Abstract
Researchers designing antitumor treatments have long focused on eliciting tumor-specific CD8 cytotoxic T lymphocytes (CTL) because of their potent killing activity and their ability to reject transplanted organs. The resulting treatments, however, have generally been surprisingly poor at inducing complete tumor rejection, both in experimental models and in the clinic. Although a few scattered studies suggested that CD4 T “helper” cells might also serve as antitumor effectors, they have generally been studied mostly for their ability to enhance the activity of CTL. In this mouse study, we compared monoclonal populations of tumor-specific CD4 and CD8 T cells as effectors against several different tumors, and found that CD4 T cells eliminated tumors that were resistant to CD8-mediated rejection, even in cases where the tumors expressed major histocompatibility complex (MHC) class I molecules but not MHC class II. MHC class II expression on host tissues was critical, suggesting that the CD4 T cells act indirectly. Indeed, the CD4 T cells partnered with NK cells to obtain the maximal antitumor effect. These findings suggest that CD4 T cells can be powerful antitumor effector cells that can, in some cases, outperform CD8 T cells, which are the current “gold standard” effector cell in tumor immunotherapy.
Introduction
Researchers designing antitumor vaccines, or treatments involving transfers of activated antitumor cells, have long focused on methods to elicit tumor-specific CD8 CTLs, envisioning that their potent ability to kill tumor targets in vitro and to reject transplants in vivo would translate into equally potent antitumor activity in vivo. Although many of the resulting treatments have indeed been able to elicit CTLs that recognize tumor cells and/or tumor antigens in vitro, complete tumor regression has been achieved in only a minority of patients.1–5 Animal models have generated similar results. In a few cases, the transfer of monoclonal T cell receptor transgenic (TCR Tg) CD8 T cells was able to clear small tumors,6 but in most, the TCR Tg CD8 cells were ineffective without the addition of other aids. In short, though CD8 CTL can clear tumors, they most often do not, unless helped by additional treatments.6–12
Over the last 25 years, a few studies have shown that CD4 T cells could also clear tumors completely independently of CD8s.13–17 Nevertheless, CD4 T cells continue to be studied mainly for their role as helpers for CD8 CTL,11,18,19 and it has even been suggested that tumor-specific CD4 T regulatory cells could act as suppressors of antitumor responses.20 Thus, their potential as CD8-independent antitumor effectors has gained only a few proponents,13–17,21–24 and only a few of the newly designed cancer vaccines incorporate antigens to stimulate CD4 cells, mostly to enhance their helper activity.25,26 Most studies using adoptive transfer of tumor-specific T cells continue to focus entirely on CD8 cells.2,3,27–30
We decided to do a direct comparison between CD4 and CD8 T cells specific for the same tumor, using TCR Tg mice containing pure populations of CD4 or CD8 T cells, in order to test each type of effector alone, without the effects of potential contaminants. To our surprise, we found that CD4 cells were actually better than CD8 cells at rejecting tumors in every case we tested (6 different tumors), even when the CD4 effectors exhibited minimal in vitro or in vivo lytic activity against the tumor cells, and even when the tumor expressed major histocompatibility complex (MHC) class I but not class II molecules. Although the CD4 T cells did not require CD8 T cells, they did partner with other host cells, because the presence of natural killer (NK) cells was essential for maximum effectiveness. All together, these results suggest that the antitumor potential of effector CD4 T cells may have been underestimated. They may not only be helper cells but also potent effector cells that can clear a wide variety of tumors.
Materials and methods
Mice
The anti-H-Y TCR transgenic mice MataHari, Marilyn, and Rachel and the H-2b or H-2k CD3KOγcKO mice have been described previously.31,32 They, and C57BL/6 (B6), C57BL/10-Rag2KO, and C57BL/10-Rag2KOγcKO mice were obtained from Taconic Farms (Germantown, NY) and housed in specific pathogen-free conditions. The National Institutes of Health is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Tumor cells
We used MB49, an H-2b bladder carcinoma,33 βTC-tet, an H-2k pancreatic β-cell tumor,34 TRAMP-C2, an H-2b prostate adenocarcinoma,35 and WR21, an H-2b salivary gland adenocarcinoma.36 IP2-E4, 3B-11, and 2F-2B are H-2k tumor cell lines obtained from a single C3H/HeJ endothelioma.37 2F-2B and B16, an H-2b melanoma, are negative for H-Y. WR21, TRAMP-C2, IP2-E4, 3B-11, and 2F-2B were from American Type Culture Collection (Manassas, VA). The lines were cultured in Iscove's modified Dulbecco's medium (IMDM) plus 10% fetal calf serum, glutamine, and antibiotics and tested to be pathogen-free (IMPACT test) before the in vivo experiments. Tumor cells were occasionally cultured for 2 days with 500 IU/mL of recombinant mouse interferon-γ (IFN-γ) (R&D Systems, Minneapolis, MN) to induce MHC class II expression.
Tumor challenge
Unless otherwise noted, 105 cells in 100 μl of phosphate-buffered saline were injected subcutaneously into the right flank. Tumor size was measured every 3 to 4 days, and the volume was calculated as length × width × height/2. Mice were killed if they became distressed or if tumor volume became > 1000 mm.3 For the βTC-tet pancreatic tumor, after 7 days of tumor challenge, blood glucose levels were measured every day and tumors were considered established when glucose levels were < 3 mM. For humanitarian reasons, mice were usually killed within 2 days after hypoglycemia detection.
Reverse transcription polymerase chain reaction
Total RNA was extracted using RNeasy Mini Kit (QIAGEN, Valencia, CA), and reverse transcribed. Polymerase chain reaction (PCR) was performed using the following primers: Dby: forward, 5′-CAATAGCAGCCGAAGTAGTGGTAGT-3′; reverse, 5′-AACTGCCTGGGAGTTATAATTTCCT-3′. Uty: forward, 5′-GCTCACTTATATGAAACCCAGAGGAA-3′; reverse, 5′-CATATTATGGTGCATCCAACCTAACT-3′. To check for genomic DNA contamination, we prepared parallel samples without reverse transcriptase (RT).
Flow cytometry
After blocking nonspecific binding with ultra-block solution (a mixture of rat, hamster and mouse sera, with 10 μg/mL 2.4G2 monoclonal antibody), cells were stained with various combinations of the following monoclonal antibodies: phycoerythrin-labeled anti-H-2Db, anti-H-2Ab, or anti-NK1.1; fluorescein isothiocyanate-labeled anti-CD4 or anti-CD8; biotin-conjugated anti-CD45.2, anti-TCRVβ6, or anti-TCRVβ8; and allophycocyanin-labeled Db-Uty tetramer (National Institute of Allergy and Infectious Diseases tetramer facility) which were followed by streptavidin-allophycocyanin incubation (all from BD Pharmingen, San Diego, CA). Dead cells were excluded by staining with 7-amino-actinomycin D (7-AAD; BD Pharmingen).
Adoptive transfer and in vitro killing assay
One day after tumor challenge, mice received 106 cells (unless otherwise noted) from freshly isolated spleen and mesenteric lymph nodes of the TCR transgenic mice, or the same cells previously primed in vitro. In vitro stimulation with or without 10% Concanavalin A supernatant (BD Biosciences, San Jose, CA) was performed as described.38 In some experiments, the in vitro primed cells were tested for killing activity using either the JAM test or the P-JAM test.38 In Figure 3d, mice that had received Marilyn cells were immunized several times (days 2, 5, 7, 9, 13, and 17 after tumor challenge) with 3 × 106 male CD3ϵ−/− splenocytes; injection route was the footpad contralateral to the tumor.
Proliferation assay
Marilyn spleen cells (105 per 0.2 mL/well) were cultured for 3 days in complete IMDM plus 2-mercaptoethanol with various numbers of mitomycin C (Sigma, St Louis, MO) treated spleen or tumor stimulators as a source of antigen. The stimulators were titrated (3-fold dilutions): the highest numbers per well were: male and female spleen cells = 5 × 105 MB49 and B16 = 1.5 × 104. [3H]Thymidine was added for the last 18 hours of culture. Cells were harvested and incorporated thymidine quantified.
CFSE labeling and in vivo killing assay
For in vivo proliferation assays, TCR transgenic cells were labeled with 4 μmol/L carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen). For target cells, male and female B6 spleen cells were labeled with 0.2 and 1μmol/L of CFSE respectively, as described.39 After washing, 3 × 106male and 3 × 106female cells were infused intravenously in 300 μl total. Between 18 and 24 hours later, we harvested the spleens (SPL), the tumor draining lymph nodes (TDLN: axillary and inguinal LN draining the flank where MB49 tumor had been injected), and the same lymph nodes from the opposite flank (LN), and determined the percentage of CFSE-positive cells by flow cytometry. The percentage of anti-H-Y—specific killing was determined as: 100 − 100 × (% male CFSE cells/% female CFSE cells).
Down-regulation of class II expression by siRNA
We evaluated 8 oligonucleotide targeting sequences of siRNA against MHC class II Aαb in transient transfection experiments (“Supplemental methods” and Figures S1 and S2), and chose one (GAAGGAGACTGTCTGGATG), corresponding to nucleotide positions 195 to 213 of Aαb coding sequence) to build short hairpin RNA (shRNA) lentiviral vectors also carrying the sequence for enhanced green fluorescent protein (GFP) (Figure S3), following published protocols.40 Generation of control viruses and viral titration are described in “Supplemental methods.” Aliquots of the MB49 cell line were then transduced with 5.9 × 105 plaque-forming units (multiplicity of infection = 4) of the empty vector, or the vector containing short interfering RNA (siRNA) for LacZ, or the siRNA for Ab, thus generating 3 cell lines (MB49∅, MB49-LacZ(i), and MB49-Ab(i), respectively). Around 95% of the cells from the 3 cell lines were GFP-positive even after several weeks of in vitro culture (Figure S4).
Depletion of NK cells
In vitro depletion of NK cells from Marilyn spleen and LN was done with Miltenyi beads (Miltenyi Biotec Inc, Auburn, CA) coated with rat anti-mouse DX5 antibodies according to the manufacturer's instructions. In vivo depletion of NK cells was achieved by intraperitoneal injections of 100 μg of the mouse IgG2a anti-NK1.1 antibody, PK136, with the mouse IgG2a anti-Ek antibody 14-4-4S as control. Both were purified from hybridoma supernatants by the NIAID antibody facility. Antibodies were given 3 days before tumor challenge, again at day 1 (together with transfer of Marilyn cells) and every 7 days until no tumor-bearing mice remained. Successful depletion was tested by fluorescence-activated cell sorting (FACS) analysis of spleen, lymph nodes, and tumors, using DX5 and NK1.1 antibodies (data not shown).
Results
The model system
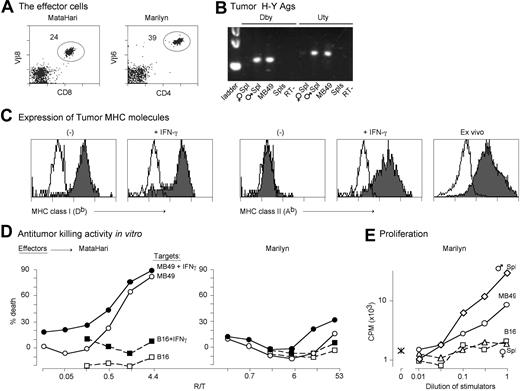
To compare the antitumor effects of pure populations of CD4 and CD8 cells against the same tumor antigen, in the absence of other T cells, we used 2 RAG.KO TCRTg mice (Figure 1A): MataHari.RAG.KO,31 whose CD8 cells are specific for the antigen Uty complexed with H-2Db; and Marilyn.RAG.KO,32 whose CD4 cells are specific for Dby complexed with Ab. Both Uty and Dby are part of the H-Y antigenic complex, expressed by almost all mammalian male cells, except red blood cells (Matzinger and Wade, unpublished data). Both antigens can be presented directly by target/stimulator cells, or indirectly by host cells, and both CD8 MataHari and CD4 Marilyn T cells can reject male skin grafts by direct or by indirect mechanisms.31,41 For the first model tumor, we chose the B6-male bladder carcinoma, MB49, which expresses both Uty (33 and Figure 1B) and Dby (Figure 1B). MB49 also expresses Db, and like most tumor cells, does not express Class II molecules (in this case, Ab) under normal in vitro conditions, though they can up-regulate Ab in vivo (Figure 1C), or when cultured with IFN-γ (Figure 1C).
In vitro characterization of the MataHari and Marilyn T cells and the MB49 tumor. (A) TCR, CD8, and CD4 expression of MataHari (Vβ8) and Marilyn (Vβ6) T cells. Numbers represent the percentage of transgenic cells in the mixed population of spleen and mesenteric lymph node cells used in the adoptive transfer experiments. (B) H-Y expression of the MB49 tumor. PCR using specific primers for the sequences of Dby and Uty that code for the peptide epitopes seen by Marilyn and MataHari, respectively. Spleens from female and male B6 mice were used as negative and positive controls. (C) Expression of MHC Class I (Db) and MHC Class II (Ab) by in vitro-grown MB49 tumor cells that were either untreated (−) or incubated for 2 days with 500 IU/mL of IFN-γ (+IFN-γ) or by tumor cells growing subcutaneously in a female B6 mouse, 7 days after inoculation (ex vivo). Unshaded area is staining seen with a control antibody (specific for Kd.) (D) In vitro antitumor activity. Eighteen-hour JAM test to measure killing activity of MataHari cells (left panel) or Marilyn cells (right panel) against MB49 (circles) or B16 (squares) tumor targets that had been incubated with IFN-γ as in C (filled symbols) or not (open symbols). R/T = responder to target ratio. (E) Proliferation of Marilyn CD4 cells in vitro. Proliferation of Marilyn cells either alone (asterisk) or in presence of mitomycin treated MB49 (circles), B16 (squares), male spleen (diamonds), or female spleen (triangles) as described in “Materials and methods.” The highest number of stimulators were 1.5 × 104 for the tumor cells and 5 × 105 for the splenocytes. D and E show one experiment that is representative of 3.
In vitro characterization of the MataHari and Marilyn T cells and the MB49 tumor. (A) TCR, CD8, and CD4 expression of MataHari (Vβ8) and Marilyn (Vβ6) T cells. Numbers represent the percentage of transgenic cells in the mixed population of spleen and mesenteric lymph node cells used in the adoptive transfer experiments. (B) H-Y expression of the MB49 tumor. PCR using specific primers for the sequences of Dby and Uty that code for the peptide epitopes seen by Marilyn and MataHari, respectively. Spleens from female and male B6 mice were used as negative and positive controls. (C) Expression of MHC Class I (Db) and MHC Class II (Ab) by in vitro-grown MB49 tumor cells that were either untreated (−) or incubated for 2 days with 500 IU/mL of IFN-γ (+IFN-γ) or by tumor cells growing subcutaneously in a female B6 mouse, 7 days after inoculation (ex vivo). Unshaded area is staining seen with a control antibody (specific for Kd.) (D) In vitro antitumor activity. Eighteen-hour JAM test to measure killing activity of MataHari cells (left panel) or Marilyn cells (right panel) against MB49 (circles) or B16 (squares) tumor targets that had been incubated with IFN-γ as in C (filled symbols) or not (open symbols). R/T = responder to target ratio. (E) Proliferation of Marilyn CD4 cells in vitro. Proliferation of Marilyn cells either alone (asterisk) or in presence of mitomycin treated MB49 (circles), B16 (squares), male spleen (diamonds), or female spleen (triangles) as described in “Materials and methods.” The highest number of stimulators were 1.5 × 104 for the tumor cells and 5 × 105 for the splenocytes. D and E show one experiment that is representative of 3.
To determine whether the TCRTg T cells could recognize the H-Y antigens expressed by MB49, we ran 18-hour killing assays against MB49 and against B16 melanoma cells (which also up-regulate class I and class II molecules after IFN-γ stimulation [Figure S5] but don't express H-Y [data not shown]). As expected, the CD8 and CD4 T cells behaved typically: activated MataHari cells killed MB49, whereas Marilyn T cells did not, even after IFN-γ-induced up-regulation of MHC class II (Figure 1D). Marilyn cells proliferated in response to male antigen-presenting cells (APCs) and approximately 5-fold less well to MB49 (presumably because of the need for antigen cross-presentation on APCs) (Figure 1E).
CD4 T cells are more effective in vivo than CD8T cells
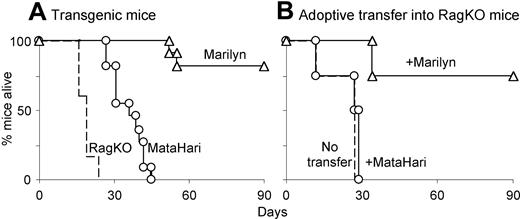
Vaccines and/or adoptive therapies that show the induction of strong in vitro CD8 activity often do not result in tumor clearance. Figure 2A shows that, in the absence of additional treatments, the same is true for MataHari. Although MataHari cells killed MB49 cells very efficiently in vitro (Figure 1D),the MataHari Tg mice did not clear the tumor in vivo (Figure 2A). To our surprise, however, 80% of the Tg Marilyn mice completely rejected MB49 tumor challenge (Figure 2A). We found similar results when we transferred splenocytes from the TCR transgenic mice into RAG.KO mice previously challenged with MB49 tumor (Figure 2B). CD4 cells unable to kill tumor cells in vitro nevertheless caused tumor rejection under in vivo conditions where CD8 cells were ineffective.
Marilyn CD4 cells reject MB49 tumor in vivo better than MataHari CD8 cells. (A) Survival of RAG.KO mice (dashed line), MataHari mice (circles) or Marilyn mice (triangles) after challenge with MB49 tumor cells. (B) Survival of RAG.KO mice that were infused one day after tumor challenge with 106 MataHari cells (circles) or 105 Marilyn cells (triangles) or no cells (dashed line).
Marilyn CD4 cells reject MB49 tumor in vivo better than MataHari CD8 cells. (A) Survival of RAG.KO mice (dashed line), MataHari mice (circles) or Marilyn mice (triangles) after challenge with MB49 tumor cells. (B) Survival of RAG.KO mice that were infused one day after tumor challenge with 106 MataHari cells (circles) or 105 Marilyn cells (triangles) or no cells (dashed line).
Analysis of the CD8 T cells' lack of in vivo antitumor effect
The poor antitumor effect of MataHari splenocytes was somewhat surprising, as they are extremely potent CTLs (Figure 1D), make IFN-γ in vitro,31 and rapidly reject male skin grafts in vivo, both by direct and indirect means.31 One possibility arose from recent studies in which CD8 T cells were refractive to secondary stimulation if they had been primed in the absence of help.42 To test whether the lack of tumor rejection was due to an absence of help during the activation phase,43 we stimulated MataHari cells in vitro in the presence of supernatants from activated CD4 T cells (CAS). Although “helped” MataHari cells were approximately 10-fold more effective in vitro (Figure 3A), they remained completely ineffective against MB49 in vivo (Figure 3B). We also provided help to MataHari cells by transferring them together with Marilyn CD4 cells into tumor-bearing mice; however, the combination of MataHari and Marilyn cells was not better than Marilyn alone (data not shown). A second possibility arose from the evidence that tumors can be immunosuppressive.44 To determine whether MB49 suppressed the killing activity of pre-activated MataHari cells,we tested the in vivo killing activity of primed MataHari cells residing in tumor-free or tumor-bearing RAG.KO mice, 20 days after adoptive transfer. We injected, as targets, male and female spleen cells labeled with CFSE, and enumerated the remaining targets 24 hours later. We found that MataHari cells killed equivalently in tumor-bearing and tumor-free mice, in both spleen and tumor-draining lymph nodes (Figure 3C). Thus, MataHari's ineffectiveness against the tumor was not because they were incapable of killing as they exhibited excellent in vivo CTL function that was clearly not inhibited by the presence of a growing tumor expressing the target Uty/Db complex.
Both helpless and helped MataHari CD8 cells are effective killers but cannot reject the MB49 tumor. (A) Concanavalin A supernatant (CAS), as a source of helper factors, improves killing activity of MataHari. MataHari cells were cultured without (open circles) or with (filled circles) CAS and then tested in vitro (one representative experiment out of 3) against MB49 or B16 tumor targets (solid or dashed lines, respectively) in a 4-hour P-JAM Test (“Materials and methods”). (B) In vivo activity of “helped” MataHari cells. MataHari cells were stimulated in vitro in the presence of CAS and then adoptively transferred into RAG.KO recipients that had been challenged with MB49 tumor cells one day previously. Data are from 2 pooled experiments, of which one is the same experiment as shown in A. (C) Presence of the MB49 tumor does not inhibit the in vivo killing activity of MataHari. In vivo killing activity was measured, at day 20 or 28, in tumor-bearing RAG.KO recipient mice (closed symbols) and in non—tumor-bearing controls (open symbols) that had been infused with either CAS-MataHari cells or naive Marilyn cells at day 1 after tumor challenge. To assess the male-specific killing activity, equal numbers of CFSE-labeled male and female target splenocytes were given, and the remaining target cells in SPL (squares), inguinal and axillary TDLN (triangles) and non—tumor-draining contralateral LN (circles) analyzed 18 to 24 hours later. Data are pooled from 2 independent experiments. (D and E) Analysis of MHC expression and MataHari trafficking in the tumor. MB49 tumors were collected 20 days after challenge, from untreated or CAS-MataHari treated RAG.KO (as in A) and analyzed by FACS, gating on 7-AAD− cells. (D) Presence of MataHari. Number represents the percentage of CD8 cells, found at the tumor site in one representative mouse. (E) Class I (Db) staining of tumor cells (gating on CD45.2− cells). Data in D and E are each from 2 mice representative of 10 analyzed. (F) Tumors growing in the presence of MataHari cells are not escape variants. Large tumors growing in RAG.KO mice (420 mm3), or in RAG.KO into which MataHari CD8 cells had been transferred (500 mm3), were excised when required by Animal Care and Use Committee protocols (19 days after adoptive transfer of the CD8 cells). Tumors were labeled with [3H]thymidine overnight and used as targets for previously in vitro activated MataHari cells in a 24-hour JAM Test. (G) Presence of anti-H-Y CD8 cells in normal B6 mice. Db/Uty tetramer staining in blood of a naive female B6 mouse (left panel) and of a moribund tumor-bearing female B6 mouse (right panel). Numbers represent the percentage of CD8 cells that bind to the tetramers. Two mice representative of 16.
Both helpless and helped MataHari CD8 cells are effective killers but cannot reject the MB49 tumor. (A) Concanavalin A supernatant (CAS), as a source of helper factors, improves killing activity of MataHari. MataHari cells were cultured without (open circles) or with (filled circles) CAS and then tested in vitro (one representative experiment out of 3) against MB49 or B16 tumor targets (solid or dashed lines, respectively) in a 4-hour P-JAM Test (“Materials and methods”). (B) In vivo activity of “helped” MataHari cells. MataHari cells were stimulated in vitro in the presence of CAS and then adoptively transferred into RAG.KO recipients that had been challenged with MB49 tumor cells one day previously. Data are from 2 pooled experiments, of which one is the same experiment as shown in A. (C) Presence of the MB49 tumor does not inhibit the in vivo killing activity of MataHari. In vivo killing activity was measured, at day 20 or 28, in tumor-bearing RAG.KO recipient mice (closed symbols) and in non—tumor-bearing controls (open symbols) that had been infused with either CAS-MataHari cells or naive Marilyn cells at day 1 after tumor challenge. To assess the male-specific killing activity, equal numbers of CFSE-labeled male and female target splenocytes were given, and the remaining target cells in SPL (squares), inguinal and axillary TDLN (triangles) and non—tumor-draining contralateral LN (circles) analyzed 18 to 24 hours later. Data are pooled from 2 independent experiments. (D and E) Analysis of MHC expression and MataHari trafficking in the tumor. MB49 tumors were collected 20 days after challenge, from untreated or CAS-MataHari treated RAG.KO (as in A) and analyzed by FACS, gating on 7-AAD− cells. (D) Presence of MataHari. Number represents the percentage of CD8 cells, found at the tumor site in one representative mouse. (E) Class I (Db) staining of tumor cells (gating on CD45.2− cells). Data in D and E are each from 2 mice representative of 10 analyzed. (F) Tumors growing in the presence of MataHari cells are not escape variants. Large tumors growing in RAG.KO mice (420 mm3), or in RAG.KO into which MataHari CD8 cells had been transferred (500 mm3), were excised when required by Animal Care and Use Committee protocols (19 days after adoptive transfer of the CD8 cells). Tumors were labeled with [3H]thymidine overnight and used as targets for previously in vitro activated MataHari cells in a 24-hour JAM Test. (G) Presence of anti-H-Y CD8 cells in normal B6 mice. Db/Uty tetramer staining in blood of a naive female B6 mouse (left panel) and of a moribund tumor-bearing female B6 mouse (right panel). Numbers represent the percentage of CD8 cells that bind to the tetramers. Two mice representative of 16.
Because the in vivo assay measures the killing activity of MataHari cells found in secondary lymphoid organs, we considered the possibility that effector MataHari cells might remain there and not traffic to the tumor itself. We therefore checked for MataHari cells in the growing tumors. They universally ranged from 1% to 10% of all the cells at the tumor site (Figure 3D), including in tumors from mice in which we had documented in vivo killing activity (Figure 3C). Thus MataHari's lack of antitumor activity was not due to an inability to traffic to the tumor. In vitro, at a ratio of one effector to 10 targets, MataHari can clear 20% of the surrounding target cells in as little as 4 hours (Figure 3A) and, in vivo, the MataHari cells easily clear male targets (Figure 3C), yet they were unable to clear the tumor.
CTL-mediated antitumor attack can select for loss variants that lack MHC class I molecules.45 To test the possibility that the tumor cells might have lost expression of Db, making them invisible to MataHari cells, we analyzed MHC expression of the same tumors that had been analyzed for MataHari trafficking, and found that all tumors from moribund mice showed high levels of Db expression (Figure 3E). The tumors had not lost the H-Y antigen or susceptibility to lysis, because they were killed in vitro by activated MataHari cells (Figure 3F). Thus, tumor growth in the treated mice is not explained by the outgrowth of escape variants.
We do not presently know why MataHari cells, which are extremely effective at rejecting male skin and at killing male targets in vitro and in vivo, cannot clear the H-Y–bearing tumor. This defect is not limited to MataHari Tg-mice. In most other models, TCRTg CD8 T cells clear tumors only when the T cells and/or the recipients are manipulated in extraordinary ways (eg, genetically manipulated to remove inhibitory surface molecules9 ) or when the host is irradiated and/or treated with nearly toxic levels of interleukin-210,11 or with anti-CTLA-4 antibody,46 and so forth. In our hands, many normal female B6 mice were equally unable to reject MB49, even though their anti-H-Y CD8 cells increased in number, as measured by staining with Uty/Db tetramers (Figure 3G). A similar disconnect between surrogate tests of effector function versus actual tumor clearance has been seen in other studies in humans1,5 and mice,7,10,47 where CD8 T cells that were effective in in vitro tests were nevertheless poor at clearing growing tumors in vivo. This is thus a widely known phenomenon that has led to the suggestion that some tumors may outpace the CD8 killers6,47,48 or that the tumor environment impairs T cell effector function.49
How then were the CD4 Marilyn cells able to reject MB49?
Marilyn does not need to directly bind the tumor
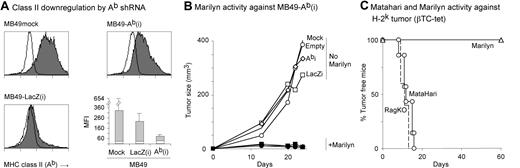
Figures 1D and 3C show that Marilyn cells exhibited a small amount of killing activity against male targets, suggesting that Marilyn might recognize Dby/Ab complexes on the tumor surface. In vivo, such recognition could lead to several outcomes. On the one hand, class II/H-Y complexes on tumor cells could act as direct targets for killing by CD4 effectors. On the other hand, class II expression by tumor cells might induce tolerance in tumor-specific CD4 T cells.50,51 To test these possibilities, we used 2 approaches. First, we created a stable transfectant of MB49, using shRNA for the α chain of Ab, to reduce the amount of MHC complexes on the tumor-cell surface (designated MB49-Ab(i); see “Materials and methods”). As controls, we generated stable transfectants carrying shRNA for LacZ (MB49-LacZ(i)) or the empty vector (MB49∅). Although the level of MHC class II Ab molecules was significantly reduced on the MB49-Ab(i) transfectants (Figure 4A), this down-regulation had neither a positive nor a negative effect on Marilyn's ability to clear the tumor (Figures 4B and S6).
Role of MHC Class II on tumor cells. (A) shRNA-mediated down-regulation of MHC class II expression by MB49. Three RAG.KO mice per group were challenged (day 0) with each of the tumor cell lines, MB49mock, MB49-LacZ(i), and MB49-Ab(i). On day 5, they received 8 × 105 Marilyn cells to induce the up-regulation of class II molecules and on day 13 the tumors were taken for analysis (gating on the CD45.2− and 7-AAD− population to exclude immigrating immune cells and dead cells). Histograms represent class II (Ab) staining of the tumor cells from one mouse from each group. Empty line is the isotype control. Bar graph represents the averages of the mean fluorescence of the 3 mice per group (gray area) compared with that of the isotype controls (white). (B) Marilyn cells reject tumors with knocked-down Ab: RAG.KO mice were challenged with either 105 MB49 mock cells (no symbol), or MB49-∅ (circles), MB49-LacZ(i) (squares), or MB49-Ab(i) (diamonds) cells. One day later, half of the mice in each group received 106 Marilyn cells (closed symbols). The average tumor size (10-20 mice total per group), from 3 individual experiments is shown. (C) Marilyn mice reject tumors that do not express Ab. Percentage of tumor free RAG.KO (broken line), MataHari (circles), or Marilyn (triangles) mice after challenge with 5 × 106 βTC-tet (H-2k) pancreatic tumor cells. Data were pooled from 2 experiments.
Role of MHC Class II on tumor cells. (A) shRNA-mediated down-regulation of MHC class II expression by MB49. Three RAG.KO mice per group were challenged (day 0) with each of the tumor cell lines, MB49mock, MB49-LacZ(i), and MB49-Ab(i). On day 5, they received 8 × 105 Marilyn cells to induce the up-regulation of class II molecules and on day 13 the tumors were taken for analysis (gating on the CD45.2− and 7-AAD− population to exclude immigrating immune cells and dead cells). Histograms represent class II (Ab) staining of the tumor cells from one mouse from each group. Empty line is the isotype control. Bar graph represents the averages of the mean fluorescence of the 3 mice per group (gray area) compared with that of the isotype controls (white). (B) Marilyn cells reject tumors with knocked-down Ab: RAG.KO mice were challenged with either 105 MB49 mock cells (no symbol), or MB49-∅ (circles), MB49-LacZ(i) (squares), or MB49-Ab(i) (diamonds) cells. One day later, half of the mice in each group received 106 Marilyn cells (closed symbols). The average tumor size (10-20 mice total per group), from 3 individual experiments is shown. (C) Marilyn mice reject tumors that do not express Ab. Percentage of tumor free RAG.KO (broken line), MataHari (circles), or Marilyn (triangles) mice after challenge with 5 × 106 βTC-tet (H-2k) pancreatic tumor cells. Data were pooled from 2 experiments.
The possibility that the residual amount of Ab expressed by MB49-Ab(i) was enough to present Dby to Marilyn led us to a second approach: testing Marilyn CD4 cells against a male tumor expressing the “wrong” MHC restriction element. The βTC-tet pancreatic tumor, from male C3H mice,34 expresses both Uty and Dby (Figure S7) but expresses H-2k, rather than H-2b, and thus cannot be recognized directly by MataHari or Marilyn T cells.41 Figure 4C shows that the CD4 Marilyn cells, but not the MataHari cells, were able to clear the H-2k male tumor even though they could not directly recognize it, demonstrating that MHC class II expression on the tumor was not necessary for tumor clearance.
Host cross presentation at the priming and effector phases
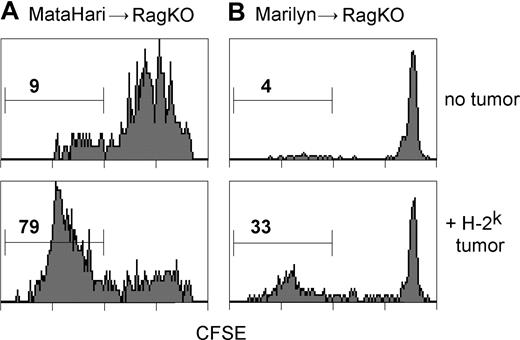
Marilyn's ability to clear the Ak tumor in an Ab host suggested that the tumor antigens were being cross-presented, and this indicated a potential explanation for the superiority of Marilyn compared with MataHari T cells. Cross presentation, which has previously been shown to be necessary for the priming of naïve antitumor CD8 CTL,52 is generally less efficient for MHC class I/Ag complexes than for class II. Perhaps, therefore, the Uty antigen was less effectively cross-presented to MataHari than the Dby to Marilyn. To test this, we labeled naive MataHari and Marilyn cells with CFSE (which is diluted 2-fold with each cell division), transferred them into H-2b recipients of an H-Y expressing H-2k tumor (tumor 3B-11; see Figures 7C and S7), where cross presentation by host cells would be the only source of stimulatory antigen, and measured the proliferative responses in the TDLNs 4 days later. Figure 5A,B show that both cells responded well; MataHari reached 79% fully divided cells (a 9-fold increase over the 9% space-induced expansion in tumor-free hosts) and Marilyn cells increased 8-fold, from 4% to 33%. Thus it seems that the efficiency of stimulatory antigen cross-presentation by host APCs was quite effective for both antigens, allowing for good priming of both cell types. Marilyn's superiority to MataHari is therefore not due to a lack of cross priming of the CD8 CTL.
H-Y antigen is cross-presented in vivo to both MataHari and Marilyn T cells. CFSE dilution of MataHari (A) or Marilyn (B) T cells 4 days after transfer into either H-2bRAG.KO tumor free (top panels) or H-2k-tumor bearing (bottom panels) mice. Histograms are gated on TCRαβ and CD8 or CD4, for MataHari or Marilyn, respectively. Cells are from the inguinal and axillary lymph nodes (the tumor-draining lymph nodes in the tumor-bearing mice). Numbers represent the percentage of TCR transgenic cells that divided more than 6 times.
H-Y antigen is cross-presented in vivo to both MataHari and Marilyn T cells. CFSE dilution of MataHari (A) or Marilyn (B) T cells 4 days after transfer into either H-2bRAG.KO tumor free (top panels) or H-2k-tumor bearing (bottom panels) mice. Histograms are gated on TCRαβ and CD8 or CD4, for MataHari or Marilyn, respectively. Cells are from the inguinal and axillary lymph nodes (the tumor-draining lymph nodes in the tumor-bearing mice). Numbers represent the percentage of TCR transgenic cells that divided more than 6 times.
To test whether there might also be a role for host-cell–mediated cross-presentation to Marilyn in the effector phase, we transferred Marilyn T cells into tumor-bearing H-2k mice, which cannot present Dby to Marilyn, and into tumor-bearing H-2b mice as controls. To prevent rejection of the Marilyn cells by host NK cells in the H-2k mice, we used C3H (H-2k) or B6 (H-2b) CD3/γc double-knockout recipients. To supply appropriate APCs for priming of the transferred CD4 Marilyn T cells, we immunized the mice repeatedly with male B6 APCs. Figure 6A shows that Marilyn cells were effective in Ab, but not in Ak recipients, even though they had clearly been activated by the multiple immunizations with Ab APCs (Figure S8). These results show that antigen presentation by host cells is required at the effector phase for tumor rejection by primed CD4 Marilyn cells, perhaps via the activation of local macrophages and/or other cells.17,22
Role of host MHC class II, and of host NK cells, in CD4-mediated tumor rejection. (A) Role of host MHC class II. RAG.KO (H-2b) mice (squares) and CD3KO/γcKO H-2b (circles) or H-2k (triangles) mice were challenged with MB49 cells. Approximately half of the mice per group were either untreated (left panel) or received 106 Marilyn cells 1 day later (right panel). To overcome the need for class II (Ab) for initiating and maintaining CD4 T-cell activation, mice receiving Marilyn cells were immunized with male splenocytes at days 2, 5, 7, 9, 13, and 17 after tumor challenge. The average tumor size (6-12 mice per group) from a total of 3 individual experiments is shown. (B) Role of NK cells. Percentage of RAG.KO/γcKO recipient mice (left panel) or RAG.KO mice (right panel) alive after receiving MB49 challenge and left untreated (broken line) or treated with 106 Marilyn cells (triangles). Some of the RAG.KO mice received Marilyn cells that had been depleted of NK cells (to deplete donor NK cells only [closed diamonds]), Marilyn cells plus either anti-NK monoclonal antibodies (to deplete both host and donor NK cells [closed circles]), or control antibodies (open circles) as described in “Materials and methods.” Data pooled from 3 independent experiments. (C) NK cells are present in the tumor. MB49 tumors were collected from Marilyn-treated RAG.KO mice 49 days after challenge and analyzed for the presence of various cell populations by FACS, gating on living 7-AAD− cells. Numbers represent the percentage of CD4 or NK cells within the CD45− population found at the tumor site. For comparison, tumors growing in untreated RAG.KO mice were stained for NK in the same way cells, 28 days after tumor challenge. Data are representative of 1 of 2 mice.
Role of host MHC class II, and of host NK cells, in CD4-mediated tumor rejection. (A) Role of host MHC class II. RAG.KO (H-2b) mice (squares) and CD3KO/γcKO H-2b (circles) or H-2k (triangles) mice were challenged with MB49 cells. Approximately half of the mice per group were either untreated (left panel) or received 106 Marilyn cells 1 day later (right panel). To overcome the need for class II (Ab) for initiating and maintaining CD4 T-cell activation, mice receiving Marilyn cells were immunized with male splenocytes at days 2, 5, 7, 9, 13, and 17 after tumor challenge. The average tumor size (6-12 mice per group) from a total of 3 individual experiments is shown. (B) Role of NK cells. Percentage of RAG.KO/γcKO recipient mice (left panel) or RAG.KO mice (right panel) alive after receiving MB49 challenge and left untreated (broken line) or treated with 106 Marilyn cells (triangles). Some of the RAG.KO mice received Marilyn cells that had been depleted of NK cells (to deplete donor NK cells only [closed diamonds]), Marilyn cells plus either anti-NK monoclonal antibodies (to deplete both host and donor NK cells [closed circles]), or control antibodies (open circles) as described in “Materials and methods.” Data pooled from 3 independent experiments. (C) NK cells are present in the tumor. MB49 tumors were collected from Marilyn-treated RAG.KO mice 49 days after challenge and analyzed for the presence of various cell populations by FACS, gating on living 7-AAD− cells. Numbers represent the percentage of CD4 or NK cells within the CD45− population found at the tumor site. For comparison, tumors growing in untreated RAG.KO mice were stained for NK in the same way cells, 28 days after tumor challenge. Data are representative of 1 of 2 mice.
NK cells play a role in the effector phase
We were surprised to find that MB49 tumors were completely inhibited in single H-2b RAG.KO recipients of Marilyn cells (Figure 6A, right panel), but grew slowly in H-2b-CD3/γc double-knockout recipients, suggesting that B cells (in CD3/γc KO mice) might inhibit the antitumor efficacy of the CD4 T cells, or that γc-binding cytokines might have a positive role in CD4 T-cell mediated tumor rejection. To distinguish between these, we compared Marilyn's antitumor efficacy in γc-positive and -negative mice lacking T and B cells (RAG.KO and Rag/γc double-knockout mice). The absence of B cells did not restore Marilyn's efficacy in γc-deficient recipients. The Marilyn cells (▵) were able to slow the growth of the MB49 tumor in the Rag/γc-deficient mice, but the tumor eventually killed the mice (Figure 6B, left panel).
Because mice lacking the common cytokine receptor γ chain are markedly deficient in NK cells, we sought to determine whether Marilyn might be partnering with NK cells to clear the tumor. For this, we transferred Marilyn cells into RAG.KO recipients and depleted the NK cells from the donor Marilyn inoculum or from both the donor inoculum and the host. Figure 6B (right panel) shows that NK-depleted recipients behaved exactly like γc-deficient recipients. In the absence of NK cells, the transferred Marilyn cells were able to delay tumor growth, but it grew out at later time points. These data suggest that the early phase of the CD4 T cell antitumor response may be NK-cell independent, whereas long-term tumor clearance requires host NK cells. To determine whether the NK cells migrated to the tumor site or might be acting only in the draining node (perhaps by supplying IFN-γ to maintain a Th1 response53 ), we stained the tumor for the presence of Marilyn CD4 cells and for NK cells. Figure 6C shows that both types of cells were present in the tumor mass, suggesting that they might be working in concert at the tumor site.
Generalizing the results to other CD4 T cells and other tumors
To determine whether Marilyn's antitumor effect could be generalized to other CD4 cells, we tested Rachel, a different TCR-Tg mouse that has slightly higher avidity for Dby/Ab.32 Although not as effective as Marilyn, Rachel cells were nevertheless more effective than MataHari (Figure 2B and 3B), delaying tumor growth for several weeks (Figure 7A).
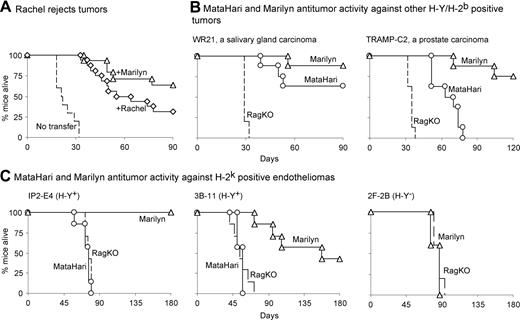
CD4 antitumor effect is generalizable. (A) Rachel, another anti-H-Y CD4 TCR Tg mouse, also rejects MB49. Percentage of RAG.KO mice alive after being challenged with MB49 tumor cells and receiving, 1 day later, either nothing (broken lines), 106 Marilyn cells (triangles), or 106 Rachel cells (an anti-H-Y CD4 transgenic mouse with a different TCR from that of Marilyn). Data from 3 pooled experiments. (B and C) Marilyn's superiority to MataHari is seen with other tumors: percentage of RAG.KO (broken line), MataHari (circles), or Marilyn (triangles) mice that survived challenge with either (B) H-2b carcinomas: 3 × 105 of WR21 (left panel, a salivary gland carcinoma), 3 × 106 of TRAMP-C2 (right panel, prostate carcinoma), or (C) H-2k endotheliomas: IP2-E4 (left panel), 3B-11 (middle panel), or H-Y–negative 2F-2B (right panel), 2 × 105 of each.
CD4 antitumor effect is generalizable. (A) Rachel, another anti-H-Y CD4 TCR Tg mouse, also rejects MB49. Percentage of RAG.KO mice alive after being challenged with MB49 tumor cells and receiving, 1 day later, either nothing (broken lines), 106 Marilyn cells (triangles), or 106 Rachel cells (an anti-H-Y CD4 transgenic mouse with a different TCR from that of Marilyn). Data from 3 pooled experiments. (B and C) Marilyn's superiority to MataHari is seen with other tumors: percentage of RAG.KO (broken line), MataHari (circles), or Marilyn (triangles) mice that survived challenge with either (B) H-2b carcinomas: 3 × 105 of WR21 (left panel, a salivary gland carcinoma), 3 × 106 of TRAMP-C2 (right panel, prostate carcinoma), or (C) H-2k endotheliomas: IP2-E4 (left panel), 3B-11 (middle panel), or H-Y–negative 2F-2B (right panel), 2 × 105 of each.
To determine whether Marilyn's antitumor effect could be generalized to tumors from different tissues, we tested 4 more tumors that were constitutively positive for Dby and Uty (Figure S7). Two expressed Ab (WR21, a salivary gland carcinoma, and TRAMP-C2, a prostate carcinoma), and 2 (the IP2-E4 and 3B-11 endotheliomas) expressed the “wrong” haplotype, H-2k. The H-Y loss variant 2F-2B, cloned from the same original tumor as IP2-E4 and 3B-11, served as an H-Y–negative control (Figure S7). Figure 7B,C show that (1) although MataHari mice showed some activity against the H-2b tumors, WR21 and TRAMP-C2, Marilyn mice were always better; (2) both Marilyn T cells (Figure 7C) and Rachel T cells (Figure S9) were more effective than MataHari against H-2k tumors they could not directly bind to (MataHari, in fact, had no effect at all on these tumors); and 3) Marilyn cells did not reject the H-Y–negative 2F-2B, showing that the CD4 cells do not act nonspecifically but that their effect is tumor-antigen-dependent, although the antigen-specific contact need not be on the tumor itself and may be mediated partially via non–antigen-specific NK cells.
Discussion
Because of their capacity to kill in vitro, the universality of MHC class I expression, and the availability of reagents to identify them, CD8 cells have been the main focus of several decades of research in antitumor immunotherapy. Our data suggest that, at least in some cases, CD4 T cells might actually do a better job than CD8 T cells. The data showed that
Tumor specific CD4 T cells were able to eliminate a wide variety of tumors that were resistant to CD8-mediated rejection.
CD4 cells partnered with NK cells for complete tumor clearance.
Lack of MHC expression by the tumor did not lessen the antitumor effect of the CD4 effector cells.
Neither the antitumoral effect of the CD4-T cells nor the ineffectiveness of the CD8-T cells was predictable from their in vitro or in vivo killing activity.
Let us take these areas in turn. First, although the last 25 years have seen occasional reports in which CD4 cells cleared tumors without the aid of CD8 cells,13–17 the relative efficacy of the 2 types of effectors has never before been evaluated, and the prevailing view is that the immune system's preeminent antitumor weapons are the CD8 T cells. The majority of antitumor therapies therefore continue to focus on CD8 killers, occasionally including CD4 cells as potential helpers. However, most CD8-oriented treatments give poor results in human trials (reviewed in 5). The finding that CD4 T cells can clear several different kinds of tumors that are resistant to CD8 cells suggests that CD4-based treatments might be worth studying. There is some support for this from clinical trials2,3 in which transfers of mixed populations containing both CD4 and CD8 cells were more effective than CD8 populations that contained no CD4s. Although the authors suggested that CD4 cells could have contributed to the in vivo persistence or activity of CD8 cells, an alternative is that the CD4 cells might themselves have had a strong antitumor effect.
Second, we found that tumor clearance by Marilyn CD4 T cells required host expression of appropriate MHC class II molecules, even when the CD4 cells were repeatedly immunized with appropriate APCs. We surmise that the T cells need to see local antigen/MHC complexes at the tumor site, either on classic APCs or on endothelial cells, which have been described to up-regulate class II molecules under inflammatory conditions.54 Two different, nonexclusive scenarios could then apply. In one, local antigen presentation activates the CD4 T cells to release cytokines that are toxic for the tumor and/or the supporting stroma.16 In other words, the CD4 T cell is the essential effector, even though its recognition of the tumor is indirect. In the second scenario, the CD4/APC interaction results in local activation of APC and/or other surrounding cells (such as host macrophages, NK cells, etc17,22 ) that in turn target the tumor or the local stromal cells. Indeed, we found that complete tumor clearance required the presence of host cells bearing NK markers, although early inhibition of tumor growth did not (Figure 6B). The essential NK1.1+ partners were not NK T cells, given that the Marilyn CD4 T cells cleared tumors in RAG.KO recipients, or NK-dendritic cells (DCs),55,56 because the CD4 T cells did not efficiently clear tumors in RAG/γc double-knockout mice (which have plenty of NK-dendritic cells56 ). The NK1.1+ partners were therefore most likely NK cells. Because NK cells and DCs can activate each other,57,58 we can picture a communication triangle in which tumor-specific CD4 cells activate NK cells via a DC bridge and/or NK cells help to sustain the CD4 response through the activation of DCs. A variation of this scenario comes from a previous report suggesting that CD4 T cells might also partner with tumor-infiltrating macrophages.17,22 At first glance, such a duo might seem less likely than that of the CD4/NK cell partnership, given that tumor-infiltrating macrophages have been shown to support tumor growth by promoting angiogenesis and tissue remodeling and repair.59,60 However, CD4 cells are effective educators of other cells: they can license DCs to activate CD8 killers,43 they can educate DCs to perpetuate oral tolerance,61 and they can induce B cells to switch to a different class of antibody. It is only a small step to suppose that tumor-specific CD4 cells could recognize tumor-infiltrating macrophages, via cross-presented tumor antigens, and alter their phenotype from “tumor-nurturing” to “tumor-rejecting.” Overall, it seems that CD4 T cells may partner with many different types of host cells to clear the tumor, something that CD8 cells may be less able to do.
Whether the CD4 T cells act alone (via T-cell–derived cytokines), or with NK and/or macrophage partners, it is not yet clear whether they target the tumor itself or the supporting stroma. There have been some rare examples where CD8 cells inhibited tumors by killing the stroma. In some cases, the CD8 cells were specific for unique stromal antigens,62,63 whereas in others they targeted tumor antigens cross-presented by the stromal cells.16,64 CD4 T cells might be expected to more efficiently recognize stromal cells cross-presenting tumor antigens, as cross presentation for MHC class II/Ag complexes is more efficient than for MHC class I.65 Indeed, although MataHari CD8 T cells can reject male H-2k skin grafts (which carry the wrong restriction element), apparently by killing cross-presenting endothelial cells and starving the graft,31 tumors are more difficult to reject than skin grafts,66 and Figure 7 shows that MataHari had virtually no effect on tumors that did not carry the correct (H-2b) restriction element. Thus, though CD8 cells, such as MataHari, can reject skin by indirect mechanisms,31,62 they seem to be less efficient than CD4 cells at rejecting tumors this way.
Finally, the finding that CD4 T cells can efficiently clear MHC class II negative tumors may allow for the return of a long-faded goal. A quarter of a century ago, when tumor antigens were first beginning to be characterized, tumor immunotherapists dreamed of activating CTL against the very protein that made a tumor tumorigenic (eg, mutated ras, hormone receptors or growth factor receptors, viral proteins, etc). The idea was that, should a tumor cell “escape” from the immune response by losing that particular protein, it would, in the process, lose its tumorigenicity. But it soon became clear that tumors could instead escape from CTL by losing MHC class I molecules while retaining the critical oncogenic proteins that maintain the tumor state—and the dream faded. The finding that CD4 T cells can efficiently induce tumor clearance using indirect measures that do not require MHC expression by the tumor cell suggests that it might be possible to resuscitate that early goal by designing strategies to elicit CD4 T cell responses against the tumorigenic proteins. Although we do not know how often CD4 cells will turn out to be more efficient than CD8 cells, we suggest that this is an understudied area. Characterization of the conditions in which CD4 effector cells are, or are not, successful might help to predict which clinical trials could take full advantage of them and allow CD4 effector cells to become an additional tool in the treatment of cancer.
The online version of this manuscript contains a data supplement.
An Inside Blood analysis of this article appears at the front of this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the NIAID tetramer facility for providing the Uty-Db tetramer, Fraia Melchionda for providing the MB49 cell line, Shimon Efrat for providing the βTC-tet cell line, and Ronald Germain and Luk Van Parijs for providing valuable reagents for the shRNA experiment. We also thank Ronald Schwartz, Jay Berzofsky, Francesco Marincola, Suzanne Topalian, and Nicholas Restifo for critically reading (and improving) the manuscript.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases. C.C.A. was funded by Canadian Institutes of Health Research and Alberta Heritage Foundation for Medical Research grants.
Authorship
Contribution: A.P.D. and P.M. conceived and designed the experiments, analyzed the data, and wrote the paper. A.P.D. performed the experiments not mentioned below. N.T.J. and O.L. designed and performed, and analyzed the data from the experiments shown in Figure 6A and S8. K.C. designed and made the lentivirus constructs and helped in experiments shown in Figures S1, S2, and S4. W.F.N.C. and C.C.A. designed and performed, and analyzed the data from the experiment shown in Figure 4C.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ainhoa Perez-Diez, Ghost Lab, Laboratory of Cellular and Molecular Immunology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; e-mail: ainhoa@nih.gov

![Figure 3. Both helpless and helped MataHari CD8 cells are effective killers but cannot reject the MB49 tumor. (A) Concanavalin A supernatant (CAS), as a source of helper factors, improves killing activity of MataHari. MataHari cells were cultured without (open circles) or with (filled circles) CAS and then tested in vitro (one representative experiment out of 3) against MB49 or B16 tumor targets (solid or dashed lines, respectively) in a 4-hour P-JAM Test (“Materials and methods”). (B) In vivo activity of “helped” MataHari cells. MataHari cells were stimulated in vitro in the presence of CAS and then adoptively transferred into RAG.KO recipients that had been challenged with MB49 tumor cells one day previously. Data are from 2 pooled experiments, of which one is the same experiment as shown in A. (C) Presence of the MB49 tumor does not inhibit the in vivo killing activity of MataHari. In vivo killing activity was measured, at day 20 or 28, in tumor-bearing RAG.KO recipient mice (closed symbols) and in non—tumor-bearing controls (open symbols) that had been infused with either CAS-MataHari cells or naive Marilyn cells at day 1 after tumor challenge. To assess the male-specific killing activity, equal numbers of CFSE-labeled male and female target splenocytes were given, and the remaining target cells in SPL (squares), inguinal and axillary TDLN (triangles) and non—tumor-draining contralateral LN (circles) analyzed 18 to 24 hours later. Data are pooled from 2 independent experiments. (D and E) Analysis of MHC expression and MataHari trafficking in the tumor. MB49 tumors were collected 20 days after challenge, from untreated or CAS-MataHari treated RAG.KO (as in A) and analyzed by FACS, gating on 7-AAD− cells. (D) Presence of MataHari. Number represents the percentage of CD8 cells, found at the tumor site in one representative mouse. (E) Class I (Db) staining of tumor cells (gating on CD45.2− cells). Data in D and E are each from 2 mice representative of 10 analyzed. (F) Tumors growing in the presence of MataHari cells are not escape variants. Large tumors growing in RAG.KO mice (420 mm3), or in RAG.KO into which MataHari CD8 cells had been transferred (500 mm3), were excised when required by Animal Care and Use Committee protocols (19 days after adoptive transfer of the CD8 cells). Tumors were labeled with [3H]thymidine overnight and used as targets for previously in vitro activated MataHari cells in a 24-hour JAM Test. (G) Presence of anti-H-Y CD8 cells in normal B6 mice. Db/Uty tetramer staining in blood of a naive female B6 mouse (left panel) and of a moribund tumor-bearing female B6 mouse (right panel). Numbers represent the percentage of CD8 cells that bind to the tetramers. Two mice representative of 16.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-10-051318/4/m_zh80120701870003.jpeg?Expires=1769129192&Signature=GO1pfyM9hU495ilqb0yxNSC-uzqYbPna6g7pLf7sqKUQz65dL7qRpLIEQom3GdWHD-gffm4S1vvjW8rty68Zg-dCaW1O2bnJ5NhDb8gU1l0pN0vaQBvgDsyKiPMDuyXsfiaPOtDOFNFiRxLLO5MLXUGe8NESXeofbsuFTN6syzxCHWipxOJ4UTMdNv4F8fUVypBBaLV0WBJJIPaYg20G1Fy2OpArMBmqhVjvjO3eVR1SskHYd1R1fiQ5wDK7gXc0Gp-iXbL8j0fK1I~KCF1FzwJDr9DGvC5EUnC4G9SJUhzYFELrxSesv0qiim0t9b~ZvRdVeaRb05UtsE3IxcoE0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Role of host MHC class II, and of host NK cells, in CD4-mediated tumor rejection. (A) Role of host MHC class II. RAG.KO (H-2b) mice (squares) and CD3KO/γcKO H-2b (circles) or H-2k (triangles) mice were challenged with MB49 cells. Approximately half of the mice per group were either untreated (left panel) or received 106 Marilyn cells 1 day later (right panel). To overcome the need for class II (Ab) for initiating and maintaining CD4 T-cell activation, mice receiving Marilyn cells were immunized with male splenocytes at days 2, 5, 7, 9, 13, and 17 after tumor challenge. The average tumor size (6-12 mice per group) from a total of 3 individual experiments is shown. (B) Role of NK cells. Percentage of RAG.KO/γcKO recipient mice (left panel) or RAG.KO mice (right panel) alive after receiving MB49 challenge and left untreated (broken line) or treated with 106 Marilyn cells (triangles). Some of the RAG.KO mice received Marilyn cells that had been depleted of NK cells (to deplete donor NK cells only [closed diamonds]), Marilyn cells plus either anti-NK monoclonal antibodies (to deplete both host and donor NK cells [closed circles]), or control antibodies (open circles) as described in “Materials and methods.” Data pooled from 3 independent experiments. (C) NK cells are present in the tumor. MB49 tumors were collected from Marilyn-treated RAG.KO mice 49 days after challenge and analyzed for the presence of various cell populations by FACS, gating on living 7-AAD− cells. Numbers represent the percentage of CD4 or NK cells within the CD45− population found at the tumor site. For comparison, tumors growing in untreated RAG.KO mice were stained for NK in the same way cells, 28 days after tumor challenge. Data are representative of 1 of 2 mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-10-051318/4/m_zh80120701870006.jpeg?Expires=1769129192&Signature=Du9~KTHsf59vynC9k9SqiS9LGnsChxAsT-n17gDivm9BAOiWUSJU9a2ba3nnM5aLHE5dpvtZaTxFgb-axa1AvcxUXzcXY0ir0aTVM6mYLu6YZL8xTEl~Bd8lR3v3rlkIoQMwnD8gHaNBxaL-7n7C9eeahqvs6rCBKhzOjIhYzrvUgcy5FBs8yKQNChuNJ5zWeusTYk6UBdc0CuvlulliiS~sMi3DOcjjaQvyjxq9UeUNhpyd2xOYbbeMv2KRDLASdk7iL8DtraBnsFiKT7wdcvnz3IjAv-4XSpYfkgqScqArzjZZ24ZsjjVE2bntHaSX56hmgayK0rvRD9He1syfEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal