Abstract
The human T-lymphotropic virus type I oncoprotein Tax is critical for T-cell transformation, acting mainly through nuclear factor kappa B essential modulator (NEMO) binding and subsequent nuclear factor-κB activation. Tax localizes to Tax nuclear bodies and to the centrosome and is subjected to ubiquitylation and small ubiquitin-like modifier (SUMO)ylation, which are both necessary for complete transcriptional activation. Using the photoconvertible fluorophore Dendra-2 coupled with live video confocal microscopy, we show for the first time that the same Tax molecule shuttles among Tax nuclear bodies and between these nuclear bodies and the centrosome, depending on its posttranslational modifications. Ubiquitylation targets Tax to nuclear bodies to which NEMO is recruited and subsequently SUMOylated. We also demonstrate that Tax nuclear bodies contain the SUMOylation machinery including SUMO and the SUMO conjugating enzyme Ubc9, strongly suggesting that these nuclear bodies represent sites of active SUMOylation. Finally, both ubiquitylation and SUMOylation of Tax control NEMO targeting to the centrosome. Altogether, we are proposing a model where both ubiquitylation and SUMOylation of Tax control the shuttling of Tax and NEMO between the cytoplasmic and nuclear compartments.
Introduction
Tax, the viral oncoprotein encoded by the pX region of the human T-lymphotropic virus type I (HTLV-I) genome is a key player in the pathogenesis of HTLV-I–associated diseases, mainly adult T-cell leukemia/lymphoma and tropical spastic paraparesis/HTLV-associated myelopathy. Tax deregulates the cellular machinery at multiple levels leading to the transformation of HTLV-I–infected T lymphocytes, predominantly through the activation of the nuclear factor-κB (NF-κB) pathway, which regulates key genes implicated in inflammation, apoptosis, and oncogenesis.1,2 A corner stone in this NF-κB activation is Tax binding to the regulatory subunit of the IκB kinase (IKK) complex, IKKγ (also known as NEMO).3,4 Tax binding to NEMO phosphorylates both IKKα and IKKβ leading to the formation of the IKK complex that phosphorylates the NF-κB inhibitors IκBs. This leads to the nuclear translocation of the active NF-κB dimers and to transcriptional activation of target genes.5 Tax/NEMO binding can also induce the IKKα-dependent processing of the NF-κB p100 precursor protein to its active p52 form.6
Tax displays a dual nuclear and cytoplasmic localization with functions that are essential for cellular transformation in each compartment.7,8 In the nucleus, Tax is predominantly located in RelA-enriched nuclear bodies.9 In the cytoplasm, a major fraction of Tax lays in perinuclear hotspots colocalizing with the centrosome or microtubule organizing center in close association with the cis-Golgi compartment.10,11
Posttranslational modification of proteins regulates protein functions by modifying their subcellular localization, stability, and/or network of interaction. We and others have described different forms of Tax posttranslational modifications including phosphorylation,12,13 acetylation,14 ubiquitylation, and small ubiquitin-like modifier (SUMO)ylation,15,16 all of which are implicated in Tax-mediated activation of gene expression. Indeed, we showed that Tax is differentially ubiquitylated by either K-48 ubiquitin chains leading to Tax degradation by the proteasome or by K-63 ubiquitin chains that mediates IKK recruitment to the centrosome and IKK activation.11 Finally, Tax SUMOylation is involved in nuclear body formation and transcriptional activation.17,18 However, whether the same or distinct Tax molecules shuttle between the centrosome and the nuclear bodies and the role of Tax posttranslational modifications in this potential dynamic trafficking of Tax and NEMO remain unclear.
In this study, we investigated the molecular determinants governing Tax shuttling as well as NEMO recruitment into different subcellular compartments. Using a photoconvertible fluorophore coupled with live video confocal microscopy, we demonstrate that the same Tax molecule shuttles among Tax nuclear bodies and between Tax nuclear bodies and the centrosome. Both ubiquitylation and SUMOylation of Tax control the bidirectional and dynamic shuttling of Tax and NEMO between nuclear bodies and the centrosome, and Tax ubiquitylation is involved in NEMO SUMOylation as well. We finally identified the presence of the SUMOylation machinery in Tax nuclear bodies, strongly suggesting that in addition to their transcriptional role, these nuclear bodies are sites of active SUMOylation.
Methods
Cell culture and transfection
HeLa, 293T, mouse embryonic fibroblasts (MEF) and human lung fibroblasts (IMR90) cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, 2mM glutamine, and antibiotics. HuT102 and Jurkat cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, 2mM glutamine, and antibiotics (all from Gibco, Invitrogen). HeLa, MEF, and IMR90 cells were transfected with DNA using Lipofectamine 2000 (Gibco, Invitrogen) or with small interfering RNA (siRNA) using HiPerFect (QIAGEN) according to the manufacturer's recommendations. 293T cells were transfected by the calcium phosphate procedure.
Plasmids and mutagenesis
Tax lysine mutants Tax K4-8R and Tax K6-8R, in which 5 or 3 central lysines, respectively, were substituted for arginines, have been described previously.17 Tax K4-8R-Ub and Tax K6-8R-Ub plasmids were generated by the fusion of the ubiquitin coding sequence to the carboxy-terminus of Tax K4-8R and Tax K6-8R, respectively, with a mutation of the carboxy-terminal glycine residue of ubiquitin to alanine to prevent possible conjugation to other substrates. K4-8R-SUMO1 and K6-8R-SUMO1 plasmids were generated by the fusion of the SUMO1 coding sequence to the carboxy-terminus of Tax K4-8R and Tax K6-8R, respectively. Tax-Dendra2 fusion was generated by fusion of the Dendra2 coding sequence derived from pDendra2-N vector (Evrogen) to the carboxy-terminus of Tax. NEMO-6His plasmid was generated by polymerase chain reaction extraction of NEMO (NM-003639) with 6 × His fused to its N terminus from pET-NEMO (Addgene) followed by ligation into pSG5-M. All mutated constructs were sequenced. Plasmids encoding hemagglutinin (HA)–ubiquitin,15 HA-SUMO1 (gift from T. Kamitani, Houston, TX), SUMO1-yellow fluorescent protein (YFP) and Ubc9-green fluorescent protein (GFP; gift from H.d.T.), and YFP-centrin (gift from C.P.) were also used.
Antibodies
Tax was detected using the monoclonal antibody (mAb) 168-A51 (National Institutes of Health [NIH] AIDS Research and Reference Reagent Program) or human anti–HTLV-I sera. HA-tagged proteins were revealed with the 12CA5 mAb (Roche). Rabbit polyclonal antibodies against IKK-γ, γ-tubulin, laminA/C, SUMO1, and Ubc9 and monoclonal antibody against HistoneH1 were obtained from Santa Cruz Biotechnology. Antibodies against SUMO1 and SUMO2 were also obtained from Zymed laboratories. γ-tubulin was also revealed using a rabbit polyclonal antibody or a mouse monoclonal antibody (Abcam). Polyubiquitylated proteins were revealed using the FK2 mAb from BIOMOL International. The lactate dehydrogenase (LDH) was revealed using a mAb from Sigma-Aldrich and the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using a mAb from Abcam. For immunofluorescence, the following secondary antibodies (Jackson Immunoresearch) were used: donkey anti–mouse conjugated to fluorescein isothiocyanate, AMCA [amino-methyl-coumarin-acetate], and Cy5. Donkey anti–rabbit immunoglobulin G (IgG)–conjugated Texas red and donkey anti–human IgG conjugated to AMCA. Goat anti–human IgG Alexa 488 and goat anti–rabbit Alexa 594 were obtained from (Molecular Probes).
siRNA
The following siRNAs were used: SUMO-1 (ggacaggauagcagugaga–dTdT), SUMO-2 (agggaugaaucuguaacuuaa–dTdT), SUMO-3 (gaggcauacaccacuuagu–dTdT), and control SiRNA directed against GFP (gcaagcugacccugaaguucau).
Cell fractionation
Cells were lysed on ice in (10mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.9, 10mM KCL, 1.5mM MgCl2, 1 mM dithiothreitol, 1× protease inhibitor cocktail [Roche]) for 10 minutes. The cytoplasmic fraction was obtained by spinning the cells for 10 minutes at 400g. The intermediate fraction was obtained by washing the pellet with (20mM Tris, pH 7.4, 100mM NaCl, 1%Triton, 0.5% sodium deoxycholate, 0.05% sodium dodecyl sulfate, 1× protease inhibitor cocktail) 3 times, and each time spinning for 10 minutes at 400g. Finally, the remaining pellet was lysed on ice in the following lysis buffer (50mM Tris, pH 7.5, 150mM NaCl, 0.5% NP-40, 0.5% Triton, 1× protease inhibitor cocktail) for 30 minutes to get the nuclear fraction. All fractions were cleared by centrifugation for 10 minutes at 12 000g.
Immunoblots
Protein concentration was determined by the Bio-Rad Laboratories Dc protein assay. Immunoblots were performed as described.17
Immunofluorescence and confocal microscopy
Twenty-four hours posttransfection, HeLa and MEF cells cultured on coverslips, were fixed with methanol at −20°C and washed with phosphate-buffered saline (PBS). Cells were blocked in PBS containing 0.5% gelatin and 0.25% bovine serum albumin, and incubated with the primary antibody. The preparations were then washed with PBS, incubated with the secondary antibody, washed then mounted with the Prolong Antifade Kit (Molecular Probes), and examined by fluorescence or confocal microscopy. Images were acquired by confocal microscopy using either a Zeiss LSM 510 META confocal laser microscope or a Zeiss LSM 710 confocal microscope (Carl Zeiss) with a Plan Apochromat 63×/1.4 numeric aperture oil-immersion objective using the LSM510v4.0sp2 or Zen 2009 (Carl Zeiss). Twenty-three z-sections (average thickness 0.3 μm) were collected per examined cell. To restore image quality and accuracy and reverse the effect of the point-spread function, and thus to produce images with increased resolution, when indicated, images or image-stacks where processed using deconvolution software (Autodeblur; ImageQuant), which uses blind iterative algorithms.
Live video microscopy was used for follow-up of intracellular distribution of the fluorescent proteins Tax-Dendra2, 37°C SUMO1-YFP, and YFP-Centrin in transfected HeLa and IMR90 cells. When indicated, green Tax-Dendra2 protein accumulated either in nuclear bodies or in perinuclear centrosomal aggregates was irreversibly photoconverted to red using a 405 laser. Cells were followed up with live video microscopy directly after photoconversion.
Ni-NTA pull-down
Ni-NTA pull-down was performed as described.15 Briefly, 36 hours posttransfection, cells were lysed in reducing and highly denaturing conditions and incubated with Ni-NTA beads. Beads were washed and bound proteins were eluted. Products were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes and blotted with specific antibodies.
Results
Tax dynamic trafficking between nuclear bodies and centrosome
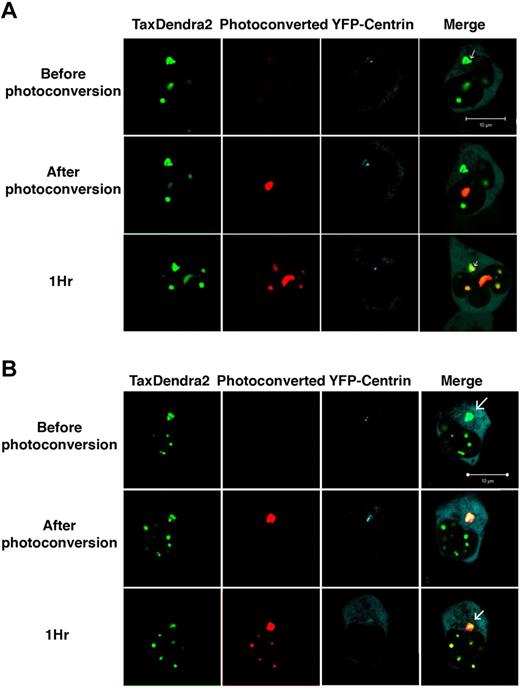
We and others have recently implicated posttranslational Tax modifications, namely ubiquitylation and SUMOylation, in Tax targeting to RelA-enriched nuclear bodies and to the centrosome.11,17,18 To investigate whether a unique molecule or distinct Tax molecules shuttle between these different subcellular compartments, we fused the photoconvertible fluorophore (Dendra-2)19 to the C-terminal end of Tax and followed the fusion protein by confocal microscopy. In transfected cells, the fusion protein maintained the same subcellular distribution as its wild-type counterpart, in addition to its ability to activate both NF-κB and CREB (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Moreover, in both human lung fibroblasts IMR90 cells (Figure 1A-B top panels) and HeLa cells (not shown), Tax-Dendra-2 was located in 2 preferential sites: Tax nuclear bodies present in 55%-60% of cells and centrosomal aggregates (white arrow) colocalizing with YFP-centrin found in 90%-95% of Tax positive cells.11
Tax dynamic trafficking between nuclear bodies and centrosome in human fibroblasts. Human fibroblasts IMR90 cells were cotransfected with Tax tagged with a green-to-red photoconvertible fluorescent protein, Dendra2 (2 μg) and YFP-Centrin (0.5 μg). Irreversible photoconversion from green to red was performed on selected nuclear body (A) or on juxta-nuclear concentrates of Tax-Dendra2 (B) 4 hours after transfection using a 405 laser. The white arrows indicate centrosomal aggregates colocalizing with YFP-centrin. Cells were imaged using a LSM710 confocal laser microscope in the green (lane 1), red (lane 2), and yellow (lane 3) channels before and after photoconversion. Original yellow color was replaced by cyan for better clarity. Scale bar, 10 μm.
Tax dynamic trafficking between nuclear bodies and centrosome in human fibroblasts. Human fibroblasts IMR90 cells were cotransfected with Tax tagged with a green-to-red photoconvertible fluorescent protein, Dendra2 (2 μg) and YFP-Centrin (0.5 μg). Irreversible photoconversion from green to red was performed on selected nuclear body (A) or on juxta-nuclear concentrates of Tax-Dendra2 (B) 4 hours after transfection using a 405 laser. The white arrows indicate centrosomal aggregates colocalizing with YFP-centrin. Cells were imaged using a LSM710 confocal laser microscope in the green (lane 1), red (lane 2), and yellow (lane 3) channels before and after photoconversion. Original yellow color was replaced by cyan for better clarity. Scale bar, 10 μm.
Four hours after transfection, 1 nuclear body was irreversibly photoconverted to red using a 405 laser (Figure 1A second panel). One hour after the initial photoconversion, the red signal was present in unphotoconverted Tax nuclear bodies and in the centrosome colocalizing with YFP-centrin (Figure 1A third panel). Importantly, no red signal was observed during follow-up of cells in the absence of photoconversion, ruling out the possibility of spontaneous photoconversion of Dendra-2 (supplemental Figure 2). Similar results were observed in HeLa cells (not shown).
To investigate whether shuttling of Tax molecules also occurs from the centrosome to nuclear bodies, perinuclear concentrates of Tax colocalizing with YFP-centrin were photoconverted 4 hours after transfection (Figure 1B second panel). Interestingly, the red signal appeared after 1 hour in Tax containing nuclear bodies, demonstrating Tax shuttling from the centrosome to nuclear bodies (Figure 1B third panel). Again, similar results were observed in HeLa cells (not shown). Altogether, these results clearly demonstrate that Tax molecules are in a dynamic bidirectional shuttling state between the centrosome and the nuclear bodies and among distinct nuclear bodies.
Tax shuttles between preexisting Ubc9 nuclear bodies and the centrosome
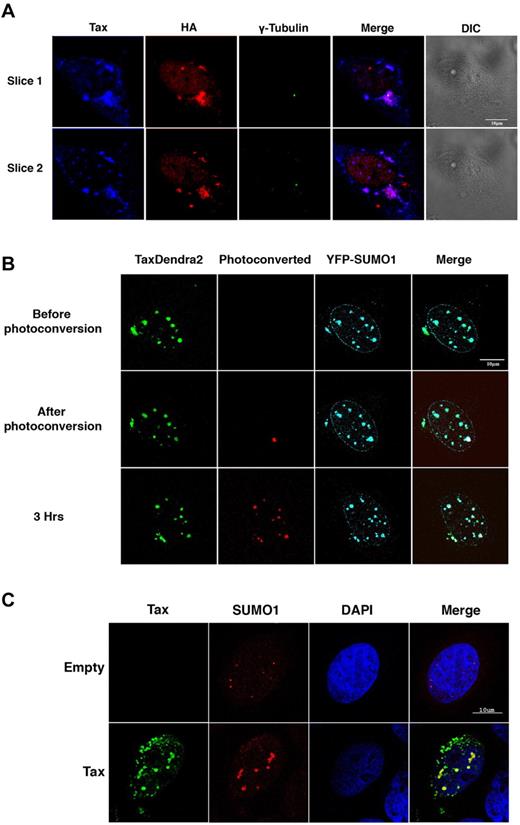
We and others have previously reported the presence of ubiquitin in centrosomal Tax aggregates and the presence of SUMO in Tax nuclear bodies.11,17,18 To confirm these findings, HeLa cells were cotransfected with Tax and HA-ubiquitin and analyzed by confocal microscopy. Overlay analysis demonstrated a complete colocalization of Tax, ubiquitin, and γ-tubulin at the centrosome (Figure 2A). Similarly, HeLa cells were cotransfected with Tax-Dendra2 and YFP-SUMO1. Overlay revealed colocalization of Tax-Dendra-2 and YFP-SUMO1 in large nuclear bodies (Figure 2B top panel). In addition, Tax-free small SUMO nuclear bodies were observed (Figure 2B) that likely represented promyelocytic leukemia (PML) nuclear bodies (Figure 3B).
Tax trafficking between SUMO-enriched nuclear bodies. (A) HeLa cells were cotransfected with Tax and HA-ubiquitin and stained by immunofluoresence with anti–HTLV-I sera (blue), anti-HA polyclonal antibody (red) and anti–γ-tubulin mAb (green). Original colors of the immunofluoresence have been changed for clarity reasons. The 2 lanes represent different sections of the same cell. (B) HeLa cells were cotransfected with Tax-Dendra2 (2 μg) and SUMO1-YFP (50 ng). Irreversible photoconversion from green to red was performed on selected nuclear bodies 4 hours after transfection using a 405 laser. Cells were imaged using a LSM510 META laser confocal microscope in the green (lane 1) and red channels (lane 2) before and after photoconversion. Scale bar, 10 μm. (C) HeLa cells were transfected with Tax or empty vector and stained by double immunofluoresence with anti-Tax mAb (green) and anti-SUMO1 polyclonal antibody (red).
Tax trafficking between SUMO-enriched nuclear bodies. (A) HeLa cells were cotransfected with Tax and HA-ubiquitin and stained by immunofluoresence with anti–HTLV-I sera (blue), anti-HA polyclonal antibody (red) and anti–γ-tubulin mAb (green). Original colors of the immunofluoresence have been changed for clarity reasons. The 2 lanes represent different sections of the same cell. (B) HeLa cells were cotransfected with Tax-Dendra2 (2 μg) and SUMO1-YFP (50 ng). Irreversible photoconversion from green to red was performed on selected nuclear bodies 4 hours after transfection using a 405 laser. Cells were imaged using a LSM510 META laser confocal microscope in the green (lane 1) and red channels (lane 2) before and after photoconversion. Scale bar, 10 μm. (C) HeLa cells were transfected with Tax or empty vector and stained by double immunofluoresence with anti-Tax mAb (green) and anti-SUMO1 polyclonal antibody (red).
Four hours after transfection, a YFP-SUMO1–containing Tax nuclear body was photoconverted to red (Figure 2B middle panel). After 3 hours of follow-up, red signal appeared in other YFP-SUMO1 containing nuclear bodies and in perinuclear aggregates (Figure 2B bottom panel), indicating Tax shuttling between SUMO-enriched Tax nuclear bodies and from nuclear bodies to the centrosome. To confirm these findings with endogenous SUMO, HeLa cells were transfected with Tax and stained with antibodies against endogenous SUMO1. In mock-transfected cells, small discrete SUMO1 containing nuclear bodies were observed. In contrast, in Tax-transfected cells, Tax and SUMO1 colocalized in large nuclear bodies (Figure 2C). Tax also colocalized with endogenous SUMO2,3 (data not shown).
SUMO recruitment into Tax nuclear bodies suggests that these bodies may be sites of active SUMOylation. To confirm this hypothesis, we checked for the presence of Ubc9 in Tax nuclear bodies. Ubc9 is a highly conserved protein known to function as an E2-SUMO conjugating enzyme with a major nuclear localization. Previous studies reported the presence of Ubc9 in PML nuclear bodies.20 We and others have shown that these PML bodies are distinct from Tax nuclear bodies.17 HeLa cells were cotransfected with Ubc9-GFP and Tax and immunostained with antibodies against Tax or PML. Confocal microscopy analysis revealed Ubc9-positive nuclear bodies in both Tax negative and Tax positive cells (Figure 3A-B). In mock-transfected cells, some Ubc9 bodies corresponded to PML nuclear bodies, whereas others were PML-free (Figure 3B). In Tax-positive cells, we observed a perfect colocalization of Tax and Ubc9 nuclear bodies, distinct from PML bodies (Figure 3A-B). Tax/Ubc9 colocalization was confirmed in Tax-transfected MEF cells immunostained with antibodies against Tax and endogenous Ubc9 (Figure 3C). Altogether, these findings suggest that Tax is recruited to preexisting Ubc9 nuclear bodies that likely represent sites of active SUMOylation.
Tax is recruited to preexisting Ubc9 nuclear bodies. (A) HeLa cells were cotransfected with Ubc9-GFP and empty vector or Tax and stained by immunofluoresence with anti-Tax mAb (red). (B) HeLa cells were cotransfected with Ubc9-GFP and empty vector or Tax and stained by immunofluoresence with anti–HTLV-I sera (blue) and anti-PML mAb (red). (C) MEFs were transfected with Tax and stained by double immunofluoresence with anti-Tax mAb (green) and anti-Ubc9 polyclonal antibody (red). Scale bar, 10 μm.
Tax is recruited to preexisting Ubc9 nuclear bodies. (A) HeLa cells were cotransfected with Ubc9-GFP and empty vector or Tax and stained by immunofluoresence with anti-Tax mAb (red). (B) HeLa cells were cotransfected with Ubc9-GFP and empty vector or Tax and stained by immunofluoresence with anti–HTLV-I sera (blue) and anti-PML mAb (red). (C) MEFs were transfected with Tax and stained by double immunofluoresence with anti-Tax mAb (green) and anti-Ubc9 polyclonal antibody (red). Scale bar, 10 μm.
Tax ubiquitylation and SUMOylation control NEMO targeting to distinct subcellular compartments
We then assessed the intracellular distribution of NEMO in Tax-positive cells. HeLa cells were transfected with Tax and stained with antibodies against endogenous SUMO1 or endogenous NEMO. In Tax-negative cells, small discrete SUMO1-positive nuclear bodies were observed, and endogenous NEMO displayed a diffuse staining. In contrast, in Tax-transfected cells, Tax colocalized with endogenous NEMO in perinuclear centrosomal aggregates as reported11 as well as with SUMO1-enriched nuclear bodies (supplemental Figure 3).
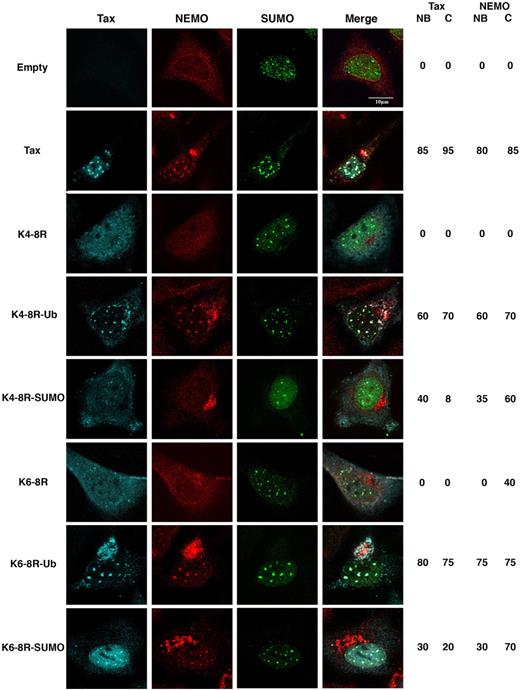
To investigate the differential role of Tax ubiquitylation and SUMOylation in NEMO recruitment to these distinct subcellular compartments, HeLa cells were cotransfected with HA-tagged SUMO1 along with wild-type Tax or the Tax K4-8R or K6-8R mutant, in which 5 and 3 central lysines of Tax, respectively were mutated to arginines. Tax K4-8R is defective for Tax ubiquitylation, Tax SUMOylation, and Tax binding to IKK, but retains the ability to activate the CREB pathway.17 Tax K6-8R is defective for Tax SUMOylation, has an attenuated ubiquitylation and NF-κB transcriptional activation, but retains a wild-type phenotype for IKK binding and for CREB activation.17 Transfected cells were immunostained with antibodies against Tax, NEMO, and HA (SUMO1). Confocal microscopy analysis showed that wild-type Tax was able to recruit NEMO both into Tax and SUMO1 containing nuclear bodies (80%) and at the centrosome (85%; Figure 4). In contrast, the K4-8R Tax mutant displayed a diffuse staining and failed to recruit NEMO, whereas the K6-8R Tax mutant retained a slight ability to recruit NEMO at the centrosome (Figure 4 and supplemental Figure 4). Fusing a ubiquitin moiety to K4-8R or to K6-8R mutants restored the intracellular distribution of Tax as well as NEMO recruitment to Tax containing nuclear bodies (60% and 75%, respectively) and at the centrosome (70% and 75%, respectively; Figure 4). On the other hand, fusing a SUMO1 moiety to both mutants only restored NEMO recruitment at the centrosome (60% for K4-8R and 70% for K6-8R) without Tax at this site, and a very weak recruitment of NEMO into small Tax containing nuclear bodies was also observed (35% for K4-8R and 30% for K6-8R; Figure 4).
Tax ubiquityaltion and SUMOylation control NEMO targeting into distinct subcellular compartments. HeLa cells were cotransfected with HA-SUMO1 and Tax, empty vector (control), or the Tax mutants K4-8R, K4-8R-Ub, K4-8R-SUMO1, K6-8R, K6-8R-Ub, or K6-8R-SUMO1, and stained by triple immunofluorescence with anti-Tax (blue), anti-HA (green), and anti-NEMO (IKK-γ) antibodies (red). Cells were examined by confocal microscopy. Scale bar, 10 μm. The percentage of cells displaying Tax or NEMO localization into nuclear bodies (NBs) and/or centrosome (C) are indicated.
Tax ubiquityaltion and SUMOylation control NEMO targeting into distinct subcellular compartments. HeLa cells were cotransfected with HA-SUMO1 and Tax, empty vector (control), or the Tax mutants K4-8R, K4-8R-Ub, K4-8R-SUMO1, K6-8R, K6-8R-Ub, or K6-8R-SUMO1, and stained by triple immunofluorescence with anti-Tax (blue), anti-HA (green), and anti-NEMO (IKK-γ) antibodies (red). Cells were examined by confocal microscopy. Scale bar, 10 μm. The percentage of cells displaying Tax or NEMO localization into nuclear bodies (NBs) and/or centrosome (C) are indicated.
To further confirm the role of Tax SUMOylation in the recruitment of NEMO at the centrosome, HeLa cells were transfected with siRNA against GFP or against SUMO1, SUMO2, and SUMO3. Successful silencing of all 3 SUMOs was assessed by Western blot analysis (data not shown). Cells were transfected with Tax and were immunostained with antibodies against Tax and NEMO. Whereas cells transfected with siRNA against GFP were able to recruit NEMO at the centrosome (59%), only (27%) of Tax-positive SUMO silenced cells displayed NEMO at the centrosome (Table 1). Altogether these data indicate that Tax ubiquitylation controls NEMO recruitment to both the centrosome and Tax nuclear bodies, while Tax SUMOylation controls NEMO recruitment to the centrosome.
The effect of SUMO silencing on NEMO subcellular distribution
| ID . | Tax nuclear bodies (%) . | NEMO nuclear bodies (%) . | Tax in the centrosome (%) . | NEMO in the centrosome (%) . | Colocalization in the nuclear bodies (%) . | Colocalization in the centrosome (%) . |
|---|---|---|---|---|---|---|
| No siRNA | 64 | 50 | 88 | 85 | 50 | 85 |
| siRNA | ||||||
| GFP | 74 | 54 | 60 | 59 | 54 | 59 |
| SUMO1,2,3 | 71 | 56 | 27 | 27 | 50 | 26 |
| ID . | Tax nuclear bodies (%) . | NEMO nuclear bodies (%) . | Tax in the centrosome (%) . | NEMO in the centrosome (%) . | Colocalization in the nuclear bodies (%) . | Colocalization in the centrosome (%) . |
|---|---|---|---|---|---|---|
| No siRNA | 64 | 50 | 88 | 85 | 50 | 85 |
| siRNA | ||||||
| GFP | 74 | 54 | 60 | 59 | 54 | 59 |
| SUMO1,2,3 | 71 | 56 | 27 | 27 | 50 | 26 |
Tax induces NEMO recruitment to the nucleus and NEMO SUMOylation
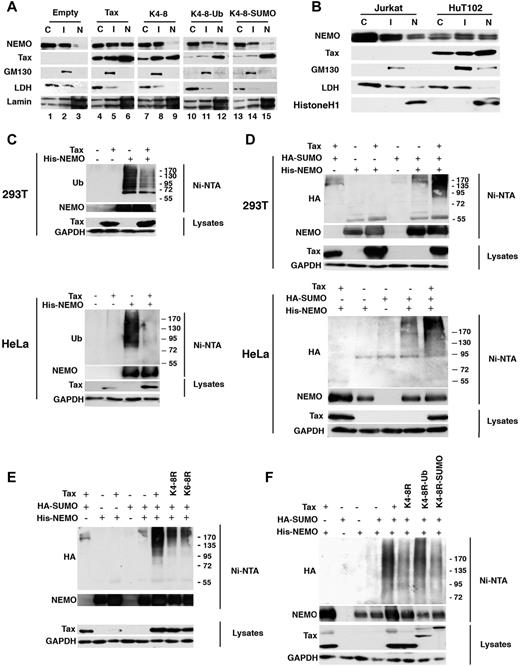
To confirm the Tax-induced NEMO recruitment to Tax nuclear bodies observed by confocal analysis, cellular fractionation approaches were performed. HeLa cells were mock- or Tax-transfected, and 3 cellular fractions were prepared: (1) nuclear fraction (N): lamin positive, LDH negative, and GM-130 negative; (2) soluble cytosolic fraction (C): LDH positive, GM-130 negative; and (3) intermediate fraction (I): the only one to contain the Golgi-associated protein GM-130 (Figure 5A). In agreement with confocal microscopy (see Figure 4), in Tax-negative cells, NEMO was mainly seen in the soluble cytosolic and intermediate fractions with negligible amounts in the nucleus (Figure 5A columns 1-3, and supplemental Figure 5A). In Tax-positive cells, a significant amount of NEMO proteins was detected in the nuclear fraction (Figure 5A column 6, and supplemental Figure 5A), indicating that Tax triggers NEMO targeting to and accumulation in the nucleus. Again and in agreement with confocal microscopy findings (see Figure 4), the Tax mutant K4-8R failed to induce nuclear accumulation of NEMO (Figure 5A column 9, and supplemental Figure 5A), whereas ubiquitin fusion but not SUMO-1 fusion to this mutant restored this accumulation (Figure 5A columns 12 and 15, and supplemental Figure 5A). Importantly, similar results were observed in HTLV-I–infected T lymphocytes. Indeed, the relative distribution of NEMO proteins in the nuclear and cytosolic fractions indicates nuclear accumulation of NEMO in HuT-102 cells but not in HTLV-I–negative Jurkat cells (Figure 5B and supplemental Figure 5B).
Tax induces nuclear accumulation and SUMOylation of NEMO. (A) HeLa cells were transfected with empty vector, Tax, or Tax mutants K4-8R, K4-8R-Ub, or K4-8R-SUMO1 plasmids. Cell fractionation was performed as described in the Methods. Lysates from the nuclear fraction (N), the soluble cytosolic fraction (C), and the intermediate fraction (I), were blotted with different antibodies as indicated. (B) Cell fractionation was performed on HTLV-I–negative Jurkat cells and on HTLV-I–infected HuT-102 cells as described in panel A. (C) 293T and HeLa cells were cotransfected with His-NEMO and Tax plasmids and proteins purified by denaturing Ni-NTA pull-down were blotted with mAb against polyubiquitylated proteins (FK2) and a polyclonal antibody against NEMO (top panel). Lysates were blotted with mAb against Tax or GAPDH (bottom panel). (D-F) 293T or HeLa cells were cotransfected with His-NEMO, HA-SUMO1, and Tax plasmids or the Tax mutants K4-8R, K6-8R, K4-8R-Ub, K4-8R-SUMO1 plasmids, and proteins purified by denaturing Ni-NTA pull-down were blotted with mAb against HA and a polyclonal antibody against NEMO. Lysates were blotted with mAb against Tax or GAPDH.
Tax induces nuclear accumulation and SUMOylation of NEMO. (A) HeLa cells were transfected with empty vector, Tax, or Tax mutants K4-8R, K4-8R-Ub, or K4-8R-SUMO1 plasmids. Cell fractionation was performed as described in the Methods. Lysates from the nuclear fraction (N), the soluble cytosolic fraction (C), and the intermediate fraction (I), were blotted with different antibodies as indicated. (B) Cell fractionation was performed on HTLV-I–negative Jurkat cells and on HTLV-I–infected HuT-102 cells as described in panel A. (C) 293T and HeLa cells were cotransfected with His-NEMO and Tax plasmids and proteins purified by denaturing Ni-NTA pull-down were blotted with mAb against polyubiquitylated proteins (FK2) and a polyclonal antibody against NEMO (top panel). Lysates were blotted with mAb against Tax or GAPDH (bottom panel). (D-F) 293T or HeLa cells were cotransfected with His-NEMO, HA-SUMO1, and Tax plasmids or the Tax mutants K4-8R, K6-8R, K4-8R-Ub, K4-8R-SUMO1 plasmids, and proteins purified by denaturing Ni-NTA pull-down were blotted with mAb against HA and a polyclonal antibody against NEMO. Lysates were blotted with mAb against Tax or GAPDH.
To investigate the molecular mechanisms underlying Tax-induced NEMO targeting, we assessed the effect of Tax on NEMO posttranslational modifications, namely ubiquitylation and SUMOylation. For that purpose, cells were cotransfected with His-NEMO and Tax. NEMO proteins were recovered following denaturing Ni-NTA pull-down and immunoblotted with anti-ubiquitin antibodies. No ubiquitin reactive species were detected in the absence of His-NEMO, indicating the specificity and the purification efficiency of the assay (Figure 5C). Interestingly, when comparable levels of NEMO were analyzed, Tax was found to reduce the amount of ubiquitylated NEMO species (Figure 5C). Similar results were obtained in cells cotransfected with His-NEMO, HA-ubiquitin, and Tax (data not shown).
Finally, to assess Tax effects on NEMO SUMOylation, cells were cotransfected with His-NEMO, HA-SUMO1, and Tax. NEMO proteins were recovered following denaturing Ni-NTA pull-down and blotted with anti-HA antibodies (Figure 5D). Few HA-reactive species were detected in the absence of His-NEMO (Figure 5D columns 1 and 4 top panel), validating the assay. Minimal baseline levels of HA-reactive species were detected in cells cotransfected with His-NEMO and HA-SUMO1 (Figure 5D column 5 top panel). In contrast, Tax expression dramatically enhanced the amount of HA-reactive species when comparable levels of NEMO were analyzed (Figure 5D column 6). This clearly demonstrates that Tax expression results in decreased NEMO ubiquitylation and massive NEMO SUMOylation. Finally, Tax K4-8R and K6-8R mutants also resulted in increased NEMO SUMOylation though to a lesser extent compared with wild-type Tax (Figure 5E and supplemental Figure 6). Ubiquitin fusion to the K4-8R Tax mutant, but not SUMO1 fusion, significantly increased NEMO SUMOylation (Figure 5F). These results strongly suggest that Tax-induced NEMO SUMOylation is positively regulated by Tax ubiquitylation.
Discussion
In this report, we show that Tax shuttles primarily among nuclear bodies, from nuclear bodies to the centrosome, and to a lesser extent in the opposite direction. This Tax trafficking is associated with NEMO targeting to the same cellular compartments and involves posttranslational protein modifications, namely ubiquitylation and SUMOylation. Indeed, Tax ubiquitylation not only targets Tax and NEMO to the centrosome as we have shown previously,11 but is also involved in the formation of Tax nuclear bodies and in NEMO recruitment to these bodies. A recent report showed that K-48 ubiquitylated Tax is present in the nuclear matrix where Tax is ubiquitylated by the E3 ligase PDLIM2 and then is destined for degradation by the proteasome.21 We show here that Tax SUMOylation, which is also implicated in nuclear body formation,17,18 is involved in targeting NEMO, but not Tax, to the centrosome. These findings and our data indicate that Tax ubiquitylation and SUMOylation dictate Tax and NEMO intracellular fate at the level of trafficking and localization.
The functional significance of the trafficking of Tax and NEMO remains to be defined. Previous reports from our group and other groups showed that Tax localization at the centrosome is implicated in Tax/IKK interaction, a mandatory step in the activation of the NF-κB pathway11 as well as in microtubule reorientation and formation of HTLV-1 the virological synapse.10,22 On the other hand, the formation of RelA/CBP/p300-enriched Tax nuclear bodies is implicated in transcriptional activation.17,18,23 Interestingly, Tax does not only target NEMO to the centrosome as shown previously,11 but also to SUMO-containing nuclear bodies. Both ubiquitylated and SUMOylated Tax are redundantly implicated in this process.
The molecular mechanisms of Tax-induced NEMO targeting remain unknown. Although classical nuclear localization signal and nuclear export signal sequences have not been described in NEMO yet, shuttling of the protein between the cytoplasmic and nuclear compartments has been reported with both activation and repression roles attributed to NEMO in the nucleus.24,25 Upon genotoxic stress, IKK-unbound NEMO localizes to the nucleus via site-specific SUMOylation by the E3 SUMO ligase PIASy and is then exported to the cytoplasm via ATM-dependent ubiquitylation mediating NF-κB activation.24,26 The same might be true in the presence of Tax. In that sense, we show that Tax expression results in a dramatic increase in SUMOylated NEMO accompanied by NEMO accumulation in the nucleus. Surprisingly, another consequence is a relative decrease in NEMO ubiquitylated species. This finding contradicts previous reports showing increased levels of ubiquitylated NEMO in NF-κB activation through tumor necrosis factor-α and the T-cell receptor pathways.27,28
Nuclear bodies are morphologically distinct substructures whose precise functions remain obscure. Although some nuclear bodies have been suggested to store specific factors, the diverse components that locate to these subdomains suggest that they constitute organized centers for assembly of functional complexes to execute specific biological processes, including DNA replication and repair, transcription and splicing, protein modification and degradation, apoptosis, and cell-cycle control.29,30 The integrity of a nuclear body could be enhanced during recruitment stages and disrupted after depletion of its component(s).29,30 In this report, we show that Tax nuclear bodies are potentially active sites of SUMOylation in addition to their transcriptional role. Indeed, Tax is targeted to Ubc9 bodies that are distinct from PML bodies. Ubc9 is a highly conserved E2 SUMO conjugating enzyme31,32 that colocalizes with SUMO1 and transcriptional regulators such as pCREB, CBP, and C-Jun in SUMO1 nuclear bodies, which are distinct from PML bodies.33
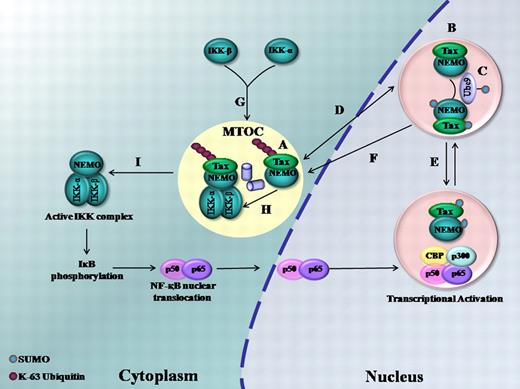
Based on our current and previous findings, we are proposing a model (Figure 6) where Tax posttranslational modifications dictate Tax and NEMO dynamic trafficking and intracellular localization. K-63 ubiquitylated Tax targets Tax and NEMO to the centrosome (Figure 6A) and to Ubc-9/SUMO-enriched nuclear bodies (Figure 6B). Tax and NEMO are subsequently SUMOylated (Figure 6C). The same Tax molecule shuttles between nuclear bodies and the centrosome (Figure 6D) and among distinct nuclear bodies (Figure 6E). Tax SUMOylation is also involved in the targeting of NEMO to the centrosome (Figure 6F), which represents the platform for Tax/IKK interaction (Figure 6G-H).11 IKK complex subsequently moves to the cytoplasm in its active Tax-free form (Figure 6I). As a consequence, IκB is phosphorylated, ubiquitylated, and degraded by the proteasome, and the NF-κB complex translocates to the nucleus.11 On the other hand, Tax SUMOylation is involved in the formation of Tax nuclear bodies and the recruitment of RelA to these bodies resulting in complete transcriptional activation.17,18 Finally, K-48 ubiquitylation targets Tax for proteasomal degradation in the nucleoplasm.11,21
A schematic representation of the role of ubiquitylation and SUMOylation in Tax and NEMO trafficking and activation of NF-κB. (A) K-63 ubiquitylated Tax targets Tax and NEMO to the centrosome. (B) Tax ubiquitylation targets Tax and NEMO to Ubc9/SUMO enriched nuclear bodies. (C) Tax and NEMO SUMOylation. (D) Bidirectional trafficking of Tax between nuclear bodies and the centrosome. (E) Tax shuttles among nuclear bodies. (F) Tax SUMOylation targets NEMO to the centrosome. (G) Recruitment of the catalytic IKK subunits to the centrosome. (H) Formation of the IKK complex. (I) Tax-free active IKK is liberated into the cytosol. (Alphabets do not necessarily indicate a chronological sequence of events.)
A schematic representation of the role of ubiquitylation and SUMOylation in Tax and NEMO trafficking and activation of NF-κB. (A) K-63 ubiquitylated Tax targets Tax and NEMO to the centrosome. (B) Tax ubiquitylation targets Tax and NEMO to Ubc9/SUMO enriched nuclear bodies. (C) Tax and NEMO SUMOylation. (D) Bidirectional trafficking of Tax between nuclear bodies and the centrosome. (E) Tax shuttles among nuclear bodies. (F) Tax SUMOylation targets NEMO to the centrosome. (G) Recruitment of the catalytic IKK subunits to the centrosome. (H) Formation of the IKK complex. (I) Tax-free active IKK is liberated into the cytosol. (Alphabets do not necessarily indicate a chronological sequence of events.)
In conclusion, the dynamic shuttling of Tax and NEMO between the nuclear and the cytoplasmic compartments, dictated by differential posttranslational Tax modifications, allows an optimal and efficient use of a relatively small viral genome. Finally, the presence of the SUMOylation machinery in Tax nuclear bodies strongly suggests that in addition to their transcriptional role, these nuclear bodies are sites of active SUMOylation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the Biological Imaging Core Facility at the American University of Beirut and the Imagery Department of the Institut Universitaire d'Hématologie IFR105.
This work was supported by the American University of Beirut Medical Practice Plan and University Research Board, the Lebanese National Council for Scientific Research, the Lady TATA Memorial Trust, le Comité de Paris de la Ligue contre le Cancer, and Fondation de France.
Authorship
Contribution: Y.K. designed the study, performed experiments, and wrote the manuscript; M.E.-S., O.H., C.P., H.d.T., A.S., and A.B. designed the study and wrote the manuscript; and N.S., A.Z., Z.D., and H.E.J., performed experiments and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ali Bazarbachi, Department of Internal Medicine, American University of Beirut, PO Box 113-6044, Beirut, Lebanon; e-mail: bazarbac@aub.edu.lb; or Ali Saib, CNRS UMR 7212, Inserm U944, Institut Universitaire d'Hématologie, Hôpital Saint-Louis, Paris, France; e-mail: ali.saib@univ-paris-diderot.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal