Abstract
Notch signaling plays both oncogenic and tumor suppressor roles, depending on cell type. In contrast to T-cell acute lymphoblastic leukemia (ALL), where Notch activation promotes leukemogenesis, induction of Notch signaling in B-cell ALL (B-ALL) leads to growth arrest and apoptosis. The Notch target Hairy/Enhancer of Split1 (HES1) is sufficient to reproduce this tumor suppressor phenotype in B-ALL; however, the mechanism is not yet known. We report that HES1 regulates proapoptotic signals by the novel interacting protein Poly ADP-Ribose Polymerase1 (PARP1) in a cell type–specific manner. Interaction of HES1 with PARP1 inhibits HES1 function, induces PARP1 activation, and results in PARP1 cleavage in B-ALL. HES1-induced PARP1 activation leads to self-ADP ribosylation of PARP1, consumption of nicotinamide adenine dinucleotide+, diminished adenosine triphosphate levels, and translocation of apoptosis-inducing factor from mitochondria to the nucleus, resulting in apoptosis in B-ALL but not T-cell ALL. Importantly, induction of Notch signaling by the Notch agonist peptide Delta/Serrate/Lag-2 can reproduce these events and leads to B-ALL apoptosis. The novel interaction of HES1 and PARP1 in B-ALL modulates the function of the HES1 transcriptional complex and signals through PARP1 to induce apoptosis. This mechanism shows a cell type–specific proapoptotic pathway that may lead to Notch agonist–based cancer therapeutics.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy diagnosed in children and is a prevalent form of adult acute leukemia.1,2 Although outcomes among children with ALL have improved dramatically, children who relapse and adults with ALL have a poor prognosis, with < 40% long-term survival.1,3 Despite dose intensification and widespread use of stem cell transplantation in relapse, little improvement in salvage rates has occurred. Hence, novel therapies, particularly those that target critical growth and survival pathways, are needed.
Numerous studies have shown that dysregulated cell signaling is extensively involved in tumor initiation, promotion, and progression. Indeed, dysregulation of the Notch pathway has been shown in a wide range of tumors, including T-cell leukemia/lymphoma, breast carcinomas, pancreatic carcinomas, and so forth.4 In T-cell ALL, Notch pathways are constitutively activated in more than one-half of all cases through activating mutations in the Notch1 receptor.5,6 In contrast, Notch1 mutations have not been found in B-cell ALL, and evidence supports an inhibitory role for Notch signaling in normal and malignant B cells.7-9 These studies show contrasting cell type–specific consequences of Notch signaling and mirror the developmental role of Notch signaling in commitment and expansion of T cells at the expense of B cells.10-12 Therefore, Notch signaling provides a highly conserved pathway that regulates lymphocyte cell lineage and plays contrasting roles in T- and B-cell leukemias.13,14
In mammals, there are 4 Notch receptors (Notch1-4) and 5 Notch ligands (Jagged1/2, Delta-like 1/2/4).15 Once bound to ligand, the Notch receptors are cleaved by γ-secretase, which leads to liberation and translocation of the Notch intracellular domain to the nucleus.16 Within the nucleus, all 4 Notch intracellular domains bind to and displace co-repressors from the transcription factor CSL (derived from CBF-1, Su(H), and Lag2, also known as RBP-Jκ) to generate a transactivation complex, thereby initiating transcription of CSL target genes such as members of the hairy/enhancer of split (HES) family.17,18
Because all 4 Notch receptors signal through the same CSL-dependent pathways, we hypothesized that downstream targets of Notch/CSL may be responsible for the contrasting roles of Notch signaling. Indeed, we found that HES1 expression is sufficient to reproduce the Notch effects in B-cell ALL (B-ALL) but does not affect growth or survival in T-cell ALL (T-ALL).9 In support of this, HES1 has been shown to mimic Notch inhibition of normal B-cell development.19
HES1 is the most commonly described Notch/CSL target gene17 and appears to contribute to T-cell leukemogenesis.20 It encodes a basic helix-loop-helix (bHLH) transcription factor, which is known to recruit co-repressors of the transducin-like enhancer (TLE, groucho) of split family.21 Through its bHLH domain, HES1, forms homodimers and heterodimers that bind to a variety of modified E-box binding sites with distinguishable affinities.22,23 Although typically considered a transcriptional repressor, evidence suggests that HES1 may lead to transactivation of some genes, depending on interacting proteins that form the HES1 transcriptional complex.24 For example, the mammalian achaete-scute homologue promoter in rodent neural stem cells may be activated by HES1 in a Ca+/calmodulin-dependent protein kinase II–dependent manner.24 In Ju et al poly (adenosine diphosphate [ADP]–ribose) polymerase-1 (PARP1) was reported to bind with HES1 in rat neural stem cells where it led to a transactivating HES1 transcriptional complex. Although the molecular mechanisms remain elusive, several reports support the existence of HES1 transcriptional complexes that may function as either repressors or activators, establishing a potential mechanism of cell type–dependent regulation of Notch/HES1 signaling.
In addition to cell type–specific modulation of HES1 function, we describe the effects of HES1 on PARP1 activity. PARP1 is a ubiquitous nuclear enzyme that catalyzes the nicotinamide adenine dinucleotide (NAD)–dependent addition of ADP-ribose polymers to a variety of nuclear proteins and has been implicated in critical stress-related functions.25 First, PARP1 is a molecular sensor of DNA breaks, and it has a key role in the spatial and temporal organization of DNA repair.26,27 Through its physical association with, and by the poly ADP ribosylation of partner proteins, it regulates chromatin structure and DNA metabolism.25 PARP1 activation can also mediate neuronal death through enhanced self-ribosylation and cleavage of PARP1 and subsequent nuclear translocation of apoptosis-inducing factor (AIF),28 resulting in activation of the caspase cascade.29 Finally, PARP1 binds to and modulates the activity of various transcription factors.24,30,31 Most relevant to this study, interaction of PARP1 with HES1 promotes phosphorylation of HES1 by Ca+/calmodulin-dependent protein kinase II with subsequent displacement of TLE co-repressors and recruitment of coactivators, resulting in formation of new transcriptional complex and result in HES1-mediated transactivation rather than repression of its target genes in rat neural stem cells.24
Here we report the interaction and functional consequences of HES1 and PARP1 in human acute lymphoblastic leukemias. Importantly, we note cell type–specific differences in B-ALL versus T-ALL in the formation of HES1 transcriptional complexes, HES1 transcriptional complex function, and activation of PARP1 and its proapoptotic consequences. Our studies provide novel evidence for a mechanism of cell-specific regulation of Notch/HES1 signaling which may play an important role in ALL and may contribute to the contrasting oncogene and tumor suppressor roles of Notch signaling in other malignancies.
Methods
Cloning, expression of plasmids
The full-length human HES1 cDNA with N-terminal FLAG tag was cloned into the MigR1 (murine stem cell virus–based retrovirus; a kind gift of Warren Pear, University of Pennsylvania) and pcDNA3.1 (cytomegalovirus [CMV] and T7 promoter expression) plasmids. Critical domains of HES1 were identified with Simple Modular Architecture Research Tool program (European Union's Scientific Data Repositories). The primers used to construct these mutants are listed in supplemental Figure 3, (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All clones were sequenced, and expression was confirmed by transient transfection in human embryonic kidney cell line (HEK-293) cells and immunoblots with anti-FLAG antibody (Sigma-Aldrich). Short hairpin RNA (shRNA) constructs against HES1 (TI349906 sequence cloned into pRFP-C-RS; Origene), PARP1 (TG315488; Origene), and AIF (TF302572l Origene) were transfected when indicated.
Cell lines and culture
We used the human precursor B-leukemia lines JM1, Nalm6, and 697 and the human T-cell leukemia lines SupT1, Molt4, and SupT1 to represent B-ALL and T-ALL, respectively. HEK-293 cells were used as a “normal” control for endogenous HES1 expression (supplemental Figure 4). Cell lines were cultured routinely at 37°C in RPMI 1640 medium (Gibco BRL) containing 10% fetal calf serum (Gibco BRL), l-glutamine and penicillin/streptomycin (hereafter referred to as complete medium) in a 5% CO2 incubator.
Proliferation assays
Cell lines were transfected with 4 μg of mRNA from a bi-cistronic FLAG-HES1/ green fluorescent protein (GFP) or control MigR1 vectors with the use of Amaxa Nucleofector kit V with programs O-17, M-01, X-05, and X-01 for SupT1, JM1, Jurkat, and 697, respectively, kit T for Nalm6 with program L-01, and kit L for Molt4 with program C-05. Aliquots from each well were stained with Trypan blue reagent, and non-blue cells were counted daily. In parallel, an aliquot was measured for GFP+ cells with the use of flow cytometry (with > 20 000 cells with the use of FL1; BD FACSCalibur), which represent the HES1 or control-transfected cells. FlowJo software (TreeStar Inc) was used to calculate the percentage of GFP+ cells in the mixed population, and that number was used with the total viable cell counts to calculate the number of viable GFP+ cells
Size-exclusion chromatography for HES1-associated PARP1 complex in the nucleus
For size exclusion chromatography analysis, a 25/30 column (Amersham Biosciences) was gravity packed with Superdex 200 and calibrated with the use of known protein standards (Gel Filtration High Molecular Weight Standards; Amersham Biosciences). Nuclear extracts containing 1 mg from ALL cells transiently expressing FLAG-HES1 was applied to the Superdex 200 column equilibrated and eluted at 0.05 mL/min and serial fractions of 500 μL were collected. The fractions were precipitated with trichloroacetic acid (TCA) and analyzed by immunoblotting for FLAG (Sigma-Aldrich).
In vitro FLAG-HES1 mRNA synthesis
The FLAG-HES1 cDNA was cloned into pcDNA3.1 that contains a T7-promoter. In vitro transcription of FLAG-HES1 was carried out with RiboMAX large-scale RNA Production System T7 per manufacturer's instructions (Promega).
HES1 reporter luciferase assay
SupT1 and JM1 cells were cotransfected with a HES1-responsive Firefly luciferase reporter construct,32 a transfection control pRL-cytomegalovirus Renilla luciferase plasmid and FLAG-HES1 or MigR1 GFP-only plasmids with the use of Nucleofector kit V and programs O-17 and M-01 for SupT1 and JM1, respectively. When indicated, cells were pretransfected with shRNA against human PARP1, HES1 proteins, or control-scrambled shRNA containing puromycin marker in pRS vector (Origene) and selected with 0.5 μg/mL puromycin. Lysates were then assayed for Firefly versus Renilla luciferase activity with the use of the Dual-Luciferase Reporter Assay System Kit (E1910; Promega).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed with the ChIP-IT Express Chromatin Immunoprecipitation Kit (Active Motif) and anti-FLAG (Sigma-Aldrich) and anti-PARP1 (Cell Signaling) antibodies. ChIP–polymerase chain reaction (PCR) was performed with primers corresponding to hHES1-500 to -324 promoter, which contain a canonical N-box HES1 binding site, were used for PCR.32 As an internal negative control, primers that amplify a random sequence in intron 3 of the HES1 gene were used.
Immunoprecipitation of FLAG-HES1 and PARP1
JM1 cells were transfected with 2 μg of in vitro–transcribed mRNA of wild-type or mutant versions of FLAG-HES1 with the use of Nucleofector kit V and program M-01. As a control, GFP mRNA was used. Lysates were immunoprecipitated with FLAG (Sigma-Aldrich) or PARP1 (Cell Signaling) antibodies and protein G magnetic beads (Active Motif). Immunoblots were probed with anti-FLAG and PARP1 (Cell Signaling).
Nuclear extracts
JM1 cells were transfected with FLAG-HES1 mRNA. After 48 hours, nuclear extracts were prepared with the Nuclear Extraction Kit (40010; Active Motif).
Poly ADP-ribosylation of PARP1
The poly(ADP) assay was carried out as published previously, with slight modifications.33 Recombinant glutathione-S-transferase (GST)–HES1 cDNA was generated in pGEX-5T. Reactions contained recombinant GST-HES1, recombinant human PARP1 (Sigma-Aldrich), NAD+ (Sigma-Aldrich) at 300mM and were incubated at 37°C for 15 minutes. poly(ADP-ribose) (PAR)–ylated PARP1 was detected with anti-PAR monoclonal antibody (Trevigen). For the detection of HES1 and PARP1, anti-GST (GE) and PARP1 (Cell Signaling) antibodies were used, respectively.
Tissue-specific AIF release assay
Mitochondria were prepared from SupT1 and JM1 cells transfected with FLAG-HES1, using a mitochondrial isolation kit for mammalian cells (Pierce). Isolated mitochondria and nuclear extracts were run on immunoblots for AIF (Cell Signaling) as well as V-DAC (a mitochondrial protein) and Laminin B (a nuclear protein), used as controls to assess purity of fractionation.
Quantification of apoptosis, adenosine triphosphate depletion, and enzyme-linked immunoabsorbent assay for PAR levels
Apoptosis was quantified by flow cytometry after incubation with annexin V–fluorescein isothiocyanate or -allophycocyanin (BD Biosciences) in 1× annexin V–binding buffer staining per manufacturer's instructions. Adenosine triphosphate (ATP) levels were measured with the 96-well OptiPlate microplates (PerkinElmer) with ATPlite Luminescence ATP detection assay system (PerkinElmer) per manufacturer's instructions. Luminescence was read with a Victor Light luminometer (PerkinElmer). Total cellular PAR levels were detected with indirect enzyme-linked immunoabsorbent assay on total cell lysates prepared from FLAG-HES1 transfection. Briefly, wells were coated with lysates, and anti-PAR antibody was used as 1:500 and incubated at 4°C for 6 hours. After washing with Tris (tris(hydroxymethyl) aminomethane)–buffered saline/0.05% Tween 20, secondary antibody was added as 1:1000 for 1 hour. The chromatic reaction was developed with substrate reagent from R&D Systems.
Inhibition of Notch and PARP1 signaling
In a panel of B- and T-ALL cells we treated with notch signaling inhibitor γ-secretase inhibitor (GSI) XVI (10μM; Calbiochem) along with control dimethyl sulfoxide and in separate experiments were also carried out with PARP1 inhibitor, 3ABA (3-aminobenzamide; 100μM; Sigma-Aldrich), an NAD+ analog.
Expression of Notch/HES1 and PARP1 in patient samples
We analyzed mRNA expression in 206 pediatric ALL samples (34 T-ALL, 172 B-ALL) run on Affymetrix U133A arrays.34 To determine differences in expression of HES1 versus PARP1 in T-ALL and B-ALL, we used LIMMA (Linear Models for Microarray Analysis),35 the empirical Bayes t test implemented in Bioconductor36 and the Benjamini-Hochberg method of false discovery rate (FDR) estimation.37 These data have been deposited at the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession no. GSE26366.
Results
HES1 inhibits ALL growth in a cell type–specific manner
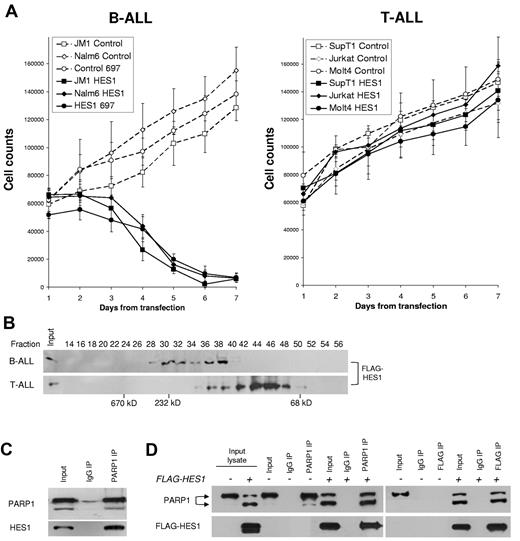
We previously reported that Notch activation inhibits B-ALL growth and survival and that HES1 is sufficient to induce this effect.9 In the present study, we reveal cell type–specific mechanisms that may contribute to Notch/HES1-mediated apoptosis in B-ALL. First, we demonstrate that transfection of human ALL cell lines with a HES1/GFP-expressing construct shows a cell type–specific growth inhibition in B-ALL but not T-ALL cells. Using flow cytometry combined with cell counts, we found that HES1/GFP expression in B-ALL cell lines led to a profound decrease (> 87%) in HES1-transfected cells over 7 days, compared with GFP-expressing controls (P < .02; Figure 1A left). In contrast, T-ALL cell lines expressing HES1/GFP showed growth at a comparable rate to GFP-transfected controls (Figure 1A right). These findings show that there are cell-specific differences in the consequences of HES1 expression in human B-ALL versus T-ALL cells. These results are consistent with our previous report and provide a model system to explore cell type–specific consequences of HES1 signaling.9 Because HES1 functions through interaction with other proteins, we hypothesized that HES1-mediated effects in B-ALL may be attributed to cell type–specific differences in the formation of HES1 transcriptional complex.
Cell-specific differences in HES1 effects, complexes, and interaction with PARP1. (A) Differential effects of HES1 on growth in B-ALL and T-ALL cell lines. Line graphs represent the cell counts of GFP+ cells up to 7 days after transfection. A panel of 3 B-ALL and 3 T-ALL cell lines were transfected with a HES1/GFP construct (solid lines, black symbols) or control GFP-only vector (dashed lines, open symbols). Numbers of GFP+ cells were calculated by combining the percentage of GFP+ cells measured by flow cytometry and Trypan blue exclusion cell counts performed daily. The results are presented as mean ± SD of 3 independent experiments. (P < .02). (B) Different HES1 complexes in B-ALL and T-ALL. Size-exclusion chromatography was performed to assess the relative size of HES1 complexes in B-ALL (JM1) and T-ALL (SupT1) cells. Freshly prepared lysates from B-ALL (JM1) and T-ALL (SupT1) cells transfected with FLAG-HES1 constructs were run through a Superdex 200 column, and 70 fractions were collected. Even fractions were used for immunoblotting to detect HES1-containing fractions with the use of anti-FLAG antibody. No signal was seen before fraction 30 or after fraction 50 in either sample. Protein mass standards were run on the same column to provide approximate molecular sizes (in kilodaltons) of complexes. Differences in fractions with FLAG-HES1 in B-ALL and T-ALL suggest that there are cell type–specific differences in HES1 complexes. Lack of FLAG-HES1 above fraction 50 suggests that no free monomers (35 kDa) are present in either cell line. (C) PARP1 binds to endogenous HES1 in B-ALL cells. IP of PARP1 in nuclear extracts from JM-1 (B-ALL) cells shows binding to endogenous HES1. (D) HES1 interacts with both full-length and cleaved PARP1. B-ALL (JM1) cells transfected with FLAG-HES1 mRNA, after 48 hours nuclear extracts were immunoprecipitated with PARP1 or FLAG beads and immunoblotted with anti-PARP1 and anti-FLAG antibodies. FLAG-IP of exogenous HES1 is associated with PARP1 cleavage and binds to both full-length and cleaved PARP1. IgG indicates immunoglobulin G.
Cell-specific differences in HES1 effects, complexes, and interaction with PARP1. (A) Differential effects of HES1 on growth in B-ALL and T-ALL cell lines. Line graphs represent the cell counts of GFP+ cells up to 7 days after transfection. A panel of 3 B-ALL and 3 T-ALL cell lines were transfected with a HES1/GFP construct (solid lines, black symbols) or control GFP-only vector (dashed lines, open symbols). Numbers of GFP+ cells were calculated by combining the percentage of GFP+ cells measured by flow cytometry and Trypan blue exclusion cell counts performed daily. The results are presented as mean ± SD of 3 independent experiments. (P < .02). (B) Different HES1 complexes in B-ALL and T-ALL. Size-exclusion chromatography was performed to assess the relative size of HES1 complexes in B-ALL (JM1) and T-ALL (SupT1) cells. Freshly prepared lysates from B-ALL (JM1) and T-ALL (SupT1) cells transfected with FLAG-HES1 constructs were run through a Superdex 200 column, and 70 fractions were collected. Even fractions were used for immunoblotting to detect HES1-containing fractions with the use of anti-FLAG antibody. No signal was seen before fraction 30 or after fraction 50 in either sample. Protein mass standards were run on the same column to provide approximate molecular sizes (in kilodaltons) of complexes. Differences in fractions with FLAG-HES1 in B-ALL and T-ALL suggest that there are cell type–specific differences in HES1 complexes. Lack of FLAG-HES1 above fraction 50 suggests that no free monomers (35 kDa) are present in either cell line. (C) PARP1 binds to endogenous HES1 in B-ALL cells. IP of PARP1 in nuclear extracts from JM-1 (B-ALL) cells shows binding to endogenous HES1. (D) HES1 interacts with both full-length and cleaved PARP1. B-ALL (JM1) cells transfected with FLAG-HES1 mRNA, after 48 hours nuclear extracts were immunoprecipitated with PARP1 or FLAG beads and immunoblotted with anti-PARP1 and anti-FLAG antibodies. FLAG-IP of exogenous HES1 is associated with PARP1 cleavage and binds to both full-length and cleaved PARP1. IgG indicates immunoglobulin G.
Distinct cell-specific complexes in ALL: HES1 and PARP1 interaction
To determine whether HES1 existed in monomers, dimers, or larger complexes, we transfected B-ALL (JM1) and T-ALL (SupT1) cells with FLAG-tagged HES1 and used nondenaturing size-exclusion chromatography to identify native HES1 protein complexes, ranging from 60 kDa to > 300 kDa. Interestingly, immunoblot for FLAG-HES1 showed differences in the size of HES1-containing complexes between B-ALL and T-ALL and the apparent absence of isolated HES1 monomers and dimers in B-ALL (Figure 1B). Using size standards on this column we estimated peak molecular mass of HES1 complexes of ∼ 140 and 230 kDa in B-ALL and ∼ 100 kDa in T-ALL. The differing sizes of the HES1 complexes in these B-ALL versus T-ALL cells suggest that cell-specific HES1 complexes exist, which we hypothesized may have different functional consequences.
To identify the components of these high molecular mass complexes, we performed IP of the FLAG-HES1 protein and sequenced the interacting proteins with the use of matrix-assisted laser desorption ionization time of flight mass spectroscopy. MASCOT protein prediction software found 40 peptides that matched the PARP1 sequence, identifying PARP1 as probably a HES1-interacting protein (P < .01; supplemental Figure 1). Because interaction between PARP1 and HES1 had been reported previously in rat neural stem cells, HES1/PARP1 interaction in human leukemia cells was plausible.24
Immunoprecipitation of PARP1 in B-ALL cells (JM1) did strongly coimmunoprecipitate endogenous HES1 from nuclear extracts, suggesting that PARP1 may be a main component of HES1 complexes in B-ALL (Figure 1C). Unfortunately, IP of endogenous HES1 was not feasible with available antibodies. Therefore, we expressed FLAG-tagged HES1 in JM1 cells and found that FLAG-HES1 and PARP1 coimmunoprecipitate each other reciprocally (Figure 1D). Interestingly, HES1 overexpression appeared to induce cleavage of PARP1 which suggested a potential proapoptotic mechanism that is addressed later in this study.
Cell-specific HES1 transcriptional repression in ALL
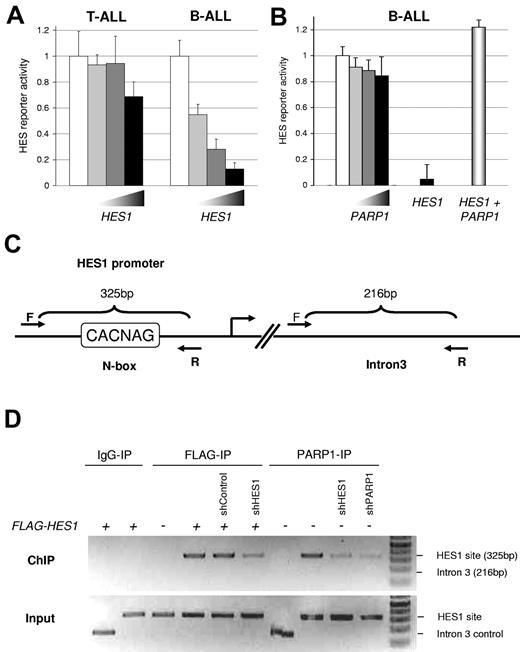
To determine whether HES1 had similar functional roles in B-ALL versus T-ALL, we transfected ALL cells with HES1 and a HES1-responsive luciferase reporter (containing 2 HES1-binding N-box [E-box variant] motifs in the context of the human HES1 promoter).32 In T-ALL (SupT1) cells, HES1 expression led to minimal repression (< 30%) of this reporter; however, similar transfection in B-ALL (JM1) cells led to significant repression (> 85%) of the reporter (P < .038; Figure 2A). These data show that increasing HES1 expression leads to different functional consequences in a cell-specific manner. Therefore, understanding the function of the HES1 transcriptional complex (including PARP1) in the context of ALLs may yield further understanding of cell type–specific consequences of Notch/HES1 signaling.
PARP1 inhibits HES1 function and localizes to a HES1 binding site. (A) Cell type–specific function of HES1. T-ALL (SupT1) and B-ALL (JM1) cells were cotransfected with HES1-responsive luciferase reporter, Renilla luciferase control, and increasing amounts of pcDNA-FLAG-HES1. After 48 hours, Firefly/Renilla luciferase activity was measured. Significant dose-dependent repression was only seen in B-ALL cells. (P < .038, mean ± SD). (B) PARP1 inhibits HES1 function. B-ALL (JM1) cells were cotransfected with HES1-responsive luciferase reporter, Renilla luciferase control, and increasing amounts of PARP1 expression plasmid or pcDNA-FLAG-HES1 with or without PARP1. No significant effect was seen with PARP1 alone. As expected, significant dose-dependent repression was seen with HES1; however, the addition of PARP1 abrogated this effect, inhibiting HES1-mediated repression of this reporter (P < .05, mean ± SD). (C) PARP1 binds to a HES1 binding site via HES1. Schematic representation of the forward and reverse primers for ChIP-PCR. Forward “F” and reverse “R” primers produce a 325-base pair (bp) product for the known HES1 site (N-box) and a 216-bp product for a random site in intron 3 as a control. (D) ChIP-PCR analysis to determine the interaction of PARP1 and HES1. JM1 cells were transfected with empty vector, FLAG-HES1, PARP1, shRNA control, shRNA to HES1, or shRNA to PARP1 as indicated. After 48 hours, the cells were fixed, and IP with anti-PARP1 or anti-FLAG antibodies was performed with the use of the ChIP-IT Express kit. PCR products were resolved on an agarose gel and visualized with ethidium bromide/UV. These results show HES1 and endogenous PARP1 binding to a known HES1 binding site. ShRNA to HES1 decreased PARP1 binding to this site, suggesting that PARP1 binding depends on endogenous HES1.
PARP1 inhibits HES1 function and localizes to a HES1 binding site. (A) Cell type–specific function of HES1. T-ALL (SupT1) and B-ALL (JM1) cells were cotransfected with HES1-responsive luciferase reporter, Renilla luciferase control, and increasing amounts of pcDNA-FLAG-HES1. After 48 hours, Firefly/Renilla luciferase activity was measured. Significant dose-dependent repression was only seen in B-ALL cells. (P < .038, mean ± SD). (B) PARP1 inhibits HES1 function. B-ALL (JM1) cells were cotransfected with HES1-responsive luciferase reporter, Renilla luciferase control, and increasing amounts of PARP1 expression plasmid or pcDNA-FLAG-HES1 with or without PARP1. No significant effect was seen with PARP1 alone. As expected, significant dose-dependent repression was seen with HES1; however, the addition of PARP1 abrogated this effect, inhibiting HES1-mediated repression of this reporter (P < .05, mean ± SD). (C) PARP1 binds to a HES1 binding site via HES1. Schematic representation of the forward and reverse primers for ChIP-PCR. Forward “F” and reverse “R” primers produce a 325-base pair (bp) product for the known HES1 site (N-box) and a 216-bp product for a random site in intron 3 as a control. (D) ChIP-PCR analysis to determine the interaction of PARP1 and HES1. JM1 cells were transfected with empty vector, FLAG-HES1, PARP1, shRNA control, shRNA to HES1, or shRNA to PARP1 as indicated. After 48 hours, the cells were fixed, and IP with anti-PARP1 or anti-FLAG antibodies was performed with the use of the ChIP-IT Express kit. PCR products were resolved on an agarose gel and visualized with ethidium bromide/UV. These results show HES1 and endogenous PARP1 binding to a known HES1 binding site. ShRNA to HES1 decreased PARP1 binding to this site, suggesting that PARP1 binding depends on endogenous HES1.
PARP1-mediated inhibition of HES1 function and binding at the HES1 promoter
To determine the role of PARP1 on HES1 function, we cotransfected a human PARP1 expression plasmid with the HES1 reporter construct, as above. Even high levels of PARP1 expression did not significantly affect the HES1 reporter (Figure 2B). Transfection of HES1 alone did cause dramatic repression of this reporter, as in Figure 2A; however, cotransfection of PARP1 with HES1 led to complete abrogation of HES1-mediated repression, with a small but significant de-repression under these conditions (P < .05; Figure 2B). This PARP1-mediated inhibition of HES1 repressor activity is similar to PARP1 effects on HES1 seen in rat neural stem cells.24 This implies that HES1 function may be modulated by binding to PARP1, providing a novel, intriguing mechanism for the regulation of Notch pathway signaling in B-ALL.
HES1-mediated PARP1 binding to a HES1 binding site in B-ALL cells
The results described above suggest PARP1 interaction with HES1 lead to modulation of HES1 transcriptional activity. To test whether PARP1 and HES1 are found bound specifically to a native HES1 binding site in genomic DNA, we performed ChIP. ChIP for HES1 in B-ALL cells shows that HES1 specifically binds to a known HES1 binding site in the promoter of the HES1 gene (ie, autoregulation) and not to a random site in intron 3 of the same gene (Figure 2C-D). This ChIP was diminished when an shRNA to HES1 was also cotransfected (Figure 2D). Most importantly, the PARP1 antibody was also able to specifically immunoprecipitate the HES1 site within the HES1 promoter, and, when the shRNA to HES1 was introduced, PARP1 binding was diminished (Figure 2D). This implies that endogenous PARP1 is present at the HES1 binding site in a native promoter in B-ALL cells and that PARP1 binding to this site is at least partially dependent on HES1. These results show that PARP1 is present at a genomic HES1 binding site, suggesting that endogenous PARP1 interacts with HES1 on native chromatin.
Critical domains of HES1
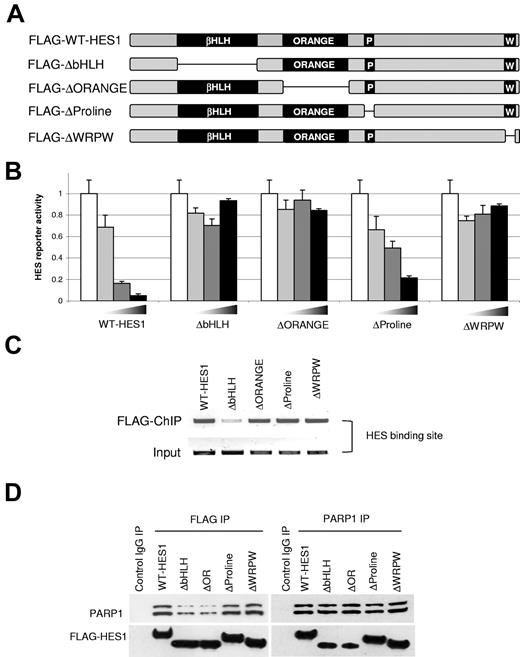
To identify the domains of HES1 that are critical for its function, we created a panel of HES1 deletions. Four domain-deleted HES1 mutants for the bHLH, orange, proline, and WRPW domains were generated (Figure 3A). Previous reports showed that the highly evolutionary conserved HES1 bHLH domain mediates specific DNA binding and homodimerization and heterodimerization with HES1, other HES family members, and other HLH proteins in different cell contexts (FLAG-ΔbHLH-HES1).38 The orange domain is a protein-protein interaction domain that regulates embryonic segmentation and participates in the ability of HES1 to block neurite outgrowth (FLAG-ΔORANGE-HES1).39 The proline-rich domain interacts with co-repressor mSin3, and similar proline-rich regions in the p53 protein protect it from proteosomal-mediated degradation (FLAG-ΔProline-HES1).40 Finally, the 4-amino acid WRPW motif, located at the far c-terminus of HES1 and all HES family members, has been shown to specifically recruit co-repressors of the TLE/groucho family (FLAG-ΔWRPW-HES1).38
HES1 domains essential for repressor function and PARP1 interaction. (A) Schematic of human HES1 domains and mutants. The conserved domains are the bHLH region, orange domain, proline-rich region, and C-terminal WRPW region. (B) HES1 mutant reporter assay. B-ALL (JM1) cells were transfected with the HES1-responsive luciferase reporter as in Figure 2A-B, with the increasing amounts of the indicated wild-type or truncated versions of FLAG-HES1 plasmids. Only the full-length HES1 and proline mutant showed repressor activity, indicating the critical role for the bHLH, orange, and WRPW domains in HES1 repressor function (P < .001, mean ± SD). (C) HES1 mutant DNA binding. B-ALL (JM1) cells were transfected with the HES1 mutants, and ChIP assays were performed as in Figure 2C-D. All mutants, except the bHLH mutant (which contains the DNA binding domain), are able to bind the native HES1 binding site. (D) Co-IP with HES1 mutants. B-ALL (JM1) cells were transfected with full-length and HES1 mutants. Co-IP was performed as in Figure 1C. The bHLH and orange domain mutants were less efficient at binding PARP1. Similar results were observed whether IP was performed with anti-FLAG or anti-PARP1.
HES1 domains essential for repressor function and PARP1 interaction. (A) Schematic of human HES1 domains and mutants. The conserved domains are the bHLH region, orange domain, proline-rich region, and C-terminal WRPW region. (B) HES1 mutant reporter assay. B-ALL (JM1) cells were transfected with the HES1-responsive luciferase reporter as in Figure 2A-B, with the increasing amounts of the indicated wild-type or truncated versions of FLAG-HES1 plasmids. Only the full-length HES1 and proline mutant showed repressor activity, indicating the critical role for the bHLH, orange, and WRPW domains in HES1 repressor function (P < .001, mean ± SD). (C) HES1 mutant DNA binding. B-ALL (JM1) cells were transfected with the HES1 mutants, and ChIP assays were performed as in Figure 2C-D. All mutants, except the bHLH mutant (which contains the DNA binding domain), are able to bind the native HES1 binding site. (D) Co-IP with HES1 mutants. B-ALL (JM1) cells were transfected with full-length and HES1 mutants. Co-IP was performed as in Figure 1C. The bHLH and orange domain mutants were less efficient at binding PARP1. Similar results were observed whether IP was performed with anti-FLAG or anti-PARP1.
To explore the importance of the various HES1 domains on HES1 function, we used the HES1 domain-deleted mutants and the HES1-responsive luciferase reporter described above. As seen in Figure 2A, the wild-type HES1 construct results in significant repression of the HES1-responsive reporter activity in B-ALL cells (P < .001; Figure 3B). In contrast, transfection with domain-deleted versions of HES1 showed loss of HES1-mediated repression by the bHLH, orange, and WRPW mutants (Figure 3B). Interestingly, the limited 4-amino acid WRPW deletion mutant was unable to function as a repressor in this assay, thus implicating TLE-mediated co-repressor mechanism in human B-ALL. At the same time deletion of the bHLH or orange domains also lost their ability to repress this reporter. Because the “basic” portion of the bHLH domain binds to DNA, function was abrogated as expected. Interestingly, we found that the orange domain deletion construct also showed no ability to repress the reporter even though it does retain DNA binding ability (Figure 3C). This suggests that it has a critical non-DNA binding role in HES1 function such as recruitment of transcriptional modifiers. In contrast, the proline domain-deleted construct retained transcriptional repressor activity, similar to wild-type HES1, suggesting that this conserved domain does not play a critical role in transcriptional repression in this cellular context. These results suggest that HES1 functions as a repressor in B-ALL cells, which appears dependent on the bHLH, orange, and WRPW domains.
PARP1 interacts with the bHLH and orange domains of HES1
To determine which domains of HES1 interact with PARP1, we transfected B-ALL (JM1) cells with the domain-deleted mutants of HES1 and performed IP and immunoblot assays with the use of antibodies specific to FLAG and PARP1. We found that the bHLH and orange deletion mutants did not pull down PARP1 as efficiently as the wild-type, proline, and WRPW domain mutants (Figure 3D). Indeed binding of bHLH and orange domain mutants was 20%-50% of wild-type HES1, depending on whether the immunoprecipitated antibody was anti-FLAG or anti-PARP1. These results suggest that PARP1 interacts with bHLH and orange domains of HES1 and does not depend on the proline-rich domain or TLE-binding WRPW motif.
Figures 1 through 3 provide a model of PARP1 interaction and modulation of HES1 function which suggests a balance of co-activator (PARP1) and co-repressor (TLE) interacting proteins. These HES1-interacting proteins bind to distinct domains of HES1 and provide a mechanism for regulation of the consequences of HES1 expression. However, there appears to be additional importance to the HES1 interaction with PARP1, as seen in Figures 1D and 3D, because expression of HES1 in B-ALL cells leads to PARP1 cleavage in a cell type–specific manner.
HES1-mediated PARP1 activation induces a proapoptotic mechanism
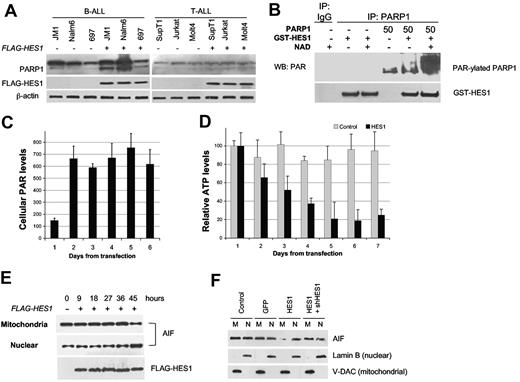
To determine whether these effects are specific for HES1 expression in B-ALL, 3 T-ALL and 3 B-ALL lines were transfected with HES1. Significant PARP1 cleavage was observed in all B-ALL lines but was minimal in all T-ALL lines (Figure 4A) despite similar transfection efficiencies. In B-ALL cells, cleaved PARP1 levels increased on average 5-fold (P = .012), in contrast to a nonsignificant 0.5-fold increase in T-ALL cells (P = .7) We note that total PARP1 levels were also higher in B-ALL versus T-ALL cells. This observation is supported by our microarray dataset of B-ALL and T-ALL patient samples (supplemental Figure 2). Therefore, higher expression of PARP1 in B-ALL cells and cell-specific cleavage of PARP1 in B-ALL cells provide a potential mechanism for HES1-induced B-ALL apoptosis.
HES1-induced activation and cleavage of PARP1. (A) Cell-specific cleavage of PARP1. A panel of 3 B-ALL and 3 T-ALL cell lines were transfected with HES1. At 48 hours, lysates were probed for PARP1 expression. Overall PARP1 levels were higher in B-ALL versus T-ALL, and HES1-mediated PARP1 cleavage was greater in B-ALL than in T-ALL. (B) HES1-induced PAR-ylation of PARP1. Cell free PAR-ylation assays were performed with recombinant HES1 (GST-HES1), PARP1, co-factor NAD+, and IP with anti-PARP1. Immunoblots for PAR and HES1 showed a > 12-fold increase in PAR-ylated PARP1 in triplicate experiments. (C) HES1-induced accumulation of cellular PAR. B-ALL (JM1) cells were transfected with FLAG-HES1 mRNA, and cellular PAR-ylated proteins were immunoprecipitated and quantitated with tetramethylbenzidine and an enzyme-linked immunoabsorbent assay reader. Intracellular PAR levels were increased and sustained from 2 to ≥ 6 days (mean ± SD). (D) HES1-induced depletion of ATP. Under similar conditions, total cellular ATP was determined with the use of the ATPlite kit as directed by the manufacturer. Luminescence was measured with a Molecular Devices luminometer. Raw data in counts are shown (n = 3). Relative ATP levels per cell decreased over time compared with vector control (P < .01, mean ± SD). (E) HES1-induced nuclear translocation of AIF in B-ALL. B-ALL (JM1) cells were transfected with FLAG-HES1 mRNA, and mitochondria and nuclear fractions were isolated at different time points. Anti-AIF immunoblot showed AIF translocation from mitochondria to nucleus. (F) HES1-induced AIF translocation can be rescued with shRNA to HES1. B-ALL (JM1) cells were transfected with HES1 mRNA, and mitochondria and nuclear fractions were isolated at 48 hours. HES1 mRNA, but not GFP control, induces nuclear translocation of AIF. Cotransfection with shRNA to HES1 inhibits effect of HES1. Lamin B (nuclear) and V-DAC (mitochondrial) confirm purity of fractions. WB indicates Western blotting.
HES1-induced activation and cleavage of PARP1. (A) Cell-specific cleavage of PARP1. A panel of 3 B-ALL and 3 T-ALL cell lines were transfected with HES1. At 48 hours, lysates were probed for PARP1 expression. Overall PARP1 levels were higher in B-ALL versus T-ALL, and HES1-mediated PARP1 cleavage was greater in B-ALL than in T-ALL. (B) HES1-induced PAR-ylation of PARP1. Cell free PAR-ylation assays were performed with recombinant HES1 (GST-HES1), PARP1, co-factor NAD+, and IP with anti-PARP1. Immunoblots for PAR and HES1 showed a > 12-fold increase in PAR-ylated PARP1 in triplicate experiments. (C) HES1-induced accumulation of cellular PAR. B-ALL (JM1) cells were transfected with FLAG-HES1 mRNA, and cellular PAR-ylated proteins were immunoprecipitated and quantitated with tetramethylbenzidine and an enzyme-linked immunoabsorbent assay reader. Intracellular PAR levels were increased and sustained from 2 to ≥ 6 days (mean ± SD). (D) HES1-induced depletion of ATP. Under similar conditions, total cellular ATP was determined with the use of the ATPlite kit as directed by the manufacturer. Luminescence was measured with a Molecular Devices luminometer. Raw data in counts are shown (n = 3). Relative ATP levels per cell decreased over time compared with vector control (P < .01, mean ± SD). (E) HES1-induced nuclear translocation of AIF in B-ALL. B-ALL (JM1) cells were transfected with FLAG-HES1 mRNA, and mitochondria and nuclear fractions were isolated at different time points. Anti-AIF immunoblot showed AIF translocation from mitochondria to nucleus. (F) HES1-induced AIF translocation can be rescued with shRNA to HES1. B-ALL (JM1) cells were transfected with HES1 mRNA, and mitochondria and nuclear fractions were isolated at 48 hours. HES1 mRNA, but not GFP control, induces nuclear translocation of AIF. Cotransfection with shRNA to HES1 inhibits effect of HES1. Lamin B (nuclear) and V-DAC (mitochondrial) confirm purity of fractions. WB indicates Western blotting.
To evaluate the consequences of HES1/PARP1 interaction on PARP1 function, we purified recombinant GST-HES1 and incubated with recombinant PARP1 protein (Sigma-Aldrich) in a cell-free system. When activated, PARP1 uses NAD and ATP to synthesize PAR which it adds to itself as well as many nuclear proteins. We found that incubation of recombinant PARP1 and HES1 in a cell-free system led to PARP1 activation as measured by 12-fold increase in PARP1 self-PAR-ylation (Figure 4B). Furthermore, we detected significantly increased PAR-ylated protein levels from lysates of B-ALL cells (JM1) after HES1 expression (P < .015; Figure 4C). Inhibition of PARP1 activity with the use of 3ABA, an NAD analog, prevented HES1-induced PAR formation (data not shown), confirming the important role of NAD in HES1-mediated PARP1 activation. These PAR-ylated proteins or the PAR polymers or both may contribute to PARP1-mediated cell death.41 Furthermore, PARP1 activation also uses significant amounts of NAD and ATP, which may also contribute to growth arrest and apoptosis.42 Consistent with this, we found that ATP levels declined after the accumulation of cellular PAR levels on day 2 (from 80% to 20%; P < .01; Figure 4D). Activation of PARP1 can also lead to the release of AIF from mitochondria to the nucleus, resulting in apoptosis.28,43 Indeed, we found that HES1-induced translocation of AIF from mitochondria to the nucleus was evident by 45 hours in JM1 B-ALL cells (Figure 4E). Furthermore, when shRNA against the HES1 coding region was cotransfected with HES1 mRNA, the nuclear translocation of AIF was inhibited, suggesting that this effect was because of HES1 expression and not because of a toxic effect of the HES1 construct (Figure 4F).
Notch ligand-mediated apoptosis, PARP1 activation, and AIF translocation in B-ALL
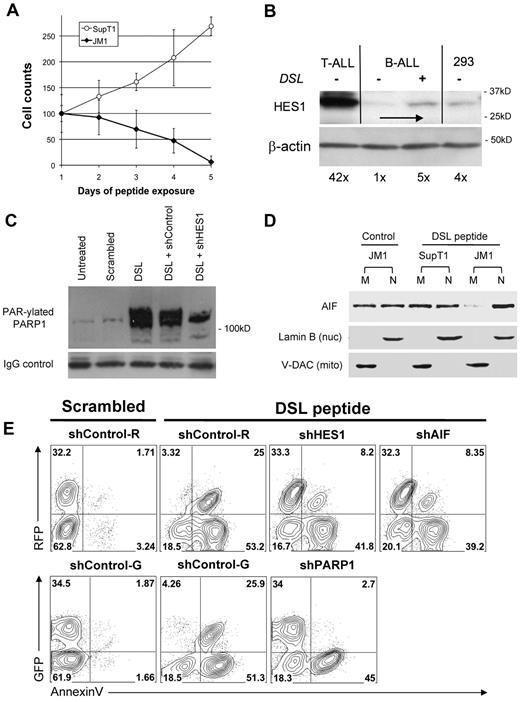
To explore the ability of ligand-mediated activation of the endogenous Notch receptors to reproduce the HES1-mediated effects in B-ALL cells, we used a Notch ligand-mimicking peptide DSL (Delta/Serrate/Lag-2).44 First, exposure to DSL peptide led to a steady decrease in B-ALL (JM1) cell number, while not effecting T-ALL (SupT1) growth (Figure 5A). To confirm that the DSL is activating Notch signaling, we demonstrate that DSL exposure in B-ALL cells leads to a 5-fold up-regulation of HES1 expression (Figure 5B). Note that the DSL ligand induces HES1 expression in B-ALL cells to levels similar to the unrelated HEK-293 cell line and still significantly less than the levels seen in T-ALL cells (42× higher than the unstimulated B-ALL). Importantly, we found that DSL exposure induced a 15-fold increase in PARP1 self–PAR-ylation that could be significantly reduced with transfection of shRNA to HES1 (Figure 5C). Furthermore, exposure to DSL peptide also induced translocation of AIF to the nucleus similar to enforced HES1 expression, as seen in Figure 4E and F (Figure 5D).
Notch ligand peptide DSL induces cell type–specific PARP1 activation, AIF translocation, and apoptosis. (A) The DSL peptide induces cell type–specific growth inhibition and cell death. T-ALL (SupT1) and B-ALL (JM1) cells were incubated with 100μM DSL peptide and were harvested at different points for Trypan blue exclusion viable cell counts. Cell counts were normalized to day 1 (control) (mean ± SD). (B) DSL induces HES1 up-regulation. B-ALL (JM1) cells were incubated with 100μM DSL peptide for 6 hours. Immunoblot for HES1 shows a 5-fold increase over baseline HES1 levels. For comparison, T-ALL (SupT1) and control HEK293 cells are shown. DSL-induced HES1 expression in B-ALL cells is far below constitutive HES1 levels in T-ALL and comparable to the nonmalignant control cells. β-actin used as a loading control. (C) DSL-induced PAR-ylation of PARP1. B-ALL (JM1) cells were incubated with 100μM DSL or scrambled peptide for 48 hours. Cell lysates were immunoprecipitated with anti-PARP1 antibody and immunoblotted with anti-PAR antibody. Increase (15-fold) in PAR-ylated PARP1 was seen after DSL exposure, whereas scrambled peptide had no effect. Transfection with shRNA to HES1 reduced PAR-ylation of PARP1 by 56%, whereas control shRNA did not. Immunoblot probed for IgG to show equal loading of immunoprecipitated protein. (D) DSL induces cell type–specific AIF translocation in B-ALL cells. T-ALL (SupT1) and B-ALL (JM1) cells were exposed to 100μM DSL peptide for 48 hours. Mitochondria and nuclear fractions were probed for AIF, showing near complete translocation of AIF. Lamin B (nuclear) and V-DAC (mitochondrial) confirm purity of fractions. (E) DSL peptide induces apoptosis in B-ALL which depends on HES1, PARP1, and AIF. B-ALL (JM1) cells were transfected with shRNAs against HES1, PARP1, AIF, and controls that coexpress either GFP or RFP, named shControl-G, shControl-R. Transfected cells were incubated with 100μM DSL or scrambled peptide for 72 hours, stained with annexin V, and flow cytometry was performed for GFP/RFP and annexin V. Percentage of events displayed in each quadrant. Nuc indicates nuclear; mito, mitochondrial.
Notch ligand peptide DSL induces cell type–specific PARP1 activation, AIF translocation, and apoptosis. (A) The DSL peptide induces cell type–specific growth inhibition and cell death. T-ALL (SupT1) and B-ALL (JM1) cells were incubated with 100μM DSL peptide and were harvested at different points for Trypan blue exclusion viable cell counts. Cell counts were normalized to day 1 (control) (mean ± SD). (B) DSL induces HES1 up-regulation. B-ALL (JM1) cells were incubated with 100μM DSL peptide for 6 hours. Immunoblot for HES1 shows a 5-fold increase over baseline HES1 levels. For comparison, T-ALL (SupT1) and control HEK293 cells are shown. DSL-induced HES1 expression in B-ALL cells is far below constitutive HES1 levels in T-ALL and comparable to the nonmalignant control cells. β-actin used as a loading control. (C) DSL-induced PAR-ylation of PARP1. B-ALL (JM1) cells were incubated with 100μM DSL or scrambled peptide for 48 hours. Cell lysates were immunoprecipitated with anti-PARP1 antibody and immunoblotted with anti-PAR antibody. Increase (15-fold) in PAR-ylated PARP1 was seen after DSL exposure, whereas scrambled peptide had no effect. Transfection with shRNA to HES1 reduced PAR-ylation of PARP1 by 56%, whereas control shRNA did not. Immunoblot probed for IgG to show equal loading of immunoprecipitated protein. (D) DSL induces cell type–specific AIF translocation in B-ALL cells. T-ALL (SupT1) and B-ALL (JM1) cells were exposed to 100μM DSL peptide for 48 hours. Mitochondria and nuclear fractions were probed for AIF, showing near complete translocation of AIF. Lamin B (nuclear) and V-DAC (mitochondrial) confirm purity of fractions. (E) DSL peptide induces apoptosis in B-ALL which depends on HES1, PARP1, and AIF. B-ALL (JM1) cells were transfected with shRNAs against HES1, PARP1, AIF, and controls that coexpress either GFP or RFP, named shControl-G, shControl-R. Transfected cells were incubated with 100μM DSL or scrambled peptide for 72 hours, stained with annexin V, and flow cytometry was performed for GFP/RFP and annexin V. Percentage of events displayed in each quadrant. Nuc indicates nuclear; mito, mitochondrial.
To determine the time course of these events and to determine whether these events occur in T-ALL cells, we exposed a B-ALL (JM1) and a T-ALL (SupT1) cell line to the DSL peptide for 1-4 days and measured PAR-ylation of PARP1 (through PARP1 IP and probing for PAR), and in nuclear extracts we measured HES1 (Notch activation), nuclear AIF, cleaved caspase-3 (proapoptotic event), and cleaved PARP1 (as a consequence of apoptosis; supplemental Figure 5). This time course shows Notch activation by day 1, PARP1 activation on days 1-3, nuclear translocation of AIF on days 2-4, and cleavage of caspase-3/PARP1 on days 3-4. Although HES1 was similarly up-regulated in the T-ALL line as expected, little PARP1 activation, nuclear AIF, or induced caspase-3/PARP1 cleavage were seen.
To determine whether these events were dependent on both Notch and PARP1 activation in a panel of ALL lines, we exposed 3 B-ALL (JM1, Nalm6, 697) and 3 T-ALL (SupT1, Jurkat, Molt4) lines to either DSL peptide or scrambled peptide for 48 or 72 hours. At 48 hours, we detected increased PAR-ylation of PARP1 in B-ALL cells which was partially abrogated by the PARP inhibitor 3ABA (supplemental Figure 5). This 3ABA treatment led to reduction in caspase-3 cleavage, suggesting a link between PARP1 activity and apoptosis in B-ALL cells. We also confirmed the Notch specificity of this phenomenon through the use of a Notch inhibiting GSI (supplemental Figure 5B). GSI treatment abrogated the DSL-mediated up-regulation of HES1 expression and diminished the PAR-ylation of PARP1, nuclear AIF, and PARP1 cleavage. Although GSI inhibited the DSL-mediated HES1 expression in T-ALL lines as expected, there was no significant effect on nuclear AIF and cleaved PARP1. These studies show the dependence of both Notch activation and PARP1 activity in the proapoptotic effect of DSL peptide treatment in B-ALL.
To more specifically determine whether HES1, PARP1, or AIF were necessary for the proapoptotic effects of Notch signaling in B-ALL cells, we transfected JM1 B-ALL cells with shRNA constructs targeting each molecule (Figure 5E). These constructs coexpress either GFP or red fluorescent protein (RFP); therefore, untransfected (parental) cells can be distinguished from transfected cells. Cells transfected with shControl or shHES1, shPARP1, shAIF vectors were exposed to either scrambled peptide or DSL peptide (100μM) for 72 hours and after stained with annexin V. As shown in column 2, both shControl-transfected (GFP+ or RFP+) and untransfected cells were equally sensitive to DSL treatment (as measured by annexin V binding) and were hardly effected by scrambled peptide (column 1; Figure 5E). Importantly, transfection with shHES1, shPARP1, and shAIF all significantly reduced the annexin V binding of GFP+ or RFP+ cells, but the untransfected cells remained sensitive to DSL treatment (columns 3-4; Figure 5E). This provides strong evidence that each of these proteins is critical for this effect and establishes a mechanism for Notch-induced apoptosis in B-ALL.
Differences in HES1 and PARP1 expression in B-ALL and T-ALL patient samples
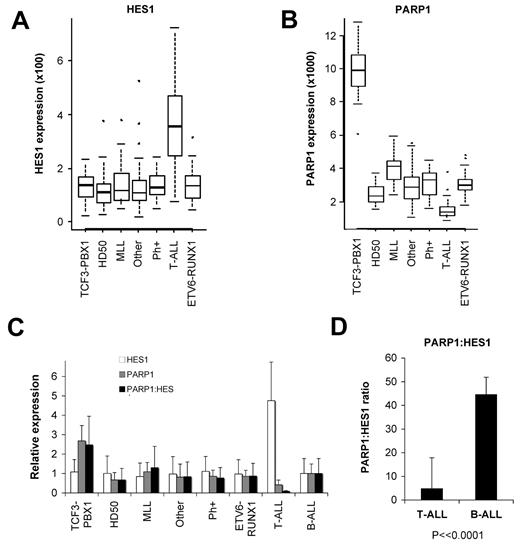
To determine whether the expression of HES1 and PARP1 in leukemia patient samples would suggest a rationale for these contrasting effects in B-ALL versus T-ALL, we analyzed mRNA expression data from 206 pediatric ALL samples (34 T-ALL, 172 B-ALL). In this cohort, Notch receptors were most strongly expressed in T-ALL samples, as expected (supplemental Figure 2). However, many B-ALL samples express Notch receptors, in particular the TCF3-PBX1 and ETV6-RUNX1 subsets. Importantly, despite Notch receptor expression, B-ALL samples show 4.8-fold lower levels of HES1 expression than T-ALL samples, suggesting lower levels of Notch activation in B-ALL samples (Figure 6A; supplemental Figure 2; P = 2.3 × 10−20, LIMMA; FDR = 1.4 × 10−18). In contrast, PARP1 is expressed at low levels in T-ALL samples, with higher levels in most B-ALL samples (Figure 6B; supplemental Figure 2; P = 4.5 × 10−19, LIMMA; FDR = 2.3 × 10−17). Interestingly, the TCF3-PBX1 t(1;19) subgroup has > 2.5-fold higher PARP1 levels than other B-ALL subgroups (P = 2.7 × 10−37, analysis of variance). In Figure 1A and in our prior publication we demonstrate that the 697 B-ALL cell line, which carries the TCF3-PBX1-generating t(1;19) translocation, is sensitive to Notch/HES1 growth inhibition and apoptosis.9
Differences in HES1 and PARP1 expression in B-ALL and T-ALL and the PARP1/HES1 ratio. Gene expression profiling was performed with U133A microarrays (Affymetrix) for 206 pediatric ALL patient samples (34 T-ALL, 172 B-ALL; see supplemental Figure 2). (A) Relative expression levels displayed with box-whisker quartile plots show higher levels of HES1 expression in T-ALL compared with multiple subgroups of B-ALL (P = 2.3 × 10−20, LIMMA; FDR = 1.4 × 10−18). (B) Expression data show that T-ALL samples express lowers levels of PARP1 than all B-ALL subgroups (P = 4.5 × 10−19, LIMMA; FDR = 2.3 × 10−17). Of note, the TCF3-PBX1 B-ALL subgroup expresses particularly high levels of PARP1 among B-ALL subgroups (P = 2.7 × 10−37, analysis of variance; FDR = 5.9 × 10−33). (C) Expression data were normalized to the combined B-ALL samples, and relative levels of HES1, PARP1, and a PARP1/HES1 ratio are shown. Note high HES1, low PARP1, and thus a low PARP1/HES1 ratio for T-ALL compared with B-ALL subgroups and combined B-ALL samples (P = 2.9 × 10−11, LIMMA). (D) Ratios of raw expression values for T-ALL and B-ALL samples (P < .0001). MLL indicates mixed-lineage leukemia; Ph+, Philadelphia chromosome (error bars represent ± 1 SD).
Differences in HES1 and PARP1 expression in B-ALL and T-ALL and the PARP1/HES1 ratio. Gene expression profiling was performed with U133A microarrays (Affymetrix) for 206 pediatric ALL patient samples (34 T-ALL, 172 B-ALL; see supplemental Figure 2). (A) Relative expression levels displayed with box-whisker quartile plots show higher levels of HES1 expression in T-ALL compared with multiple subgroups of B-ALL (P = 2.3 × 10−20, LIMMA; FDR = 1.4 × 10−18). (B) Expression data show that T-ALL samples express lowers levels of PARP1 than all B-ALL subgroups (P = 4.5 × 10−19, LIMMA; FDR = 2.3 × 10−17). Of note, the TCF3-PBX1 B-ALL subgroup expresses particularly high levels of PARP1 among B-ALL subgroups (P = 2.7 × 10−37, analysis of variance; FDR = 5.9 × 10−33). (C) Expression data were normalized to the combined B-ALL samples, and relative levels of HES1, PARP1, and a PARP1/HES1 ratio are shown. Note high HES1, low PARP1, and thus a low PARP1/HES1 ratio for T-ALL compared with B-ALL subgroups and combined B-ALL samples (P = 2.9 × 10−11, LIMMA). (D) Ratios of raw expression values for T-ALL and B-ALL samples (P < .0001). MLL indicates mixed-lineage leukemia; Ph+, Philadelphia chromosome (error bars represent ± 1 SD).
In general, we found high PARP1 and low HES1 expressions in B-ALL, with a high PARP1/HES1 ratio, especially in the t(1;19) TCF3-PBX1 subgroup (Figure 6C; P = 2.9 × 10−11, LIMMA). In contrast, T-ALL cells have low PARP1 and high HES1 levels, leading to a > 10-fold lower PARP1/HES1 ratio than the combined B-ALL samples (Figure 6D; P < .0001, t test). Given our data which reveal bidirectional functional effects of the PARP1/HES1 interaction, we hypothesize that differing PARP1/HES1 ratios may influence the functional consequences of PARP1 and HES1 in B-ALL versus T-ALL.
Discussion
The mechanisms responsible for the cell-specific effects of Notch signaling are not well understood. In the current study we describe one mechanism that may contribute to the proapoptotic effects of Notch activation seen in B-cell ALL. We demonstrate that the interaction between PARP1 and HES1 in B-ALL has consequences both for HES1-mediated repression and PARP1 activity. First, PARP1 binds HES1 by the critical bHLH and orange domains and subverts the HES1-negative feedback loop by inhibiting the HES1 transcriptional repressor function. Second, HES1 induces activation of PARP1, leading to self and global PAR-ylation, reduction in NAD and ATP levels, nuclear translocation of AIF, and subsequent apoptosis-positive feedback loop resulting in B-ALL apoptosis. As a potential therapeutic approach, we demonstrate that activation of Notch signaling through Notch ligand-mimicking peptide DSL causes induction of apoptosis that is dependent on Notch receptor cleavage, HES1 expression, PARP1 expression and activity, and AIF nuclear expression. Thus, our results suggest that in B-ALL cells the activation of Notch signaling resulting in HES1-mediated PARP1 activation and subsequent AIF-induced apoptosis may in part explain the contrasting effects of Notch signaling in B-ALL versus T-ALL and may represent a mechanism of cell-specific consequences of Notch activation.
Although we have defined the HES1/PARP1/AIF mechanism as outlined above, the consequences of Notch/HES1-mediated PARP1 activation may be diverse. Indeed, activation of PARP1 plays several critical roles in DNA repair and transcriptional regulation which may contribute to a potential therapeutic effect. For example, PAR-ylation of DNA repair enzymes by PARP1 leads to inhibition of DNA repair, resulting in genomic instability and apoptosis.42 In addition, PARP1 activation causes NAD(P)H dehydrogenase, quinone 1 (NQO1)–dependent nucleotide depletion and increased cell death by depleting energy substrates,45 reminiscent of the Notch/HES1-induced decrease in ATP levels we observe in B-ALL. Therefore, we postulate that the proapoptotic effect of Notch/HES1 signaling may be due in part to depletion of NAD+ and ATP, rendering B-ALL cells susceptible to cell death. Recent findings have also shown that PARP1 activation can inhibit DNA methyltransferase (DNMT1) activity that results in global hypomethylation,46 potentially providing an epigenetic mechanism to Notch/HES1 effects in B-ALL. Indeed, we have found that promoter-associated cytosine-phosphate-guanosine islands of Notch receptors and HES genes are heavily methylated in B-ALL cells in contrast to normal CD19+ B cells and T-ALL cells (Guillermo Garcia-Manero, personal communication). Treatment with the DNA methyltransferase inhibitor decitabine led to up-regulation of HES genes and cell death. These additional mechanisms require further study; however, they highlight that the novel finding of Notch/HES1-mediated activation of PARP1 in B-ALL may have diverse consequences.
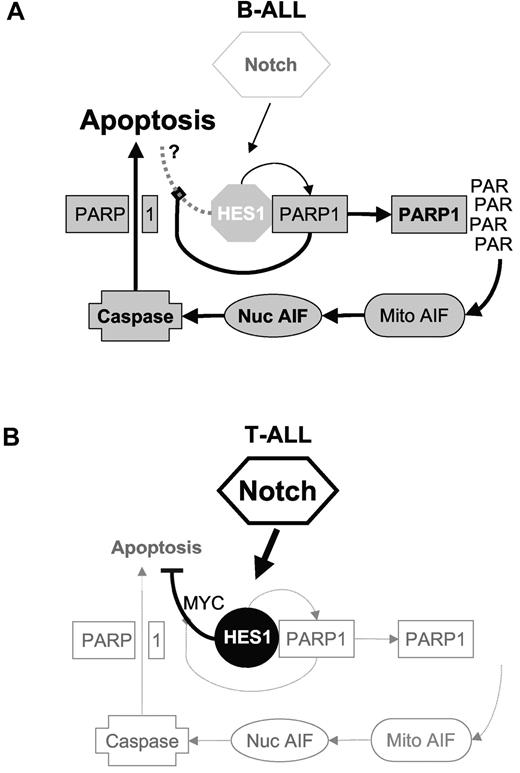
In this study we reveal a cell-specific effect of Notch/HES1 activation that contributes to Notch-mediated tumor-inhibiting effects in B-ALL (Figure 7). We found that high PARP1/HES1 ratios in B-ALL patient samples may promote this mechanism, whereas low PARP1/HES1 ratios might limit the effect of this proapoptotic mechanism in T-ALL. In addition, Notch signaling in T-ALL induces several prosurvival pathways, including c-MYC, AKT, nuclear factor κB, and cyclin D3 signaling, which have not been seen in B-ALLs.47-49 Although it is likely that Notch signaling induces multiple pathways in both B- and T-ALL cells, cell-specific differences do exist, and PARP1/HES1 interaction appears to shift the balance toward tumor suppression in B-ALL, providing a potential therapeutic opportunity. As such Notch agonist peptides are an interesting possibility but warrant further preclinical investigation.
Models of HES1 and PARP1 effects in B-ALL versus T-ALL. On the basis of our findings, we hypothesize that differences in the ratio of PARP1 to HES1 in B-ALL versus T-ALL may partially explain the differences seen in response to Notch/HES1 signaling in these 2 cell types. (A) In B-ALL, low Notch activation and thus low HES1, with higher levels of PARP1, lead to a high PARP1/HES1 ratio. Notch activation leads to HES1 expression, but high PARP1 levels inhibit HES1 function. In addition, increased HES1 induces activation of PARP1, resulting in high PAR levels, leading to nuclear translocation of AIF, caspase activation, PARP1 cleavage, and apoptosis. (B) In contrast, T-ALL have constitutive Notch signaling with high HES1 expression and low PARP1 expression, resulting in a low PARP1/HES1 ratio. Thus, because of a relative lack of PARP1, HES1 repressor function is maintained, and increased HES1 does not lead to significant PARP1 activation or apoptosis. Rather HES1 appears to inhibit apoptosis through repression of phosphatase with tensin homology (PTEN).50
Models of HES1 and PARP1 effects in B-ALL versus T-ALL. On the basis of our findings, we hypothesize that differences in the ratio of PARP1 to HES1 in B-ALL versus T-ALL may partially explain the differences seen in response to Notch/HES1 signaling in these 2 cell types. (A) In B-ALL, low Notch activation and thus low HES1, with higher levels of PARP1, lead to a high PARP1/HES1 ratio. Notch activation leads to HES1 expression, but high PARP1 levels inhibit HES1 function. In addition, increased HES1 induces activation of PARP1, resulting in high PAR levels, leading to nuclear translocation of AIF, caspase activation, PARP1 cleavage, and apoptosis. (B) In contrast, T-ALL have constitutive Notch signaling with high HES1 expression and low PARP1 expression, resulting in a low PARP1/HES1 ratio. Thus, because of a relative lack of PARP1, HES1 repressor function is maintained, and increased HES1 does not lead to significant PARP1 activation or apoptosis. Rather HES1 appears to inhibit apoptosis through repression of phosphatase with tensin homology (PTEN).50
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the members of our laboratory and the Department of Pediatric Research. We also thank Robert M. Sutphin, Michelle C. Barton, and David H. Hawke for their helpful contributions to this project.
This work was supported by the National Cancer Institute (grants K08CA101934 and R01CA138816; principal investigator, P.A.Z.-M.).
National Institutes of Health
Authorship
Contribution: S.K. designed research, performed experiments, and wrote the manuscript; W.F. and R.H. performed experiments; G.S. performed experiments and performed statistical analysis; C.G.M. provided critical data; J.M. provided critical reagents; P.A.Z.-M. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrick A. Zweidler-McKay, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 853, Houston, TX 77030; e-mail: PZweidler@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal