Abstract
We designed a whole tumor cell vaccine by “loading” lymphoma tumor cells with CG-enriched oligodeoxynucleotide (CpG), a ligand for the Toll-like receptor 9 (TLR9). CpG-loaded tumor cells were phagocytosed, delivering both tumor antigen(s) and the immunostimulatory CpG molecule to antigen-presenting cells (APCs). These APCs then expressed increased levels of costimulatory molecules and induced T-cell immunity. TLR9 was required in the APCs but not in the CpG-loaded tumor cell. We demonstrate that T cells induced by this vaccine are effective in adoptive cellular therapy for lymphoma. T cells from vaccinated mice transferred into irradiated, syngeneic recipients protected against subsequent lymphoma challenge and, remarkably, led to regression of large and established tumors. This therapeutic effect could be transferred by CD4+ but not by CD8+ T cells. A CpG-loaded whole-cell vaccination is practical and has strong potential for translation to the clinical setting. It is currently being tested in a clinical trial of adoptive immunotherapy for mantle-cell lymphoma.
Introduction
CG-enriched oligodeoxynucleotides (CpGs) have been shown to interact with Toll-like receptor 9 (TLR9) in antigen-presenting cells (APCs) and in B cells, where they induce the expression of costimulatory molecules such as CD80 and CD86, major histocompatibility complex (MHC) class II molecules, and pro-inflammatory cytokines.1-4 Malignant B cells also express TLR9 and respond to CpG in a similar fashion. We have previously shown that systemic antitumor immunity can be induced by the combination of cytotoxic chemotherapy with local, intratumoral injection of CpG. This therapy can eliminate established, metastatic lymphoma tumors.5,6 In these studies, we found that it was necessary to inject CpG directly into the tumor. Using a model system in which both the host and the tumor lack TLR9 (TLR9KO), we found that TLR9 was required for this therapy, but that its expression could be restricted either to the host or to the tumor cell. Therefore, we concluded that CpG can act either on the tumor B cells or on the host APCs to enhance the uptake and presentation of tumor antigens, thereby leading to a cytotoxic CD8+ antitumor T-cell response. CD8+ T cells thus induced were especially effective when adoptively transferred to tumor-bearing animals.5
In an attempt to extend the use of CpG as an immunotherapy and to make it more practical, we exposed tumor B cells to CpG ex vivo, and subsequently injected them into the host as a whole tumor cell vaccine. This approach obviates the need for an accessible, injectable tumor site. We show that vaccination with such CpG-loaded tumor B cells also induces antitumor T-cell immunity, but in this case it is a CD4+ and not a CD8+ T-cell response.
We demonstrate that CpG loads into tumor B cells independently of TLR9. Loaded tumor cells can release CpG into their environment and are more highly phagocytosed by both macrophages and dendritic cells (DCs). In turn, these APCs take on an activated phenotype with high expression of costimulatory molecules, a response that is dependent on TLR9. This vaccination maneuver induces CD4+ antitumor T cells that can be adoptively transferred to cure large, established tumors.
Methods
Reagents
CpG 1826 with sequence 5′-TCCATGACGTTCCTGACGTT was provided by Coley Pharmaceutical Group. Fluorescein isothiocyanate (FITC)–conjugated CpG 1826 was purchased from InvivoGen. The following monoclonal antibodies (mAbs) were used for flow cytometry: rat anti–mouse CD4-Pacific blue, rat anti–mouse CD19-PE, rat anti–mouse CD25-FITC, rat anti–mouse CD40-PE, rat anti–mouse FoxP3-PE, rabbit anti–mouse caspase-3-PE, hamster anti–mouse CD80-PE, H-2Kb-PE, I-Ab-PE, rat isotype controls-PE, hamster anti–mouse CD11c-PE, and rat anti–mouse F4/80-PE. Antibodies were purchased from either BD Biosciences or eBioscience. Alexa Fluor 700 dye (Ax700) was purchased from Invitrogen. BBL Thioglycollate Medium Brewer Modified was purchased from BD Biosciences.
Cell lines and mice
H11 is a pre–B-cell line in the C57BL/6 background that was generated as follows. Primary bone marrow cells were isolated from C57BL/6 mice and transfected with the retrovirus vector murine stem cell virus (MSCV)–neo/p190Bcr-Abl, which carries the oncogene Bcr-Abl7 (a gift from M. Cleary and K. Smith, Stanford University School of Medicine). A cell line was generated from the bone marrow of a TLR9KO BALB/C mouse as described previously.6 A20 (BALB/c B-cell lymphoma line) and EL4 (C57BL/6 T-cell lymphoma line) cells were obtained from ATCC. MC-38 (C57BL/6 colon carcinoma), LLC1 (C57BL/6 lung carcinoma), and B16 (C57BL/6 melanoma) cells were gifts of S. Strober (Stanford University School of Medicine). Tumor cells were cultured in complete Roswell Park Memorial Institute 1640 medium (cRPMI; Invitrogen) containing 10% fetal bovine serum (FBS; Thermo Scientific), 100 U/mL penicillin, 100 μg/mL streptomycin (both from Invitrogen), and 50μM 2-ME (Sigma-Aldrich). Six- to 8-week-old female C57Bl/6J mice were purchased from The Jackson Laboratory. TLR9KO mice on a C57BL/6 background were obtained from Lawrence Steinman (Stanford University) with permission from S. Akira. All studies were approved by the Stanford University Administrative Panel on Laboratory Animal Care.
Tumor inoculation and animal studies
H11 tumor cells (0.5 × 106/mL) were incubated in the presence of 3 μg/mL CpG at 37°C and 5% CO2. After 24 hours, cells were washed 3 times with wash buffer to remove unbound CpG. H11 cells that were loaded with CpG (CpG/H11) or H11 control cells (H11) were irradiated (50 Gy) and used to vaccinate C57BL/6 donor mice subcutaneously for 5 consecutive days at a dose of 1 × 106 cells/vaccination. On day 13, bone marrow and splenocytes of donor mice were transferred by intravenous injection into irradiated C57BL/6 recipient mice (9.5-Gy total body irradiation, Philips X-ray unit, 250 kV, 15 mA) along with 1 × 106 irradiated tumor cells as a posttransplantation “booster” vaccine.5 Recipient mice were challenged with H11 tumor cells subcutaneously at a dose of 1 × 107 cells in 50 μL of serum-free RPMI on day 16. Tumor growth was monitored by caliper measurement. For therapeutic experiments, recipient mice were challenged with 1 × 107 H11 tumor cells subcutaneously 9 days after inoculation, and the tumors were approximately 3 cm2. Recipients were administered 9.5-Gy total body irradiation and given transplantation as described above.
Flow cytometry was used to sort CD4+ and CD8+ T cells from splenocytes of CpG/H11- or H11-vaccinated donor mice. Purified donor CD4+ or CD8+ cells were administered to irradiated recipient mice. Recipient mice were challenged with tumor and followed as above.
Flow cytometry
Cells were surface stained in wash buffer (phosphate-buffered saline [PBS], 1% FBS, and 0.01% sodium azide), fixed in 2% paraformaldehyde, and analyzed by flow cytometry on an FACSCalibur or LSR II system (BD Biosciences). Data were analyzed using Cytobank (Stanford University).8
CpG-loading studies
H11 or TLR9KO cells were loaded with FITC-labeled CpG for 30 seconds, 30 minutes, 1 hour, 6 hours, 12 hours, or 24 hours, as described in “Tumor inoculation and animal studies.” Cells were immediately washed and fixed in 2% paraformaldehyde to stop the CpG interaction at precise time points. CpG loading was analyzed by flow cytometry.
CpG-leaking studies
H11 cells were loaded with FITC-labeled CpG for 24 hours and washed thoroughly in cRPMI. Macrophages or splenocytes from either wild-type C57BL/6 or TLR9KO mice were used as responder cells. Responder cells were assayed for expression of the costimulatory molecules CD40, CD80, and CD86 after 2 and 24 hours of exposure.
Confocal microscopy
H11 or TLR9KO cells were suspended in RPMI and incubated for 24 hours with 3 μg/mL FITC-conjugated CpG. The stained cells were fixed in 2% paraformaldehyde for 10 minutes, washed, transferred to coverslips pretreated with Cell-Tak cell and tissue adhesive (BD Biosciences), permeabilized for 10 minutes with 0.1% Triton-X 100 in PBS, and blocked for 1 hour with 5% goat serum and 1% bovine serum albumin (BSA) in PBS. To detect CD19, cells were incubated as indicated, then stained with CD19-APC for 30 minutes. Cells were mounted in antifade reagent (ProLong Gold; Invitrogen) with or without 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). Images were obtained with a confocal microscope (LSM 510 NLO; Carl Zeiss) using a Plan-Apochromat 100×/1.4 oil objective (Carl Zeiss). LSM 510 AIM software (Version 4.0; Carl Zeiss) was used to acquire images. All experiments were performed in triplicate.
Generation of thioglycollate-induced macrophages
Thioglycollate solution was prepared as described previously.9 Thioglycollate solution (500 μL) was injected intraperitoneally into each mouse. After 72 hours, macrophages were isolated from the peritoneal cavity by lavage. Cells were adhered to 6-well plates for 4 hours, at which point nonadherent cells were washed away. The remaining adherent cells were > 95% macrophages (F4/80+), as assessed by flow cytometry.
Generation of bone marrow–derived DCs
DCs were generated from either C57BL/6 or TLR9KO bone marrow by 7-day culture in cRPMI supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF).10
Phagocytosis assay
Macrophages or DCs were prepared as described in the 2 previous sections. Ax700-labeled H11 or CpG/H11 tumor cells (5 × 106) were added to the macrophage- or DC-containing wells for 24 hours. Adherent cells were harvested from the plate by scraping, stained with anti-F4/80-PE or CD11c-PE, and analyzed by flow cytometry.
Statistical analysis
Prism 5.0 software (GraphPad) was used to analyze tumor growth and to determine statistical significance of differences between groups by applying an unpaired Student t test. Kaplan-Meier plots were used to analyze survival. Comparisons of survival curves were made using the log-rank test. Significance of difference in phagocytosis assays was determined by applying an unpaired Student t test. P values < .05 were considered significant.
Results
CpG loading is required for effective vaccination in TLR9-competent hosts
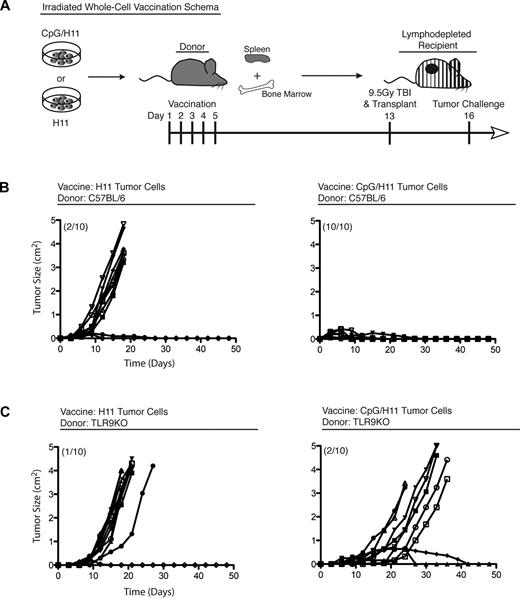
Our prior work with direct intratumor injection of CpG suggested that malignant B cells may be activated by CpG to present their own tumor antigens and induce a therapeutically effective T-cell immune response.5,6 Accordingly, we hypothesized that tumor B cells could be exposed to CpG ex vivo and serve as a whole-cell vaccine. Tumor B cells (H11) were stimulated with CpG for 24 hours in vitro (CpG/H11), washed, irradiated, and administered to donor animals at a dose of 1 × 106 cells/vaccination. Splenocytes from these vaccinated donor mice were transferred to irradiated recipient mice (10 × 106 splenocytes/mouse) that were subsequently challenged with a lethal dose of H11 tumor cells (Figure 1A). Adoptively transferred splenocytes from CpG/H11-vaccinated donor mice protected 100% of recipients from tumor challenge for more than 100 days (Figure 1B right panel). In contrast, cell transfer from mice vaccinated with untreated H11 cells protected only 20% of recipients (Figure 1B left panel). The transfer of cells from unvaccinated mice was similarly ineffective (data not shown).
A CpG-loaded, whole-cell vaccine generates robust antitumor immunity. (A) Vaccination schema: C57BL/6 donors were vaccinated with either CpG/H11 or H11. (B) Cohorts of C57BL/6 recipient mice (n = 10) and (C) TLR9KO (C57BL/6 background) recipient mice were followed for tumor growth and survival. Numbers in parentheses represent the number of mice that survived more than 100 days. Results are representative of 2 independent experiments.
A CpG-loaded, whole-cell vaccine generates robust antitumor immunity. (A) Vaccination schema: C57BL/6 donors were vaccinated with either CpG/H11 or H11. (B) Cohorts of C57BL/6 recipient mice (n = 10) and (C) TLR9KO (C57BL/6 background) recipient mice were followed for tumor growth and survival. Numbers in parentheses represent the number of mice that survived more than 100 days. Results are representative of 2 independent experiments.
To determine whether TLR9 is required for effective vaccination, we vaccinated TLR9KO mice and adoptively transferred their splenocytes into irradiated wild-type recipients. These TLR9KO donor cells failed to protect against tumor challenge (Figure 1C). This result established that TLR9 was necessary for the induction of immunity in the donor and raised the question of which donor cells were responding to the vaccine. Notably, the H11 tumor cell line lacks expression of MHC class II (I-Ab), regardless of stimulation with CpG (Figure 2).
H11 tumor cells lack expression of MHC class II (I-Ab). H11 tumor cells were incubated with CpG and assayed after 24 hours for expression of the indicated molecules.
H11 tumor cells lack expression of MHC class II (I-Ab). H11 tumor cells were incubated with CpG and assayed after 24 hours for expression of the indicated molecules.
Vaccination induces CD4+ T cell–mediated antitumor immunity
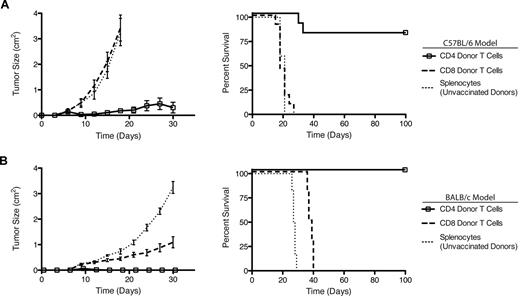
Next, we purified CD4+ and CD8+ T cells from vaccinated mice and transferred them separately into irradiated recipients.5 We chose cell doses that reflect the natural ratio of CD4+:CD8+ T cells in donor spleens (∼ 2:1): CD4+ = 2 × 106 cells/recipient mouse; CD8+ = 1 × 106 cells/recipient mouse. Three days after transfer, recipient mice were challenged with a lethal dose of H11 tumor cells. The purified CD4+ T cells from vaccinated donors were sufficient to protect 80% of recipient mice for more than 100 days (Figure 3). Conversely, the purified CD8+ T cells had no effect either on tumor growth rate or on overall survival.
Vaccine-induced CD4+ T cells mediate antitumor immunity. (A) T-cell subsets were isolated by flow cytometric cell sorting from CpG/H11-vaccinated C57BL/6 donors and transferred into lympho-depleted recipients. Cohorts of C57BL/6 recipient mice (n = 10) were followed for tumor growth (left panel) and survival (right panel). (B) T-cell subsets were isolated by flow cytometric cell sorting from CpG/A20-vaccinated BALB/c donors and transferred into lympho-depleted recipients. Cohorts of BALB/c recipient mice (n = 10) were followed for tumor growth (left panel) and survival (right panel). Results are representative of 2 independent experiments.
Vaccine-induced CD4+ T cells mediate antitumor immunity. (A) T-cell subsets were isolated by flow cytometric cell sorting from CpG/H11-vaccinated C57BL/6 donors and transferred into lympho-depleted recipients. Cohorts of C57BL/6 recipient mice (n = 10) were followed for tumor growth (left panel) and survival (right panel). (B) T-cell subsets were isolated by flow cytometric cell sorting from CpG/A20-vaccinated BALB/c donors and transferred into lympho-depleted recipients. Cohorts of BALB/c recipient mice (n = 10) were followed for tumor growth (left panel) and survival (right panel). Results are representative of 2 independent experiments.
To generalize our conclusions, we studied a second mouse tumor (A20 of BALB/c origin). As before, we loaded A20 tumor cells with CpG (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and used them to vaccinate BALB/c mice (supplemental Figure 1B). T-cell subsets from these donor mice were purified and adoptively transferred into irradiated, syngeneic recipient mice. Recipients were challenged with a lethal dose of A20 tumor cells 3 days after transfer. As seen in the H11-C57BL/6 system, CD4+ T cells from vaccinated BALB/c donors were both necessary and sufficient to protect recipient mice from tumor challenge (Figure 3B). CD8+ T cells from these same donors had little impact on tumor growth or on overall survival. These findings confirm that the requirement for CD4+ T cells induced by this vaccine maneuver was neither strain nor tumor model specific.
Loading of lymphoma B cells with CpG is TLR9 independent
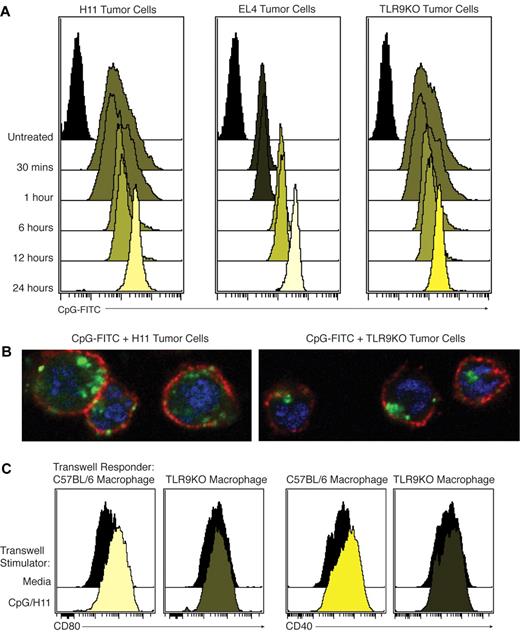
As described previously by others,2 we found that CpG is taken up into cells rapidly after exposure in vitro. Using FITC-labeled CpG (CpG-FITC), we observed that loading of tumor B cells with CpG occurs effectively after 24 hours of in vitro incubation. In both H11 and A20 mouse B-cell lymphoma lines, CpG was bound and sequestered in a time-dependent manner (Figure 4A and supplemental Figure 1A). Confocal microscopy of CpG-loaded H11 revealed that CpG was extensively endocytosed (Figure 4B).
CpG can be loaded into tumor cells independently of TLR9 and leaked into the immediate microenvironment. (A) H11, EL4, or TLR9KO tumor cells were incubated with CpG-FITC. Cells were thoroughly washed and analyzed by flow cytometry. Results were consistent across 3 independent experiments. (B) Representative confocal microscopy image of tumor cells loaded with CpG-FITC for 24 hours. Blue = DAPI, red = CD19, and green = CpG-FITC. (C) CpG/H11 tumor cells or media were plated in the upper portion of a permeable supports. Either C57BL/6 or TLR9KO macrophages were plated as responder cells in the base of the permeable supports. After 24 hours, macrophages were collected and CD40 and CD80 expression were determined by flow cytometry. All plots were gated on live cells.
CpG can be loaded into tumor cells independently of TLR9 and leaked into the immediate microenvironment. (A) H11, EL4, or TLR9KO tumor cells were incubated with CpG-FITC. Cells were thoroughly washed and analyzed by flow cytometry. Results were consistent across 3 independent experiments. (B) Representative confocal microscopy image of tumor cells loaded with CpG-FITC for 24 hours. Blue = DAPI, red = CD19, and green = CpG-FITC. (C) CpG/H11 tumor cells or media were plated in the upper portion of a permeable supports. Either C57BL/6 or TLR9KO macrophages were plated as responder cells in the base of the permeable supports. After 24 hours, macrophages were collected and CD40 and CD80 expression were determined by flow cytometry. All plots were gated on live cells.
We next tested whether loading is dependent on functional TLR9. We incubated a TLR9KO B-cell tumor line and a TLR9-negative T-cell lymphoma line (EL4) with CpG-FITC, as above. CpG entered and was retained equally well by these TLR9-deficient cell lines (Figure 4A). Further, confocal microscopy studies showed that CpG was similarly endocytosed in TLR9-deficient cells (Figure 4B). These results suggest that any tumor cells, even those lacking TLR9, can be effectively loaded with CpG by in vitro incubation.
CpG-loaded tumor cells leak CpG into their immediate microenvironment
We sought to determine whether loaded CpG is irreversibly taken up by tumor cells or if it could be released, or “leaked,” into the immediate microenvironment and activate nearby immune cells. H11 tumor cells were loaded with CpG-FITC, washed thoroughly, and plated on permeable supports (Transwell; Corning Life Sciences) with splenocytes on the opposite side of the membrane. We observe that the splenocytes became FITC positive, suggesting that CpG leaks and crosses the membrane (supplemental Figure 2).
To demonstrate that leaked CpG can activate nearby APCs, we plated CpG-loaded H11 cells in a permeable support with wild-type or TLR9KO macrophages plated on the other side of the membrane. Macrophages were assayed for expression of CD40 and CD80. Wild-type macrophages exhibited increased expression of CD40 and CD80 after culture with CpG-loaded H11. TLR9KO macrophages showed no evidence of this activation, confirming that leaked CpG activates local APCs in a TLR9-dependent manner (Figure 4C).
To activate T cells, APCs must present 2 concurrent signals, the first via cognate antigen (signal 1) and the second by costimulatory molecules (signal 2). Based on our in vivo data, we hypothesized that CpG-loaded tumor cells would enhance the phagocytic ability of APCs and also induce these same APCs to express higher levels of costimulatory molecules.
CpG-loaded tumor cells enhance the phagocytic potential of APCs
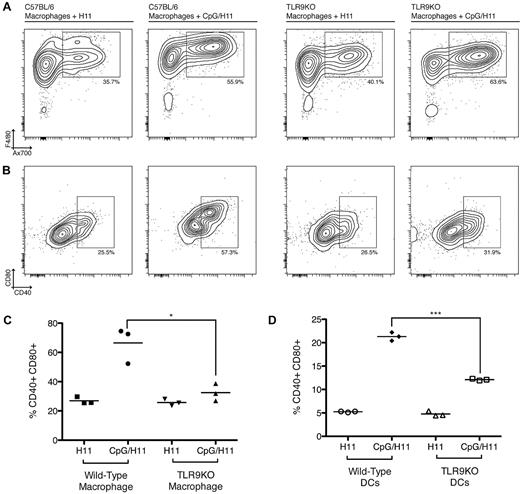
To address whether CpG-loaded tumor cells are more effectively phagocytosed by APCs, we labeled CpG/H11 or H11 tumor cells with Ax700, and placed them in coculture with adherent macrophages for 24 hours. At the end of the incubation time, the culture wells were vigorously washed to remove any cells that were not phagocytosed (nonadherent cells), and the remaining macrophages were harvested and assayed by flow cytometry. F4/80+ macrophages that phagocytosed tumor cells appeared as Ax700+F4/80+ cells. CpG/H11 cells were more highly phagocytosed than H11 tumor cells alone (Figure 5A top row).
Preincubation of H11 cells with CpG enhances phagocytosis and activation of macrophage and DCs. (A) Ax700-labeled CpG/H11 or H11 tumor cells were added to macrophage-containing wells for 24 hours. Nonadherent cells were washed off the plate, and the remaining macrophages were harvested and analyzed by flow cytometry. Phagocytosis was assessed by the percentage of Ax700+F4/80+ cells (top row). (B) Activation was assessed by expression of CD40 and CD80 (bottom row). Plots are representative of 3 independent wells per condition. (C) Data from 3 independent wells shows the percentage of CD40+CD80+ macrophages from either wild-type C57BL/6 or TLR9KO mice after incubation with CpG/H11 or H11. *P < .05 (Student unpaired t test). (D) Bone marrow–derived DCs were assayed for phagocytosis and activation as above. Data from 3 independent wells show the percentage of CD40+CD80+ DCs from either wild-type C57BL/6 or TLR9KO mice after incubation with CpG/H11 or H11. ***P < .0001. All plots are gated on live cells.
Preincubation of H11 cells with CpG enhances phagocytosis and activation of macrophage and DCs. (A) Ax700-labeled CpG/H11 or H11 tumor cells were added to macrophage-containing wells for 24 hours. Nonadherent cells were washed off the plate, and the remaining macrophages were harvested and analyzed by flow cytometry. Phagocytosis was assessed by the percentage of Ax700+F4/80+ cells (top row). (B) Activation was assessed by expression of CD40 and CD80 (bottom row). Plots are representative of 3 independent wells per condition. (C) Data from 3 independent wells shows the percentage of CD40+CD80+ macrophages from either wild-type C57BL/6 or TLR9KO mice after incubation with CpG/H11 or H11. *P < .05 (Student unpaired t test). (D) Bone marrow–derived DCs were assayed for phagocytosis and activation as above. Data from 3 independent wells show the percentage of CD40+CD80+ DCs from either wild-type C57BL/6 or TLR9KO mice after incubation with CpG/H11 or H11. ***P < .0001. All plots are gated on live cells.
To determine whether leaked CpG was interacting with TLR9 and enhancing phagocytosis, we analyzed phagocytosis by macrophages from TLR9KO mice that were similarly incubated with CpG/H11 or H11 labeled with Ax700. Surprisingly, we observed that CpG/H11 cells were still more highly phagocytosed than H11 cells by TLR9KO macrophages (Figure 5A top row).
DCs are the most potent APCs in eliciting T-cell immunity. Therefore, we performed a similar set of experiments using DCs. Once again, uptake of CpG/H11 cells by DCs was higher that that of H11 cells (supplemental Figure 3). This increased uptake was not dependent on TLR9, because TLR9KO DCs displayed a similar preference for the CpG-loaded tumor cells (supplemental Figure 3). Increased phagocytosis of CpG/H11 cells was not dependent on TLR9, suggesting that CpG stimulation of the tumor B cell induces phenotypic changes, making them better targets of phagocytosis by APCs.
CpG-loaded H11 tumor cells enhance the activation state of APCs
Next, we assessed whether CpG-loaded tumor cells could enhance the expression of costimulatory molecules by the engulfing APCs. Macrophages were analyzed for expression of costimulatory molecules after exposure to CpG-loaded tumor cells. Macrophages exposed to CpG/H11 expressed higher CD40 and CD80 than macrophages exposed to H11 alone (Figure 5A-C). Despite their increased phagocytosis, there was not a similar enhancement in CD40 or CD80 expression by TLR9KO macrophages exposed to CpG/H11 (Figure 5A-C). Similarly, DCs exposed to CpG/H11 expressed higher CD40 and CD80 (Figure 5D), but not when they were derived from TLR9KO mice. Therefore, CpG loaded into tumor cells and delivered to APCs after engulfment provided an important stimulatory signal that was dependent on TLR9.
CpG/H11 vaccine-induced T cells can eradicate large and established tumors
We evaluated the ability of CpG/H11 vaccine-induced lymphocytes to treat large and established tumors after adoptive transfer. Recipient mice were inoculated with H11 tumor cells subcutaneously, and 9 days after inoculation, the tumor volume averaged 3 cm.2 Irradiation was then administered, followed by transfer of 25 × 106 splenocytes from CpG/H11-vaccinated donors, unvaccinated donors, or donors vaccinated with an irrelevant tumor cell, CpG/EL4 (Figure 6A). Splenocytes from CpG/H11-vaccinated donor mice cured 100% of recipient mice. In contrast, splenocytes from unvaccinated mice or mice vaccinated with irrelevant tumor cells protected none of the recipient mice (Figure 6B-C).
Vaccine-induced T cells treat large and established tumors. (A) Vaccination and treatment schema: C57BL/6 donors were vaccinated with CpG/H11. (B) Cohorts of C57BL/6 recipient mice (n = 5) received 25 × 106 splenocytes from CpG/H11 vaccinated, CpG/EL4-vaccinated, or unvaccinated donors, and were followed for tumor growth. (C) Images of representative recipient mice on day 9 after tumor inoculation and on day 21. Results are representative of 2 independent experiments. (D) IFN-γ response assay to detect antitumor immune responses in recipient mice 15 days after transfer. Peripheral blood lymphocytes were cocultured with irradiated H11 tumor cells or the irrelevant tumor cell lines MC-38, LLC1, or B16.
Vaccine-induced T cells treat large and established tumors. (A) Vaccination and treatment schema: C57BL/6 donors were vaccinated with CpG/H11. (B) Cohorts of C57BL/6 recipient mice (n = 5) received 25 × 106 splenocytes from CpG/H11 vaccinated, CpG/EL4-vaccinated, or unvaccinated donors, and were followed for tumor growth. (C) Images of representative recipient mice on day 9 after tumor inoculation and on day 21. Results are representative of 2 independent experiments. (D) IFN-γ response assay to detect antitumor immune responses in recipient mice 15 days after transfer. Peripheral blood lymphocytes were cocultured with irradiated H11 tumor cells or the irrelevant tumor cell lines MC-38, LLC1, or B16.
Antitumor immune responses in donor mice were analyzed at the time of transplantation. We evaluated whether these cells were tumor reactive by in vitro interferon-γ (IFN-γ) production assay. Whole peripheral blood lymphocytes were collected and placed in coculture with irradiated tumor cells. We were unable to detect any IFN-γ production in the donor cells in response to coculture with tumor cells. We similarly evaluated antitumor immune responses in recipient mice 15 days after transfer, and IFN-γ responses were detected in these mice. Approximately 3.1% of CD4 T cells and 9.0% of CD8 T cells produced IFN-γ in response to coculture with H11 tumor cells (Figure 6D). This response was specific, because coculture with the irrelevant tumor lines MC-38, LLC1, and B16 did not induce IFN-γ expression above background. To address whether this T-cell response was due to reactivity against FBS antigens,11 donors were vaccinated as described in “Tumor inoculation and animal studies.” One group of recipient mice received tumor challenge (subcutaneously) with 10 × 106 H11 tumor cells cultured in cRPMI (“in vitro passage”). The other group received tumor challenge (subcutaneously) with 10 × 106 H11 tumor cells freshly isolated from a growing tumor on a mouse (“in vivo passage”). Splenocytes from vaccinated mice equally protected recipients from tumor challenge with both “in vitro” and “in vivo” H11. Both tumors grew similarly in mice receiving splenocytes from unvaccinated donors (supplemental Figure 4).
Our experimental design is a model for an ongoing clinical trial of autologous transplantation for patients with mantle cell lymphoma (see www.ClinicalTrials.gov, identifier NCT00490529). As the protocol for this trial describes, patients will have undergone cytoreduction and will be in a state of minimal residual disease before vaccination. We have tested whether vaccination of tumor-bearing mice can induce antitumor immunity. Briefly, donor mice were inoculated subcutaneously with 10 × 106 H11 tumor cells and, 6 days after inoculation, tumor size was approximately 1 cm.2 Donor mice were then given a single dose of cytoxan (100 mg/kg), followed by 5 subcutaneous injections of the CpG/H11 vaccine. Untreated and cytoxan-treated donors served as controls. Three days after the last vaccination, donor mice were killed and their splenocytes harvested. Then, 25 × 106 splenocytes were injected into lethally irradiated recipient mice. Because cytoreduction was minimally effective in reducing tumor burden, a significant number of tumor cells were carried over in the transplantation, resulting in a rapid dissemination of disease in recipient mice. However, we did observe a statistically significant difference in overall survival in mice that received cytoxan plus vaccination compared with mice that received cytoxan alone (supplemental Figure 5). This result suggests that vaccination is effective at inducing antitumor immunity, even in the setting of a tumor-bearing donor.
Discussion
Our results indicate that vaccination with CpG-loaded whole-tumor cells can induce a robust antitumor T-cell immune response. Furthermore, we have uncovered several findings that relate to the mechanism of this vaccine: (1) CpG can be effectively loaded into any tumor cell independent of its expression of TLR9; (2) uptake by APCs of CpG-loaded tumor cells is more efficient; (3) engulfing APCs are induced to express costimulatory molecules, an effect that is dependent on TLR9; and (4) CpG-loaded whole-cell vaccines induce CD4+ T cells that can eradicate tumors when adoptively transferred to syngeneic recipients.
Many studies support an effective role for TLR ligands in the induction of antitumor immune responses.5,6,12,13 The “danger model” of Matzinger suggests that tumor antigens present in the context of bacterial signals may be viewed by the immune system as dangerous, leading to tumor elimination.14 Indeed, reports from Speiser et al15 and Kochenderfer et al12 have shown that the addition of CpG to tumor antigens (in these cases, a melanoma peptide) stimulates the expansion and function of peptide-specific CD8+ T cells in humans and mice. Our strategy of using a CpG-loaded whole-cell tumor vaccine builds upon this prior work. Our finding that CpG loading of tumor cells is independent of TLR9 suggests the broad applicability of this strategy to many tumor types.
Our in vivo and in vitro studies support the notion that CpG-loaded cells efficiently deliver both CpG and tumor antigens to host APCs. This CpG has a stimulatory effect on the APCs, leading to enhanced antigen presentation and in turn better stimulation of an antitumor immune response (Figure 7). Studies by Sotomayor et al showed that in the absence of a stimulatory signal, host APCs took up B-cell lymphoma tumor antigens, presented them to CD4+ T cells, and induced T-cell tolerance against the tumor.16 In contrast to direct antigen presentation by the lymphoma B cell itself, this “cross-presentation” by host APCs was the central mechanism for tolerance induction. Accordingly, a significant body of work has focused on manipulating APCs to better stimulate antitumor immunity. Hsu et al demonstrated that autologous DCs pulsed ex vivo with lymphoma-derived idiotype proteins stimulate host antitumor immunity in patients with B-cell lymphoma.17 Most recently, Dendreon Corporation has demonstrated in prostate cancer patients that autologous DCs pulsed ex vivo with a tumor-associated antigen, prostatic acid phosphatase, induce both humoral and T-cell immune responses and increase overall survival in patients with hormone-refractory prostate cancer. This was the first therapeutic cancer vaccine approved by the Food and Drug Administration.18 Song et al demonstrated in an animal model that direct injection of autologous DCs into tumor sites could lead to a systemic antitumor T-cell immune response.19 Using TLR9 ligands instead of DCs, Li et al found a similar therapeutic effect.6 In the present article, we have described the delivery of both tumor antigens and the TLR9 ligand stimulatory signal to APCs by first loading tumor cells ex vivo with CpG and then injecting them into random subcutaneous sites. One potential limitation of this approach is the restriction of TLR9 expression to plasmacytoid DCs in humans compared with myeloid DCs in mice.20 However, Tel et al21 and Hoeffel et al22 have shown that plasmacytoid DCs can uptake exogenous antigens in the context of apoptotic cells and induce both CD4+ and CD8+ T-cell responses against these antigens. Therefore, we believe that in humans, CpG-loaded tumor cells will be taken up by plasmacytoid DCs and activate these cells to induce antitumor T-cell responses.
Role of CpG in mediating effective vaccination with a CpG-loaded whole tumor cell vaccine. At left (1), CpG loading of tumor cells is independent of TLR9, suggesting broad applicability of this strategy to many tumor types. In center (2), CpG-loaded tumor cells function to specifically and efficiently deliver CpG to APCs. This CpG has a stimulatory effect on the APCs, leading to (3, right) more efficient antigen presentation and, in turn, better stimulation of an antitumor T-cell response.
Role of CpG in mediating effective vaccination with a CpG-loaded whole tumor cell vaccine. At left (1), CpG loading of tumor cells is independent of TLR9, suggesting broad applicability of this strategy to many tumor types. In center (2), CpG-loaded tumor cells function to specifically and efficiently deliver CpG to APCs. This CpG has a stimulatory effect on the APCs, leading to (3, right) more efficient antigen presentation and, in turn, better stimulation of an antitumor T-cell response.
Rosenberg et al have demonstrated impressive clinical outcomes using ex vivo–generated T cells in adoptive immunotherapy for metastatic melanoma.23-25 Our prior work illustrated how antitumor T cells induced by intratumoral injection of CpG could be used in mouse models for adoptive immunotherapy.5 Here, we demonstrate in 2 different tumor models (H11-C57BL/6 and A20-BALB/c) that a vaccine composed of CpG-loaded whole tumor cells can also generate antitumor T cells that, when transferred to adoptive hosts, both protect against (Figures 1,3; supplemental Figure 1) and eradicate large, established tumors (Figure 6).
Interestingly, our results establish that CpG-loaded whole-cell vaccines induce an antitumor CD4+ T-cell response. These CD4+ T cells were both necessary and sufficient to transfer antitumor immunity. To date, the field of adoptive cell therapy has focused primarily on CD8+ cytotoxic T lymphocytes.23,25,26 However, Hunder et al demonstrated durable clinical remissions in melanoma patients treated with ex vivo–expanded, antigen-specific CD4+ T cells.27 Most tumors express MHC class I but lack expression of MHC class II. CD8+ T cells offer a direct mechanism to kill these tumor cells via recognition of peptide-MHC I complexes.23,25,26 Regardless, several reports have identified important roles for CD4+ T cells in antitumor immunity.27-30 Studies by Antony et al31 and Wang et al32 have described the classic “helper” function of CD4+ T cells in priming CD8+ antitumor T-cell responses. Other studies have demonstrated that antitumor CD4+ T cells can clear tumors independently of CD8+ cytotoxic T lymphocytes.27-36 Both Hung et al30 and Corthay et al33 have shown that CD4+ T cells recruit macrophages and eosinophils to tumor sites and are essential for successful tumor rejection. Perez-Diez et al suggested that CD4+ T-cell antitumor responses are dependent on the presence of natural killer cells for long-term tumor clearance.36
A relevant concern in using CD4+ T cells for adoptive therapy is the potential for cotransfer of regulatory T cells. Our prior work has demonstrated that antitumor T cells proliferate 3.6-fold more than FoxP3+ regulatory T cells when transferred into lympho-depleted recipient mice,5 thereby addressing this concern. A second concern is that vaccination may stimulate regulatory T-cell expansion. We have not observed a significant increase in the percentage of FoxP3+CD25+ regulatory T cells in response to vaccination compared with naive donors (data not shown).
Despite these concerns, the concept of CD4+ T cells coordinating broad antitumor responses is important for the field of adoptive therapy. CD4+ cells play central roles in nearly all aspects of the adaptive immune response, including the recruitment of other immune cell types and the activation of B cells and APCs.37 This role is particularly pertinent in responding to tumors that have down-regulated their MHC molecules, a common mechanism for tumor evasion of immune responses.38 Our model demonstrates that CD4+ T cells can mediate tumor rejection independently of MHC class II expression on the tumor. Therefore, adoptive therapy with CD4+ cells is a powerful approach to generating adaptive antitumor immunity.
The work reported here demonstrates that a CpG-loaded whole tumor cell vaccine is an effective and practical strategy to generate an antitumor T-cell response. Our studies have shown that CpG can be carried by the loaded tumor cell into the APC, where it interacts with TLR9 to enhance antigen presentation. The lack of requirement for TLR9 in the tumor cell suggests that a similar approach may be effective for many tumor types. A critical component of this maneuver relies on the ability to isolate ample numbers of tumor cells to prepare the vaccine. Accordingly, leukemia or other malignancies with leukemic presentation would facilitate this isolation. We are currently enrolling patients in a clinical trial using this approach for the treatment of mantle cell lymphoma.
The online version of this article contains a data supplement.
Presented in oral form at the 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 8, 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank K. Sachen and R. Houot for their review of this manuscript.
This work was supported by the National Institutes of Health (grants CA33399 and CA34233). M.J.G. is supported by a Howard Hughes Medical Institute Research Training Fellowship. B.V. is supported by a Ruth L. Kirschstein Award (grant 5 T32 AI07290 B.V.). R.L. is an American Cancer Society clinical research professor.
National Institutes of Health
Authorship
Contribution: M.J.G., B.V., R.R., J.D.B., H.K., and D.K.C. designed the research, performed experiments, analyzed results, made the figures, and wrote the paper; and S.L. and R.L. designed the research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Levy, Chief, Division of Oncology, Department of Medicine, Stanford University School of Medicine, 269 Campus Dr, CCSR 1105, Stanford, CA 94305; e-mail: levy@stanford.edu.
References
Author notes
M.J.G. and B.V. contributed equally to this study.