GVHD histopathology scores in the liver, colon, and small intestine of these recipients given naive cells or total CD8+ T cells were significantly increased compared with recipients given TCD BM cells alone. In contrast, none of the recipients given memory CD8+ CD44hi T cells died during the 100-day observation period, and the GVHD scores were not significantly different from those given TCD BM cells alone. The liver GVHD scores were significantly higher in the hosts given naive compared with memory CD8+ T cells. The latter result is consistent with a previous report regarding the lack of GVHD with CD8+CD44hi cells,12 but is different from that reported in a recent study in which purified central memory CD62LhiCD44hi CD8+ T cells were injected into lethally irradiated, MHC-mismatched recipients of BMTs.15 In the latter study, the central memory CD8+ T cells induced significantly higher GVHD scores than the TCD BM controls.15 A possible explanation is that a combination of central and effector memory CD8+ T cells were used in the current CD8+CD44hi studies, and that the addition of the effector memory cells attenuated the potency of the GVHD of the central memory T cells. Effector memory T cells have been reported to induce little or no GVHD even after exposure to alloantigens,10,12-14 and regulation of GVHD by this subset has not been studied. Recent studies indicate that CD8+CD122hi T cells that express a memory phenotype have potent regulatory activity.26-28
Both naive and memory CD8+ T cells had the capacity to facilitate the establishment of complete chimerism and tumor eradication despite the lack of GVHD by the memory cells. The increased potency of GVHD of the naive compared with memory cells was associated with more rapid accumulation and expansion in the lymphoid tissues, liver, and intestines as judged by BLI and CFSE staining. The increased IL-2 secretion of the naive T cells may contribute to the increased early proliferation,29 and the increased expression of CCR9 and α4β7 integrin gut homing receptors30 may contribute to the increased naive T cell early trafficking to the intestines.
It is likely that the ability of memory CD8+ T cells to eradicate tumor cells is dependent on their alloreactivity, because C57BL/6 CD8+ T cells tolerized to BALB/c alloantigens lose their graft antitumor activity against BCL1 lymphoma.31 Alloreactivity of memory CD8+ T cells can be explained by cross-reactivity with viral antigens in the environment that have been shown to enhance immune responses to alloantigens on organ transplants.32-34 In addition, naive CD8+ T cells can masquerade as memory phenotype cells after homeostatic proliferation with maintenance of the naive T-cell TCR repertoire.35
The current study also investigated CD8+CD44hi memory and unseparated total T cells as after-transplantation infusion therapy in recipients given TCD BM cells and tumor cells. The infusion was administered at day 16, a time point at which tumor expansion in the lymphoid tissues was apparent by BLI. When unseparated total T cells were used for infusion, acute lethal GVHD was observed in all recipients. In contrast, purified CD8+ memory T cells allowed for the survival of 100% of the recipients, and survivors remained free of tumors for at least 100 days. The controls given TCD BM without the infusion all succumbed to tumor growth. In conclusion, CD8+ memory T cells containing both central and effector memory subsets were able to separate GVHD and antilymphoma activity when added to TCD BMTs.
Presented in abstract form at the 51st annual meeting of the American Society of Hematology, New Orleans, LA, December 6, 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr R. Levy for providing the hybridoma for the anti-idiotype BCL1 antibody and Dr Kondala Atkuri and Ms Sue Shepperd of the L.A. Herzenberg laboratory for helping with the fluorochrome conjugation of the antibody.
This work was supported by grants from the National Institutes of Health (HL-58250, HL-57743, and CA-49605) and from the Leukemia & Lymphoma Society.
National Institutes of Health
Authorship
Contribution: S.D. designed and performed experiments, analyzed data, and wrote the manuscript; J.B. helped design experiments, performed research, analyzed data, and helped write the manuscript; H.E.K. performed experiments and helped write the manuscript; N.K. performed histopathologic scoring and prepared figures; M.S. purified and helped conjugate the anti-idiotype BCL1 antibody and helped write the manuscript; R.S.N. provided luciferase-labeled cells and supervised bioluminescence studies; and S.S. provided overall research supervision and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samuel Strober, MD, Stanford University School of Medicine, CCSR Bldg, Rm 2215-C, 300 Pasteur Dr, Stanford, CA 94305-5166; e-mail: sstrober@stanford.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal