Tumor antigen-specific T-cell tolerance may limit the efficacy of therapeutic cancer vaccines. Direct presentation of antigens by tumor cells incapable of providing adequate costimulation to tumor-specific T cells has been suggested as the basis for this unresponsiveness. Using parent-into-F1 bone marrow (BM) chimeras, this study unambiguously demonstrates that the induction of this tolerant state requires T-cell recognition of tumor antigen presented by BM-derived antigen-presenting cells (APCs), not tumor cells themselves. In the absence of host APC presentation, tumor-specific T cells remained functional, even in the setting of antigen expressed by B-cell lymphomas residing in secondary lymphoid tissues. The intrinsic APC capacity of tumor cells has therefore little influence over T-cell priming versus tolerance, a decision that is regulated at the level of host APCs.
Introduction
The 2-signal model of T-cell activation1,2 is frequently evoked in the field of tumor immunology to account for the failure of the immune system to successfully reject antigenic cancer cells in vivo. Cancer cells, it is argued, typically being the transformed counterparts of “nonprofessional” antigen-presenting cells (APCs), provide signal 1, but not signal 2, on their encounter with tumor-specific T cells, leading to the induction of T-cell tolerance.3
Numerous studies, however, have shown that cells from at least one type of malignancy, that is, B-cell lymphoma, can efficiently activate T cells in vitro, including CD4+ T cells specific for epitopes derived from their own unique immunoglobulin idiotype.4,5 These tumor cells, being derived from APCs, constitutively express major histocompatibility complex (MHC) class I and II molecules as well as low but inducible levels of intercellular adhesion molecule 1, leukocyte function–associated antigen 3, and the costimulatory molecules B7-1 and B7-2 and therefore seem to be well equipped to provide the necessary signals required for T-cell activation. Indeed, cross-linking of CD40 on lymphoma cells has been shown to up-regulate their expression of adhesion and costimulatory molecules, resulting in a markedly enhanced T-cell response to B-cell tumors in vitro.6 It is therefore paradoxical that malignant B cells, residing in the very compartment where T-cell responses are initiated, fail to be eliminated in immunocompetent hosts. In fact, it has been suggested that the consequence of antigen presentation by lymphoma cells in vivo may be T-cell tolerance rather than activation, due to the provision of partial but inadequate costimulatory signals by the lymphoma cells.7
We have previously shown that a murine B-cell lymphoma (A20) transfected to express the model antigen influenza hemagglutinin (HA), is capable of activating HA-specific CD4+ T cells from T-cell receptor (TCR) transgenic mice in vitro.8 However, when these same transgenic T cells are transferred into mice bearing lymphoma expressing the model antigen (A20HA), the observed outcome is the induction of HA-specific CD4+ T-cell anergy.9 These results suggest either that (1) B-cell tumors are not efficient APCs in vivo, and their direct encounter with T cells is responsible for the induction of T-cell tolerance or (2) tumor antigens are captured and presented by host APCs to T cells in a context that favors the development of T-cell tolerance. In support of the first hypothesis, nonmalignant B cells have been shown to not only fail to activate naive T cells in vivo,10 but to induce tolerance as well, even when the B cells had been activated with lipopolysaccharide (LPS).11 An alternate explanation—supporting the second hypothesis—has been provided by the results of studies emphasizing the role of host bone marrow (BM)-derived cells in the induction and establishment of peripheral tolerance to self-antigens.12-14
Therefore, to assess the relative contribution of antigen presentation by B-lymphoma cells versus host's APCs, we compared T-cell recognition of a model tumor antigen in 2 sets of BM chimeras. In the first set (H-2d→H-2dxb), host APCs express the restricting element (I-Ed) required for presentation of the model antigen to TCR transgenic T cells, whereas in the second set (H-2b→H-2dxb), they do not. After immune reconstitution, anti-HA TCR transgenic CD4+ T cells from H-2dxb F1 donors were transferred to both sets of chimeras, which were then challenged with A20HA lymphoma (H-2d). With this experimental design, the effects of direct antigen presentation by the B-cell lymphoma should be evident in both sets of chimeras. However, if tolerance requires tumor antigen–presentation by BM-derived cells, it would only be seen in chimeras in which the APCs express the required restricting element.
The results of this analysis provide a clear demonstration of the requirement for antigen processing and presentation by host APCs in the development of lymphoma-specific CD4+ T-cell tolerance. Specifically, clonotypic T cells isolated from tumor-bearing mice are tolerant only in the H-2d→H-2dxbchimeras. In sharp contrast, in H-2b→H-2dxb chimeras (where only lymphoma cells are able to present H-2d-restricted HA epitopes), T cells remain functional. These findings unambiguously demonstrate that tumor antigen– processing and presentation by the host's APCs (not lymphoma cells) is the dominant mechanism underlying the development of antigen-specific T-cell tolerance.
Materials and methods
Mice
Male BALB/c severe combined immunodeficiency (SCID) or C57B6/SCID mice, aged 6 to 8 weeks, were purchased from Jackson Laboratories (Bar Harbor, ME). BALB/cxC57BL/6 F1 mice, BALB/c, and C57BL/6 mice were obtained from the National Institutes of Health (Frederick, MD). TCR transgenic mice expressing an αβ-TCR specific for amino acids 110 to 120 from influenza HA presented by I-Ed were a generous gift of H. von Boehmer.15
Construction of BM chimeric mice
The femurs and tibiae were obtained from either BALB/c SCID mice or C57B6/SCID mice and BM was harvested by flushing the bones with RPMI at 4°C. Single-cell suspensions were obtained by passing BM cells through a cell strainer (Becton Dickinson, Franklin Lakes, NJ). Cells were washed 3 times in sterile Hanks balanced salt solution (HBSS) and 4 × 106 BM cells from either BALB/c SCID mice (H-2d) or C57B6/SCID mice (H-2b) were injected into the tail vein of irradiated (1000 rads) BALB/cxC57BL/6 F1 (H-2dxb) recipients.
Three months after BM transplant, one mouse from each group was killed to assess donor chimerism. Splenocytes were stained for I-Ed and I-Ab using the monoclonal antibody (MoAb) 14.4.4 and MoAb Y3P, respectively, followed by fluorescein isothiocyanate (FITC)–goat anti–mouse IgG2a secondary antibody. Splenocytes were analyzed for MHC-II expression by flow cytometry.
Tumor cells
The A20 cells were obtained from American Type Culture Collection (Rockville, MD). A20HA was generated by electroporation-mediated plasmid transfection, and transfected cells were selected and grown as previously reported.8
Adoptive transfer of antigen-specific T cells
For the adoptive transfers of anti–HA-specific CD4+T cells, we mated the BALB/c TCR transgenics to C57BL/6 mice to generate H-2dxb F1 TCR transgenic offspring. It is necessary to use F1 TCR transgenic donors to ensure that any nontransgenic T cells that are transferred into the chimeras are not alloreactive to the recipient resulting in graft-versus-host reaction. Transferred T cells were detectable in the lymphoid organs of the chimeric recipients up to 6 months after transfer (data not shown).
The transgenic donor population was obtained from the thymus of H-2dxb F1 TCR transgenic animals to avoid any contaminating MHC class II–bearing APCs. CD8+ T cells and double-negative thymocytes were depleted using the antibodies 3.155 and J.11.d.2, respectively. The percentage of T cells doubly positive for CD4 and the clonotypic TCR was determined by flow cytometry as described below. Cells were washed 3 times in sterile HBSS and 1 × 106 CD4+ anti-HA TCR+ T cells were injected into the tail vein of immune reconstituted chimeric mice.
Reisolation of clonotypic T cells after in vivo transfer
Clonotypic CD4+ T cells injected into tumor-free chimeric mice or mice challenged with A20HA tumors were reisolated from the spleen or lymph nodes of these animals 22 days after T-cell transfer. In all experiments, unless otherwise noted, 3 mice per group were analyzed individually. On the day of analysis, mice were euthanized and spleen cells were obtained by passage over nylon mesh and centrifugation on a Ficoll gradient (Amersham Pharmacia Biotech, Piscataway, NJ). Lymphoma cells were depleted by passage over nylon wool followed by complement lysis with the MoAb J11.d.2 specific for heat-stable antigen (HSA) expressed by A20 cells. Inguinal and axillary lymph nodes were also harvested from tumor-free and tumor-bearing chimeric mice. Lymph node cells from 3 animals per group were pooled and cell suspensions were made by passage over nylon mesh. Contaminating lymphoma cells were eliminated by complement killing using the MoAb J11.d.2.
Flow cytometric analysis
The T cells were stained with FITC-conjugated goat anti–mouse CD4 (Caltag) and biotinylated rat anti–clonotypic TCR antibody MoAb 6.5 followed by phycoerythrin (PE)-conjugated streptavidin (Caltag, Burlingame, CA). Gated events (50 000) were collected on a FACScan (Becton Dickinson) and analyzed using CellQuest software (Becton Dickinson). Values represent the mean ± SE of the percentage of cells expressing the clonotypic TCR. Background staining of splenocytes from naive F1 mice is usually less than 0.10%. Expression of CD45Rb or CD62L on clonotype-positive T cells was determined by 3-color flow cytometric analysis, staining with cychrome-labeled anti–mouse CD4 (Pharmingen, San Diego, CA), biotinylated anti–TCR clonotype MoAb 6.5 followed by PE-labeled streptavidin and FITC-conjugated anti–mouse CD45RB or CD62L (Pharmingen). Live gating on CD4+ T cells was used to collect a total of 100 000 events.
Splenic dendritic cells (DCs) and B-lymphoma cells were stained for MHC class II expression (I-Ed) with a biotinylated MoAb 14.4.4 followed by PE-conjugated streptavidin (Caltag). Ten thousand gated events were collected on a FACScan and analyzed using Flow-Jo software. The expression of B7 costimulatory molecules by these APCs was determined by staining with either a FITC-conjugated anti-CD80 (Pharmingen) or a biotinylated anti-B7.2 antibody GL-1 followed by PE-conjugated streptavidin.
Antigen-specific proliferation
Purified T cells (4 × 104/well) from the different experimental groups were mixed with fresh F1 splenocytes (8 × 104/well) to which different concentrations of the MHC class II–restricted HA peptide (amino acids 110-120; SFERFEIFPKE; single-letter amino acid codes) were or were not added. The cells were pulsed with 3H-thymidine (1 mCi/well, Amersham) after 3 days in culture. Cells were harvested 18 hours later with a Packard Micromate cell harvester. 3H-thymidine incorporation into DNA was measured as counts per minute (cpm) on a Packard Matrix 96 direct beta counter. Data are calculated as cpm in the peptide-pulsed group minus cpm from cells cultured in medium alone divided by the number of clonotype-positive cells in the well as determined by FACS. Values are displayed as the mean ± SE cpm/100 clonotype-positive T cells per well.
Cytokine release
T cells purified and plated as above were cultured with media alone or HA peptide (100 μg/mL) plus fresh F1 splenocytes. Forty-eight hours later, supernatants were collected and stored at −70°C until assayed for interleukin-2 (IL-2) by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Data represent mean ± SE of triplicate cultures from 3 mice in each group and are expressed as the amount of IL-2 produced per 100 clonotype-positive T cells.
To assess the ability of B-lymphoma cells to present antigen to naive anti-HA transgenic T cells in vitro, irradiated (10 000 rads) A20WT, A20HA, as well as splenic DCs (1 × 105 cells/well) were mixed with 5 × 104 highly purified T cells from 6.5 TCR transgenic splenocytes. Different concentrations of HA peptide were added to the cultures containing splenic DCs or A20WT. No HA-peptide was added to the A20HA–T-cell cultures. After 48 hours, supernatants were collected and assayed for IL-2 by ELISA.
Isolation of splenic DCs
Splenic DCs were enriched according to the method of Steinman and colleagues.16 Briefly, to release DCs, spleens were injected with collagenase D, torn apart, and spleen fragments were subjected to further collagenase digestion. Low-density cells including DCs, were selected by centrifugation over a 30% dense bovine serum albumin (BSA) gradient (Sigma, St Louis, MO), cultured on plastic dishes for 1 to 2 hours after which the nonadherent cells (mostly lymphocytes) were washed away. The adherent cells were cultured overnight and DCs that detach from the plates during the incubation period were collected.
Results
A20 B-lymphoma cells function as potent APCs in vitro
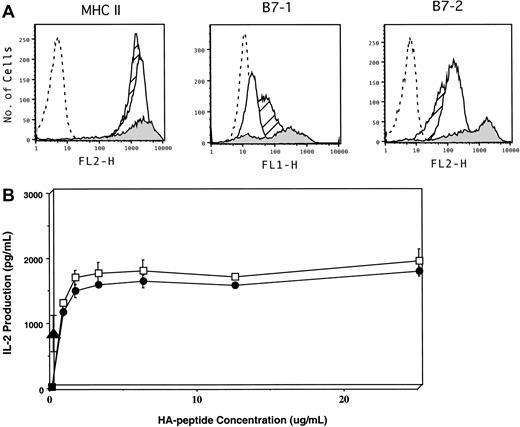
To examine the relative contribution of direct presentation of tumor antigen by lymphoma cells versus presentation by host APCs, we first compared the intrinsic capacity of A20 cells to activate naive antigen-specific T cells in vitro to that of DCs. A20 cells express abundant levels of MHC class II antigens, similar to splenic DCs (Figure 1A). In addition, this tumor constitutively expresses low to intermediate levels of B7-1 and B7-2, although as expected, the level of expression is significantly less than that present on DCs. Despite these phenotypic differences, A20 cells functioned equivalently to DCs in stimulating naive HA-specific CD4+ T cells in vitro, as measured by IL-2 release across a full range of peptide concentrations (Figure 1B, open squares versus closed circles). Furthermore, in the absence of any exogenous APCs, A20HA cells (which express HA as a transmembrane protein) are highly efficient at presenting MHC class II epitopes directly to CD4+ HA-specific T cells (closed triangle). These in vitro findings demonstrate that this B-cell lymphoma has a significant intrinsic capacity to process and present endogenous antigen to antigen-specific CD4+ T cells, resulting in their activation.
Phenotypic and functional characterization of A20 B-cell lymphoma as an APC.
(A) Flow cytometric analysis of MHC class II, CD80 (B7.1), and CD86 (B7.2) expression on A20WT (open histogram), A20HA (hatched histogram), and splenic DCs (shaded histogram). Isotype control staining of A20WT is shown as the dashed-line histogram. (B) IL-2 production by anti-HA transgenic CD4+ T cells in response to cognate antigen presented by lymphoma cells or splenic DCs. The 1 × 105 irradiated (10 000 rads) A20 WT (●), A20HA (▴), or splenic DCs (■) were mixed in vitro with 5 × 104 highly purified naive anti-HA/I-EdTCR transgenic T cells. Increasing concentrations of HA-peptide were added to the cultures containing either splenic DCs or A20WT. No HA-peptide was added to the A20HA–T-cell cultures. After 48 hours, supernatants were collected and assayed for IL-2 by ELISA. Values are the mean ± SE of triplicate cultures. No measurable IL-2 was produced from cultures of purified T cells pulsed with peptide in the absence of added APCs. IL-2 production by T cells cultured with A20HA alone is approximately 956 pg/mL. Shown is a representative experiment of 3 independent experiments with similar results.
Phenotypic and functional characterization of A20 B-cell lymphoma as an APC.
(A) Flow cytometric analysis of MHC class II, CD80 (B7.1), and CD86 (B7.2) expression on A20WT (open histogram), A20HA (hatched histogram), and splenic DCs (shaded histogram). Isotype control staining of A20WT is shown as the dashed-line histogram. (B) IL-2 production by anti-HA transgenic CD4+ T cells in response to cognate antigen presented by lymphoma cells or splenic DCs. The 1 × 105 irradiated (10 000 rads) A20 WT (●), A20HA (▴), or splenic DCs (■) were mixed in vitro with 5 × 104 highly purified naive anti-HA/I-EdTCR transgenic T cells. Increasing concentrations of HA-peptide were added to the cultures containing either splenic DCs or A20WT. No HA-peptide was added to the A20HA–T-cell cultures. After 48 hours, supernatants were collected and assayed for IL-2 by ELISA. Values are the mean ± SE of triplicate cultures. No measurable IL-2 was produced from cultures of purified T cells pulsed with peptide in the absence of added APCs. IL-2 production by T cells cultured with A20HA alone is approximately 956 pg/mL. Shown is a representative experiment of 3 independent experiments with similar results.
In vivo kinetics of tumor growth in parent-into-F1 BM chimeras
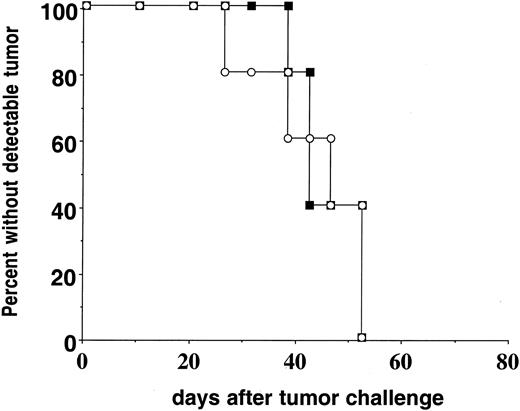
Preliminary studies of tumor growth in conventional parent-into-F1 BM chimeras revealed delayed kinetics of A20HA tumor progression in H-2b→H-2dxb F1 relative to H-2d→H-2dxb F1 chimeras (data not shown). Consequently, a direct comparison of tumor-specific T-cell function in the 2 chimeras would be confounded by the greater tumor antigen load into H-2d→H-2dxb F1 recipients with this experimental design. We therefore created parent-into-F1 chimeras using BM from BALB/c or C57BL/6 SCID donors. The use of SCID BM resulted in hematopoietic reconstitution (including myeloid-derived APC populations) without the development of mature T- or B-cell compartments. Three months after BM transplantation, the chimeras received anti-HA CD4+ T cells from TCR transgenic mice (H-2dxb) and were challenged 1 day later with A20HA tumor cells. The transfer of HA-specific T cells did not prevent the systemic progression of A20HA cells because no differences in tumor growth rates were observed in H-2dSCID→ H-2dxb F1 as compared to H-2bSCID→H-2dxb F1 chimeras (Figure 2). Tumor growth was not associated with loss of HA expression, because explanted tumor was recognized by fresh anti-HA TCR transgenic T cells equivalently to A20HA tumor that was maintained in vitro (data not shown).
Kinetics of A20HA tumor growth in reconstituted BM chimeras.
H-2dxb F1 mice were lethally irradiated (1000 rads) and received a graft consisting of 4 × 106 BM cells from either BALB/cSCID (H-2d, ▪) or C57B6/SCID (H-2b, ○) donors. Three months after BM reconstitution, either group of chimeric mice received 1 × 106CD4+ TCR transgenic T cells (H-2dxb) specific for HA/IEd intravenously (IV). One day later (day zero) all the animals were challenged IV with 1 × 106 A20HA tumor cells and they were inspected twice weekly for the development of tumors. Ten mice were included in each group.
Kinetics of A20HA tumor growth in reconstituted BM chimeras.
H-2dxb F1 mice were lethally irradiated (1000 rads) and received a graft consisting of 4 × 106 BM cells from either BALB/cSCID (H-2d, ▪) or C57B6/SCID (H-2b, ○) donors. Three months after BM reconstitution, either group of chimeric mice received 1 × 106CD4+ TCR transgenic T cells (H-2dxb) specific for HA/IEd intravenously (IV). One day later (day zero) all the animals were challenged IV with 1 × 106 A20HA tumor cells and they were inspected twice weekly for the development of tumors. Ten mice were included in each group.
Phenotypic characterization of reconstituted BM chimeras
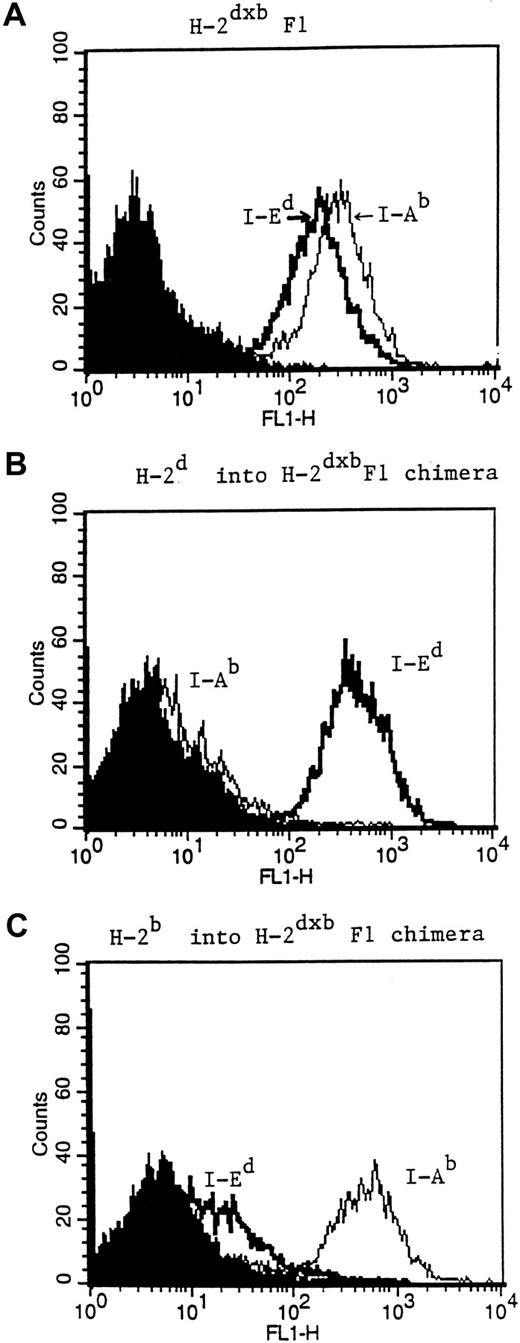
A major concern in the generation of H-2d→H-2dxb and H-2b→ H-2dxb chimeras is the possibility that residual H-2dxbF1 APCs may be present after reconstitution of the recipient animal. This is especially relevant for the reconstituted H-2b→H-2dxb F1 chimeras, where the presence of residual host APCs may be a confounding factor. Therefore, mice from each of the 2 groups of BM chimeras were killed after immune reconstitution and the percentage of MHC class II–positive cells of donor haplotype was determined. As seen in Figure3, staining of splenocytes from H-2d→H-2dxb chimeras (middle panel) revealed the presence of only I-Ed+ cells and absence of I-Ab+ splenocytes. Similarly, splenocytes from H-2b→H-2dxb chimeras were only positive for I-Ab with no detectable contaminating I-Ed+cells (lower panel). Furthermore, anti-HA (F1) transgenic CD4+ T cells transferred into H-2b→ H-2dxb chimeras failed to respond to immunization with a recombinant vaccinia virus encoding HA, providing functional confirmation of the absence of APCs expressing the restricting element (I-Ed) required for presenting the nominal peptide antigen to the clonotypic T cells (data not shown).
Phenotypic characterization of reconstituted BM chimeras.
Three months after BM reconstitution, mice from either H-2d→H-2dxb (B) or H-2b→H-2dxb chimeras (C) were killed. Splenocytes from these animals were stained for the expression of I-Ed using the MoAb 14.4.4 (IgG2a), or for the expression of I-Ab with the MoAb Y3P (IgG2a). FITC goat anti–mouse IgG2a secondary antibody was added and the stained splenocytes were analyzed for MHC class II expression by FACS. Splenocytes from an H-2bxd mouse (A) were used as a positive control. Shaded histogram in each figure represents background staining.
Phenotypic characterization of reconstituted BM chimeras.
Three months after BM reconstitution, mice from either H-2d→H-2dxb (B) or H-2b→H-2dxb chimeras (C) were killed. Splenocytes from these animals were stained for the expression of I-Ed using the MoAb 14.4.4 (IgG2a), or for the expression of I-Ab with the MoAb Y3P (IgG2a). FITC goat anti–mouse IgG2a secondary antibody was added and the stained splenocytes were analyzed for MHC class II expression by FACS. Splenocytes from an H-2bxd mouse (A) were used as a positive control. Shaded histogram in each figure represents background staining.
Loss of the naive phenotype and clonal expansion of antigen-specific T cells is seen only in H-2d→H-2dxb tumor-bearing chimeras
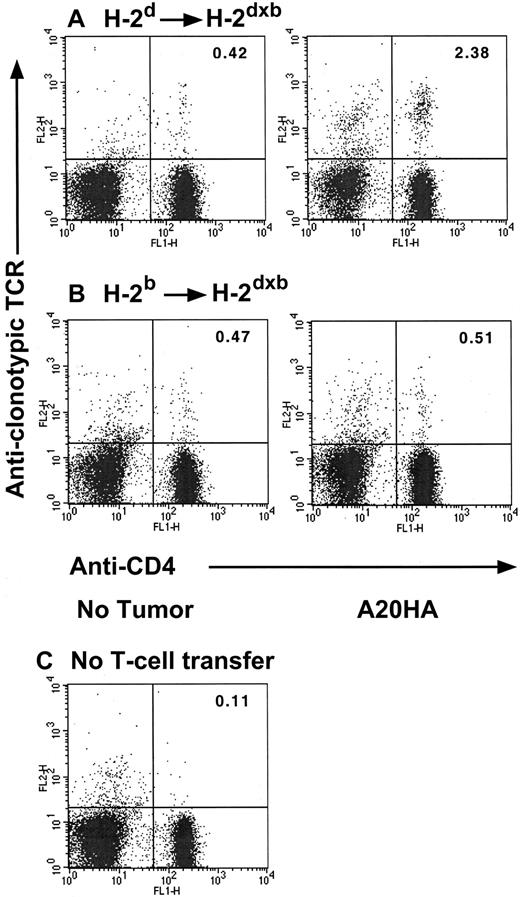
In previous studies we have shown that antigen-specific transgenic CD4+ T cells transferred into A20HA tumor-bearing hosts undergo clonal expansion and have a phenotype associated with antigen recognition (CD45Rblow, CD62low, and CD44high). However, functional analysis demonstrates that these cells have a diminished response to peptide antigen in vitro and are unable to be primed in vivo. This T-cell unresponsiveness is antigen specific and is fully established 3 weeks (day +22) after T-cell transfer into tumor-bearing mice.9 We therefore used this adoptive transfer system to assess the fate of anti-HA CD4+ transgenic T cells in 2 different contexts: (1) in the setting where both APCs as well as B-lymphoma cells can present HA-antigen to clonotypic CD4+ T cells (H-2dSCID→H-2dxb chimeras) and (2) when antigen can be presented only by malignant B cells (H-2bSCID→H-2dxb chimeras). As shown in Figure 4, 3 weeks after tumor challenge, there was a significant clonal expansion of HA-specific, clonotype-positive T cells in H-2d→H-2dxbtumor-bearing mice relative to their tumor-free counterparts (2.38% versus 0.42%, respectively). In sharp contrast, no change in the percentage of clonotypic CD4+ T cells was observed in H-2b→H-2dxb tumor-bearing chimeras (where only the tumor cells can present the antigen) relative to tumor-free H-2b→H-2dxb chimeras (0.51% versus 0.47%, respectively). It should be emphasized that at this time point, a similar tumor burden was present in both sets of chimeras, consistent with the equivalent growth kinetics displayed in Figure 2.
Changes in clonotype-positive T cells in H-2d→H-2dxb tumor-bearing chimeras.
Three months after BM reconstitution, H-2d→H-2dxb (A) or H-2b→ H-2dxb (B) chimeras received 1 × 106 anti-HA CD4+ TCR transgenic T cells IV. One day later, half the animals in each set of chimeras were challenged with 1 × 106 A20HA tumor cells given IV. On day +22 after tumor challenge, all the animals were killed, and purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 versus anti-HA TCR clonotype (MoAb 6.5). (C) Background staining was done using this antibody combination on spleen cells from BALB/c mice without transgenic T-cell transfer. Three mice were included per group. Shown are representative FACS plots from individual mice in each group. Upper right quadrant numbers: percentage of double-positive T cells.
Changes in clonotype-positive T cells in H-2d→H-2dxb tumor-bearing chimeras.
Three months after BM reconstitution, H-2d→H-2dxb (A) or H-2b→ H-2dxb (B) chimeras received 1 × 106 anti-HA CD4+ TCR transgenic T cells IV. One day later, half the animals in each set of chimeras were challenged with 1 × 106 A20HA tumor cells given IV. On day +22 after tumor challenge, all the animals were killed, and purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 versus anti-HA TCR clonotype (MoAb 6.5). (C) Background staining was done using this antibody combination on spleen cells from BALB/c mice without transgenic T-cell transfer. Three mice were included per group. Shown are representative FACS plots from individual mice in each group. Upper right quadrant numbers: percentage of double-positive T cells.
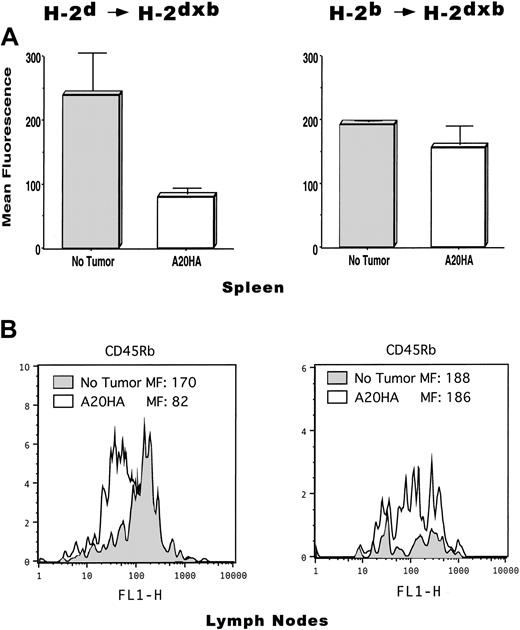
FACS analysis of activation/memory markers on clonotype-positive CD4+ T cells revealed that T cells from the spleen of H-2d→H-2dxb tumor-bearing chimeras displayed decreased expression of CD45Rb compared to T cells from tumor-free chimeras, indicative of having encountered HA-antigen in vivo (Figure5A, left panel). In sharp contrast, T cells from H-2b→H-2dxb tumor-bearing chimeras (where only the tumor can present the antigen) displayed similar expression of CD45Rb compared to T cells from tumor-free mice (Figure5A, right panel).
Phenotypic changes associated with antigen recognition on CD4+ TCR clonotype+ T cells is only seen in H-2d→H-2dxb tumor-bearing chimeras.
T cells from the mice in Figure 4 were isolated from their spleen or lymph nodes as described in “Materials and methods.” Cells were stained with cychrome-labeled anti–mouse CD4, biotinylated anti–TCR clonotype MoAb 6.5 followed by PE-labeled streptavidin and FITC-conjugated anti–mouse CD45RB. Live gating on CD4+ T cells was set, and 100 000 events collected per sample. (A) Mean fluorescence intensity ± SE is shown for CD45Rb expressed by CD4+TCR clonotype-positive T cells isolated from the spleen of tumor-free (No tumor) or A20HA-bearing chimeric mice (3 mice per group). (B) Representative FACS profile of CD45Rb expression by anti-HA CD4+ T cells isolated from lymph nodes. Inguinal and axillary lymph nodes were harvested from tumor-free and tumor-bearing chimeric mice. Lymph node cells from 3 animals per group were pooled and T cells were isolated and stained as described above. Shaded histograms in each figure correspond to clonotype-positive T cells from tumor-free mice. Open histograms correspond to clonotype-positive T cells from tumor-bearing mice. MF indicates mean fluorescence intensity. Shown is a representative experiment of 3 independent experiments with similar results.
Phenotypic changes associated with antigen recognition on CD4+ TCR clonotype+ T cells is only seen in H-2d→H-2dxb tumor-bearing chimeras.
T cells from the mice in Figure 4 were isolated from their spleen or lymph nodes as described in “Materials and methods.” Cells were stained with cychrome-labeled anti–mouse CD4, biotinylated anti–TCR clonotype MoAb 6.5 followed by PE-labeled streptavidin and FITC-conjugated anti–mouse CD45RB. Live gating on CD4+ T cells was set, and 100 000 events collected per sample. (A) Mean fluorescence intensity ± SE is shown for CD45Rb expressed by CD4+TCR clonotype-positive T cells isolated from the spleen of tumor-free (No tumor) or A20HA-bearing chimeric mice (3 mice per group). (B) Representative FACS profile of CD45Rb expression by anti-HA CD4+ T cells isolated from lymph nodes. Inguinal and axillary lymph nodes were harvested from tumor-free and tumor-bearing chimeric mice. Lymph node cells from 3 animals per group were pooled and T cells were isolated and stained as described above. Shaded histograms in each figure correspond to clonotype-positive T cells from tumor-free mice. Open histograms correspond to clonotype-positive T cells from tumor-bearing mice. MF indicates mean fluorescence intensity. Shown is a representative experiment of 3 independent experiments with similar results.
A representative FACS profile displaying a decreased CD45Rb expression on T cells from lymph nodes of H-2d→H-2dxbtumor-bearing mice—compared to T cells from tumor-free mice—is shown in Figure 5B (left panel). Similar to our findings in the spleen, no changes in CD45Rb expression were observed on T cells isolated from the lymph nodes of H-2b→H-2dxb tumor-bearing chimeras (Figure 5B, right panel).
Therefore, BM-derived APCs are absolutely required for the induction of clonal expansion and loss of the naive phenotype observed in tumor antigen–specific CD4+ T cells during lymphoma progression.
Induction of antigen-specific CD4+ T-cell tolerance is mediated by cross-presentation of tumor-antigens by host APCs
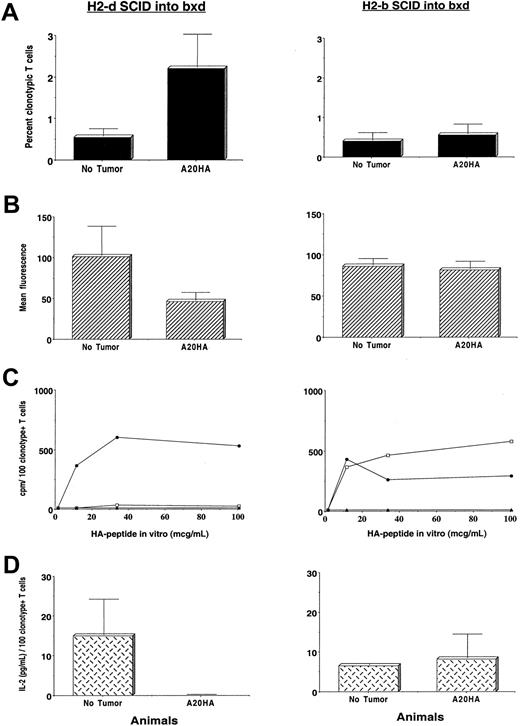
The phenotypic changes in clonotype-positive T cells from H-2d→H-2dxb tumor-bearing chimeras described above can be observed subsequent to both T-cell priming as well as tolerance induction.17 Therefore, in the next set of experiments we evaluated the function of clonotype-positive T cells from the different experimental groups in response to incubation with the cognate HA-antigenic peptide in vitro. Figure6 shows the phenotypic as well as functional characteristics of clonotype-positive T cells from H-2d→H-2dxb chimeras (left panel) and H-2b→H-2dxb chimeras (right panel). Despite the significant expansion of HA-specific CD4+ T cells in H-2d→H-2dxb tumor-bearing chimeras (Figure6A, left panel) and decreased expression of CD62L (Figure 6B, left panel), T cells from this group had a blunted HA-specific proliferative response (Figure 6C, left panel) and were unable to produce IL-2 in response to in vitro incubation with HA-peptide (Figure 6D, left panel). Therefore, as previously observed in a syngeneic BALB/c system,9 antigen-specific CD4+ T cells from H-2d→H-2dxb tumor-bearing chimeras have been rendered fully unresponsive during A20 lymphoma progression.
Induction of tumor antigen–specific CD4+ T-cell tolerance requires presentation by BM-derived APCs.
Three months after BM reconstitution, H-2dSCID→H-2dxb (left panel) or H-2bSCID→H-2dxb chimeras (right panel) received 1 × 106 anti-HA CD4+ TCR transgenic T cells IV. One day later, half the animals in each set of chimeras were challenged with 1 × 106 A20HA tumor cells given IV. On day +22 after tumor challenge, all the animals were killed and T cells were purified from their spleens as described. (A) Purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 versus anti-HA TCR clonotype as in Figure 4. Values represent mean ± SE of percentage of T cells expressing the clonotypic TCR for 3 mice per group. (B) T cells from tumor-free or tumor-bearing chimeras were stained with cychrome-labeled anti–mouse CD4, biotinylated anti–TCR clonotype MoAb 6.5 followed by PE-labeled streptavidin and FITC-conjugated anti–mouse CD62 as in Figure 5. Mean fluorescence intensity ± SE is shown (2 mice per group). (C) In vitro proliferative response of clonotype-positive T cells to stimulation with HA110-120 peptide. Purified T cells (4 × 104/well) from tumor-free (no tumor, ●), tumor-bearing chimeras (A20HA, ■), or from F1 naive mice (no T-cell transfer, ▴) were mixed with fresh splenocytes (8 × 104/well) from F1 mice to which different concentrations of HA peptide were added. 3H incorporation was assayed after 3 days of incubation, and is shown as counts per minute (cpm) from which values for medium alone were subtracted. Values represent the mean of the cpm/100 clonotype-positive T cells per well from 2 to 3 mice in each group. (D) Purified T cells (4 × 104 /well) from the mice in panel C were mixed with fresh splenocytes (8 × 104/ well) from naive F1 mice to which HA peptide (100 μg/mL) was added. Forty-eight hours later, supernantants were collected and assayed for IL-2 by ELISA. Data represent mean ± SE of the cpm/100 clonotype-positive T cells per well from 2 mice in each group.
Induction of tumor antigen–specific CD4+ T-cell tolerance requires presentation by BM-derived APCs.
Three months after BM reconstitution, H-2dSCID→H-2dxb (left panel) or H-2bSCID→H-2dxb chimeras (right panel) received 1 × 106 anti-HA CD4+ TCR transgenic T cells IV. One day later, half the animals in each set of chimeras were challenged with 1 × 106 A20HA tumor cells given IV. On day +22 after tumor challenge, all the animals were killed and T cells were purified from their spleens as described. (A) Purified splenic T cells were analyzed by 2-color flow cytometry staining for CD4 versus anti-HA TCR clonotype as in Figure 4. Values represent mean ± SE of percentage of T cells expressing the clonotypic TCR for 3 mice per group. (B) T cells from tumor-free or tumor-bearing chimeras were stained with cychrome-labeled anti–mouse CD4, biotinylated anti–TCR clonotype MoAb 6.5 followed by PE-labeled streptavidin and FITC-conjugated anti–mouse CD62 as in Figure 5. Mean fluorescence intensity ± SE is shown (2 mice per group). (C) In vitro proliferative response of clonotype-positive T cells to stimulation with HA110-120 peptide. Purified T cells (4 × 104/well) from tumor-free (no tumor, ●), tumor-bearing chimeras (A20HA, ■), or from F1 naive mice (no T-cell transfer, ▴) were mixed with fresh splenocytes (8 × 104/well) from F1 mice to which different concentrations of HA peptide were added. 3H incorporation was assayed after 3 days of incubation, and is shown as counts per minute (cpm) from which values for medium alone were subtracted. Values represent the mean of the cpm/100 clonotype-positive T cells per well from 2 to 3 mice in each group. (D) Purified T cells (4 × 104 /well) from the mice in panel C were mixed with fresh splenocytes (8 × 104/ well) from naive F1 mice to which HA peptide (100 μg/mL) was added. Forty-eight hours later, supernantants were collected and assayed for IL-2 by ELISA. Data represent mean ± SE of the cpm/100 clonotype-positive T cells per well from 2 mice in each group.
In sharp contrast, CD4+ HA-specific T cells remained responsive in H-2b→H-2dxb tumor-bearing chimeras (Figure 6C,D, right panel). In fact, the absence of clonal expansion (Figure 6A, right panel) and persistence of the naive phenotype (Figure 6B, right panel) is consistent with these cells never having encountered antigen in vivo. These differences in phenotype and function of HA-specific T cells in the 2 sets of chimeras were observed in the setting of comparable tumor burdens and they were manifest as early as 1 week after tumor challenge (data not shown).
Therefore, because T-cell tolerance is only seen in the H-2d→ H-2dxb chimeras but not in the H-2b→H-2dxb chimeras (which lack the appropriate APC population), induction of tolerance to tumor antigens requires APCs capable of taking up antigens shed from the tumor, and presenting processed antigenic peptides to antigen-specific T cells. Furthermore, the dominant influence of host APCs on T-cell recognition of tumor antigen in vivo is demonstrated despite the intrinsic capacity of lymphoma cells to directly present tumor antigens as measured in vitro (Figure 1).
Discussion
These findings unambiguously demonstrate that the induction of tumor antigen–specific T-cell tolerance by B-cell lymphomas in vivo requires BM-derived APCs capable of taking up antigens shed from the tumor and presenting processed antigenic peptides to antigen-specific CD4+ T cells (“cross-presentation”) rather than direct presentation by lymphoma cells themselves.
A number of studies in recent years have postulated that tolerance to tumor antigens results from a direct encounter of T cells with tumor cells that are ill-equipped to provide the necessary “signals” for T-cell activation. Because most tumors of nonhematopoietic origin fail to express costimulatory molecules, the current model of tumor-induced T-cell tolerance (“signal 1 without signal 2”) predicts that tumor-specific T cells are rendered anergic on encountering antigen on tumor cells that cannot provide costimulation.18
There are, however, several problems with this model in terms of explaining the induction of tolerance to tumor antigens. First, if all that is required for tolerance induction is the inability of tumor cells to deliver costimulatory signals to T cells, then the induction of such a state of unresponsiveness should not be seen in tumors derived from APCs, which are reasonably well equipped to deliver “signal 1” and “signal 2” to antigen-specific T cells. As an example, A20 B-cell lymphoma, being a tumor derived from cells with antigen-presenting capabilities, has been used extensively for in vitro studies of antigen processing and presentation. Indeed, we have shown that in vitro culture of A20HA with highly purified HA-specific, MHC class II–restricted TCR transgenic T cells resulted in activation and IL-2 production by antigen-specific T cells (Figure 1 and reference 8). Yet, despite these in vitro properties, growth of this tumor in vivo resulted in the induction of antigen-specific CD4+ T-cell tolerance rather than T-cell priming.9 This state of unresponsiveness was not the result of B-cell lymphomas losing their ability to express costimulatory molecules in vivo—as it has been proposed by others7—because A20HA explants from tumor-bearing mice still express high levels of MHC class II molecules as well as costimulatory molecules and are indeed capable of inducing the activation of naive antigen-specific T cells (data not shown). Therefore, the demonstration that antigen-specific T-cell tolerance ensues during the in vivo growth of tumor cells capable of providing several known signals required for T-cell activation indicates that the intrinsic APC capacity of the tumor cell themselves is not the primary determinant of the outcome of the host T-cell response in vivo. Instead, the results of these studies consistently point toward the context of antigen presentation by host APCs as playing a dominant role in the regulation of T-cell activation in vivo.
Although in some forms of human lymphoma, abundant T-cell populations can be seen histologically surrounding the malignant B-cell population, proof of cognate T-cell/B-cell tumor interaction as well as assessment of the functional status of these antigen-specific T cells are lacking. It is plausible that the differential expression of lymphoid homing receptors and chemokines by lymphoma cells would influence both the microenvironment occupied by the tumor and the surrounding T-cell infiltrate. In any case, although some forms of lymphoma may favor direct T-cell/tumor interactions, our study indicates that such interactions are not required for the development of tumor-specific T-cell tolerance that is mediated by BM-derived APCs.
The demonstration of the critical role of APCs in the induction of tolerance to tumor antigens is consistent with previous studies of T-cell tolerance to peripheral self-antigens.12-14,19 APCs have been shown to capture tissue-specific self-antigens, migrate to the lymphoid organs, and present antigens to either naive CD8+ T cells12 or CD4+ T cells13 in a way that resulted in tolerance induction rather than priming of T cells. In these studies, the induction of T-cell unresponsiveness to self-antigens represented an “active process” characterized by the loss of the naive phenotype as well as proliferation and clonal expansion of antigen-specific T cells. Interestingly, similar phenotypic and functional changes in antigen-specific CD4+ T cells are described in the present report (Figures 3A, 4A, and 5) suggesting that the induction of tolerance to tumor antigens is mediated by a similar mechanism. Furthermore, these similarities raise the likelihood that the same mechanisms that normally prevent inappropriate attack against self-antigens may also impose significant barriers for the development of effective immune responses against antigens expressed by tumors. Strikingly, our model demonstrates that this can occur even for a fully xenogeneic protein expressed exclusively by the tumor cells (clinically, a “best case” scenario). This barrier may be even greater in the case of nonmutated tissue-restricted self-antigens that are overexpressed by tumors and have been targeted for active immunotherapy in the clinic.20
Our recent observations that similar tolerogenic events can be seen in mice harboring a metastatic renal cell carcinoma expressing a model tumor antigen (RencaHA) (E.M.S. and colleagues, manuscript in preparation, July 2001) also support a role for BM-derived APCs in tolerance induction to tumor antigens expressed by solid malignancies. Despite the fact that this tumor does not express either MHC class II molecules or costimulatory molecules, HA-specific T cells from the spleen and lymph nodes of RencaHA-bearing mice lose their naive phenotype (indicative of having encountered the antigen in vivo) and were found to be fully unresponsive. Furthermore, similar to the findings in other solid tumor systems,21 the loss of the naive phenotype of T cells during RencaHA progression was seen in the absence of tumor metastasis to the lymphoid organs, suggesting that cells—other than tumor cells themselves—carry the tumor antigens from the tumor site to the lymphoid organs and are responsible for tolerance induction. Indeed, Doan and coworkers have recently found that transgenic CD8+ T cells specific for the human papillomavirus E7 oncoprotein expressed in epithelial cells are rendered tolerant by a mechanism that involved presentation of the antigen by BM-derived APCs.22
Given the above reasoning, we propose a model in which host APCs capture tumor antigens at the tumor site and then migrate to the lymphoid organs for presentation of the antigen to tumor-specific T cells. TCR engagement with tumor antigen/MHC class II molecules presented by APCs results in loss of the naive phenotype as well as a modest clonal expansion of antigen-specific T cells (Figures 3A and4A). Although this APC–T-cell encounter induces a “partial activation” state, by itself this appears to be insufficient to trigger the effector function necessary for achieving tumor rejection. Instead, in the face of persisting antigen, this state of “partial activation” is followed by the development of unresponsiveness (Figure 5, left panel), suggesting perhaps that in the absence of additional signals capable of sustaining and/or amplifying this initial response,23 the normal default of T-cell responses to tumor antigens is tolerance induction rather than priming.
The requirement for BM-derived APCs in both the induction of tolerance to tumor antigens, as shown in the present report, as well as in priming effective antitumor responses,24 places APCs at the crossroads of these highly divergent outcomes. It is plausible that the differentiation or activation state of the APC population may be the central determinant of T-cell priming versus tolerance. Indeed, it has been suggested that depending on their maturational state, DCs are able to either tolerize (immature DCs) or induce T-cell activation (mature DCs).25,26 Furthermore, we have recently demonstrated that in vivo activation of APCs through CD40 triggering could not only provide the signal(s) needed to induce T-cell activation rather than tolerance to tumor antigens, but also led to an enhanced response to vaccination in tumor-bearing animals.27 Using a similar strategy, Diehl and coworkers have shown that in vivo triggering of CD40 can also overcome peptide-induced peripheral cytotoxic T-lymphocyte tolerance and markedly enhanced the efficacy of peptide-based antitumor vaccine.28 Therefore, modulating the activation/maturational state of APCs may represent a useful strategy to overcome APC-mediated tolerance to tumor antigens.
It is also possible that discrete subpopulations of BM-derived APCs could be responsible for tolerance induction, and several potential candidates have been proposed. Huang and colleagues have recently identified a DC subset (CD4−/OX41− DCs) that constitutively transport apoptotic intestinal epithelial cells remnants to T-cell areas of mesenteric lymph nodes in vivo, suggesting a role for these DCs in inducing peripheral self-tolerance.19 The possibility that other subpopulation(s) of APCs may be involved in tolerance induction is plausible because macrophages29-31as well as B cells10,11 32 have been shown, under certain circumstances, to induce T-cell tolerance in vitro and in vivo. Perhaps, similar to the well-known hierarchy in antigen presentation and priming displayed by different APC populations (DCs > B cells > macrophages), a hierarchy may also exist in the capacity of these APC subsets to induce antigen-specific tolerance. In our model, B cells seem not to play a major role, because tolerance induction was seen in tumor-bearing chimeras (H-2dSCID→H-2dxb) that lack normal B cells. Therefore, either DCs or macrophages or both may represent the APC population mediating tumor-induced antigen-specific tolerance and studies to address this important question are ongoing.
These studies have elucidated, therefore, the dominant role that BM-derived APCs (and not lymphoma cells themselves) play in the induction and maintenance of tolerance to B-cell lymphoma–associated tumor antigens. Furthermore, they have identified a potential target that, if appropriately manipulated, may lead to approaches to overcome tumor-specific tolerance, a critical barrier that needs to be faced in the design of an effective immunotherapy against B-cell malignancies.
The authors wish to thank Sara Cooke for expert technical assistance and Drew Pardoll and Ephraim Fuchs for helpful discussions and careful review of the manuscript.
Supported by Public Health Service grants CA78658 R01 (H.I.L.) and CA078656 K08 (E.M.S). H.I.L. is a Scholar of the Leukemia and Lymphoma Society of America.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hyam I. Levitsky, Johns Hopkins University School of Medicine, 452 Bunting Blaustein Cancer Research Bldg, 1650 Orleans St, Baltimore, MD 21231; e-mail: hy@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal