Abstract
Aberrant nuclear factor κB (NF-κB) signaling has been found to be of particular importance in diffuse, large B-cell lymphoma (DLBCL) cell survival and proliferation. Although the canonical NF-κB signaling pathway has been studied in some detail, activation of the alternative NF-κB pathway in DLBCL is not well characterized. Important insights into the regulation of the alternative NF-κB pathway in B lymphocytes has recently revealed the regulatory importance of the survival kinase NIK (NF-κB–inducing kinase) in genetically engineered murine models. Our studies demonstrate that both the canonical and alternative NF-κB pathways are constitutively activated in DLBCL. We also demonstrate that NIK kinase aberrantly accumulates in DLBCL cells due to constitutive activation of B-cell activation factor (BAFF)–R (BR3) through interaction with autochthonous B-lymphocyte stimulator (BLyS) ligand in DLBCL cells. Activation of BR3 in DLBCL induces recruitment and degradation of tumor necrosis factor receptor-associated factor 3, which results in NIK kinase accumulation, IκBα phosphorylation, and NF-κB p100 processing, thereby resulting in continuous activation of both NF-κB pathways in DLBCL cells, leading to autonomous lymphoma cell growth and survival. These results further elucidate mechanisms involved in abnormal NF-κB activation in DLBCL, and should contribute to better future therapeutic approaches for patients with DLBCL.
Introduction
Non-Hodgkin lymphomas (NHL), the fifth most common cancer in the United States, are a group of tumors of the immune system, of which most are of B-lymphocyte lineage.1 Diffuse, large B-cell lymphoma (DLBCL) is the most common type of B-cell NHL, accounting for 30% to 40% of cases, but this is also a highly heterogeneous group.2 DLBCLs are currently considered to consist of at least 3 genetic “signatures” based on various methods of gene expression profiling.3 Although these putative DLBCL subtypes have validity, considerable sharing and overlapping of phenotypic/genotypic characteristics clearly exist. Expression of MUM1/IRF-4 and CD138, postgerminal, center-associated antigens with constitutive activation of the nuclear factor κB1 (NF-κB1) pathway is considered a specific gene array “signature,” defined as an activated-B cell type (ABC-like DLBCL); whereas the t (14;18)(q32;q21)/bcl2 translocation, with expression of the bcl-6 and CD10 GCB biomarkers, is considered to represent the GCB-like LBCL. These subgroups of DLBCL, in pre-rituximab cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) and RCHOP therapy clinical trials, are reported to have disparate clinical outcomes and significantly different 5-year survival rates.3,4
Many aspects of the pathophysiology of DLBCL, particularly regarding tumor cell growth and survival, are still poorly understood, hampering new therapeutic approaches. B-lymphocyte stimulator (BLyS; also called B-cell activation factor, BAFF), is a prominent member of the tumor necrosis factor (TNF) ligand family. The BAFF receptor (BAFF-R, hereafter called BR3), a TNF-R family member, is the most dominant BLyS receptor in B lymphocytes.5 The role of the BLyS/BR3 signaling dyad in neoplastic B cells has been shown to be of critical importance for B-lymphocyte survival, maturation, migration, and proliferation, but the nature of key regulatory mechanisms in B-cell physiology are just beginning to be clarified.6-8 Several studies in B-cell NHL and chronic lymphocytic leukemia have shown that BLyS receptors are expressed in virtually all human B-cell lymphomas and leukemias, and that the tumor cells also usually express/produce the BLyS ligand.9-11 This suggests that positive feedback and possible autocrine mechanisms are involved in the expression and activation of BLyS /BR3 ligand-receptor complex in neoplastic B cells.
Downstream mediators of BLyS/BR3 activation include the NF-κB, AKT, and extracellular signal-regulated kinase (ERK) signaling pathways.9,12-16 BR3 activation of the canonical (classic, NF-κB1) NF-κB pathway is similar to other TNF-R, such as CD40, but perhaps even more important is the alternative (non-canonical, NF-κB2) NF-κB pathway, which is the dominant signaling pathway activated by the BLyS/BR3 dyad.9,12-16 Recent studies have reported that the NF-κB–inducing kinase (NIK), a mitogen-activated protein kinase kinase kinase (MAP3K), is an important regulatory kinase in the BR3-induced survival pathway in murine B cells.17,18 However, mechanism(s) involving BLyS/BR3 signaling functions involving NIK-regulated NF-κB pathway activation in human normal and NHL-B cells remain mostly undefined.
Recent results in genetically engineered mouse models demonstrate that alternative NF-κB pathway activation is controlled through negative feedback mechanisms involving negative regulation of the key upstream NIK by the adaptor/regulator proteins TNF receptor-associated factor 2 and 3 (TRAF2/3) and the cellular inhibitors of apoptosis ubiquitin ligases (c-IAP1/2).19,20 Overexpression of wild-type NIK kinase in genetically engineered mouse models leads to B-cell hyperplasia through amplification of the BLyS-induced alternative NF-κB pathway signals involved in mediating B-cell survival.17 Interruption of the cognitive interaction between TRAF3 and NIK proteins induces constitutive BLyS-independent activation of the alternative NF-κB pathway, leading to a large accumulation of mature B cells in lymphoid organs, disrupting their structural integrity.17 Other recent studies have proposed a model in which interactions between TRAF2 and TRAF3 adaptor/regulatory proteins constitutively inhibit B-cell survival through inhibition of activation in the alternative NF-κB pathway. This mechanism, through which NIK protein accumulation is prevented, is based on the finding that the NIK kinase bears a TRAF3 interaction site that can result in ubiquitin-mediated NIK proteosomal degradation through interaction with TRAF3 protein.18,21 When the plasma membrane BR3 receptor is bound by its cognate ligand BLyS, however, the BR3 receptor sequesters TRAF3 protein by binding to a C-terminal cytoplasmic binding site on the receptor, preventing interactions between NIK and the inhibitory TRAF3/TRAF2/c-IAP1/2 ubiquitin ligase multimeric complex.17,19-21 Loss of this quaternary inhibitory complex can lead to increased NIK protein accumulation, subsequent NF-κB2 (p100) processing, and increased B-cell survival.19,20,22,23 These studies imply that deregulated NIK kinase expression and cellular accumulation can function as an important mechanistic component in the pathophysiology of B-cell neoplasia, particularly in aggressive lymphomas.
Although its role in B-cell neoplasia has gone mostly unrecognized to date, recently, the alternative NF-κB pathway has been implicated in a variety of B-cell malignancies.9,24-28 Recent studies in multiple myeloma, a plasma cell neoplasm arising from terminally differentiated B cells, described genetic aberrations affecting several key components of NF-κB activation, primarily involving the alternative NF-kB pathway.29 These aberrations/mutations led to the absence of important negative regulators of NF-κB signaling, including TRAF3, TRAF2, and c-IAP1/2, or to overexpression of NIK.30,31 These studies also indicated that elevated canonical and alternative NF-κB activity, through mechanisms involving deregulation of NIK kinase, directly contributed to disease progression in multiple myeloma, and also suggested that TRAF3, at least in this context, can function as an important suppressor of lymphoid neoplasia through the negative regulation of both the canonical and alternative NF-κB pathways.23,29,30
In this study, we report that NIK kinase protein is overexpressed and accumulates in both GCB-like and ABC-like DLBCL cell lines and in patient DLBCL samples, dysregulating and contributing to constitutive NF-κB pathway activation and abnormal lymphoma cell proliferation. Autonomous activation of the BR3 receptor in DLBCL cells also promotes lymphoma cell growth and survival by protecting the NIK kinase protein from negative regulation through TRAF3-dependent protein degradation. Association of the BR3 receptor with the negative regulation factors TRAF3 and TRAF2 and the c-IAP1/2 ubiquitin ligases represents a key mechanism for mediating BR3-induced TRAF3 degradation in DLBCL cells. Our studies identify a molecular mechanism that explains how the activated BR3 receptor functions in NIK-induced NF-κB pathways in DLBCL, which contributes to a better understanding of the pathophysiologic mechanisms in aggressive B-cell lymphomagenesis and provides a potentially important target(s) for future therapeutic strategies in patients with DLBCL.
Methods
Cells
Human DLBCL cell lines (MS, DS, DB, JM, FN, EJ, CJ, HF, HB, MZ, PL, LR, LP and SF) were established from diagnostic biopsy tissue or lymphomatous effusions from DLBCL patients as described previously.32 The SUDHL4, SUDHL6, OCI-LY3, and OCI-LY10 DLBCL cell lines were obtained from Dr Michael Rosenblum (M. D. Anderson Cancer Center). The Pfeifer DLBCL cell line was purchased from the ATCC. This study was conducted in accordance with the Helsinki protocol and was approved by our institutional review board. Informed consent was obtained from all patients whose tumor samples were used. The cells were cultured in RPMI medium (Gibco) containing 15% fetal calf serum (Hyclone). Normal human B lymphocytes were purified from blood donor buffy coats using the human B-cell enrichment cocktail from StemCell Technologies. Purified B cells were activated by incubating for 48 hours with recombinant human BLyS (50 ng/mL; Peprotech) with or without anti-IgM (3.5 μg/mL; ICN).
Antibodies and reagents
Unless otherwise specified, monoclonal and polyclonal antibodies against the following molecules were used: BLyS, p52, p50, p65, and phospho-p65 (Millipore); NIK and phospho-IκBα (Cell Signaling Technology); polyclonal TRAF3, c-IAP1, and c-IAP2 (Santa Cruz Biotechnology); TRAF3 (BD Pharmingen); TRAF2 (Imgenex); BR3 (Alexis Biochemicals); BR3 antibody 9.1 (Genentech); polyclonal BR3 (ProSci); and human BLyS antibody for neutralization (R&D Systems).
Transfection, shRNAs, and plasmids
Transient transfections in cultured lymphoma cells were conducted using the Nucleofector protocol from Amaxa Biosystems or the Neon transfection system from Invitrogen. Transfection experiments in DLBCL cells were performed in vitro in representative, transfectable DLBCL cells, as described previously,9,33-35 using validated green fluorescent protein (GFP)–short hairpin RNAs (shRNAs), and were repeated at least twice to verify reproducible experimental results. Twenty-four hours after transfection, GFP+ cells were sorted by a fluorescence-activated cell sorter (FACS) flow cytometer and plated. Cell proliferation was measured 96 hours after sorting, while some cells were lysed for Western blot analysis. BR3, NIK, TRAF3, and cIAP1/2 shRNAs and control shRNA were from SABiosciences. Each target was validated with 4 shRNAs, and the optimal shRNA was chosen to perform the experiments in this manuscript. TRAF3 expression plasmid was kindly provided by Dr Bryant G. Darnay (M. D. Anderson Cancer Center).
Proliferation assays, co-immunoprecipitation procedures, and confocal microscopic analysis
Analysis of NF-κB DNA-binding activity
Electromobility gel shift assays were performed according to procedures described previously.38 The DNA-binding activity of NF-κB subunits was analyzed using an enzyme-linked immunosorbent assay (ELISA)–based assay according to the manufacturer's instructions (TransAM NF-κB Family Transcription Factor Assay Kit; Active Motif). Briefly, nuclear extracts were added into wells of a 96-well plate that contained an immobilized oligonucleotide carrying an NF-κB consensus site. NF-κB proteins bound to this immobilized oligonucleotide were detected by incubating with a primary antibody that recognizes active p50, p52, p65, c-rel, or relB, followed by horseradish peroxidase-conjugated secondary antibody, and binding was quantified by spectrophotometry at 450 nm with a reference wavelength of 650 nm.
DLBCL tissue microarray analysis and immunohistochemistry
A tissue microarray analysis (TMA) was composed of 43 DLBCL patient tumors that were fixed, paraffin-embedded archival tissue specimens.39 The pathologic diagnosis of these tumors was confirmed by standard histologic and immunophenotypic analysis performed in the Department of Hematopathology at M. D. Anderson Cancer Center. Tissue sections (5-μ-thick) prepared from the TMA were deparaffinized in xylene and rehydrated in ethanol. After blocking nonspecific binding with a protein block serum-free solution (DakoCytomation), primary antibodies from various commercial and academic sources were used. Immunostaining was performed using epitope retrieval with Diva Decloaker buffer and a decloaking chamber (Biocare Medical), and the Mach 3 system (Biocare Medical). Staining was performed using the Autostainer Plus (DakoCytomation). The washing buffer used was 0.05M Tris-buffered saline supplemented with 0.05% Tween. 3,3′-Diaminobenzidine tetrahydrochloride was used as the chromogen (Liquid DAB+ Subtrate Chromogen System; DakoCytomation), and all tissue sections were counterstained with hematoxylin. The Applied Imaging Ariol automated analysis system (Genetix) was used for quantification of the results with antibodies specific for pIκBα, serine 276 phosphorylated p65 (pp65), p52, NIK, and BR3 TMA slides. TMA photomicrographs were captured using an Olympus BX41 dual head light microscope equipped with an Olympus Q-Color 5 digital camera (Olympus America), with a 20× plan-apochromat objective. Digital images were obtained and adjusted using Adobe Photoshop CS3.
Statistical analysis
The software used for statistical analysis was Prism 5b (GraphPad Software). Statistical significance was determined by the Student t test. P values < .05 were considered statistically significant.
Results
Identification and characterization of NF-κB family members in DLBCL subsets
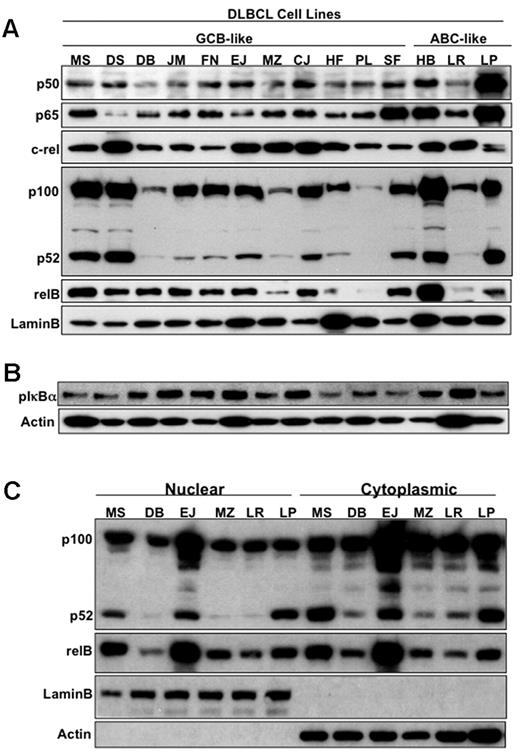
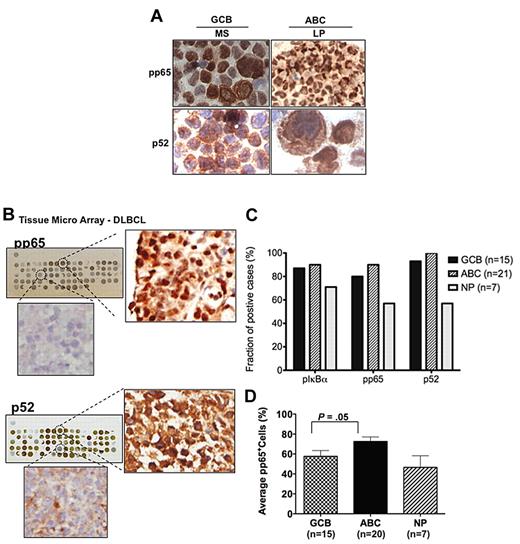
The NF-κB family consists of 5 transcription factor members, and 2 main signaling pathways control its activation: the classical (canonical) and the alternative. To determine which NF-κB pathway is activated in DLBCL cell lines, nuclear extracts were purified from 14 representative DLBCL cell lines (11 GCB-like and 3 ABC-like; Table 1), and immunoblotted to determine the presence of the canonical NF-κB components (p50, p65, and c-rel) and the alternative NF-κB components (p52 and relB). As shown in Figure 1A, there was differential nuclear expression of all 5 members of the NF-κB family in the 14 DLBCL cell lines. Phosphorylated IκBα was also constitutively expressed in cytoplasmic extracts of all 14 DLBCL cell lines, suggesting that the canonical NF-κB pathway is constitutively active (Figure 1B). Interestingly, further cellular fractionation studies demonstrated that NF-κB2-p100 was also present in the nuclear compartment, along with p52 and relB of the alternative NF-κB pathway, suggesting that p100 may potentially play a functional role in the nucleus of DLBCL (Fig 1C). Immunohistochemical analysis of nuclear p52 and phospho-p65 expression in representative ABC-like and GCB-like DLBCL cell lines (LP and MS, respectively; Figure 2A), further confirmed the activation of both NF-κB pathways in DLBCL cell lines.
Characterization of DLBCL cell lines
| DLBCL cell lines . | Type . | CD10 . | Bcl-6 . | IRF4/MUM-1 . | NF-κB1/2 . |
|---|---|---|---|---|---|
| MS | GCB | + | + | − | + |
| DS | GCB | + | + | + | + |
| DB | GCB | + | + | − | + |
| JM | GCB | + | + | + | + |
| FN | GCB | + | + | + | + |
| CJ | GCB | + | − | + | + |
| EJ | GCB | + | + | + | + |
| HF | GCB | − | + | − | + |
| PL | GCB | + | + | + | + |
| SF | GCB | + | − | + | + |
| MZ | GCB | + | − | − | + |
| HB | ABC | − | − | + | + |
| LR | ABC | − | − | + | + |
| LP | ABC | − | − | + | + |
| DLBCL cell lines . | Type . | CD10 . | Bcl-6 . | IRF4/MUM-1 . | NF-κB1/2 . |
|---|---|---|---|---|---|
| MS | GCB | + | + | − | + |
| DS | GCB | + | + | + | + |
| DB | GCB | + | + | − | + |
| JM | GCB | + | + | + | + |
| FN | GCB | + | + | + | + |
| CJ | GCB | + | − | + | + |
| EJ | GCB | + | + | + | + |
| HF | GCB | − | + | − | + |
| PL | GCB | + | + | + | + |
| SF | GCB | + | − | + | + |
| MZ | GCB | + | − | − | + |
| HB | ABC | − | − | + | + |
| LR | ABC | − | − | + | + |
| LP | ABC | − | − | + | + |
Fourteen DLBCL cell lines were used to characterize the expression of bcl-6, CD10, IRF-4 (MUM1), and NF-κB. CD10, Bcl-6, and IRF-4 expression was analyzed using immunohistochemistry and Western blot analysis. NF-κB expression was analyzed by electromobility gel-shift assays and DNA-binding ELISA.
+ indicates positive; and −, negative.
Identification and characterization of NF-κB family members in DLBCL subsets. (A) Nuclear extracts purified from DLBCL cell lines (GCB-like and ABC-like, as indicated) were subjected to Western blot analysis with p50, p52, p65, c-rel, relB, and lamin B antibodies. (B) Cytoplasmic extracts purified from DLBCL cell lines (GCB-like and ABC-like, as indicated) were analyzed by Western blot analysis with phospho-IκBα and actin antibodies. (C) 6 DLBCL cell lines (4 GCB-like and 2 ABC-like) were used to purified cytoplasmic and nuclear extracts and were subjected to Western blot analysis with p52, relB, lamin B (nuclear marker), and actin (cytoplasmic marker) antibodies.
Identification and characterization of NF-κB family members in DLBCL subsets. (A) Nuclear extracts purified from DLBCL cell lines (GCB-like and ABC-like, as indicated) were subjected to Western blot analysis with p50, p52, p65, c-rel, relB, and lamin B antibodies. (B) Cytoplasmic extracts purified from DLBCL cell lines (GCB-like and ABC-like, as indicated) were analyzed by Western blot analysis with phospho-IκBα and actin antibodies. (C) 6 DLBCL cell lines (4 GCB-like and 2 ABC-like) were used to purified cytoplasmic and nuclear extracts and were subjected to Western blot analysis with p52, relB, lamin B (nuclear marker), and actin (cytoplasmic marker) antibodies.
Immunohistochemical analysis of NF-κB components in DLBCL cell lines and primary DLBCL samples. (A) Representative GCB-like (MS) and ABC-like (LP) DLBCL cell lines were used for immunohistochemical staining for pp65 and p52. (B) Representative immunohistochemical analysis of DLBCL tissue arrays of 43 primary DLBCL biopsies with high/low p52, and high/low pp65. Tissue arrays with duplicate samples were scored using an automated analysis system for percentage of positive cell staining for p-IκBα and for the presence of the NF-κB components, pp65 and p52 (supplemental Figure 1). (C) Prevalence of cases that are positive (> 30% staining) for pIκBα, pp65, and p52 in the DLBCL subgroups GCB, ABC, and non-profiled (NP). (D) Analysis of pp65 expression (average of pp65-positive cells) in the DLBCL subgroups GCB, ABC, and non-profiled (NP).
Immunohistochemical analysis of NF-κB components in DLBCL cell lines and primary DLBCL samples. (A) Representative GCB-like (MS) and ABC-like (LP) DLBCL cell lines were used for immunohistochemical staining for pp65 and p52. (B) Representative immunohistochemical analysis of DLBCL tissue arrays of 43 primary DLBCL biopsies with high/low p52, and high/low pp65. Tissue arrays with duplicate samples were scored using an automated analysis system for percentage of positive cell staining for p-IκBα and for the presence of the NF-κB components, pp65 and p52 (supplemental Figure 1). (C) Prevalence of cases that are positive (> 30% staining) for pIκBα, pp65, and p52 in the DLBCL subgroups GCB, ABC, and non-profiled (NP). (D) Analysis of pp65 expression (average of pp65-positive cells) in the DLBCL subgroups GCB, ABC, and non-profiled (NP).
We used TMA sections of the 43 DLBCL cases to immunohistochemically assess CD10, BCL-6, and MUM-1 (30% cutoff) to determine the DLBCL subtypes: ABC-like (21 cases), GCB-like (15 cases), and 7 undetermined cases (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We also quantified by immunohistochemistry the percentage of positive cells in each biopsy core for the canonical NF-κB pathway components pIκBα and phospho-p65 (pp65) and the alternative NF-κB2 component p52 (supplemental Figure 1). Figure 2B shows immunohistochemical staining results of a TMA slide for pp65 and p52, as well as their representative biopsy cores. In most cases (> 80%), both GCB and ABC subtypes highly expressed pIκBα, pp65, and p52 (Figure 2C), suggesting that both NF-κB pathways are activated in primary DLBCL samples. Further analysis of pp65 expression in DLBCL tumors indicates that the ABC subtype, on the average, has more pp65-positive cells than the GCB subtype (Figure 2D).
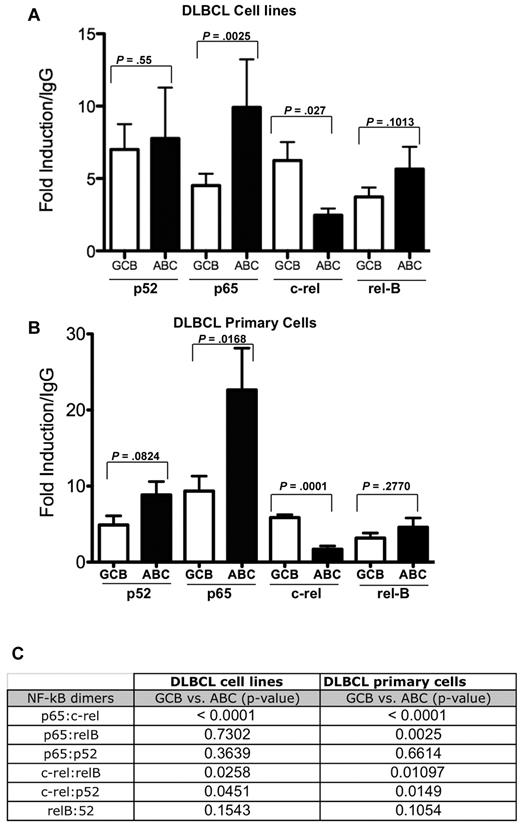
To confirm that constitutive nuclear NF-κB components in DLBCL were functional, DNA-binding ELISA was performed using nuclear extracts from 19 DLBCL cell lines: 5 additional DLBCL cell lines from other sources (3 GCB: Pfeiffer, SUDHL-4, and SUDHL-6; and 2 ABC: OCI-Ly 3 and OCI-Ly 10) and an additional 14 primary DLBCL cells (see Table 2 for immunophenotype) that were different from those used in the tissue microarray analysis. All members of the NF-κB family were found to constitutively bind to a consensus NF-κB DNA binding site in DLBCL cell lines (supplemental Figure 2A) and DLBCL tumors (supplemental Figure 2B). The data from both cell lines and primary cells indicated that the canonical NF-κB member p65, while activated in both subtypes, was more significantly activated in the ABC-like DLBCL compared with GCB-like DLBCL, whereas c-rel DNA-binding activity was higher in GCB compared with the ABC-DLBCL subtype (Figure 3A-B). Statistical analysis using the independent samples Student t test showed a significant difference in the p65:c-rel ratio between the GCB and the ABC DLBCL subtypes as determined by DNA-binding ELISA, in both cell lines (P < .0001) and tumor cells (P < .0001; Figure 3C).
Characterization of primary DLBCL cells
| DLBCL PS . | Type . | CD10 . | Bcl-6 . | IRF4/MUM-1 . | NF-κB1/2 . |
|---|---|---|---|---|---|
| 1 | GCB | + | + | + | + |
| 2 | GCB | − | + | − | + |
| 3 | GCB | + | + | N/A | + |
| 4 | GCB | − | + | − | + |
| 5 | GCB | + | + | N/A | + |
| 6 | GCB | − | + | − | + |
| 7 | GCB | + | + | − | + |
| 8 | GCB | + | + | + | + |
| 9 | GCB | + | + | + | + |
| 10 | ABC | − | − | + | + |
| 11 | ABC | − | − | + | + |
| 12 | ABC | − | − | + | + |
| 13 | ABC | − | − | + | + |
| 14 | ABC | − | − | + | + |
| DLBCL PS . | Type . | CD10 . | Bcl-6 . | IRF4/MUM-1 . | NF-κB1/2 . |
|---|---|---|---|---|---|
| 1 | GCB | + | + | + | + |
| 2 | GCB | − | + | − | + |
| 3 | GCB | + | + | N/A | + |
| 4 | GCB | − | + | − | + |
| 5 | GCB | + | + | N/A | + |
| 6 | GCB | − | + | − | + |
| 7 | GCB | + | + | − | + |
| 8 | GCB | + | + | + | + |
| 9 | GCB | + | + | + | + |
| 10 | ABC | − | − | + | + |
| 11 | ABC | − | − | + | + |
| 12 | ABC | − | − | + | + |
| 13 | ABC | − | − | + | + |
| 14 | ABC | − | − | + | + |
Fourteen DLBCL primary samples were used to characterize the expression of bcl-6, CD10, IRF-4 (MUM1), and NF-κB. CD10, bcl-6, and IRF-4 expression was analyzed using immunohistochemistry and Western blot analysis. NF-κB expression was analyzed by DNA-binding ELISA.
+ indicates positive; −, negative; and PS, patient samples.
NF-kB DNA-binding analysis in DLBCL cell lines and primary samples. Statistical comparison of each NF-kB component DNA-binding activity in GCB versus ABC DLBCL cell lines (A) and DLBCL primary cells (B) were assessed by the Student t test of average value from the ELISA data in supplemental Figure 2A and B. P < .05 was considered statistically significant. (C) Six possible NF-kB dimer ratios obtained from the ELISA data in supplemental Figure 2A and B for DLBCL cell lines and primary samples, respectively, were compared in GCB versus ABC DLBCL subtypes by the Student t test. P < .05 was considered statistically significant.
NF-kB DNA-binding analysis in DLBCL cell lines and primary samples. Statistical comparison of each NF-kB component DNA-binding activity in GCB versus ABC DLBCL cell lines (A) and DLBCL primary cells (B) were assessed by the Student t test of average value from the ELISA data in supplemental Figure 2A and B. P < .05 was considered statistically significant. (C) Six possible NF-kB dimer ratios obtained from the ELISA data in supplemental Figure 2A and B for DLBCL cell lines and primary samples, respectively, were compared in GCB versus ABC DLBCL subtypes by the Student t test. P < .05 was considered statistically significant.
NIK kinase protein accumulates in DLBCL tumor cells at steady state in vitro
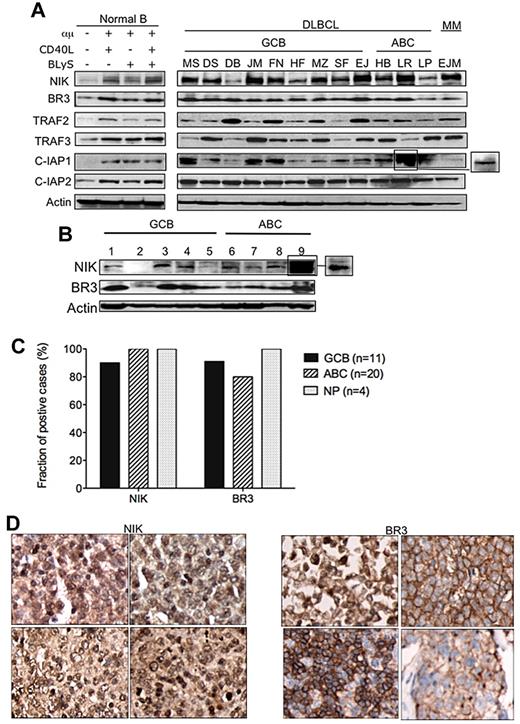
To study the pathologic mechanisms involved in the constitutive activation of the less-studied alternative NF-κB pathway promoting abnormal DLBCL cell growth and survival, we studied the protein expression status of key regulatory molecules in the NIK kinase-induced alternative NF-κB pathway, including BR3, NIK, TRAF2, TRAF3, and the c-IAP1 and c-IAP2 ubiquitin ligases in normal peripheral blood B cells, representative DLBCL cell lines, and in tumor samples from patients with DLBCL. A multiple myeloma cell line, EJM, previously shown to express high levels of NIK, was used as a positive control for NIK protein expression.40 The results of these studies showed that the NIK protein was highly expressed in DLBCL cell lines (Figure 4A) and primary cells (Figure 4B). We also performed immunohistochemical analysis of the TMA of DLBCL tumors to further confirm the characteristics of NIK kinase and BR3 receptor protein expression in a larger group of representative DLBCL patient biopsy samples, similar to the one used in Figure 2B. As shown in Figure 4C, NIK and BR3 proteins were found to be co-expressed in GCB- and ABC-DLBCL biopsy specimens. Figure 4D shows representative examples of immunohistochemical analysis of NIK and BR3 in DLBCL tumors studied. Previous studies have shown in genetically engineered mouse lymphoid cells that NIK protein was barely detectable, presumably due to constant TRAF3-induced NIK proteosomal degradation.18-20 NIK accumulation in human DLBCL cell lines and patient tumor samples suggested that constitutive NIK kinase activation is likely to be a key signaling mechanism involved in abnormal lymphoma tumor cell proliferation and survival through the alternative NF-κB pathway, but also possibly through the canonical NF-κB pathway.23
NIK kinase protein accumulates in DLBCL tumor cells. (A) Cell lysates of normal/activated B cells and DLBCL cell lines were probed with NIK, TRAF2, TRAF3, BR3, c-IAP1, or c-IAP2 antibodies in Western blot analyses. Actin was used as a loading control. Box to the right indicates lighter exposure. (B) Cell lysates of DLBCL lymphoma patient biopsies were probed with NIK and BR3 antibodies in Western blot analysis. Actin was used as a loading control. Box to the right indicates lighter exposure. (C) DLBCL TMA analyzed for NIK and BR3 protein expression. Immunohistochemical analysis of DLBCL patient sample TMA of 43 primary DLBCL biopsies using antibodies against NIK and BR3 proteins. Tissue arrays with duplicate samples were scored using an automated analysis system for percentage of positive cells (> 30%) staining for NIK and BR3 protein in the DLBCL subgroups GCB, ABC, and non-profiled (NP; supplemental Figure 1). (D) Representative DLBCL TMA stained for NIK and BR3. Images are representative of the biopsy cores from the DLBCL TMA slides.
NIK kinase protein accumulates in DLBCL tumor cells. (A) Cell lysates of normal/activated B cells and DLBCL cell lines were probed with NIK, TRAF2, TRAF3, BR3, c-IAP1, or c-IAP2 antibodies in Western blot analyses. Actin was used as a loading control. Box to the right indicates lighter exposure. (B) Cell lysates of DLBCL lymphoma patient biopsies were probed with NIK and BR3 antibodies in Western blot analysis. Actin was used as a loading control. Box to the right indicates lighter exposure. (C) DLBCL TMA analyzed for NIK and BR3 protein expression. Immunohistochemical analysis of DLBCL patient sample TMA of 43 primary DLBCL biopsies using antibodies against NIK and BR3 proteins. Tissue arrays with duplicate samples were scored using an automated analysis system for percentage of positive cells (> 30%) staining for NIK and BR3 protein in the DLBCL subgroups GCB, ABC, and non-profiled (NP; supplemental Figure 1). (D) Representative DLBCL TMA stained for NIK and BR3. Images are representative of the biopsy cores from the DLBCL TMA slides.
NIK protein stabilization through the BR3 receptor has important functions in both alternative and canonical NF-κB pathway activations in DLBCL cells
To determine the functions of NIK kinase in NF-κB pathway activation in DLBCL, we evaluated the effect of inhibition of NIK protein expression in vitro, using validated specific shRNA in representative GCB-like (MS) and ABC-like DLBCL (HB) cell lines. As shown in Figure 5A, transfection of validated NIK shRNA into these DLBCL cells resulted in significant inhibition of NIK protein expression compared with cells transfected with a non-targeted control shRNA. NIK-transfected cells also showed down-regulation of phosphorylated IκBα and p52 protein levels, key components of both the canonical and alternative NF-κB pathway, respectively. DNA-binding ELISA also showed inhibition of both p52 and p65 DNA-binding activity after NIK shRNA transfection (Figure 5B), suggesting that components of both the canonical and the alternative NF-κB pathways are downstream targets of NIK kinase in DLBCL cells. Subsequent proliferation assays showed that inhibition of NIK kinase protein expression in GCB- and ABC-like DLBCL cells decreased lymphoma cell growth in vitro (Figure 5C), implicating NIK-induced NF-κB pathway activation as having a significant role in DLBCL proliferation.
BR3 receptor activation promotes DLBCL cell growth through NIK protein stabilization. (A) Cell lysates from representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with NIK shRNA or negative control (NC) shRNA for 48 hours were analyzed by Western blot analysis for NIK, p52, and pIkBα. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (B) Nuclear extracts purified from GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with NIK shRNA or negative control (NC) shRNA for 48 hours were analyzed by DNA-binding ELISA for p52 and p65. (C) Proliferation of DLBCL cells (GCB-MS and SUDHL-4; ABC-HB and OCI-Ly3) transfected with NIK shRNA or negative control non-targeting sequence was analyzed by [3H] thymidine incorporation assays in vitro for 72 hours. The data shown are the means and ranges of triplicate samples of 2 independent experiments. Error bars represent SD. (D) Cell lysates from a representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with BR3 shRNA or negative control (NC) shRNA for 48 hours were probed with BR3, NIK, pIκBα, p52, or caspase 3 antibodies by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (E). DLBCL cells (GCB-MS and SUDHL-4; ABC-HB and OCI-Ly3) were transfected with BR3 shRNA or negative control non-targeting sequence and cell proliferation was analyzed by [3H]-thymidine incorporation assays in vitro for 72 hours. The data shown are the means and ranges of triplicate samples relative to control samples of 2 independent experiments. Error bars represent SD.
BR3 receptor activation promotes DLBCL cell growth through NIK protein stabilization. (A) Cell lysates from representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with NIK shRNA or negative control (NC) shRNA for 48 hours were analyzed by Western blot analysis for NIK, p52, and pIkBα. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (B) Nuclear extracts purified from GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with NIK shRNA or negative control (NC) shRNA for 48 hours were analyzed by DNA-binding ELISA for p52 and p65. (C) Proliferation of DLBCL cells (GCB-MS and SUDHL-4; ABC-HB and OCI-Ly3) transfected with NIK shRNA or negative control non-targeting sequence was analyzed by [3H] thymidine incorporation assays in vitro for 72 hours. The data shown are the means and ranges of triplicate samples of 2 independent experiments. Error bars represent SD. (D) Cell lysates from a representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with BR3 shRNA or negative control (NC) shRNA for 48 hours were probed with BR3, NIK, pIκBα, p52, or caspase 3 antibodies by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (E). DLBCL cells (GCB-MS and SUDHL-4; ABC-HB and OCI-Ly3) were transfected with BR3 shRNA or negative control non-targeting sequence and cell proliferation was analyzed by [3H]-thymidine incorporation assays in vitro for 72 hours. The data shown are the means and ranges of triplicate samples relative to control samples of 2 independent experiments. Error bars represent SD.
Several previous studies have shown that the BR3 receptor promotes normal murine B lymphocyte survival and proliferation through activation of NIK protein kinase and the downstream alternative NF-κB pathway.17,22,23 Our previous studies reported that BLyS/BR3 signaling promotes DLBCL cell growth and survival through an internal, autonomous cellular mechanism,9,33 suggesting that BR3 receptor protein activation is a key molecule contributing to NIK protein steady-state accumulation and function in this type of lymphoma. To further support our hypothesis, we transfected representative GCB-like and ABC-like DLBCL cells with specific BR3 shRNA for 48 hours, and then determined NIK protein levels in these lymphoma cells. As shown in Figure 5D, the protein level of NIK kinase, as well as pIκBα and p52, were decreased when BR3 expression was inhibited through specific shRNA transfection in GCB- and ABC-like DLBCL cells compared with similarly transfected control tumor cells. Down-regulation of BR3 also inhibited cell proliferation (Figure 5E) and cell survival through caspase 3 cleavage (Figure 5D) in both GCB and ABC cell types.
NIK kinase protein is negatively regulated by TRAF3 in DLBCL cells
Several studies have shown that the TRAF3 adaptor/regulatory protein is a key inhibitory molecule in NIK-induced alternative NF-κB activation in murine B cells. To determine whether the TRAF3 protein functions similarly in the alternative NF-κB pathway activation in DLBCL cells, we transfected representative GCB-like (MS) and ABC-like (HB) DLBCL cell lines with a TRAF3 expression vector, and then assayed for the effects on the NIK protein level in lymphoma cells. Overexpression of TRAF3 in these cell lines resulted in decreased NIK protein expression levels, as shown by Western blot analysis (Figure 6A). To confirm these observations, we transfected both MS and HB cells with validated TRAF3 shRNAs to inhibit TRAF3 expression. In these experiments, NIK protein level was increased in TRAF3 shRNA-transfected DLBCL cells relative to appropriate control samples (Figure 6B). Modulation of TRAF3 in DLBCL cells also effected cell proliferation (Figure 6C). These results suggest that TRAF3 functions as a critical inhibitory protein in these DLBCL cells, and that TRAF3 negatively regulates the NIK kinase–dependent NF-κB pathway activation in vitro.
The role of TRAF3 in the stabilization of NIK in DLBCL cells. (A) Cell lysates from a representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with a TRAF3 expression vector or a control empty vector for 48 hours were probed with TRAF3 or NIK antibodies by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (B) Cell lysates from a representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with TRAF3 shRNA or negative control shRNA were probed with TRAF3 or NIK antibodies by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (C) DLBCL cells (GCB-MS and SUDHL-4; ABC-HB and OCI-Ly3) were transfected with TRAF plasmid, TRAF3 shRNA, or negative control shRNA and cell proliferation was analyzed by [3H]-thymidine incorporation assays in vitro for 72 hours. The data shown are the means and ranges of triplicate samples relative to control samples of 2 independent experiments. Error bars represent SD.
The role of TRAF3 in the stabilization of NIK in DLBCL cells. (A) Cell lysates from a representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with a TRAF3 expression vector or a control empty vector for 48 hours were probed with TRAF3 or NIK antibodies by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (B) Cell lysates from a representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with TRAF3 shRNA or negative control shRNA were probed with TRAF3 or NIK antibodies by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (C) DLBCL cells (GCB-MS and SUDHL-4; ABC-HB and OCI-Ly3) were transfected with TRAF plasmid, TRAF3 shRNA, or negative control shRNA and cell proliferation was analyzed by [3H]-thymidine incorporation assays in vitro for 72 hours. The data shown are the means and ranges of triplicate samples relative to control samples of 2 independent experiments. Error bars represent SD.
Constitutive BLyS/BR3 signaling stimulates NIK-induced NF-κB pathway activation through induction of TRAF3 degradation in DLBCL
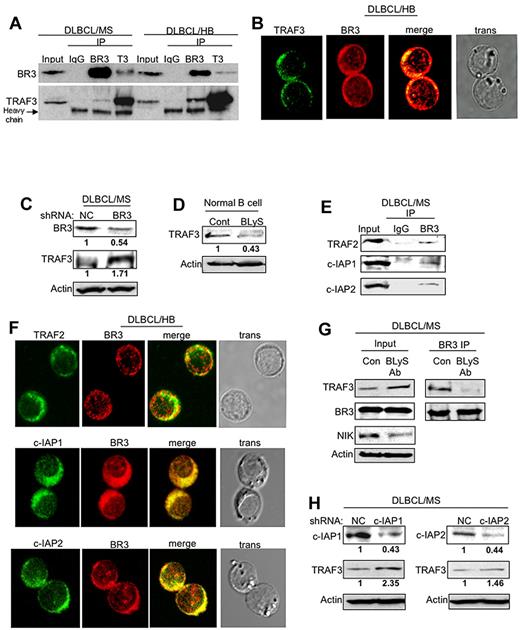
Several recent studies in genetically engineered mouse models have suggested that the BR3 receptor functions primarily in alternative NF-κB pathway activation by rescuing NIK kinase protein from TRAF3-induced proteosomal degradation in mouse B cells.17 To identify possible similar mechanisms to explain how activated BR3 receptor protein may protect NIK kinase from degradation in DLBCL cells, we investigated whether the BR3 receptor could function in the regulation of TRAF3 protein degradation in DLBCL cells. We discovered that BR3 receptor protein bound TRAF3 in cytoplasmic extracts of DLBCL cells in co-immunoprecipitation experiments using BR3 and TRAF3 antibodies (Figure 7A). Confocal microscopy analysis also demonstrated co-localization of BR3 and TRAF3 in DLBCL cells (Figure 7B). Down-regulation of BR3 protein in BR3-shRNA–transfected DLBCL cells (MS) resulted in the accumulation of TRAF3 protein level (Figure 7C), suggesting that the activated BR3 receptor may stimulate NIK-induced alternative NF-κB pathway activation by promoting TRAF3 degradation in DLBCL cells. To confirm our initial observation we treated normal purified human peripheral blood B cells with human recombinant BLyS protein to activate BR3 signaling, and then determined TRAF3 protein level in vitro in normal, activated B cells. As shown in Figure 7D, the TRAF3 protein level decreased by approximately 50% in the normal B cells stimulated with recombinant BLyS compared with untreated quiescent B cells. These results suggest that the BLyS-stimulated BR3 receptor activates NIK kinase-induced NF-κB signaling pathway activation by promoting TRAF3 regulatory protein degradation in normal peripheral blood B cells, confirming our observation in DLBCL cells. Because the TRAF2/cIAP1/2 complex has ubiquitin ligase activity that can induce NIK protein ubiquitination followed by proteosomal degradation,18-20 we next determined whether the ubiquitin ligase trimeric complex, TRAF2/cIAP1/2, was also involved in TRAF3 degradation, as has been suggested in recent murine B-cell experiments. Results of our immunoprecipitation experiments showed that the TRAF2/cIAP1/2 complex was associated with the BR3 receptor-bound TRAF3 regulatory protein in the cytoplasmic extracts of DLBCL cells (Figure 7E). Confocal microscopy analysis demonstrated the co-localization of TRAF2 and cIAP1/2 with BR3 in DLBCL cells (Figure 7F). Next, we evaluated whether constitutive BLyS signaling contributes to NIK kinase stabilization through TRAF3 degradation in DLBCL cells. For these experiments, we treated a representative DLBCL cell line (MS) with monoclonal BLyS antibodies to inhibit constitutive BLyS bioactivity. We then assayed for BR3 and TRAF3 protein interactions, as well as for TRAF3 and NIK protein levels, in the treated DLBCL cells using immunoprecipitation and Western blot analyses. As shown in Figure 7G, cells treated with BLyS monoclonal antibody accumulate TRAF3 proteins; however, less TRAF3 protein was recruited to the BR3 receptor compared with controls. Inhibiting BLyS in this manner also resulted in a lower NIK protein level in the treated DLBCL cells compared with controls. These studies suggest that constitutive BLyS stimulation functions in maintaining (stabilizing) NIK kinase activity in DLBCL cells by promoting BR3 receptor activation-induced TRAF3 protein degradation.
Constitutive BLyS/BR3 signaling activates NIK-induced NF-κB pathway activation through induction of TRAF3 degradation in DLBCL. (A) Cytoplasmic cell lysates from MS-GCB or HB-ABC DLBCL cells were immunoprecipitated with BR3 or TRAF3 antibodies and probed with TRAF3 or BR3 antibodies. IgG was used as a negative control. (B) DLBCL-HB cells were co-stained with TRAF3 (FITC, green) or BR3 (Texas Red, red) antibody and analyzed by confocal microscopy. Yellow color indicates co-localization of BR3 and the other probed proteins. (C) Total cell lysates from DLBCL cells (MS) transfected with specific BR3 shRNA or control shRNA were probed with TRAF3 or BR3 antibodies by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (D) Total cell lysates from normal Go B cells stimulated with or without human recombinant BLyS ligand were probed with TRAF3 antibody by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (E) Cytoplasmic extract from DLBCL cells (MS) was subjected to immunoprecipitation analysis with BR3 antibody and then probed with TRAF2, TRAF3, c-IAP1, c-IAP2, or BR3 antibodies by Western blot analysis. (F) DLBCL cells (HB) were co-stained with BR3 (Texas Red, red), along with TRAF2 (FITC, green), c-IAP1 (FITC, green), or c-IAP2 (FITC, green) antibodies, and then analyzed by confocal microscopy. Yellow color indicates co-localization of BR3 and the other probed proteins. (G) Cytoplasmic extracts purified from DLBCL cells (MS) treated with BLyS antibody or control cells were probed with TRAF3 BR3 or NIK antibody in Western blot analysis (left). These samples were also subjected to immunoprecipitation analysis with BR3 antibodies and then probed with TRAF3 or BR3 antibodies for Western blot analysis (right). (H) Cell lysates purified from DLBCL-MS cells transfected with c-IAP1, c-IAP2 shRNA, or control shRNA were analyzed by Western blot analysis with c-IAP1, c-IAP2, or TRAF3 antibody. Actin was used as loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level.
Constitutive BLyS/BR3 signaling activates NIK-induced NF-κB pathway activation through induction of TRAF3 degradation in DLBCL. (A) Cytoplasmic cell lysates from MS-GCB or HB-ABC DLBCL cells were immunoprecipitated with BR3 or TRAF3 antibodies and probed with TRAF3 or BR3 antibodies. IgG was used as a negative control. (B) DLBCL-HB cells were co-stained with TRAF3 (FITC, green) or BR3 (Texas Red, red) antibody and analyzed by confocal microscopy. Yellow color indicates co-localization of BR3 and the other probed proteins. (C) Total cell lysates from DLBCL cells (MS) transfected with specific BR3 shRNA or control shRNA were probed with TRAF3 or BR3 antibodies by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (D) Total cell lysates from normal Go B cells stimulated with or without human recombinant BLyS ligand were probed with TRAF3 antibody by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (E) Cytoplasmic extract from DLBCL cells (MS) was subjected to immunoprecipitation analysis with BR3 antibody and then probed with TRAF2, TRAF3, c-IAP1, c-IAP2, or BR3 antibodies by Western blot analysis. (F) DLBCL cells (HB) were co-stained with BR3 (Texas Red, red), along with TRAF2 (FITC, green), c-IAP1 (FITC, green), or c-IAP2 (FITC, green) antibodies, and then analyzed by confocal microscopy. Yellow color indicates co-localization of BR3 and the other probed proteins. (G) Cytoplasmic extracts purified from DLBCL cells (MS) treated with BLyS antibody or control cells were probed with TRAF3 BR3 or NIK antibody in Western blot analysis (left). These samples were also subjected to immunoprecipitation analysis with BR3 antibodies and then probed with TRAF3 or BR3 antibodies for Western blot analysis (right). (H) Cell lysates purified from DLBCL-MS cells transfected with c-IAP1, c-IAP2 shRNA, or control shRNA were analyzed by Western blot analysis with c-IAP1, c-IAP2, or TRAF3 antibody. Actin was used as loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level.
To further support this model, additional experiments showed that when c-IAP1/2 expression was inhibited with specific shRNAs in a representative DLBCL cell line (HB), TRAF3 protein level increased in these cells (Figure 7H), indicating that the c-IAP1/2 ubiquitin ligase(s) are likely to be involved in the TRAF3 protein degradation mechanism.
Discussion
Abnormal activation of NF-κB pathways, particularly through the classic (canonical) pathway, has been shown in several studies to be critical in DLBCL cell survival and proliferation.11-14,25 In contrast, the mechanisms governing activation of the alternative NF-κB pathway in lymphoma cells, such as DLBCL, are poorly documented and understood.26 The critical NF-κB–inducing kinase, NIK, was recently found to be a key upstream regulatory molecule of the alternative NF-κB signaling pathway, and probably also the canonical NF-κB pathway; it is maintained at very low levels in unstimulated B cells, and is often barely detectable in normal murine B cells.17,19,20 Two groups of investigators have recently reported that NIK kinase was overexpressed in human multiple myeloma cells.30,31 However, the expression status of NIK in B-cell lymphomas, while of great potential interest, has not been reported, although recent studies have shown genetic abnormalities involving growth and survival and NF-κB inhibitory (A20) genes.25,40 In the present study, we show that the NIK kinase is in fact overexpressed/accumulated in DLBCL cells compared with normal human peripheral blood B cells, suggesting that uninhibited/stabilized NIK kinase continuously stimulates DLBCL growth and survival. Inhibition of NIK protein expression in DLBCL cells significantly decreased both p52 and IκBα proteins, leading to diminished tumor cell growth capacity in treated lymphoma cells, which suggests that constitutive activation of NIK kinase is likely to be involved in inducing both the canonical and alternative NF-κB pathways that are constitutively activated in DLBCLs.41
Our results also show that NF-κB components are differentially expressed in ABC-like and GCB-like DLBCL. Based on the results of protein-expression and DNA-binding assays, the p65/c-rel ratio is significantly higher in ABC-like DLBCL than in GCB-like DLBCL. These findings are consistent with previous findings in which the ABC-like DLBCL was found to have high expression of known NF-κB target genes compared with GCB-type DLBCL42 ; c-rel was found to be amplified in some GCB-like tumors.43 Our findings suggest that the NF-κB target genes identified in ABC-like DLBCL are most likely controlled through the p65 subunit, a transcription factor that is known to control cell growth and survival through the regulation of anti-apoptotic proteins.44 It is possible that relA (p65) and c-rel have differential roles in controlling gene expression, given that both appear to regulate distinct sets of genes45 depending on the relative levels of each subunit, as seen in TNF-related apoptosis-inducing ligand (TRAIL) signaling.46 Peripheral mononuclear blood leukocyte (PMBL)–DLBCL also has activated NF-κB (mostly c-rel), but responds favorably to chemotherapy, unlike the ABC-like subsets, suggesting that different NF-κB member (or dimer sets) regulate different sets of target genes.
Studies from our laboratory and from other investigators have shown that BLyS/BR3 signaling is critical in stimulating and maintaining both canonical and alternative NF-κB signaling pathway activation in both normal and neoplastic murine and human B cells.9,12-15,47 Recent studies also indicated that NIK is a key regulatory survival kinase involved in BR3 receptor-induced alternative NF-κB pathway activation in murine lymphoid cells, but whether the BR3 receptor functions directly in mediating and maintaining NIK protein activity in human normal or malignant B-lymphoid cells has only recently begun to be elucidated.48,49 Our studies show that inhibition of BR3 receptor protein expression decreases NIK protein levels in DLBCL, as well as lymphoma cell proliferation, indicating that constitutive BLyS/BR3 signaling is critical in NIK-induced NF-κB pathway activation and survival mechanisms in DLBCL cells.
Because NIK appears to be a key regulatory molecule in NF-κB pathway activation in both normal and malignant B cells, the mechanisms controlling NIK protein degradation have become important in understanding the pathophysiology and experimental therapeutics of DLBCL. NIK kinase protein is tightly regulated in normal and neoplastic human B cells; however, the mechanisms in involved have not previously been determined. Here we demonstrate that the critical NF-κB signaling adaptor/regulatory protein TRAF3 plays a dominantly negative role in NIK protein signaling and accumulation in both GCB-like and ABC-like human DLBCL cells. Inhibition of TRAF3 by specific shRNA or overexpression of TRAF3 by transfection in DLBCL cells increased or decreased NIK protein levels, respectively, in these lymphoma cells (Figure 4). Our observations suggest that TRAF3 functions as considerably more than simply as a signaling adaptor molecule involved in signal transduction. Dysregulation of this important regulator protein, together with its sequestration through binding to the activated cell membrane BR3 receptor, likely plays a critical role in abnormal DLBCL cell growth and survival.
Previous studies have demonstrated that BR3 receptor activation promotes normal and malignant B-cell survival through both alternative and canonical NF-κB pathway activation, but the mechanism of BR3 receptor-induced NF-κB pathway activation has not been studied in detail or in normal compared with neoplastic human B-lymphoid cells. In this study, we demonstrate that BR3 receptor proteins are primarily located in lipid rafts that reside in the DLBCL plasma membranes, where the BR3 receptor attracts and binds to the TRAF3 protein. This finding suggests that the BR3 receptor and TRAF3 protein form a complex within the plasma membrane to stabilize and further stimulate downstream NF-κB pathways in DLBCL cells, similar to the CD40 signalosome described previously.38
TRAF3 is the only TRAF molecule that has been previously shown to bind directly to the BR3 receptor,50 and recruitment of TRAF3 by the BR3 receptor has previously been reported as a mechanism to explain how BLyS/BR3 signaling promotes NIK-induced alternative NF-κB pathway activation in B-lymphoid cells.17,48 In our study, we have determined that the BR3 receptor most likely contributes to TRAF3 protein degradation, in addition to protecting and stabilizing the NIK kinase protein from proteasomal degradation by recruiting, binding, and sequestering the TRAF3 molecule. The TRAF2/cIAP1/2 ubiquitin ligase complex has been recently reported to be involved in TRAF3-induced NIK degradation in murine genetically engineered B cells.19,20 In our study, we provide evidence that the BR3 receptor forms a multimeric complex with TRAF3, TRAF2, c-IAP1, and c-IAP2 in DLBCL cells, suggesting that BLyS/BR3 signaling may also induce TRAF3 degradation through a TRAF2/c-IAP1/2 complex-mediated ubiquitination mechanism. Our findings in human DLBCL cells are generally consistent with pathophysiologic dysregulation affecting the TRAF3-NIK axis, possibly as a result of constitutive/continuous feedback activation of the BLyS/BR3 receptor/ligand dyad described previously.9,33 These findings also appear to be consistent with the murine model of alternative NF-κB pathway regulation in normal B lymphocytes from genetically engineered mouse models recently proposed by Karin et al.19,20 Interestingly, as Wallach and Kovalenko have also recently pointed out,18 NIK activity can become “seriously harmful” when any of the various ubiquitin ligases that are involved in its regulation are inhibited/mutated. In addition, there are also potential opportunities for developing therapeutic agents that can inhibit NIK functions or protect from the pathologic effects of mechanisms that compromise the function(s) of these critical ubiquitin ligases. Although TRAF2, c-IAP1, and c-IAP2 were found in BR3/TRAF3 complexes in DLBCL cells, the structure, detailed composition, and function(s) of these binding complexes in the pathophysiology of lymphoid tumors such as DLBCL will require additional studies for further mechanistic characterization.
In summary, we have further delineated molecular mechanisms demonstrating how activated BR3 receptors in DLBCL function in mediating NIK kinase-induced dysregulation of NF-κB activation, leading to increased growth and survival in DLBCL cells. These studies contribute to deciphering the complex pathophysiologic mechanisms involved in aggressive B-cell NHLs, and provide a basis for future targeted therapeutic strategies for patients with DLBCL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by National Cancer Institute grant CA-R01-100836 (to R.J.F.), Cancer Center support grant CA-16672-26 (to R.J.F.), a grant from the Lymphoma Research Foundation of America (to R.J.F), and a grant from the Leukemia & Lymphoma Society (LSS 6087-08 to R.J.F.). L.V.P. was supported by the Odyssey program and a Kimberly-Clark Foundation Award for Scientific Achievement at The University of Texas M. D. Anderson Cancer Center.
National Institutes of Health
Authorship
Contribution: L.V.P. and L.F. designed and performed experiments and contributed to writing the manuscript; A.T.T., C.B.-R., E.D., and F.V. helped perform experiments; L.J.M. contributed to writing the manuscript; and R.J.F. supervised the project and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard J. Ford, MD, PhD, Department of Hematopathology, Unit 72, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: rjford@mdanderson.org.
References
Author notes
L.V.P. and L.F. contributed equally to this study.

![Figure 5. BR3 receptor activation promotes DLBCL cell growth through NIK protein stabilization. (A) Cell lysates from representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with NIK shRNA or negative control (NC) shRNA for 48 hours were analyzed by Western blot analysis for NIK, p52, and pIkBα. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (B) Nuclear extracts purified from GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with NIK shRNA or negative control (NC) shRNA for 48 hours were analyzed by DNA-binding ELISA for p52 and p65. (C) Proliferation of DLBCL cells (GCB-MS and SUDHL-4; ABC-HB and OCI-Ly3) transfected with NIK shRNA or negative control non-targeting sequence was analyzed by [3H] thymidine incorporation assays in vitro for 72 hours. The data shown are the means and ranges of triplicate samples of 2 independent experiments. Error bars represent SD. (D) Cell lysates from a representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with BR3 shRNA or negative control (NC) shRNA for 48 hours were probed with BR3, NIK, pIκBα, p52, or caspase 3 antibodies by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (E). DLBCL cells (GCB-MS and SUDHL-4; ABC-HB and OCI-Ly3) were transfected with BR3 shRNA or negative control non-targeting sequence and cell proliferation was analyzed by [3H]-thymidine incorporation assays in vitro for 72 hours. The data shown are the means and ranges of triplicate samples relative to control samples of 2 independent experiments. Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/1/10.1182_blood-2010-06-290437/4/m_zh89991063130005.jpeg?Expires=1769990939&Signature=ttt0f04ZqttR7k6u7z~B1I6~3dvGDuvTY-cRwmQqIvoidxxcNVJxGEZ0rv-a7VApArjTMlzD8883OWvaaKafNBjPnYDmg0MNxOuCTep6~WTMDx4wMEyQFslbYNs7Hn4-zPSoOoTaVMVAfsQzi5hwk263yTbzM9Q3Z9IK7sWNh7PryGpu9yHUi5j0equvMNGooyOLNZNyjn1o6G4dkhoPvyyfWekAXfOh4~5y6GfI70lmiD1tOyca5PG~3dTNIsUum1i9dNg7UUUiTEwVkBXGL9kv2gvCuTWaxI9fYMURJ0g3xGBnzdE6KdeRX9F9dA77fd7DPeGRS2WTqYdIXx0Flw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. The role of TRAF3 in the stabilization of NIK in DLBCL cells. (A) Cell lysates from a representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with a TRAF3 expression vector or a control empty vector for 48 hours were probed with TRAF3 or NIK antibodies by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (B) Cell lysates from a representative GCB-like (MS) or ABC-like (HB) DLBCL cells transfected with TRAF3 shRNA or negative control shRNA were probed with TRAF3 or NIK antibodies by Western blot analysis. Actin was used as a loading control. Relative protein level of each target molecule was measured with ImageJ densitometer software and normalized to the actin level. (C) DLBCL cells (GCB-MS and SUDHL-4; ABC-HB and OCI-Ly3) were transfected with TRAF plasmid, TRAF3 shRNA, or negative control shRNA and cell proliferation was analyzed by [3H]-thymidine incorporation assays in vitro for 72 hours. The data shown are the means and ranges of triplicate samples relative to control samples of 2 independent experiments. Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/1/10.1182_blood-2010-06-290437/4/m_zh89991063130006.jpeg?Expires=1769990939&Signature=jt-T7qIV6~xvKOJSTZ-MHIEIpRXRzvnbc6qjNZZPj6IIBMxyN5jfoCNbVs3nqbn7pjIWJ0TkKbI9j75Yl-EA-SRuBpUeiUxxqi73XUF9~qnFq1qxTKrUEIUoG~4HArBByJzBL6t3qw6V9Vc8O1QcA3xC-FQT8HnnYaOsJpqhKbkvNR3jBDxWUsCbpu~~dIq~-Qqzyjx3~V5HL1u0KZVvec20fsoprmNGi2dujKnBtfNiREAmKhrSB0of9M0PXu~B4TPieTl-704JpbTM54-Os16ZLjgFpvMgvc6hdGznOdXKtQEp5kJjjec1VR2tcyAB8Y3u1LcI4zJBFbcCCuCg7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal