Cancer and Leukemia Group B 19808 (CALGB 19808) is the only randomized trial of a second-generation P-glycoprotein (Pgp) modulator in untreated patients with acute myeloid leukemia (AML) younger than age 60 years. We randomly assigned 302 patients to receive induction chemotherapy regimens consisting of cytosine arabinoside (Ara-C; A), daunorubicin (D), and etoposide (E), without (ADE) or with (ADEP) PSC-833 (P). The incidence of complete remission was 75% with both regimens. Reversible grade 3 and 4 liver and mucosal toxicities were significantly more common with ADEP. Therapy-related mortality was 7% and did not differ by induction arm. Excess cardiotoxicity was not seen with high doses of D in ADE. The median disease-free survival was 1.34 years in the ADE arm and 1.09 years in the ADEP arm (P = .74, log-rank test); the median overall survival was 1.86 years in the ADE arm and 1.69 years in the ADEP arm (P = .82). There was no evidence of a treatment difference within any identifiable patient subgroup. Inhibition of Pgp-mediated drug efflux by PSC-833 did not improve clinical outcomes in younger patients with untreated AML. This trial was registered at www.clinicaltrials.gov as #NCT00006363.
Introduction
Preclinical and correlative science data have consistently underlined the importance of drug resistance associated with p-glycoprotein (encoded by MDR1; Pgp) as a major mechanism by which malignant cells evade cytotoxicity mediated by antineoplastic agents.1,2 Despite the biologic rationale, few clinical benefits have emerged to support the use of Pgp inhibitors in the treatment of acute myeloid leukemia (AML) or other malignant diseases.2 Studies that used first-generation Pgp inhibitors showed a response and survival advantage for the use of cyclosporine A in relapsed and refractory AML,3 whereas quinine increased the incidence of complete remission (CR) among a subset of patients with untreated AML and in vivo drug efflux.4 Clinical trials of PSC-833 (valspodar; Novartis), a non–immunosuppressive cyclosporine analog that is a substantially more potent inhibitor of Pgp,5 have been disappointing in older patients with AML. Phase 3 trials comparing induction chemotherapy with or without PSC-833 in older patients with relapsed/refractory6 and chemotherapy-naive7,8 AML have been negative, despite significant correlations between outcomes and Pgp expression and function.8,9
This Cancer and Leukemia Group B (CALGB) phase 3 trial was designed to test the hypothesis that younger patients would benefit more than older patients from Pgp blockade despite having a lower incidence of Pgp expression. Among younger patients with AML, Pgp expression and inhibition of Pgp-mediated drug efflux do, although expression of multidrug resistance-associated and lung resistance proteins (MRP-1 and LRP) do not, correlate with clinical outcomes.1 We postulated that AML blasts in younger patients would be less likely to be associated with other adverse prognostic features seen in older patients and independently associated with treatment failure, particularly unfavorable cytogenetics.10 Younger patients have clonogenic AML progenitor cells marked by low Pgp expression but high levels of Pgp-mediated drug efflux.11 Furthermore, we expected that younger patients would tolerate intensive chemotherapy regimens in combination with Pgp-reversal agents significantly better than older patients, thereby allowing these hypotheses to be adequately tested in a randomized trial.
In addition to modulating Pgp-mediated multidrug resistance (MDR) present in pretreatment cells, incorporation of modulators into induction regimens may also prevent emergence of MDR at a subsequent time point. In vitro exposure of Pgp-negative cells to doxorubicin induces Pgp-mediated resistance, but the rate of mutation to the Pgp-expressing resistant phenotype is 10-fold lower in cells exposed to doxorubicin and PSC-833 together.12 In addition, Pgp expression and function increase in both Pgp-negative and Pgp-positive AML blasts after short in vitro exposure to anthracyclines or cytosine arabinoside (Ara-C), but not after exposure to these agents in the presence of PSC-833.13 These in vitro data provide a compelling rationale for focusing clinical trials of Pgp modulation in settings in which the incidence of preexisting drug resistance is low, such as de novo AML patients younger than 60 years of age.
Preliminary data supporting this hypothesis were the improvements in disease-free survival (DFS) and overall survival (OS) in patients treated with chemotherapy plus PSC-833 in our previous study, CALGB 9621, among patients younger than 45 years.14 That study was a dose-escalation study in which we developed the 2 remission induction regimens, 1 with and 1 without PSC-833 (P), which were compared in the phase 3 trial we are reporting. A fixed dose of cytarabine (A) given by continuous intravenous (CIV) infusion was combined with escalating doses of daunorubicin and etoposide, with (ADEP) and without (ADE) PSC-833.
CALGB 19808 was designed to compare ADE and ADEP with both regimens given at the highest clinically feasible doses. The daunorubicin dose in ADE was 1.5 to 2 times that generally used in induction therapy of AML, whereas the etoposide dose was comparable to that reported in other trials for untreated AML.15 Induction therapy outcomes are described here for the 302 patients who were randomly assigned between ADE and ADEP before the discontinuation of the development of PSC-833 by its sponsor (Novartis).
Methods
Previously untreated patients with AML younger than 60 years were eligible. The diagnosis of AML used World Health Organization criteria and required the presence of at least 20% myeloblasts in bone marrow. Patients with therapy-related AML or AML arising from a prior myeloproliferative disorder were ineligible, as were patients with acute promyelocytic leukemia. The presence of a myelodysplastic syndrome (MDS) excluded patients only if a diagnosis of MDS had been established by bone marrow examination more than 3 months before diagnosing AML. Prior use of hematopoietic growth factors, hydroxyurea for leukocytosis, or a single dose of emergency cranial radiotherapy for central nervous system leukostasis was permitted. Bone marrow slides were centrally reviewed. Beyond these explicit eligibility criteria, physicians were to exercise their best judgment about the suitability of patients for intensive induction chemotherapy, taking into account adequate organ function, availability of appropriate facilities and personnel, as well as the potential need for later myeloablative therapy for those patients with defined cytogenetic risk after achieving CR. Because this was a randomized trial to evaluate the cyclosporine A analog PSC-833 during induction therapy, use of medications that could interact with cyclosporines was restricted before and after randomization.
Induction therapy
ADE consisted of Ara-C 100 mg/m2 given by CIV infusion daily on days 1 through 7, and daunorubicin 90 mg/m2 by short IV bolus and etoposide 100 mg/m2 intravenously over 2 hours, given on days 1, 2, and 3. ADEP included a loading dose of PSC-833 of 2.8 mg/kg intravenously given over 2 hours on day 1, followed by 10 mg/kg CIV infusion for 72 hours. After the completion of the loading dose, Ara-C 100 mg/m2 was given by CIV infusion daily on days 1 through 7, and daunorubicin 40 mg/m2 by short IV bolus and etoposide 40 mg/m2 intravenously over 2 hours were each given on days 1, 2, and 3. A bone marrow examination was required on day 14; presence of persistent leukemia, defined as at least 5% blasts in the biopsy with at least 20% cellularity, called for a second course of induction therapy. Uncertainty about the presence of residual leukemia, particularly in the setting of marrow cellularity less than 20%, called for repeat bone marrow examinations, as clinically indicated, with the stipulation that a decision about the need for further therapy could not be delayed beyond day 42. Second inductions of ADE used identical doses as those used in the first induction except that Ara-C was given over 5 days, and daunorubicin and etoposide were given for 2 days each. A second ADEP induction also used identical doses of the cytotoxic agents as those given in the first ADEP induction, with the same 5 + 2 +2 schedule. The PSC-833 loading dose and schedule was identical to that given in the first ADEP induction, except that the daily dose was infused between hours 2 and 50.
Experimental design and statistical methods
The primary objective of the induction part of the trial was to determine whether the use of the Pgp-modulating agent PSC-833 in the ADEP regimen improved DFS and OS compared with patients treated with ADE alone. Patients were centrally registered and randomly assigned at the CALGB Statistical Center between ADE and ADEP in a 1:1 ratio with no stratification. The intent-to-treat (ITT) principle, in which all patients are included in the analysis on the treatment arm to which they were randomly assigned, was followed for all analyses addressing the primary study objectives. However, there were also some exploratory secondary analyses that were on subgroups of patients. Follow-up of surviving patients was current through January 2010.
The null hypothesis tested was that patients in both arms have identical distributions of OS and DFS times. On the basis of prior CALGB trials in this patient population, if the null hypothesis is true, the estimated median OS was expected to be approximately 21 months and the median DFS was expected to be approximately 12 months. A proportional hazards model was assumed. To observe a hazard ratio (ADE to ADEP) of 1.4 for OS, 374 deaths would need to occur among the originally planned 600 randomly assigned patients (2-sided log-rank test; α = 0.05; power = 0.90). Unfortunately, PSC-833 was withdrawn from clinical development in August 2003 after the random assignment of the initial 302 patients. The trial remained open with all subsequent patients receiving the ADE induction regimen to complete the second primary objective of the phase 3 study, assessing the effect of recombinant human interleukin-2 (rIL-2) therapy on DFS and OS. These latter patients are not included in the current analysis because there was no randomization between induction regimens for these patients. In the analysis reported here, there were 193 deaths, only 52% of the required number by the original design. This reduction lowered the power to detect a hazard ratio of 1.4 to approximately 0.64, rather than the originally planned 0.90.
Survival times were calculated from date of random assignment to date of death from any cause. DFS was calculated from date of CR to relapse or to death from any cause if relapse had not occurred. Patients alive at last follow-up were right-censored for the survival analysis, and patients alive without relapse were right-censored for DFS analysis. The distributions of overall survival and DFS were estimated with the Kaplan-Meier method for right-censored data. Estimates of hazard ratios and related confidence intervals were based on fitting proportional hazards models.
Criteria for response and toxicity
National Cancer Institute Workshop criteria (1990) were used to define CR.16 Revised criteria were published when the study was nearing completion,17 consequently flow cytometric and/or cytogenetic data were not used for CR assessments. Patients who relapsed within 4 weeks of achieving CR were considered treatment failures. Relapse was defined by the presence of more than 5% marrow blasts in a patient who previously achieved CR, the presence of blasts with Auer rods in marrow or blood, sustained presence of blasts in the blood, or the emergence of extramedullary leukemia. The National Cancer Institute Common Toxicity Criteria were used to grade toxicity. Induction death was defined as any death occurring within 30 days of completing a first or second induction course.
Postremission therapy
Postremission therapy depended on the cytogenetic risk status, determined for all patients at diagnosis. As part of CALGB study 8461, cytogenetic and molecular genetic studies were performed on AML samples obtained at diagnosis to identify patients with core binding factor (CBF) leukemia, ie, those patients with t(8;21)(q22q22), inv(16)(p13q22), or t(16;16)(p13q22). Reverse transcription–polymerase chain reaction assays were performed centrally to confirm the presence of CBF leukemia-associated transcripts.18 Patients with CBF AML were then defined as favorable risk after review of all available cytogenetic and reverse transcription–polymerase chain reaction data and, when necessary, ancillary data such as fluorescence in situ hybridization and morphology. Presence of additional cytogenetic abnormalities, including complex karyotypes, did not alter a favorable risk designation in patients otherwise confirmed to have CBF AML. For remission consolidation chemotherapy, patients with CBF received 3 courses of high-dose cytarabine (HiDAC).19
All other patients were designated as having non-CBF AML. These patients had intermediate or unfavorable risk karyotypes according to the European LeukemiaNet (ELN) criteria.20 Unfortunately, insufficient data about molecular markers were available to fully categorize cytogenetic subsets. Thus, in this study, the 4 groupings in the ELN criteria are defined as follows: Favorable consisted solely of patients with CBF, intermediate-I of patients with normal cytogenetics, and intermediate-II and adverse with karyotypes as described in the criteria.
The patients without CBF in CR were assigned to receive a 2-step autologous stem cell transplantation (ASCT) protocol consisting of HiDAC and high-dose infusional etoposide given for in vivo purging and peripheral blood stem cell mobilization followed by an etoposide and busulfan conditioning regimen. This therapy has been used in patients with AML in first CR21 as well as in second CR in CALGB 9620.22 If unable to undergo transplantation, a novel 3-course consolidation sequence was given, consisting of the HiDAC/etoposide sequence used in the 2-step transplantation regimen, followed by 2 courses of HiDAC, as previously described.23 The intent was to have all patients with non-CBF AML undergo protocol-specified autologous transplantation. At the discretion of treating physicians, a small number of patients, generally younger patients with highly adverse karyotypes, were removed from protocol therapy to undergo allogeneic transplantation (see Figure 1 Consolidated Standards of Reporting Trials [CONSORT] flow diagram).
After consolidation, continuing patients were randomly assigned (stratified by induction regimen and CBF status) either to observation or to a 90-day immunotherapy course with subcutaneous rIL-2, using a regimen similar to that used for patients 60 years or older in CALGB studies 9420 and 9720 and previously described.24 The CONSORT flow diagram (Figure 1) shows the progression of patients in CR to the immunotherapy randomization, with substantial numbers of patients not reaching that point for multiple reasons, including relapse and refusal. The preliminary findings of the immunotherapy portion of this trial have been reported.25
Quality control, quality assurance, and auditing
Institutional review board approval was required for every participating institution. Written, informed consent was secured from each treated patient in accordance with the Declaration of Helsinki. Members of the CALGB Audit Committee visit all participating institutions at least once every 3 years to verify compliance with federal regulations and protocol requirements for CALGB studies, including those pertaining to eligibility, treatment, response, and follow-up. Patient registration, data collection, and all statistical analyses were carried out by the CALGB Statistical Center. Data quality was ensured by careful review of the data by Statistical Center staff and by the study chairperson following standard CALGB policies. The medical records of 168 patients (56% of the 302 patients) were selected and audited by members of the CALGB Audit Committee; records from each participating institution were reviewed.
Results
Patient characteristics
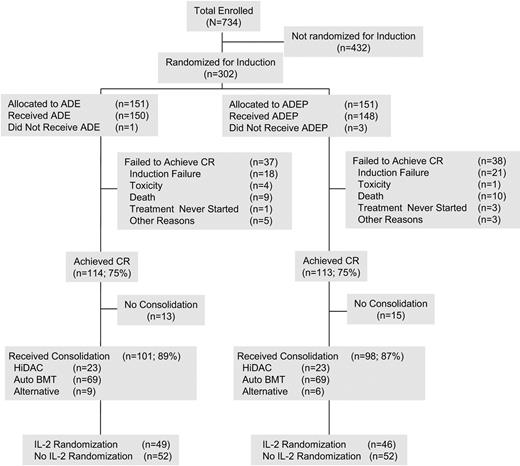
Between January 2001 and August 2003, a total of 302 patients were randomly assigned before the unavailability of PSC-833. A CONSORT flowchart showing the distribution of all patients is given in Figure 1. This figure also covers post-CR therapy. By the ITT principle, all 302 patients are included in the primary analyses. Patient characteristics are described in Table 1. There were no major differences in these characteristics between randomized treatment arms.
CONSORT flow sheet. The first 302 patients enrolled were randomly assigned between ADE and ADEP. After the unavailability of PSC-833, all subsequent patients received induction chemotherapy with ADE. All 302 patients randomly assigned are included in the analysis, as required by the ITT principle. The flow sheet outlines the therapies received by patients in CR. BMT indicates bone marrow transplantation.
CONSORT flow sheet. The first 302 patients enrolled were randomly assigned between ADE and ADEP. After the unavailability of PSC-833, all subsequent patients received induction chemotherapy with ADE. All 302 patients randomly assigned are included in the analysis, as required by the ITT principle. The flow sheet outlines the therapies received by patients in CR. BMT indicates bone marrow transplantation.
Demographic and clinical characteristics
| Characteristic . | ADE (n = 151) . | ADEP (n = 151) . | Total (n = 302) . |
|---|---|---|---|
| Age, y | |||
| Median | 46 | 46 | 46 |
| Range | 19-59 | 17-59 | 17-59 |
| Sex | |||
| Male, n (%) | 83 (55) | 77 (51) | 160 (53) |
| Female, n (%) | 68 (45) | 74 (49) | 142 (47) |
| Race or ethnicity | |||
| White, n (%) | 123 (81) | 128 (85) | 251 (83) |
| Hispanic, n (%) | 9 (6) | 5 (3) | 14 (5) |
| African American, n (%) | 10 (7) | 9 (6) | 19 (6) |
| Oriental, n (%) | 3 (2) | 4 (3) | 7 (2) |
| Unknown/Other, n (%) | 6 (4) | 5 (3) | 11 (4) |
| ELN categories, n (%) | |||
| Favorable, n (%) | 22 (15) | 27 (18) | 49 (16) |
| Intermediate-I, n (%) | 62 (41) | 58 (38) | 120 (40) |
| Intermediate-II, n (%) | 17 (11) | 19 (13) | 36 (12) |
| Adverse, n (%) | 26 (17) | 18 (12) | 44 (15) |
| Unknown, n (%) | 24 (16) | 29 (19) | 53 (18) |
| FAB classification | |||
| M0, n (%) | 8 (5) | 7 (5) | 15 (5) |
| M1, n (%) | 31 (21) | 35 (23) | 66 (22) |
| M2, n (%) | 55 (36) | 51 (34) | 106 (35) |
| M4, n (%) | 27 (18) | 37 (25) | 64 (21) |
| M5, n (%) | 12 (8) | 11 (7) | 23 (8) |
| M6, n (%) | 10 (7) | 1 (<1) | 11 (4) |
| M7, n (%) | 1 (<1) | 4 (3) | 5 (2) |
| RAEB_T, n (%) | 3 (2) | 4 (3) | |
| Other (Ineligible), n (%) | 4 (3) | 1 (<1) | 5 (2) |
| LDH,U/L | |||
| Minimum | 98 | 91 | 91 |
| 25th percentile | 195 | 241 | 221 |
| Median | 336 | 450.5 | 389 |
| 75th percentile | 763 | 898 | 801 |
| Maximum | 2517 | 14 784 | 14 784 |
| WBC count, ×103/μL | |||
| Minimum | 0.7 | 0.2 | 0.2 |
| 25th percentile | 2.0 | 3.6 | 2.3 |
| Median | 4.5 | 16.0 | 10.4 |
| 75th percentile | 38.3 | 50.2 | 47.2 |
| Maximum | 345.0 | 302.3 | 345.0 |
| Characteristic . | ADE (n = 151) . | ADEP (n = 151) . | Total (n = 302) . |
|---|---|---|---|
| Age, y | |||
| Median | 46 | 46 | 46 |
| Range | 19-59 | 17-59 | 17-59 |
| Sex | |||
| Male, n (%) | 83 (55) | 77 (51) | 160 (53) |
| Female, n (%) | 68 (45) | 74 (49) | 142 (47) |
| Race or ethnicity | |||
| White, n (%) | 123 (81) | 128 (85) | 251 (83) |
| Hispanic, n (%) | 9 (6) | 5 (3) | 14 (5) |
| African American, n (%) | 10 (7) | 9 (6) | 19 (6) |
| Oriental, n (%) | 3 (2) | 4 (3) | 7 (2) |
| Unknown/Other, n (%) | 6 (4) | 5 (3) | 11 (4) |
| ELN categories, n (%) | |||
| Favorable, n (%) | 22 (15) | 27 (18) | 49 (16) |
| Intermediate-I, n (%) | 62 (41) | 58 (38) | 120 (40) |
| Intermediate-II, n (%) | 17 (11) | 19 (13) | 36 (12) |
| Adverse, n (%) | 26 (17) | 18 (12) | 44 (15) |
| Unknown, n (%) | 24 (16) | 29 (19) | 53 (18) |
| FAB classification | |||
| M0, n (%) | 8 (5) | 7 (5) | 15 (5) |
| M1, n (%) | 31 (21) | 35 (23) | 66 (22) |
| M2, n (%) | 55 (36) | 51 (34) | 106 (35) |
| M4, n (%) | 27 (18) | 37 (25) | 64 (21) |
| M5, n (%) | 12 (8) | 11 (7) | 23 (8) |
| M6, n (%) | 10 (7) | 1 (<1) | 11 (4) |
| M7, n (%) | 1 (<1) | 4 (3) | 5 (2) |
| RAEB_T, n (%) | 3 (2) | 4 (3) | |
| Other (Ineligible), n (%) | 4 (3) | 1 (<1) | 5 (2) |
| LDH,U/L | |||
| Minimum | 98 | 91 | 91 |
| 25th percentile | 195 | 241 | 221 |
| Median | 336 | 450.5 | 389 |
| 75th percentile | 763 | 898 | 801 |
| Maximum | 2517 | 14 784 | 14 784 |
| WBC count, ×103/μL | |||
| Minimum | 0.7 | 0.2 | 0.2 |
| 25th percentile | 2.0 | 3.6 | 2.3 |
| Median | 4.5 | 16.0 | 10.4 |
| 75th percentile | 38.3 | 50.2 | 47.2 |
| Maximum | 345.0 | 302.3 | 345.0 |
ADE indicates cytosine arabinoside, daunorubicin, and etoposide; ADEP, cytosine arabinoside, daunorubicin, etoposide, and PSC-833; ELN, European LeukemiaNet; FAB, French-American-British; RAEB_T, refractory anemia with excess blasts in transformation; LDH, lactate dehydrogenase; and WBC, white blood cell.
The median age was 46 years (range, 17-59 years); 160 patients (53%) were male; and 251 patients (83%) were white. There were 119 patients (39%) with normal cytogenetics and 49 patients (16%) with CBF AML. Of the 302 patients randomly assigned, 249 (82%) had karyotypes that were centrally reviewed and found to be adequate. The remaining patients are categorized as “unknown.” The 249 patients with adequate centrally reviewed cytogenetics were categorized by the ELN20 classification scheme, modified as described above, with the following results: 49 (20%) favorable; 120 (48%) intermediate-I, 36 (14%) intermediate-II; and 44 (18%) adverse. The French-American-British classifications given in Table 1 are based on the central review of the available pathology; otherwise, they are based on the institutional pathology as reported on the on study form. Central review was available for 226 of the 302 cases (75%). Five patients (2%) were identified as ineligible after randomization: 2 patients with chronic myeloid leukemia in blast phase and 1 patient each with acute promyelocytic leukemia, MDS, or AML after a history of myelofibrosis; these patients were included in the ITT analyses. There were no substantive differences in the conclusions when these ineligible patients were excluded from the analyses.
Outcomes
Table 2 gives a summary of CR, DFS, and OS in the 2 treatment groups. Although a comparison of CR rates was not an objective of the study, the CR rate was 75% on each arm, 114 of 151 patients on ADE and 113 of 151 on ADEP. CR was achieved after a single course of induction therapy in 90% of the responding patients on each arm. Among patients with CBF leukemia, 46 of 49 (94%) entered CR: 21 of 21 (100%) on ADE; 25 of 28 (89%) on ADEP. CR occurred after a single course in 41 of the 46 CRs in the patients with CBF: 20 of 21 (95%) on ADE; 21 of 25 (84%) on ADEP. Nearly identical proportions of patients in both arms with persistent leukemia after 1 course received a second induction: 27 of 151 (ADE) and 26 of 151 (ADEP). Among patients surviving induction, primary resistance was observed at comparable rates in the 2 regimens: 22 of 151 (15%) with ADE and 21 of 151 (14%) with ADEP. The time from achieving CR to initiating postremission therapy did not differ by induction arm.
Patient outcomes
| End point . | ADE (n = 151) . | ADEP (n = 151) . | P . |
|---|---|---|---|
| CR, n (%) | 114 (75) | 113 (75) | .999 |
| DFS | .740 | ||
| Median, y | 1.34 | 1.09 | |
| Percentage of DFS at 5 y (95% CI) | 36 (28-45) | 36 (27-45) | |
| OS | .824 | ||
| Median, y | 1.86 | 1.69 | |
| Percentage alive at 5 y (95% CI) | 37 (30-45) | 37 (29-45) |
| End point . | ADE (n = 151) . | ADEP (n = 151) . | P . |
|---|---|---|---|
| CR, n (%) | 114 (75) | 113 (75) | .999 |
| DFS | .740 | ||
| Median, y | 1.34 | 1.09 | |
| Percentage of DFS at 5 y (95% CI) | 36 (28-45) | 36 (27-45) | |
| OS | .824 | ||
| Median, y | 1.86 | 1.69 | |
| Percentage alive at 5 y (95% CI) | 37 (30-45) | 37 (29-45) |
ADE indicates cytosine arabinoside, daunorubicin, and etoposide; ADEP, cytosine arabinoside, daunorubicin, etoposide, and PSC-833; CR, complete remission; DFS, disease-free survival; CI, confidence interval; OS, overall survival.
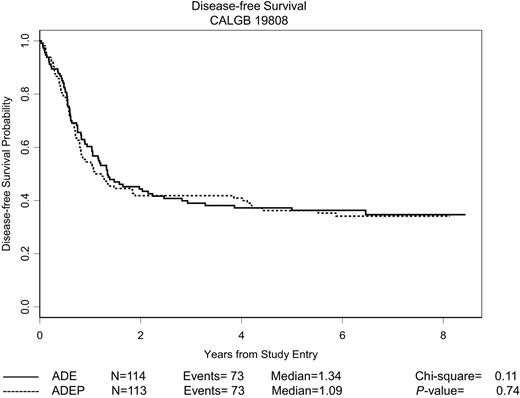
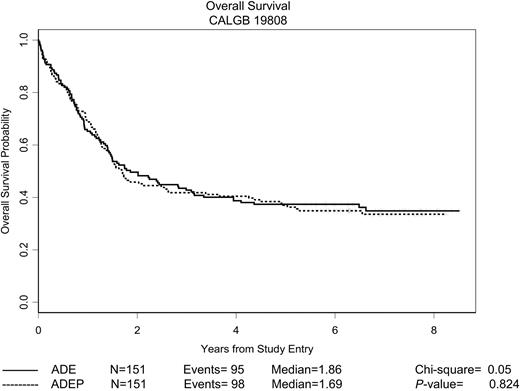
As is also evident from Table 2, there was no difference between the 2 treatment groups with respect to DFS or OS. Kaplan-Meier curves depicting the estimated DFS and OS distributions for all patients randomly assigned are shown in Figures 2 and 3, respectively. The median follow-up of patients still alive is 6.5 years, with a maximum follow-up of 8.5 years. There was no difference between treatment arms with respect to either DFS (P = .74, log-rank test) or OS (P = .82, log-rank test). The median DFS for all responding patients was 1.3 years, and the 5-year DFS was 36% (95% confidence interval [CI], 30%-43%). The median OS for all patients was 1.7 years, and the 5-year OS was 37% (95% CI, 32%-43%).
DFS of randomly assigned patients. The estimated probability of DFS for all 227 patients randomly assigned who achieved a CR is shown according to the assigned treatment regimen.
DFS of randomly assigned patients. The estimated probability of DFS for all 227 patients randomly assigned who achieved a CR is shown according to the assigned treatment regimen.
OS of patients randomly assigned patients. The estimated probability of OS for all 302 patients randomly assigned is shown according to the assigned treatment regimen.
OS of patients randomly assigned patients. The estimated probability of OS for all 302 patients randomly assigned is shown according to the assigned treatment regimen.
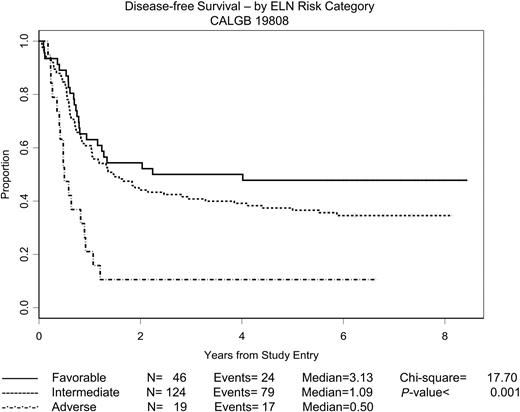
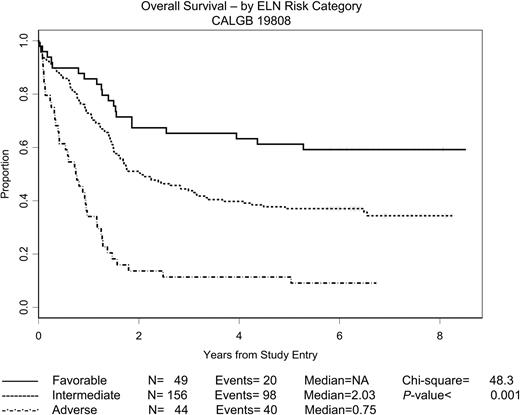
ELN cytogenetic groupings are the only pretreatment characteristics significantly associated with outcome. Estimated DFS and OS distributions according to the ELN risk categories described earlier are depicted in Figures 4 and 5, respectively, with intermediate-I and intermediate-II patients lumped into a single “intermediate” category. The 2 intermediate categories were virtually indistinguishable with respect to both DFS and OS. The OS figure includes only the 249 patients who underwent central cytogenetic review and had satisfactory karyotypes; the DFS figure includes only the 189 patients in this group with a CR. The 53 patients with unknown ELN risk are omitted from the figures, but the DFS and OS distributions for these patients were quite similar to what one would expect if the ELN status was missing at random. As expected, for both DFS and OS, the ELN categories were strong prognostic variables (P < .001 in both cases; 3-sample log-rank test). The estimated median DFS was 3.13 years for patients with favorable risk; 1.09 years for patients with intermediate risk; and 0.50 years for patients with adverse risk. The median OS was undefined for patients with favorable risk (the estimated probability of surviving 5 years is approximately 0.60), 2.03 years for patients with intermediate risk, and 0.75 years for patients with adverse risk.
DFS by ELN risk category. The estimated probability of DFS for the 189 patients randomly assigned who achieved a CR and for whom the ELN risk category is known is shown according to risk category. ELN risk categories have been adapted as described in the text.
DFS by ELN risk category. The estimated probability of DFS for the 189 patients randomly assigned who achieved a CR and for whom the ELN risk category is known is shown according to risk category. ELN risk categories have been adapted as described in the text.
OS by ELN risk category. The estimated probability of OS for the 249 patients randomly assigned for whom the ELN risk category is known is shown according to risk category. ELN risk categories have been adapted as described in the text.
OS by ELN risk category. The estimated probability of OS for the 249 patients randomly assigned for whom the ELN risk category is known is shown according to risk category. ELN risk categories have been adapted as described in the text.
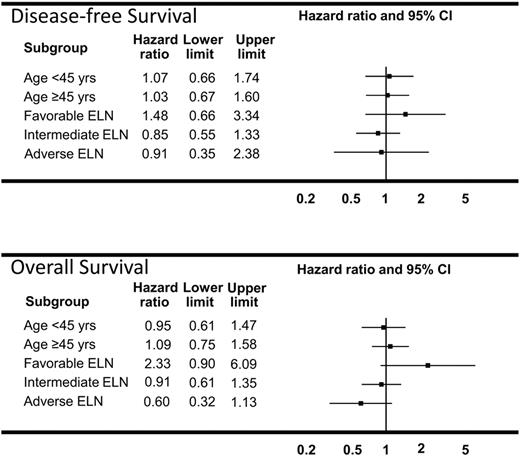
In exploratory regression analyses we also looked for possible “treatment-covariate interactions” (ie, evidence that the treatment effect might differ among certain subgroups of patients, in particular, that an identifiable subgroup of patients might benefit from ADEP). We considered all variables in Table 1, even though only the ELN variable was prognostic by itself. Results for age and ELN category are presented in Forest plot format in Figure 6. These give the estimated hazard ratios in each subgroup with the associated 95% CIs. Because all of the intervals include the value 1, there is no evidence of a treatment covariate or subgroup effect in any of these analyses. The result for OS in the ELN categories is intriguing in that the ADEP group did somewhat better in the adverse ELN group and somewhat worse in the patients with favorable ELN. But this is an exploratory analysis, and, even without accounting for multiplicity, none of the results reached nominal statistical significance. In addition, in 1 case, OS in patients with adverse ELN, the proportional hazards assumption is not supported by the data so the estimated hazard ratio in this case should be interpreted as an overall or “average” ratio.
Forest plots of DFS and OS models in subgroups of patients. Estimated hazard ratios (ADEP vs ADE) and the 95% CIs from a fitted proportional hazards regression model are provided for each category. Estimated hazard ratios less than 1 favor ADEP, whereas those greater than 1 favor ADE.
Forest plots of DFS and OS models in subgroups of patients. Estimated hazard ratios (ADEP vs ADE) and the 95% CIs from a fitted proportional hazards regression model are provided for each category. Estimated hazard ratios less than 1 favor ADEP, whereas those greater than 1 favor ADE.
Postremission therapy
All but 1 of the 46 patients with CBF AML in CR received consolidation chemotherapy with HiDAC on protocol. Of the 180 patients with non-CBF AML achieving CR, 112 (62%) completed intensification treatment as planned; 40 (22%) started but did not complete treatment; and 28 (16%) never started treatment. The distribution of patients receiving the specified postremission therapies was similar between induction treatment arms. Twenty patients, 10 in each arm, received allogeneic transplants in first CR at the discretion of treating physicians. The induction regimens did not differ in the proportion of patients undergoing autologous or allogeneic transplantation, or the alternative intensification regimen.
Patients remaining in CR after completion of all planned consolidation chemotherapy or ASCT, excluding those receiving allografts, were then eligible for random assignment between the 90-day rIL-2 immunotherapy sequence and observation. These outcomes will be reported separately after longer follow-up.
In an exploratory analysis similar to the one described in “Outcomes” above for baseline characteristics, we also analyzed the treatment effects in various subgroups defined by post-CR treatment. In no case was there a significant treatment effect. For example, for the 138 patients who received ASCT as post-CR consolidation therapy, the estimated treatment hazard ratios were 0.99 (95% CI, 0.66-1.49) for DFS and 0.93 (95% CI, 0.60-1.44) for OS. There was no evidence that the outcomes for patients receiving ADEP differed from those for patients receiving ADE depending on the post-CR therapy received.
Treatment-related toxicity
Table 3 gives grade 3, 4, and 5 toxicities observed in more than 10% of treated patients by treatment arm. Toxicity data are available for 298 of the 302 randomized patients (99%). Three patients were never treated on study. Treatment-related mortality, defined as death occurring within 30 days after starting induction chemotherapy, was 7% on both arms, 10 patients on the ADE arm, and 11 patients on the ADE arm. Compared with ADE, ADEP had increased gastrointestinal toxicity, including anorexia, nausea, stomatitis, esophagitis, and dysphagia, as well as generally reversible hyperbilirubinemia. Increased neurotoxicity that could be attributed to PSC-833 in ADEP was not observed.
Nonhematologic toxicity by treatment arm
| . | Grade of adverse event . | Total . | ||
|---|---|---|---|---|
| 3, n (%) . | 4, n (%) . | 5, n (%) . | ||
| Nonhematologic adverse events | ||||
| Fatigue (asthenia, lethargy, malaise) | ||||
| ADE | 12 (8) | 1 (<1) | 0 | 150 |
| ADEP | 14 (9) | 4 (3) | 0 | 148 |
| Rash/desquamation | ||||
| ADE | 15 (10) | 1 (<1) | 0 | 150 |
| ADEP | 19 (13) | 1 (<1) | 0 | 148 |
| Anorexia | ||||
| ADE | 9 (6) | 5 (3) | 0 | 150 |
| ADEP | 17 (11) | 24 (16) | 0 | 148 |
| Diarrhea | ||||
| ADE | 15 (10) | 0 | 1 (<1) | 150 |
| ADEP | 14 (9) | 0 | 0 | 148 |
| Esophagitis | ||||
| ADE | 6 (4) | 6 (4) | 0 | 150 |
| ADEP | 19 (13) | 9 (6) | 0 | 148 |
| Mucositis/stomatitis | ||||
| ADE | 9 (6) | 3 (2) | 0 | 150 |
| ADEP | 20 (14) | 15 (10) | 0 | 148 |
| Nausea | ||||
| ADE | 12 (8) | 0 | 0 | 150 |
| ADEP | 26 (18) | 0 | 0 | 148 |
| Bilirubin | ||||
| ADE | 7 (5) | 2 (1) | 0 | 150 |
| ADEP | 25 (17) | 10 (7) | 0 | 148 |
| Infection with unknown ANC | ||||
| ADE | 9 (6) | 1 (<1) | 0 | 150 |
| ADEP | 3 (2) | 0 | 1 (<1) | 148 |
| Febrile neutropenia | ||||
| ADE | 79 (53) | 2 (1) | 0 | 150 |
| ADEP | 66 (45) | 7 (5) | 0 | 148 |
| Infection (documented) | ||||
| ADE | 63 (42) | 9 (6) | 2 (1) | 150 |
| ADEP | 66 (45) | 8 (5) | 1 (<1) | 148 |
| Hypocalcemia | ||||
| ADE | 15 (10) | 7 (5) | 0 | 150 |
| ADEP | 15 (10) | 3 (2) | 0 | 148 |
| Hypokalemia | ||||
| ADE | 18 (12) | 9 (6) | 0 | 150 |
| ADEP | 19 (13) | 10 (7) | 0 | 148 |
| Hyponatremia | ||||
| ADE | 9 (6) | 0 | 0 | 150 |
| ADEP | 14 (9) | 2 (1) | 0 | 148 |
| Adult respiratory distress syndrome | ||||
| ADE | 0 | 8 (5) | 1 (<1) | 150 |
| ADEP | 0 | 3 (2) | 0 | 148 |
| Dyspnea | ||||
| ADE | 9 (6) | 7 (5) | 0 | 150 |
| ADEP | 9 (6) | 4 (3) | 1 (<1) | 148 |
| Pulmonary-Other | ||||
| ADE | 1 (<1) | 3 (2) | 1 (<1) | 150 |
| ADEP | 2 (1) | 1 (<1) | 0 | 148 |
| Thrombosis/thrombus/embolism | ||||
| ADE | 3 (2) | 0 | 0 | 150 |
| ADEP | 1 (<1) | 0 | 1 (<1) | 148 |
| Maximum nonhematologic adverse events | ||||
| ADE | 98 (65) | 37 (25) | 5 (3) | 150 |
| ADEP | 82 (55) | 59 (40) | 4 (3) | 148 |
| . | Grade of adverse event . | Total . | ||
|---|---|---|---|---|
| 3, n (%) . | 4, n (%) . | 5, n (%) . | ||
| Nonhematologic adverse events | ||||
| Fatigue (asthenia, lethargy, malaise) | ||||
| ADE | 12 (8) | 1 (<1) | 0 | 150 |
| ADEP | 14 (9) | 4 (3) | 0 | 148 |
| Rash/desquamation | ||||
| ADE | 15 (10) | 1 (<1) | 0 | 150 |
| ADEP | 19 (13) | 1 (<1) | 0 | 148 |
| Anorexia | ||||
| ADE | 9 (6) | 5 (3) | 0 | 150 |
| ADEP | 17 (11) | 24 (16) | 0 | 148 |
| Diarrhea | ||||
| ADE | 15 (10) | 0 | 1 (<1) | 150 |
| ADEP | 14 (9) | 0 | 0 | 148 |
| Esophagitis | ||||
| ADE | 6 (4) | 6 (4) | 0 | 150 |
| ADEP | 19 (13) | 9 (6) | 0 | 148 |
| Mucositis/stomatitis | ||||
| ADE | 9 (6) | 3 (2) | 0 | 150 |
| ADEP | 20 (14) | 15 (10) | 0 | 148 |
| Nausea | ||||
| ADE | 12 (8) | 0 | 0 | 150 |
| ADEP | 26 (18) | 0 | 0 | 148 |
| Bilirubin | ||||
| ADE | 7 (5) | 2 (1) | 0 | 150 |
| ADEP | 25 (17) | 10 (7) | 0 | 148 |
| Infection with unknown ANC | ||||
| ADE | 9 (6) | 1 (<1) | 0 | 150 |
| ADEP | 3 (2) | 0 | 1 (<1) | 148 |
| Febrile neutropenia | ||||
| ADE | 79 (53) | 2 (1) | 0 | 150 |
| ADEP | 66 (45) | 7 (5) | 0 | 148 |
| Infection (documented) | ||||
| ADE | 63 (42) | 9 (6) | 2 (1) | 150 |
| ADEP | 66 (45) | 8 (5) | 1 (<1) | 148 |
| Hypocalcemia | ||||
| ADE | 15 (10) | 7 (5) | 0 | 150 |
| ADEP | 15 (10) | 3 (2) | 0 | 148 |
| Hypokalemia | ||||
| ADE | 18 (12) | 9 (6) | 0 | 150 |
| ADEP | 19 (13) | 10 (7) | 0 | 148 |
| Hyponatremia | ||||
| ADE | 9 (6) | 0 | 0 | 150 |
| ADEP | 14 (9) | 2 (1) | 0 | 148 |
| Adult respiratory distress syndrome | ||||
| ADE | 0 | 8 (5) | 1 (<1) | 150 |
| ADEP | 0 | 3 (2) | 0 | 148 |
| Dyspnea | ||||
| ADE | 9 (6) | 7 (5) | 0 | 150 |
| ADEP | 9 (6) | 4 (3) | 1 (<1) | 148 |
| Pulmonary-Other | ||||
| ADE | 1 (<1) | 3 (2) | 1 (<1) | 150 |
| ADEP | 2 (1) | 1 (<1) | 0 | 148 |
| Thrombosis/thrombus/embolism | ||||
| ADE | 3 (2) | 0 | 0 | 150 |
| ADEP | 1 (<1) | 0 | 1 (<1) | 148 |
| Maximum nonhematologic adverse events | ||||
| ADE | 98 (65) | 37 (25) | 5 (3) | 150 |
| ADEP | 82 (55) | 59 (40) | 4 (3) | 148 |
ADE indicates cytosine arabinoside, daunorubicin, and etoposide; ADEP, cytosine arabinoside, daunorubicin, etoposide, and PSC-833; ANC, absolute neutrophil count.
Data collection about cardiac effects of the induction regimen was not incorporated into the trial design, and pretherapy measurements of cardiac function were not mandatory. Such assessments were required before autologous transplantation. On the basis of the adverse event reports received, 6 of 151 (4%) patients receiving ADE developed abnormal left ventricular function. Three of the 6 received 2 inductions, 1 of whom had cardiac dysfunction after recovering from respiratory failure after a third postremission course of HiDAC. Another had autopsy-proven viral myocarditis. Among the 151 patients treated with ADEP, 2 instances of impaired left ventricular function were recorded (1.3%).
Discussion
Leukemia stem cells express Pgp and multiple other drug resistance mechanisms. We chose to test the hypothesis that Pgp modulation during induction chemotherapy would improve outcomes in younger, untreated patients with de novo AML, perhaps by achieving greater cytoreduction of AML stem cells that have functional Pgp expression. We report the results of a phase 3 trial that compared induction chemotherapy with and without Pgp modulation with the use of PSC-833. This study is the first to assess a second-generation Pgp modulator in untreated, younger adults with AML. Negative outcomes from 2 prior phase 3 trials in untreated older patients with AML have been reported.7,8 Our study (CALGB 9720) for patients at least 60 years old showed no difference in OS by treatment despite a higher early death rate on the PSC-833 arm.7 Our prior phase 1 trial in patients younger than 60 years with de novo AML showed the feasibility of using higher than conventional doses of daunorubicin in the ADE regimen, which served as the control arm of the phase 3 trial described in this report.14 ADE has acceptable toxicity with respect to mucosal and cardiac effects and induction mortality. This may be partly because almost 90% of patients who achieved CR did so with a single course of induction chemotherapy.
Recently reported phase 3 trials in younger and older patients in which daunorubicin-containing induction regimens of 90 mg/m2 and 45 mg/m2 were compared showed a significant benefit favoring higher dose daunorubicin therapy.26,27 Our findings showing clinical equivalence for the higher and lower chemotherapy dose arms suggest that serum levels of daunorubicin and etoposide were probably similar in both regimens, as has been previously shown in trials combining mitoxantrone, etoposide, cytarabine, and PSC-833.28,29 We cannot exclude the possibility that ADEP could be superior to an ADE regimen that used lower doses of daunorubicin (eg, 60 mg/m2), whether because of PSC-833–related pharmacokinetic or pharmacodynamic (Pgp modulation) effects.
Despite the negative clinical results in phase 3 trials of PSC-833 in older patients with AML, correlative science data continue to highlight the importance of Pgp-mediated drug efflux. High efflux predicts for a significantly lower likelihood of achieving CR.9 An integrated Pgp score reflecting both Pgp expression and function was strongly correlated with incidence of CR and with DFS and OS in a phase 3 trial of PSC-833–containing induction therapy in older patients.8 The observation that Pgp may inhibit apoptosis independent of its effect on drug efflux provides another rationale for continued efforts to develop strategies directed at Pgp inhibition.30 Pretherapy blasts were collected from patients in this trial and will be analyzed for the presence of Pgp expression and modulation of drug efflux in vitro by PSC-833. Once these experiments are completed, the findings will be correlated with the clinical outcomes observed in this trial (M.R.B.).
Although PSC-833 has been withdrawn, clinical studies continue with Pgp inhibitors, including zosuquidar31,–33 and tariquidar,34 which do not alter the pharmacokinetics of daunorubicin and etoposide. Zosuquidar is a Pgp-reversal agent that does not interact with Pgp substrates, thereby allowing for the safe administration of full anthracycline doses. However, a phase 3 trial in older patients with previously untreated AML conducted by the Eastern Cooperative Oncology Group failed to show an advantage from using zosuquidar with standard daunorubicin and cytarabine induction chemotherapy.33 Because Pgp inhibition may depend on length of exposure to Pgp-reversal agents, a prolonged CIV infusion schedule of the Pgp inhibitor in combination with induction chemotherapy is undergoing evaluation.31
The antileukemia effects of the immunotoxin gemtuzumab ozogamicin are also affected by Pgp, with clinical responses inversely associated with Pgp expression.35 Strategies aimed at inhibiting Pgp may thus augment the effects of such targeted therapies. Finally, novel Pgp inhibitors with unique pharmacokinetic and toxicity profiles continue to be developed36,37 ; even as a cytotoxic agent, amonafide l-maleate, which is not a substrate for Pgp-mediated efflux, is being tested in poor risk AML.38 As long as the anthracyclines, epidophyllotoxins, and other natural products continue to be mainstays of AML therapy, countering Pgp-mediated drug efflux will remain an important therapeutic goal.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients who consented to participate in this study and the many physicians, nurses, and data coordinators who helped to complete it. We thank Michael Kelly, the senior protocol coordinator in the CALGB Central Office, for his management of the study from its inception.
This work was supported by the National Cancer Institute to the Cancer and Leukemia Group B (grant CA31946; Richard L. Schilsky, MD, chairman) and to the CALGB Statistical Center (grant CA33601; Stephen George, PhD) and by The Leukemia Clinical Research Foundation. Institutions were supported by the National Cancer Institute (grant CA35279, North Shore University Hospital, New York University School of Medicine; grant CA33601, CALGB Statistical Center; grant CA77658, The Ohio State University; grant CA77440, Washington University School of Medicine; grant CA03927, Comprehensive Cancer Center of Wake Forest University; grant CA35279, North Shore University Hospital, Albert Einstein College of Medicine; grant CA32291, Dana-Farber Cancer Institute; grant CA47559, University of North Carolina at Chapel Hill; grant CA41287, University of Chicago; grant CA31983, University of Maryland Greenebaum Cancer Center; grant CA02599, Roswell Park Cancer Institute).
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: J.E.K. and R.A.L. designed and performed research, analyzed data, and wrote the paper; S.L.G. designed research, analyzed data, and wrote the paper; V.H., E.H., K.M., J.W.V., and C.D.B. analyzed data; and G.M., R.V., B.L.P., S.L.A., D.J.D., T.C.S., W.S., and M.R.B. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Cancer and Leukemia Group B study 19808 appears as a supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Jonathan E. Kolitz, Don Monti Division of Oncology/Hematology, Department of Medicine, Monter Cancer Center, North Shore University Hospital, 450 Lakeville Rd, Lake Success, NY 11042; e-mail: kolitz@nshs.edu.