The most frequent mutation causing Glanzmann thrombasthenia in Iraqi-Jews (IJ-1) is an 11-bp deletion in exon 13 of the glycoprotein (GP) IIIa gene. This deletion predicts a frameshift that results in the elimination of the C406-C655 disulfide bond and a premature termination codon shortly before the transmembrane domain. To determine the contribution of each of these alterations to the thrombasthenic phenotype, Chinese hamster ovary or baby hamster kidney cells were cotransfected with normal GPIIb complementary DNA (cDNA) and the following GPIIIa cDNAs: normal, cDNA bearing IJ-1 mutation, 2011T>A mutated cDNA predicting C655S (single-letter amino acid codes) substitution, and 2019A>T mutated cDNA predicting Stop657. Elimination of the C406-C655 disulfide bond by C655S substitution did not affect GPIIb/IIIa surface expression or binding of the transfected cells to immobilized fibrinogen, whereas elimination of the transmembrane and cytoplasmic domains in IJ-1 and Stop657 mutants prevented both surface expression and binding of the transfected cells to immobilized fibrinogen. Immunohistochemical staining and immunoprecipitation demonstrated that the elimination of amino acids 657-762 in IJ-1 and Stop657 prevented intracellular GPIIb/IIIa complex formation, and differential immunofluorescence staining of GPIIIa and cellular organelles suggested that the truncated uncomplexed GPIIIa protein was retained in the endoplasmic reticulum. Because the use of GPIIIa Stop693 and normal GPIIb cDNAs yielded GPIIb/IIIa complex formation, though with lower efficiency, it is suggested that amino acids 657-692 of GPIIIa are essential for the intracellular association of GPIIb and GPIIIa.
Introduction
Platelet aggregation is essential for normal hemostasis and depends on fibrinogen binding to the glycoprotein (GP) IIb/IIIa complex, a calcium-dependent heterodimer that is expressed exclusively in megakaryocytes and platelets.1 Soon after their production in megakaryocytes, GPIIb (αIIb) and GPIIIa (β3) are introduced into the endoplasmic reticulum (ER), where they form a complex, undergo N-linked glycosylation, and form disulfide bonds. The complex is transported to the Golgi apparatus for final oligosaccharide processing and then to the open canalicular system, α granules, and plasma membrane.2,3 The assembly of GPIIb and GPIIIa as a complex is a prerequisite for cell surface expression.3
A diminished amount or dysfunction of the GPIIb/IIIa complex causes Glanzmann thrombasthenia (GT).4 Affected patients exhibit a lifelong moderate-to-severe bleeding tendency that can be diagnosed by absent platelet aggregation in response to all physiologic agonists, absent or diminished clot retraction, prolonged bleeding time, and normal platelet count. GT is transmitted in an autosomal recessive manner and is caused by mutations in either GPIIb or GPIIIa genes. More than 50 mutations causing GT have been published, and most of them are listed in a database.5 Expression of some of these mutations in heterologous cell cultures has shed light on the functional importance of particular domains within GPIIb and GPIIIa.
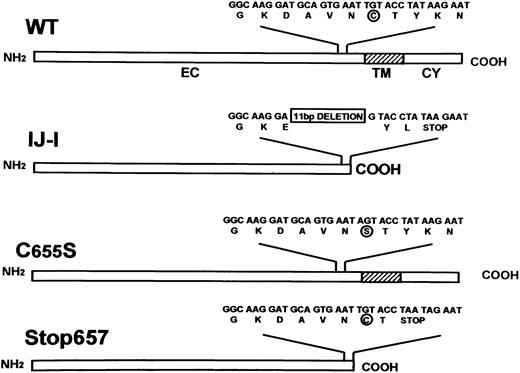
GT is a rare disease worldwide, but it was found to be relatively frequent in several ethnic groups such as Iraqi-Jews,6among whom 40 patients belonging to 21 unrelated families were ascertained.7 The predominant mutation causing GT in Iraqi-Jews is an 11-bp deletion within exon 13 (the previous mutation nomenclature designated this exon as numner 12) of the GPIIIa gene.8 This mutation, designated IJ-1, leads to a shift in the reading frame with substitution of 3 amino acids, including C655 (single-letter amino acid code), and predicts a premature termination of translation at amino acid 657, which is located in the extracellular domain shortly before the transmembrane domain. Hypothetically, the phenotypic expression of the 11-bp deletion can be caused by the absence of the long-range C406-C655 disulfide bond, by the elimination of the transmembrane and cytoplasmic domains, or by both. Wang et al9 studied one of these alternative mechanisms and showed that the expression of mutant GPIIIa C655Y in Chinese hamster ovary (CHO) cells, together with normal GPIIb, did not hamper GPIIb/IIIa complex formation and membrane expression. It thus seemed that truncation of GPIIIa was the main cause for GT resulting from the IJ-1 mutation.
The aim of this study was to further explore the mechanism by which truncation of GPIIIa abolishes surface expression of the GPIIb/IIIa complex. It will be shown that GPIIIa containing IJ-1 mutation is retained in the ER of transfected CHO cells and that there is no intracellular GPIIb/IIIa complex formation.
Materials and methods
Antibodies
Rabbit polyclonal antibody recognizing both GPIIb and GPIIIa was produced in our laboratory.10 Sheep polyclonal antibody recognizing both GPIIb and GPIIIa was purchased from Enzyme Research (South Bend, IN). Murine monoclonal antibodies AP3 and AP5 (both specific for GPIIIa) were generously provided by Dr Kunicki (Scripps Research Institute, La Jolla, CA). Murine monoclonal antibody 10E5 specific for GPIIb/IIIa complex was a gift from Dr Coller (Mount Sinai School of Medicine, NY). LM609 monoclonal antibody against CD51 (vitronectin receptor) was purchased from Serotec (Oxford, United Kingdom). Murine monoclonal antibody SZ22 specific for GPIIb heavy chain was purchased from Immunotech (Marseilles, France). Rabbit anti–rat mannosidase II antiserum was purchased from Dr Moremen (University of Georgia, Athens), and rabbit anticanine calnexin carboxyl terminus IgG was purchased from Stressgene Biotechnologies (Victoria, BC, Canada). Goat anti–mouse IgG fluorescein isothiocyanate (FITC) conjugate, goat anti–mouse IgG agarose conjugate, goat anti–rabbit IgG agarose conjugate, donkey antisheep peroxidase conjugate, and goat antimouse peroxidase conjugate were purchased from Sigma (St Louis, MO). Goat anti–rabbit IgG rhodamine conjugate was purchased from Boehringer Mannheim GmbH (Mannheim, Germany).
Purification of platelet messenger RNA and synthesis of first-strand complementary DNA
Messenger RNA (mRNA) was prepared from platelets of an Iraqi-Jewish patient with GT bearing the IJ-1 mutation by using QuickPrep mRNA purification kit (Pharmacia Biotech, Piscataway, NJ) and was reverse transcribed into first-strand complementary DNA (cDNA) using the reverse transcription system (Promega, Madison, WI) and oligo(dT) primer.
Preparation of GPIIIa mutant cDNA constructs
The GPIIIa cDNA in pGem7 vector (pGem7/IIIa) was generously provided by Dr Newman (The Blood Center of Southeastern Wisconsin, Milwaukee). A polymerase chain reaction (PCR)-amplified fragment containing exons 11-14 of IJ-1 cDNA and a PCR fragment with a T-to-A substitution at position 2011 predicting C655S substitution of GPIIIa (Figure 1) were introduced into GPIIIa cDNA. This fragment was generated by PCR site-directed mutagenesis11 using 2 overlapping oligonucleotide primers incorporating the single base pair substitution (underlined): sense, 5′ GAATAGTACCTATAAGAATGAGG 3′; antisense, 5′CTTATAGGTACTATTCACTGCATCC 3′. A fragment with an AT-to-TA substitution at position 2019-2020, leading to premature termination at the codon that usually codes for amino acid 657, was also introduced into GPIIIa cDNA. This fragment was similarly generated by using a set of overlapping oligonucleotide primers incorporating a dinucleotide base pair substitution (underlined): sense, 5′GAATTGTACCTAATAGAATGAGG3′; antisense, 5′TTCTATTAGGTACAATTCACTGC3′. PCR products containing each of the 3 mutations were digested with NotI andAccI restriction enzymes and cloned into pGem7/IIIa, replacing the corresponding normal cDNA fragment. Wild-type (WT) and mutated cDNAs were subcloned into the PvuII site of Pcep4 mammalian expression vector carrying the hygromycin resistance gene as a selection marker (Invitrogen, San Diego, CA).
Schematic representation of the structures of normal GPIIIa (WT) and GPIIIa in IJ-1, C655S, and Stop657 mutants.
Extracellular (EC), transmembrane (TM), and cytoplasmic (CY) domains are depicted. Nucleotide and amino acid sequences are shown. C/S at 655 is circled, and the 11-bp deletion of IJ-1 mutation is boxed.
Schematic representation of the structures of normal GPIIIa (WT) and GPIIIa in IJ-1, C655S, and Stop657 mutants.
Extracellular (EC), transmembrane (TM), and cytoplasmic (CY) domains are depicted. Nucleotide and amino acid sequences are shown. C/S at 655 is circled, and the 11-bp deletion of IJ-1 mutation is boxed.
A Stop693 mutant lacking the transmembrane and intracellular domains was constructed as follows: a DNA fragment containing a TAA (stop) codon instead of ATC (Ile693) at positions 2175-2177 was amplified using a sense primer corresponding to WT GPIIIa cDNA 5′CCTGTGTGACTCCGACTGGACCG3′, together with an antisense primer carrying the trinucleotide-substitution (underlined) 5′TTAGTCAGGGCCCTTGGGACACT3′. The PCR product was digested with AflII restriction enzyme, and the fragment (containing nucleotides 2029-2177 with the stop codon) was introduced into pCep4/GPIIIa expression vector whose nucleotides 2029-2665 were deleted by AflII andSse8387I restriction enzymes.
Cell culture and cotransfection of GPIIb and GPIIIa cDNAs in mammalian cells
CHO cells were grown in modified Eagle minimum essential medium supplemented by 2 mg/mL L-glutamine and 5% fetal calf serum (FCS). Baby hamster kidney (BHK) and COS-7 cells were grown in Dulbecco modified Eagle medium supplemented by 2 mg/mL L-glutamine and 5% FCS for BHK cells or 10% FCS for COS cells. All media and FCS were purchased from Biological Industries (Beit-Haemek, Israel). Cells were cotransfected (using lipofectamine reagent [Gibco BRL, Paisley, United Kingdom]) with 1 μg WT or the 4 mutated forms of Pcep4/GPIIIa and 1 μg pcDNA3/GPIIb, a mammalian expression vector carrying the normal cDNA of GPIIb and G418 resistance gene as a selection marker (generously provided by Dr Newman). Mock cells were produced by transfecting CHO or BHK cells with 1 μg Pcep4 and 1 μg pcDNA3. Transfected cells were selected with medium containing 0.7 mg/mL G418 (Gibco BRL) and 0.5 mg/mL hygromycin (Boehringer Mannheim GmbH) for 3 weeks. Transient transfection was carried out in the same manner, and the cells were not selected but were analyzed 48 hours after transfection.
Assessment of surface expressed GPIIb/IIIa complex by flow cytometry
Transfected cells were harvested with phosphate-buffered saline (PBS)/5 mM EDTA, pelleted, and incubated in growth medium for 30 minutes at room temperature. The cells were pelleted again, resuspended in PBS (5 × 105 cells/100 μL) and incubated for 30 minutes at room temperature with monoclonal antibodies against either GPIIIa (AP3, 15 μg/mL), GPIIb (SZ22, 15 μg/mL), GPIIb/IIIa complex (10E5, 30 μg/mL), or the vitronectin receptor (LM609, 30 μg/mL). Cells were then washed with PBS and incubated for 30 minutes at room temperature with FITC conjugate goat anti–mouse IgG diluted 1:50 in PBS. Finally, the cells were diluted to 5 × 104 cells/mL and analyzed for surface fluorescence in a flow cytometer (Coulter EPICS XL, Louton, United Kingdom).
Cell adhesion to immobilized fibrinogen
Sixteen-millimeter microtiter wells were precoated with fibrinogen (Sigma) 100 μL/well of 100 μg/mL solution overnight at 4°C, blocked by PBS containing 1% (wt/vol) bovine serum albumin (BSA) for 1 hour at room temperature, and then washed with PBS. Transfected cells were labeled with 3H-thymidine (0.37 MBq/mL in growth medium) overnight at 37°C. Cells were harvested as described, pelleted, and incubated in growth medium for 30 minutes at room temperature. Aliquots of 5 × 105cells/300 μL were added to the wells in quadruplicate and incubated for 15 to 30 minutes at 37°C. Wells were washed twice with PBS, adherent cells were lysed in 200 μL 2% sodium dodecyl sulfate (SDS), and radioactivity was counted using a liquid scintillation analyzer 16TR (Packard, Meriden, CT). Percentage adherent cells was calculated as cpm adherent cell lysate compared to cpm original cell sample × 100.
Immunoprecipitation of GPIIb and IIIa
CHO or BHK cells transfected with WT GPIIb and WT or mutant forms of GPIIIa were grown to subconfluence (5 × 106cells/100-mm plate), washed with PBS, and harvested in lysis buffer (20 mM Tris HCl, pH 7.5, 150 mM NaCl, 1% Nonident P40, 1% deoxycholic acid, 5 mM CaCl2, and Complete protease inhibitor mix [Boehringer]). Cell lysates were incubated on ice for 30 minutes and centrifuged for 20 minutes at 16 000g to remove particulate debris. The supernatant was incubated with 20 μg rabbit polyclonal antibodies against GPIIb and GPIIIa overnight at 4°C and then was incubated with agarose-bound goat anti–rabbit IgG for 5 hours at 4°C. In other experiments, cell lysates were incubated with 10 μg 10E5 monoclonal antibody against GPIIb/IIIa complex overnight at 4°C, followed by incubation with agarose-conjugated goat anti–mouse IgG for 5 hours at 4°C. After 5 washes with TBS (20 mM Tris HCl, pH 7.5, 150 mM NaCl) containing 1% Triton X-100, the immunoprecipitated proteins were eluted at 100°C with nonreducing loading buffer (40 mM Tris pH 6.8, 1.5% SDS, 8% glycerol, and 0.01% bromophenol blue). For immunoblot analyses, samples were run on 7.5% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA) that was incubated with either anti-GPIIIa AP5 monoclonal antibody or sheep polyclonal antibodies against GPIIb and GPIIIa. Immunoreactive bands on the membrane were detected using peroxidase conjugated anti–mouse IgG or anti–sheep IgG, respectively, enhanced chemiluminescence kit (Amersham, Buckinghamshire, United Kingdom) and x-ray film exposure. Prestained standards (Bio-Rad Laboratories, Richmond, CA) were used to determine the molecular weights of the precipitated proteins.
Pulse-chase analysis
Transfected cells were metabolically labeled with [35S]methionine12 and chased for 1, 3, 6, and 20 hours. Cell lysates were prepared and precleared by sequential addition of 3 μg nonimmune mouse IgG and 60 μL 10% Pansorbin cells (Calbiochem-Novabiochem, La Jolla, CA). Precleared lysates were incubated with the anti-GPIIIa AP5 monoclonal antibody (3 μg) overnight at 4°C, followed by incubation with agarose-conjugated goat anti–mouse IgG (50 μL) for 5 hours at 4°C. Recombinant proteins were electrophoresed by SDS-PAGE, and gel was fixed in isopropanol–acetic acid–water (25:10:65), vacuum dried, and exposed to Biomax MS film using Biomax Transcreen-LE (Kodak, Rochester, NY) at −70°C. In some experiments, when we tried to immunoprecipitate recombinant proteins from the culture medium, the chase was carried out using serum-free medium followed by medium concentration by Centricon YM-30 (Millipore) and analysis by immunoprecipitation and SDS-PAGE as described.
Immunohistochemistry
Transfected cells were grown on glass slides to subconfluence, washed in PBS, dried for 24 hours, and fixed in acetone for 15 minutes at 4°C. They were then incubated in Tris-buffered saline (pH 7.6) containing AP5 or 10E5 monoclonal antibodies (10-20 μg/mL) followed by development using an alkaline phosphatase–antialkaline phosphatase kit (APAAP, Dako, Glostrup, Denmark).
Immunofluorescence microscopy
Transfected cells were grown on 12-mm coverslips to subconfluence, washed in PBS, and fixed in acetone:methanol (1:1) for 4 minutes on ice. After a 20-minute incubation in blocking solution (2.5% BSA, 0.05% NP-40 in PBS), the cells were incubated for 1 hour at room temperature with 1:1000 AP5 monoclonal antibody against GPIIIa and either rabbit anti–rat mannosidase II or rabbit anticanine calnexin carboxyl terminus. Cells were washed 3 times with PBS/0.5% BSA and incubated for 1 hour at room temperature with 1:50 FITC conjugate goat anti–mouse IgG and rhodamine conjugate goat anti–rabbit IgG. Coverslips were coated with antifade solution (Oncor, Gaithersburg, MD) and analyzed using an Olympus BH2 fluorescent light microscope equipped with a PlanApo objective 100 × /1.4 oil, an appropriate spectral filter (BH2-TFC1 triple-band filter cufor DAPI/FITC/TRITC), and a 100-W mercury lamp. Photography was carried out using SD-200 Spectratube system (Applied Spectral Imaging, Migdal Haemek, Israel).
Results
Surface expression of GPIIIa in transfected cells
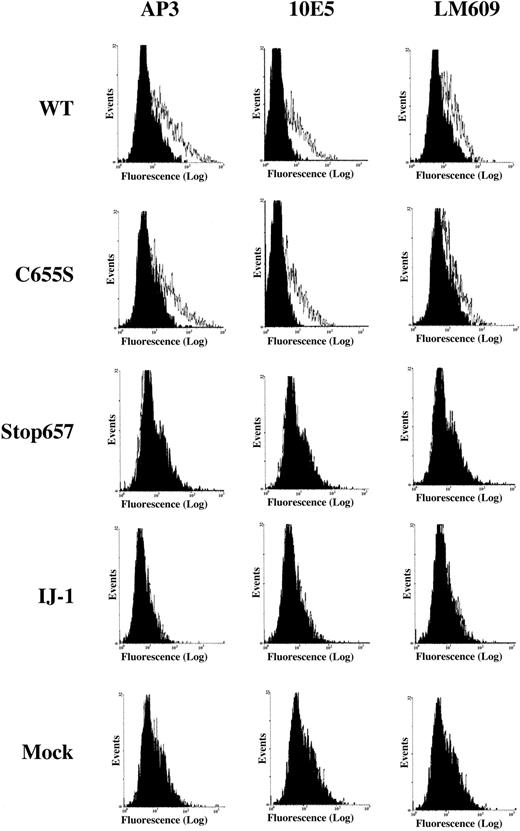
Most experiments described were carried out in CHO cells. However, some experiments were carried out in BHK cells. To evaluate the relative significance of the elimination of C406-C655 bond and the truncation of GPIIIa after amino acid 657 in the IJ-1 mutation, we expressed in BHK cells normal GPIIb cDNA together with one of the following GPIIIa cDNAs: WT, IJ-1, a mutant yielding C655S, and a mutant producing Stop657 (Figure 1). Flow cytometric analysis of BHK cells transfected with normal GPIIb and normal GPIIIa or mutant GPIIIa producing C655S revealed significant binding of AP3 monoclonal antibody against GPIIIa and 10E5 monoclonal antibody against GPIIb/IIIa complex, whereas cells transfected with normal GPIIb and IJ-1 or Stop657 constructs and mock cells showed no binding (Figure2). Similarly, binding of SZ22 antibody against GPIIb was detected in WT and C655S cells but not in IJ-1, Stop657, or mock cells (data not shown).
Flow cytometric analysis of normal, mutant, and mock-transfected cells.
Surface expression of GPIIIa, GPIIb/IIIa complex, and vitronectin receptor (VnR) in BHK cells transfected with normal GPIIb cDNA and either normal or mutant GPIIIa cDNAs. Monoclonal antibodies used were AP3 against GPIIIa, 10E5 against GPIIb/IIIa, and LM609 against vitronectin receptor. LM609 detects a complex of hamster αv and human GPIIIa. The black histogram represents the binding of FITC-labeled secondary antibody. It can be seen that binding of all 3 antibodies is similar for WT and C655S cells, whereas no binding is detectable for Stop657, IJ-1, and mock cells. The x-axis represents fluorescence intensity, and the y-axis represents total events.
Flow cytometric analysis of normal, mutant, and mock-transfected cells.
Surface expression of GPIIIa, GPIIb/IIIa complex, and vitronectin receptor (VnR) in BHK cells transfected with normal GPIIb cDNA and either normal or mutant GPIIIa cDNAs. Monoclonal antibodies used were AP3 against GPIIIa, 10E5 against GPIIb/IIIa, and LM609 against vitronectin receptor. LM609 detects a complex of hamster αv and human GPIIIa. The black histogram represents the binding of FITC-labeled secondary antibody. It can be seen that binding of all 3 antibodies is similar for WT and C655S cells, whereas no binding is detectable for Stop657, IJ-1, and mock cells. The x-axis represents fluorescence intensity, and the y-axis represents total events.
Hamster cells produce an αv subunit that can form a complex with human GPIIIa detectable by the monoclonal antibody LM609.13 14 Binding of LM609 monoclonal antibody to BHK cells transfected with normal GPIIb and WT GPIIIa cDNA or mutant GPIIIa cDNA producing C655S was similar (Figure 2). In contrast, BHK cells transfected with normal GPIIb and IJ-1 cDNA or cDNA producing Stop657 and mock cells did not bind LM609. These data indicate that the C655S substitution prevented neither GPIIIa surface expression nor complex formation with GPIIb or with αv. In contrast, the mutant IJ-1 and Stop657 forms of GPIIIa were not expressed on the cell surface as GPIIb/IIIa complexes or as GPIIIa complexed with αv.
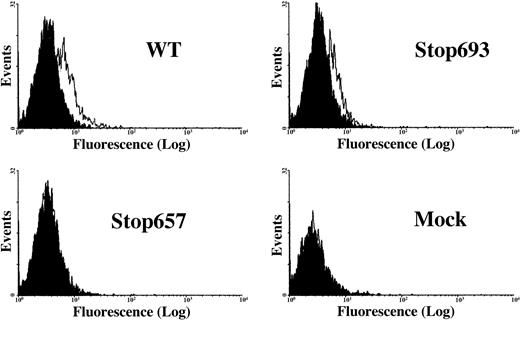
A previous study in transiently transfected COS cells showed that a GPIIIa/Stop693 mutant, which lacks the transmembrane and intracellular domains (preserving the whole extracellular domain), formed a surface-expressed complex with WT GPIIb, though with a lower efficiency.15 In the present study, flow cytometric analysis demonstrated binding of 10E5 monoclonal antibody against GPIIb/IIIa complex to BHK cells transfected with normal GPIIb and either normal or mutant Stop693 GPIIIa, but not to BHK cells transfected with normal GPIIb and mutant Stop657 GPIIIa (Figure3). Similar results were obtained in COS cells (data not shown).
Flow cytometric analysis of transiently transfected cells.
Surface expression of the GPIIb/IIIa complex in BHK cells transfected with normal GPIIb cDNA and either normal or mutant Stop693 or Stop657 GPIIIa cDNAs. Binding of 10E5 antibodies is demonstrated for WT and Stop693 cells but not for Stop657 and mock cells. The x-axis represents fluorescence intensity, and the y-axis represents total events.
Flow cytometric analysis of transiently transfected cells.
Surface expression of the GPIIb/IIIa complex in BHK cells transfected with normal GPIIb cDNA and either normal or mutant Stop693 or Stop657 GPIIIa cDNAs. Binding of 10E5 antibodies is demonstrated for WT and Stop693 cells but not for Stop657 and mock cells. The x-axis represents fluorescence intensity, and the y-axis represents total events.
Adhesion of transfected CHO cells to immobilized fibrinogen
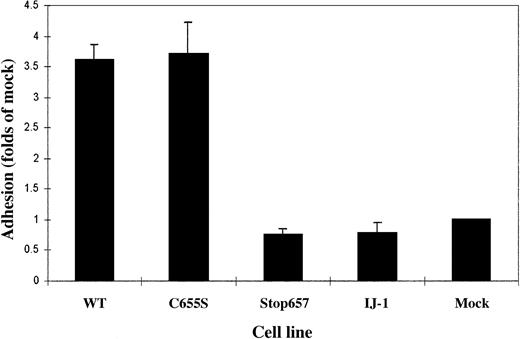
To determine whether the expressed receptors were functional, adhesion to immobilized fibrinogen was measured. Cells transfected with normal GPIIb and GPIIIa cDNAs and cells transfected with normal GPIIb and GPIIIa C655S cDNAs adhered to fibrinogen, whereas cells containing IJ-1 and Stop657 did not (Figure 4).
Adhesion of normal, mutant, and mock-transfected cells labeled by 3H-thymidine to immobilized fibrinogen.
Adherence of each cell clone is expressed in proportion to the adherence of mock cells. Bars represent the mean ± SD of 3 experiments, each performed in quadruplicate. It can be seen that cells transfected with normal GPIIb and GPIIIa cDNAs and cells transfected with normal GPIIb and GPIIIa C655S cDNAs adhered to fibrinogen, whereas cells containing IJ-1 and Stop657 did not.
Adhesion of normal, mutant, and mock-transfected cells labeled by 3H-thymidine to immobilized fibrinogen.
Adherence of each cell clone is expressed in proportion to the adherence of mock cells. Bars represent the mean ± SD of 3 experiments, each performed in quadruplicate. It can be seen that cells transfected with normal GPIIb and GPIIIa cDNAs and cells transfected with normal GPIIb and GPIIIa C655S cDNAs adhered to fibrinogen, whereas cells containing IJ-1 and Stop657 did not.
Detection of intracellular GPIIIa and GPIIb/IIIa complexes in transfected cells
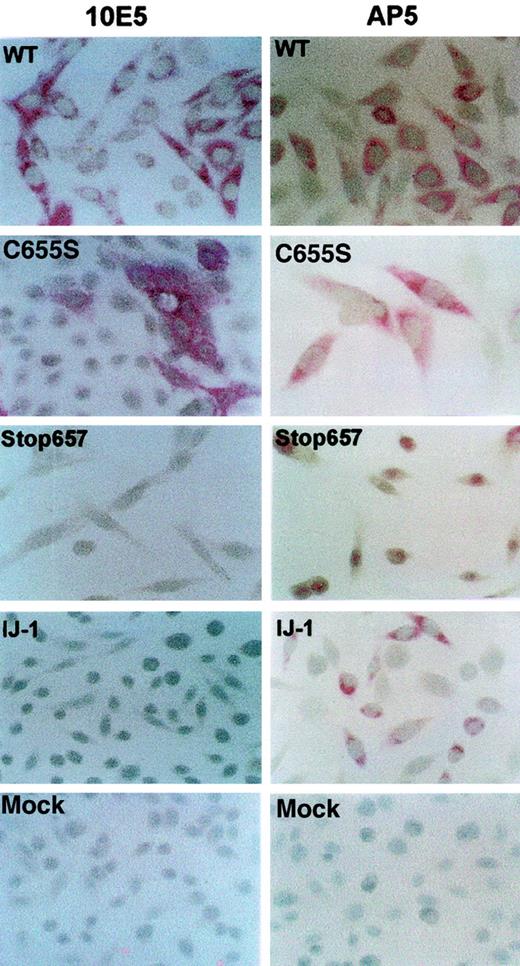
Because GPIIb/IIIa complexes were not detected on the plasma membrane of cells transfected with cDNA containing IJ-1 and Stop657 mutations, we addressed whether GPIIIa was produced in these cells. For this purpose, we used immunohistochemical staining of the cells with monoclonal antibodies directed against GPIIIa (AP5) and GPIIb/IIIa complex (10E5). The results demonstrated that GPIIIa was present in cells transfected with IJ-1 and Stop657 cDNA and in WT and C655S cDNA. In contrast, GPIIb/IIIa complex was expressed in WT and C655S cells but not in IJ-1 and Stop657 cells (Figure 5).
Detection of GPIIIa and GPIIb/IIIa complexes within transfected cells using immunohistochemical staining.
Transfected cells were stained with monoclonal antibodies AP5 and 10E5 against GPIIIa and GPIIb/IIIa complex, respectively. Antibody binding was determined by alkaline phosphatase–antialkaline phosphatase staining. It can be seen that GPIIIa was detected in cells containing IJ-1, Stop657, C655S, and WT, whereas GPIIb/IIIa was detected in WT and C655S but not in IJ-1 and Stop657. Original magnification, × 200-400..
Detection of GPIIIa and GPIIb/IIIa complexes within transfected cells using immunohistochemical staining.
Transfected cells were stained with monoclonal antibodies AP5 and 10E5 against GPIIIa and GPIIb/IIIa complex, respectively. Antibody binding was determined by alkaline phosphatase–antialkaline phosphatase staining. It can be seen that GPIIIa was detected in cells containing IJ-1, Stop657, C655S, and WT, whereas GPIIb/IIIa was detected in WT and C655S but not in IJ-1 and Stop657. Original magnification, × 200-400..
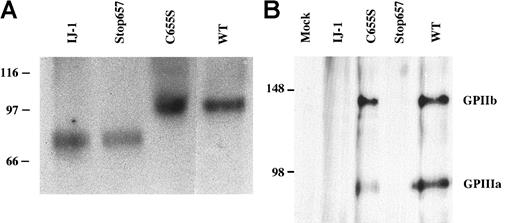
To biochemically confirm the production of GPIIIa and the formation of GPIIb/IIIa complex in the transfected cells, we carried out immunoprecipitation experiments (Figure6). Immunoprecipitation of cell lysates with polyclonal antibodies against GPIIb and GPIIIa, followed by Western blot analysis using the anti GPIIIa monoclonal antibody AP5, revealed that GPIIIa from WT and C655S cells migrated normally at approximately 90 kd under nonreducing conditions. This observation indicates that the absence of Cys406-Cys655 does not change the migration of GPIIIa under nonreducing conditions. GPIIIa from cells containing IJ-1 and Stop657 migrated more rapidly, consistent with the presence of truncated forms of GPIIIa (Figure 6A). Given that previous studies showed that truncated forms of GPIIb and GPIIIa still formed soluble complexes and that these complexes as well as truncated GPIIIa were secreted by cells,16 17 we examined the culture medium of IJ-1 and Stop657 cells for GPIIIa. Neither by immunoprecipitation and Western blotting nor by metabolic labeling of the cells and immunoprecipitation with AP5 antibody could we detect any secreted form of GPIIIa in the medium (data not shown). None of the GPIIIa forms examined exhibited decreased stability in pulse-chase experiments (data not shown). Collectively, these results suggest that IJ-1 GPIIIa and Stop657 GPIIIa were produced in the transfected cells but were not secreted or surface expressed.
Detection of GPIIIa and GPIIb/IIIa complex in lysates of transfected cells.
(A) Transfected CHO cell lysates were immunoprecipitated by using polyclonal antibodies against GPIIb and GPIIIa and then detected by immunoblotting using AP5 antibody against GPIIIa. GPIIIa of WT and C655S-containing cells migrated normally at approximately 90 kd, whereas GPIIIa of IJ-1 and Stop657 cells migrated more rapidly, consistent with a truncated protein. (B) Transfected BHK cell lysates were immunoprecipitated using 10E5 monoclonal antibody against GPIIb/GPIIIa complex and detected by immunoblotting using polyclonal antibodies against GPIIb and GPIIIa. GPIIb and GPIIIa were detected in WT and C655S cells, indicating the presence of GPIIb/IIIa complex in these cells, but not in IJ-1, Stop657, and mock cells. Numbers on the left represent molecular weight standards.
Detection of GPIIIa and GPIIb/IIIa complex in lysates of transfected cells.
(A) Transfected CHO cell lysates were immunoprecipitated by using polyclonal antibodies against GPIIb and GPIIIa and then detected by immunoblotting using AP5 antibody against GPIIIa. GPIIIa of WT and C655S-containing cells migrated normally at approximately 90 kd, whereas GPIIIa of IJ-1 and Stop657 cells migrated more rapidly, consistent with a truncated protein. (B) Transfected BHK cell lysates were immunoprecipitated using 10E5 monoclonal antibody against GPIIb/GPIIIa complex and detected by immunoblotting using polyclonal antibodies against GPIIb and GPIIIa. GPIIb and GPIIIa were detected in WT and C655S cells, indicating the presence of GPIIb/IIIa complex in these cells, but not in IJ-1, Stop657, and mock cells. Numbers on the left represent molecular weight standards.
Immunoprecipitation of cell lysates with 10E5 monoclonal antibody against GPIIb/IIIa followed by Western blot analysis using the anti GPIIb and GPIIIa polyclonal antibodies showed a 140-kd band consistent with WT GPIIb and a 90-kd band consistent with WT GPIIIa. These bands were absent in IJ-1, Stop657, and mock cells (Figure 6B).
Cellular localization of IJ-1 and Stop657 truncated GPIIIa
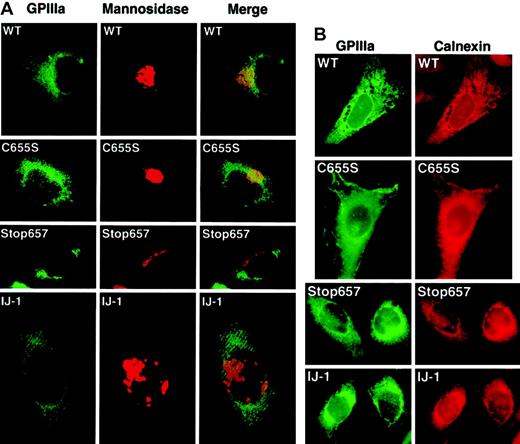
To determine the cellular localization of the mutant GPIIIa (IJ-1) and GPIIIa (Stop657), immunofluorescence staining was performed using the AP5 monoclonal antibody against GPIIIa and rabbit polyclonal antibodies against calnexin, an ER marker, or mannosidase II, a Golgi marker. Secondary antibodies were antimouse FITC conjugate and antirabbit rhodamine conjugate. Under a fluorescence microscope, AP5-binding sites were depicted in green, and the ER and Golgi were depicted in red. In all transfected cells, GPIIIa was distributed evenly in the cell, whereas Golgi mannosidase II was aggregated in clumps (Figure 7A). This suggested that the main fraction of GPIIIa was not accumulated in the Golgi apparatus. Merging of the AP5 and mannosidase staining images demonstrated some dotted yellow images in WT and C655S, which might have represented a small amount of GPIIIa processed in the Golgi apparatus. No such yellow image was observed in cells expressing GPIIIa (IJ-1) and GPIIIa Stop657, suggesting that truncated GPIIIa did not reach the Golgi. The distribution of GPIIIa in normal and mutated transfectants was similar to the distribution of calnexin (Figure 7B). This suggests that in IJ-1 and Stop657 cells, GPIIIa was retained in the cells and did accumulate in the ER. Because we already knew that GPIIb/IIIa complex was expressed on the cell membrane of WT and C655S cells, most of the observed scattered staining in these cells probably represented the GPIIIa on the cell membrane with perhaps some representing GPIIIa in the ER.
Cellular localization of GPIIIa by immunofluorescence staining.
Transfected cells were stained simultaneously with AP5, a monoclonal antibody against GPIIIa, and rabbit polyclonal antibodies against mannosidase II (a Golgi marker) or calnexin (an ER marker). (A) Staining with AP5 (green) and anti–mannosidase II (red) shows diffuse GPIIIa in all transfected cells, with no visible aggregation of GPIIIa in the Golgi. The merge, however, does detect small amounts of GPIIIa (yellow color) only in WT and C655S. Original magnification, × 1000. (B) Staining with AP5 (green) and anti-calnexin (red) shows a similar distribution of GPIIIa and ER marker in all types of transformed cells. The substantial AP5 staining in WT and C655S cells probably represents GPIIIa on the cell surface. Original magnification, × 1000.
Cellular localization of GPIIIa by immunofluorescence staining.
Transfected cells were stained simultaneously with AP5, a monoclonal antibody against GPIIIa, and rabbit polyclonal antibodies against mannosidase II (a Golgi marker) or calnexin (an ER marker). (A) Staining with AP5 (green) and anti–mannosidase II (red) shows diffuse GPIIIa in all transfected cells, with no visible aggregation of GPIIIa in the Golgi. The merge, however, does detect small amounts of GPIIIa (yellow color) only in WT and C655S. Original magnification, × 1000. (B) Staining with AP5 (green) and anti-calnexin (red) shows a similar distribution of GPIIIa and ER marker in all types of transformed cells. The substantial AP5 staining in WT and C655S cells probably represents GPIIIa on the cell surface. Original magnification, × 1000.
Discussion
The 11-bp deletion mutation in exon 13 of the GPIIIa gene, which is the predominant mutation in patients with GT of Iraqi-Jewish origin (IJ-1), predicts a frameshift resulting in the removal of C655 and leading to the absence of the long-range disulfide bond C406-C655. The importance of this long-range disulfide bond in GPIIb/IIIa complex formation, surface expression, and function was addressed in the present study by cotransfection of CHO or BHK cells with a GPIIIa cDNA construct that predicts a C655S change and normal GPIIb cDNA. It was shown that the cells produced GPIIIa, which complexed with GPIIb and hamster αv (Figure 2), and that the surface-expressed GPIIb/IIIa complexes mediated normal binding of the cells to immobilized fibrinogen (Figure 4). These results confirm and extend the observations of Wang et al,9 who showed that the C406-C655 disulfide bond was unnecessary for GPIIb/IIIa complex formation and function. Interestingly, the second long-range disulfide bond C5-C435 was also found to be unnecessary for GPIIb/IIIa complex formation and surface expression.18 Collectively, these data strongly suggest that the loss of C655 in the IJ-1 mutation is not the mechanism for the phenotypic expression of GT in these patients.
In this study, we demonstrated that the IJ-1 mutation produced a truncated protein that is probably retained in the ER. Fluorescence-activated cell sorting analysis disclosed that BHK cells transfected with IJ-1 GPIIIa, and normal GPIIb cDNA constructs did not express GPIIIa or GPIIb/IIIa complexes on their surfaces, and, as a result, these cells did not bind to immobilized fibrinogen. These data were consistent with our previous data, which failed to show any binding of labeled monoclonal antibodies against GPIIb/IIIa complex 10E5 to the platelet membrane of patients with GT bearing the IJ-1 mutation.19 Immunohistochemical staining, immunoprecipitation, and pulse-chase experiments showed that GPIIIa was produced in these cells but appeared truncated and stable. We also showed that, unlike cells transfected with normal GPIIb and GPIIIa cDNAs, cells bearing IJ-1 GPIIIa and normal GPIIb did not contain GPIIb/IIIa complexes but did contain GPIIIa that was localized in the ER (Figures 5-7).
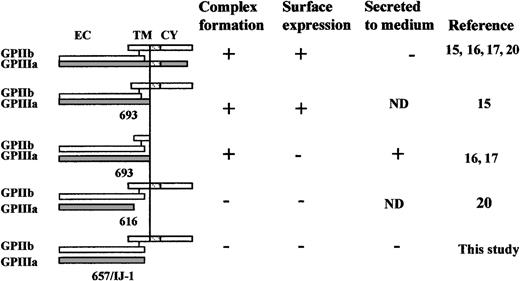
All results obtained in IJ-1–bearing cells were reproduced in cells bearing a Stop657 mutation, which predicted the formation of a similarly truncated GPIIIa but with preservation of the C406-C655 disulfide bond. These observations further confirm that the lack of C406-C655 disulfide bond in GPIIIa is not the mechanism responsible for the defect in the mutation IJ-1 and that the region following amino acid 656 is essential for GPIIb/IIIa complex formation and its subcellular transport from the ER (Figures 5-7). Previous studies15 and the data presented in Figure 3 demonstrated that cells transfected with normal GPIIb cDNA and Δ693-762 GPIIIa cDNA (lacking the transmembrane and cytoplasmic domains of GPIIIa but maintaining the extracellular domain) did form GPIIb/IIIa complexes that were surface expressed, though with a reduced efficiency. Moreover, cells transfected with cDNA constructs that excluded the transmembrane and cytoplasmic domains of both GPIIIa and GPIIb still produced GPIIb/IIIa complexes, which were secreted from the cells but were not surface expressed.16,17 Another study showed that cells transfected with normal GPIIb cDNA and Δ616-762 GPIIIa cDNA with a predictable exclusion of the transmembrane, the intracellular domains, and a segment of the extracellular domain did not form GPIIb/IIIa complexes.20 Our data demonstrate that the lack of an even smaller segment of the extracellular domain (Δ657-762) results in the lack of intracellular GPIIb/IIIa complex formation. The effects of these truncations on GPIIb/IIIa complex formation, surface expression, or secretion are depicted in Figure8. It can be seen that full-length GPIIIa and GPIIb form a surface-expressed complex. A truncated form of GPIIIa that lacks the cytoplasmic and transmembrane domains but preserves the whole extracellular domain forms a surface-expressed complex with WT GPIIb and forms a soluble complex when the GPIIb is similarly truncated. Truncated forms of GPIIIa that lack parts of the extracellular domain (ie, Stop616 and Stop657) fail to form a complex with GPIIb. Taken together, the data strongly suggest that the region between amino acids 657 and 692 is essential for intracellular GPIIb/IIIa complex formation and transport from the ER to the Golgi.
Schematic representation of the effects of various truncations in GPIIIa and GPIIb on GPIIb/IIIa complex formation, surface expression, and secretion.
CY, cytoplasmic domain; EC, extracellular domain; ND, not determined; TM, transmembrane; WT, wild-type.
Schematic representation of the effects of various truncations in GPIIIa and GPIIb on GPIIb/IIIa complex formation, surface expression, and secretion.
CY, cytoplasmic domain; EC, extracellular domain; ND, not determined; TM, transmembrane; WT, wild-type.
We thank Ester Rosenthal for expert technical assistance in flow cytometric analysis, Miriam Biniaminov for help with immunohistochemical staining, and Deborah L. French for her critique and suggestions.
Supported by the Israeli Science Foundation, administered by Israel Academy of Sciences and Humanity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Uri Seligsohn, Institute of Thrombosis and Hemostasis, Sheba Medical Center, Tel-Hashomer, Israel 52621; e-mail:zeligson@post.tau.ac.il.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal