The JAK2 V617F mutation is found in most patients with a myeloproliferative neoplasm and is sufficient to produce a myeloproliferative phenotype in murine retroviral transplantation or transgenic models. However, several lines of evidence suggest that disease phenotype is influenced by the level of mutant JAK2 signaling, and we have therefore generated a conditional knock-in mouse in which a human JAK2 V617F is expressed under the control of the mouse Jak2 locus. Human and murine Jak2 transcripts are expressed at similar levels, and mice develop modest increases in hemoglobin and platelet levels reminiscent of human JAK2 V617F–positive essential thrombocythemia. The phenotype is transplantable and accompanied by increased terminal erythroid and megakaryocyte differentiation together with increased numbers of clonogenic progenitors, including erythropoietin-independent erythroid colonies. Unexpectedly, JAK2V617F mice develop reduced numbers of lineage−Sca-1+c-Kit+ cells, which exhibit increased DNA damage, reduced apoptosis, and reduced cell cycling. Moreover, competitive bone marrow transplantation studies demonstrated impaired hematopoietic stem cell function in JAK2V617F mice. These results suggest that the chronicity of human myeloproliferative neoplasms may reflect a balance between impaired hematopoietic stem cell function and the accumulation of additional mutations.
Introduction
A gain-of-function JAK2 V617F mutation is found in the majority of patients with polycythemia vera (PV) and in 50% to 60% of those with essential thrombocythemia (ET) or idiopathic myelofibrosis.1,,–4 Early studies using X-linked polymorphisms suggested that PV and ET resulted from transformation within the hematopoietic stem cell (HSC) compartment.5,6 More recently, the mutation was detected in Lin−CD34+CD38−CD90+ cells7 and also in CD34+CD38− SCID repopulating cells8 but, although clearly a critical and early event, the JAK2 mutation is not always the initiating lesion because it may be preceded by a 20q deletion or TET2 mutation in some patients.9,10
It is not yet clear how a single point mutation in JAK2 is associated with different diseases. It has been suggested that modifier alleles may play a role together with the copy number of the mutant allele and/or the strength of mutant JAK2 signaling.11 Consistent with this model, most patients with PV harbor subclones homozygous for the JAK2 mutation, although this is rare in ET.12,13 Moreover, JAK2 exon 12 mutations are found in patients with a variant form of PV, but not in patients presenting with ET, and are associated with enhanced downstream signaling.14
A role for mutation dose has also been suggested by studies of mouse models. Retroviral transplantation studies demonstrated that the JAK2 V617F mutation is capable of producing a marked myeloproliferative phenotype with substantial erythrocytosis and subsequent myelofibrosis.1,15,,–18 These models result in high levels of mutant JAK2; and, importantly, thrombocytosis was not generally observed. Inducible BAC transgenic mice displayed a variable phenotype that correlated with JAK2 V617F transgene copy number.19 Mice in which mutant JAK2 was induced by a VavCre transgene developed thrombocytosis and had relatively low numbers of transgenes, whereas mice induced by MxCre developed erythrocytosis as well as thrombocytosis and retained higher numbers of transgenes. Noninducible transgenic mice also displayed a variable phenotype.20,21 However, interpretation of results obtained using retroviral or transgenic approaches is complicated by position effects reflecting variable integration sites together with the fact that expression levels are not under physiologic control.
We have therefore generated inducible knock-in mice in which a mutant JAK2 is expressed under the control of the intact endogenous mouse Jak2 locus.
Methods
Generation of conditional JAK2V617F mice
A 10-kb region spanning the mouse exon 2 of Jak2 was retrieved into a vector (PL253) from BAC clone bMQ-261O4 using an Escherichia coli recombineering approach.22 A cassette containing a floxed PGKNeo-poly(A) minigene followed by a mutant human JAK2 V617F and a SV40-poly(A) was then introduced into the translation start site in exon 2 using recombineering (Image clone accession no: BC039695, the missing 9-bp sequence starting at position 203 in the image clone was introduced and the G to T mutation [V617F] at position 1930 was mutated using Quick Change II XL site-directed mutagenesis, Stratagene). This generated 6.4 kb upstream and 3.3 kb downstream of the cassette as the 5′ and the 3′ homologous regions of the targeting vector. The targeting vector was electroporated into AB2.2 ES cells. Targeted ES clones with a floxed JAK2 (JAK2F/+) allele were identified by Southern blot analysis using both 5′ and 3′ external probes. Three correctly targeted ES cell clones were injected into albino C57Bl/6 blastocysts, and chimeras were bred with C57Bl/6 to produce heterozygous JAK2F/+ mice, which were then bred with C57Bl/6 Mx1Cre mice to generate cohorts of conditional JAK2F/+ Mx1Cre+ mice. All mice were kept in specific pathogen-free conditions, and all procedures were performed according to the United Kingdom Home Office regulations.
Induction of JAK2 V617F expression
Six-week-old mice were injected intraperitoneally with sterile polyinosinic-polycytidylic acid (pIpC; Sigma-Aldrich) dissolved to 2.5 mg/mL in phosphate-buffered saline (PBS; Invitrogen). Cohorts, including JAK2F/+ Mx1Cre+ and littermate controls (JAK2+/+ Mx1Cre+, JAK2+/+ Mx1Cre−, and JAK2F/+ Mx1Cre−), were injected with 100 μL (250 μg/dose) every other day for a total of 3 injections.
Real-time quantitative PCR analysis
RNA was extracted using Tri Reagent (Sigma-Aldrich). Real-time quantitative polymerase chain reaction (PCR) was performed using QPCR SYBR Green (Agilent Technologies) and Mx3000P Real-Time PCR system (Stratagene). Expression of human JAK2 and mouse Jak2 was derived from standard curves generated using serial dilutions containing 103 to 109 copies of mouse Jak2 and human JAK2, respectively. Expression of Id1, Pim1, and Fos was assessed in E15.5 fetal liver or bone marrow burst-forming units-erythroid (BFU-E) colonies relative to the levels of Gapdh. Sequences of the primers are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Blood and tissue histologic analysis
Peripheral blood was taken from tail vein into ethylenediaminetetraacetic acid–coated tubes, and automated total and differential blood cell counts were determined using a Woodley ABC blood counter (Woodley). Plasma erythropoietin (EPO) levels were measured with the Quantikine Mouse/Rat Epo Immunoassay kit (R&D Systems). For histologic analysis, mice were examined after sacrifice for the presence of tumors or other abnormalities. Specimens were collected into 4% buffered formaldehyde (CellPath PLC). Iliac crest bones were decalcified in 25mM ethylenediaminetetraacetic acid for 2 to 3 weeks, and sections were stained with hematoxylin and eosin or Gömöri for reticulin fibers. Images were taken using Olympus BX51 microscope with a Pixera 600ES camera and the software for image acquisition was Viewfinder Version 3.01 (Pixera).
Flow cytometric analysis
Single-cell suspensions from spleen or BM were stained with Gr-1 and Mac-1 for myeloid analysis and CD71 and Ter119 for erythroid analysis (BD Biosciences), and washed in PBS/2% fetal bovine serum. For megakaryocytic ploidy analysis, BM cells were cultured for 5 days in serum-free media containing murine SCF (20 ng/mL) and increasing concentrations of murine thrombopoietin (TPO; 0, 0.5, 5, and 50 ng/mL, PeproTech). Cells were fixed in 4% paraformaldehyde and stained with CD41 and propidium iodide (PI). Ploidy was assessed by gating on CD41+ cells and assessing amounts of PI staining. For lineage−Sca-1+c-Kit+ (LSK) and progenitor population analysis, nucleated BM cells were stained with CD3, CD4, CD8, B220, Gr1, Ter119, CD19, IgM, and IL-7Rα chain to eliminate lineage+ cells in subsequent fluorescence-activated cell sorter (FACS) analysis. Cells were further stained with FcγRII/III, CD34, Sca-1, and c-Kit antibodies (BD Biosciences). For analysis of LSK subpopulations, lineage-depleted (lineage−) cells were isolated using a lineage cell depletion kit (Miltenyi Biotec), and were then stained with Sca-1 (Biolegend), c-Kit, CD34, and Flt3 (eBioscience) followed by FACS. Flow cytometric analysis was performed with CyAn ADP analyzer (Beckman Coulter). Data were analyzed using FlowJo Version 7.2 software (TreeStar).
BM transplantation assays
For all transplantation assays, recipients were F1 C57Bl6/129SvEvBrd (CD45.1/CD45.2) mice that were sublethally irradiated (2 × 450 cGy). Donor cells were derived 6 weeks or 26 weeks after pIpC, from JAK2F/+ Mx1Cre mice (termed JAK2V617F) or littermate controls obtained after 2 generation backcross into C57Bl/6 background. For noncompetitive transplantation assays, 1 × 106 nucleated BM cells were injected into the recipient mice. Peripheral blood was obtained at 4 weeks after transplantation and examined by FACS analysis for percentage chimerism using CD45.1 and CD45.2 antibodies (BD Biosciences), and at 12 weeks for full blood count analysis.
For competitive repopulation assays, competitor BM cells were obtained from wild-type C57Bl/6 (CD45.1) mice. A total of 1 × 106 nucleated BM cells at a test/competitor ratio of 1:1 were injected into the recipient mice. For secondary BM transplantation, 2 × 106 nucleated BM cells from primary recipients were injected into recipient mice. Blood was obtained at 5 and 16 weeks after transplantation for repopulation levels (CD45.1/2) and staining with Gr1 and B220 for lineage analysis.
Cell-cycle and apoptosis analysis
HSC-enriched LSK cells were obtained by FACS sorting using lineage− BM cells. The cell-cycle status of LSK population was then determined by 2-parameter analysis using DNA content (as measured by PI) versus Ki-67 expression with an anti–Ki-67 kit (BD Biosciences). Similarly obtained LSK cells were stained with annexin V and 7AAD (BD Biosciences) to assess their apoptotic status.
DNA damage analysis
Cytospins of nucleated bone marrow (BM) or sorted LSK cells were prepared. Cells were fixed and permeabilized with 1:1 mixture of methanol and acetone and blocked with 1% bovine serum albumin (BSA) in PBS containing 0.05% Tween 20 (PBST). Primary antibody against γ-H2AX (Millipore) was applied for 1 hour in PBST with 1% BSA at room temperature. The slides were then stained with fluorescein isothiocyanate-conjugated secondary antibody (Santa Cruz Biotechnology) for 45 minutes at room temperature in PBST containing 1% BSA. Nuclei were stained with 4′,6-diamidino-2-phenylindole (Vectashield). Images were captured using an inverted Zeiss Axioskop2 mot plus fluorescence microscope equipped with a 40× Plan NEOFLUAR lens and an AxioCam MRm camera. Fluorescent signal intensities of γ-H2AX foci from at least 70 nuclei per animal were measured using Isis software (Oxford Isis).
Statistics
The statistical significance between control and JAK2V617F mice was determined by a 2-tailed, unpaired Student t test. The repeated blood sample results were analyzed using linear mixed-effects models to deal with the repeated measures structure of the dataset. The fixed time points of blood sampling (6, 12, 16, 20, and 26 weeks) were treated as discrete dummy variables, allowing nonlinearity in the change of counts over time. Autoregressive models were fitted to allow for the correlation of counts within individual mice. The hypotheses of interest were whether there were differences between JAK2V617F mice compared with controls in blood count variables, and whether these changed over time (genotype-time interaction term). Hypothesis tests were performed with likelihood ratio tests.
Results
Generation of JAK2V617F knock-in mice
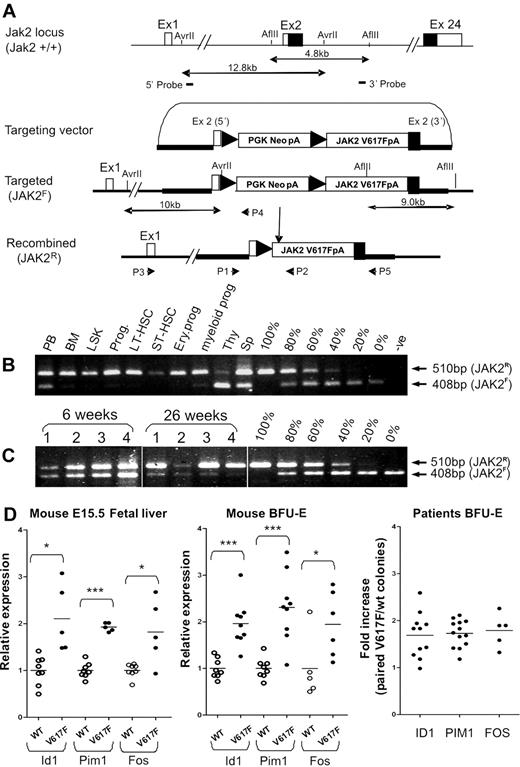
To better understand the role of the JAK2 V617F mutation in the pathogenesis of myeloproliferative neoplasms (MPNs), we generated a conditional allele in murine ES cells using homologous recombination (Figure 1A). The targeted allele (JAK2F) contains a floxed PGKNeo-poly(A) minigene together with a mutant human JAK2 cDNA inserted into the translation start codon of Jak2 exon 2 and resulted in a mouse Jak2 null allele. Removal of the PGKNeo-polyA minigene by Cre-mediated recombination generates the JAK2R allele, which is predicted to express human JAK2 V617F under the control of murine Jak2 regulatory elements. Three correctly targeted ES clones were identified by Southern blot analysis (supplemental Figure 1A) and injected into C57Bl/6 albino blastocysts to produce germline transmission. The resulting chimeric mice were bred to C57Bl/6 mice to generate germline transmission.
Generation of a conditional human JAK2 V617F knock-in allele. (A) Diagram showing the endogenous mouse Jak2 allele, the targeting vector, the knock-in allele resulting from homologous recombination, and the activated recombined allele after excision of the PGKNeo-poly (A) cassette. (B) PCR showing high levels of recombination in BM, stem cells, and progenitors and relatively lower levels in peripheral blood, spleen, and thymus. PCR was performed using P1 + P2 + P4 (A) on genomic DNA from peripheral blood (PB), BM, LSK, total progenitors (Prog), LT-HSC (lineage−Sca-1+c-Kit+CD34−), ST-HSC (lineage−Sca-1+c-Kit+CD34+), myeloid progenitors (lineage−Sca-l−c-Kit+CD34+ CD71−), and erythroid progenitors (lineage−Sca-l−c-Kit+CD34− CD71+), thymus (Thy), and spleen cells (Sp). Serial dilutions were made by mixing the corresponding amount of genomic DNA from JAK2F/+ and JAK2R/+ ES cells. Top (P1 + P2) and bottom (P1 + P4) bands represent the recombined and floxed allele, respectively. (C) PCR (performed as in panel B) showing that the proportion of recombined allele in peripheral blood samples from JAK2V617F mice increases with time. Vertical lines have been inserted to indicate removal of gel lanes. (D) Real-time quantitative PCR analysis showing comparable up-regulation of Stat5 and Erk1/2 target genes in erythroid cells (both in fetal and adult erythroid cells) from JAK2V617F mice compared with results obtained from ET patients. In ET patient samples, individual BFU-E were genotyped, pooled according to genotypes, and transcript levels of target genes in JAK2 V617F mutant and wild-type colonies were compared (far right panel). The fold increase represents the ratio of gene expression in V617F and WT pools with each data point representing an individual patient. Transcript levels of the same target genes were increased by a similar amount in both fetal liver and adult BM BFU-E from JAK2V617F mice (V617F) compared with littermate controls (WT). BFU-E colonies were derived from 3 JAK2V617F and 3 wild-type control mice. Individual colonies from JAK2V617F mice were genotyped to distinguish those carrying the active recombined allele. Colonies were pooled according to the genotype (4-6 colonies/pool). Expression of target genes in a pooled BFU-E sample was calculated relative to the mean of the wild-type pooled samples, which was defined as 1. *P < .05. ***P < .001.
Generation of a conditional human JAK2 V617F knock-in allele. (A) Diagram showing the endogenous mouse Jak2 allele, the targeting vector, the knock-in allele resulting from homologous recombination, and the activated recombined allele after excision of the PGKNeo-poly (A) cassette. (B) PCR showing high levels of recombination in BM, stem cells, and progenitors and relatively lower levels in peripheral blood, spleen, and thymus. PCR was performed using P1 + P2 + P4 (A) on genomic DNA from peripheral blood (PB), BM, LSK, total progenitors (Prog), LT-HSC (lineage−Sca-1+c-Kit+CD34−), ST-HSC (lineage−Sca-1+c-Kit+CD34+), myeloid progenitors (lineage−Sca-l−c-Kit+CD34+ CD71−), and erythroid progenitors (lineage−Sca-l−c-Kit+CD34− CD71+), thymus (Thy), and spleen cells (Sp). Serial dilutions were made by mixing the corresponding amount of genomic DNA from JAK2F/+ and JAK2R/+ ES cells. Top (P1 + P2) and bottom (P1 + P4) bands represent the recombined and floxed allele, respectively. (C) PCR (performed as in panel B) showing that the proportion of recombined allele in peripheral blood samples from JAK2V617F mice increases with time. Vertical lines have been inserted to indicate removal of gel lanes. (D) Real-time quantitative PCR analysis showing comparable up-regulation of Stat5 and Erk1/2 target genes in erythroid cells (both in fetal and adult erythroid cells) from JAK2V617F mice compared with results obtained from ET patients. In ET patient samples, individual BFU-E were genotyped, pooled according to genotypes, and transcript levels of target genes in JAK2 V617F mutant and wild-type colonies were compared (far right panel). The fold increase represents the ratio of gene expression in V617F and WT pools with each data point representing an individual patient. Transcript levels of the same target genes were increased by a similar amount in both fetal liver and adult BM BFU-E from JAK2V617F mice (V617F) compared with littermate controls (WT). BFU-E colonies were derived from 3 JAK2V617F and 3 wild-type control mice. Individual colonies from JAK2V617F mice were genotyped to distinguish those carrying the active recombined allele. Colonies were pooled according to the genotype (4-6 colonies/pool). Expression of target genes in a pooled BFU-E sample was calculated relative to the mean of the wild-type pooled samples, which was defined as 1. *P < .05. ***P < .001.
Several targeted ES cell clones were transfected with a Cre recombinase expression vector, and subclones were examined to confirm that Cre expression resulted in removal of the PGKNeo-poly(A) minigene and expression of human JAK2 transcript (supplemental Figure 1B). Quantitative real-time PCR demonstrated that ES cells containing the recombined JAK2 allele (JAK2R/+) expressed similar levels of murine and human JAK2 transcripts (supplemental Figure 1C). Individual JAK2F/+ and JAK2R/+clones were induced to undergo hematopoietic differentiation, and colony assays were performed using progenitors from day 6 embryoid bodies. Substantial numbers of EPO-independent erythroid colonies were generated by all 4 JAK2R/+ clones but not by JAK2F/+ or wild-type control clones (AB2.2; supplemental Figure 1D). Together, these data demonstrate that the JAK2R/+ allele expresses JAK2 V617F transcripts at physiologic levels and that the mutant kinase is biologically active.
JAK2V617F mice develop a cell-intrinsic myeloproliferative phenotype resembling JAK2 V617F-positive ET
Heterozygous mice carrying the JAK2F/+ allele were characterized by Southern blotting (supplemental Figure 2) and were crossed to Mx1Cre transgenic mice23 to generate multiple cohorts of offspring representing between 2 and 6 generation backcrosses onto a C57Bl6 background. At 6 weeks after pIpC injection, analysis of JAK2F/+Mx1Cre mice (henceforth termed JAK2V617F) revealed high levels of recombination (> 80%) in various BM cell populations, including LSK stem/progenitor cells, more committed progenitors (lineage−c-Kit+), long-term repopulating HSCs, short-term repopulating HSC (ST-HSCs), common myeloid progenitors, and erythroid progenitors (Figure 1B). Lower levels of recombination were observed in peripheral blood, thymus, and spleen, suggesting reduced efficiency of recombination in the lymphoid compartment24 and/or impaired lymphoid differentiation of JAK2 V617F–positive cells. Consistent with the former explanation, analysis of paired samples indicated that the levels of recombination in peripheral blood increased with time with no change in the differential nucleated cell counts (Figure 1C).
Quantitative real-time PCR demonstrated that BM-derived BFU-E cells from JAK2V617F mice expressed similar levels of murine and human JAK2 transcripts (supplemental Figure 3A). Sequencing the entire human cDNA obtained from fetal liver cells of induced JAK2V617F mice showed it to be completely as expected. Immunoblotting using an antibody that detects both human and murine Jak2 showed similar levels of total Jak2 in E15.5 fetal liver cells from JAK2V617F and wild-type control mice. However, an antibody specific for human JAK2 only detected JAK2 protein in JAK2V617F mice (supplemental Figure 3B). In the absence of cytokine stimulation, pStat5 was not detected, but EPO stimulation resulted in higher levels of pStat5 in JAK2V617F mice compared with littermate controls (supplemental Figure 3B). Importantly, in the absence of added cytokine, pSTAT5 was similarly undetectable in erythroid cells or platelets obtained from JAK2 V617F–positive patients.25,26
To further confirm activity of the mutant human protein, transcript levels of Stat5 and Erk1/2 target genes in erythroid cells from JAK2V617F mice were compared with results obtained from ET patients (Figure 1D). In ET patient samples, individual BFU-E were genotyped, colonies from a given patient were pooled according to genotypes, and transcript levels of target genes in JAK2 V617F mutant and wild-type pools were compared, thus circumventing the problem of interindividual variability in expression levels. The results demonstrated a significant 1.5- to 2-fold increase of target genes in mutant colonies. In JAK2V617F mice, transcript levels of the same genes were increased by a similar amount compared with littermate controls, both in fetal and adult erythroid cells. These data unambiguously demonstrate activity of the kinase in the knock-in mice and furthermore show that activation of target genes is quantitatively similar to that seen in ET patients.
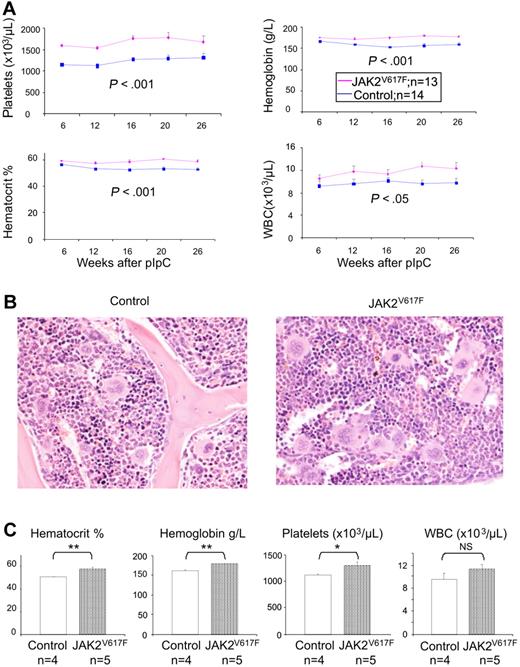
The same phenotype was observed in multiple cohorts (Figure 2; supplemental Figure 4). In each case, JAK2V617F test mice were compared with littermate controls (JAK2+/+Mx1Cre+, JAK2+/+Mx1Cre−, or JAK2F/+Mx1Cre−) and pIpC was administered to both test and control mice to control for any effect of interferon induction or Cre expression. As shown in Figure 2A, JAK2V617F mice showed significantly increased platelet counts compared with littermate controls. The estimated average difference is 389 × 103/μL (95% confidence interval [CI], 219-560 × 103/μL; P < .001). There was no effect of time or sex on the difference between the genotypes in platelet count. There were also significantly higher hemoglobin levels in JAK2V617F mice compared with controls. The estimated mean difference is 17.1 g/L (95% CI, 11.9-22.3 g/L; P < .001). There was no effect of sex on the difference between JAK2V617F mice and controls. White blood cell counts were significantly higher in JAK2V617F mice. The estimated mean difference is 1.5 × 103/μL (95% CI, 0.0-3.1 × 103/μL; P = .05). There was no effect of time or sex on the difference between the genotypes in white cell counts. Plasma EPO levels at 6 and 26 weeks after pIpC were not significantly different between JAK2V617F and control littermates (supplemental Figure 5). Histologic analysis of BM from JAK2V617F mice at 6 and 26 weeks after pIpC showed megakaryocytic hyperplasia with large and hyperlobated forms, which showed increased clustering. There was no increase in reticulin staining at either time point (Figure 2B; and data not shown). Spleen histology was normal at both 6 and 26 weeks (data not shown).
JAK2V617F mice develop a myeloproliferative disease. (A) Time course of blood parameters of JAK2V617F and control mice showing significantly increased platelets, hematocrit, and hemoglobin, and moderately elevated white blood cell counts (JAK2V617F, JAK2F/+Mx1Cre+; controls, JAK2+/+Mx1Cre+). Data are mean ± SEM. (B) Hematoxylin and eosin staining of BM with megakaryocytic hyperplasia with increased clustering and hyperlobated nuclei. (C) Myeloproliferative phenotype is transplantable. Histograms show blood counts of recipient mice 12 weeks after transplantation of 1 × 106 BM cells from either JAK2V617F or control mice. *P < .05. **P < .01. NS indicates not significant. Data are mean ± SEM.
JAK2V617F mice develop a myeloproliferative disease. (A) Time course of blood parameters of JAK2V617F and control mice showing significantly increased platelets, hematocrit, and hemoglobin, and moderately elevated white blood cell counts (JAK2V617F, JAK2F/+Mx1Cre+; controls, JAK2+/+Mx1Cre+). Data are mean ± SEM. (B) Hematoxylin and eosin staining of BM with megakaryocytic hyperplasia with increased clustering and hyperlobated nuclei. (C) Myeloproliferative phenotype is transplantable. Histograms show blood counts of recipient mice 12 weeks after transplantation of 1 × 106 BM cells from either JAK2V617F or control mice. *P < .05. **P < .01. NS indicates not significant. Data are mean ± SEM.
To test whether or not the phenotype of JAK2V617F mice is cell intrinsic, BM cells from mice 26 weeks after pIpC were transplanted into sublethally irradiated CD45.1/CD45.2 recipients. Four weeks after transplantation, FACS analysis demonstrated that approximately 70% of peripheral blood nucleated cells were of donor origin (CD45.2). Compared with recipients of normal BM, recipients of JAK2V617F BM developed increased hemoglobin levels, red blood cell counts, and platelet counts by 12 weeks after transplantation (Figure 2C), indicating that the phenotype observed in JAK2V617F mice is mediated by cells derived from BM.
JAK2V617F mice develop PV and BM fibrosis
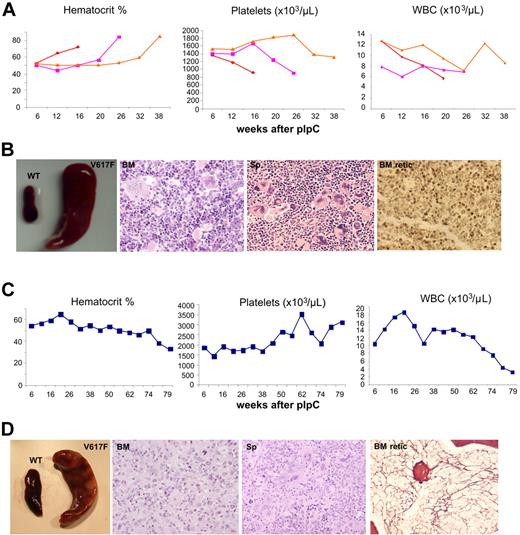
Approximately 10% of JAK2V617F mice observed over 26 weeks after pIpC developed marked erythrocytosis or BM fibrosis. Mice with a PV-like phenotype displayed a marked increase in hematocrit, splenomegaly, and a fall in their platelet counts together with increased erythroid and megakaryocytic hyperplasia of both BM and spleen (Figure 3A-B). Reticulin was not increased in BM or spleen (Figure 3B; and data not shown). FACS analysis demonstrated an increase in Ter119+ erythroid cells in BM, an increase in CD71+Ter119+ erythroid cells in the spleen, and an increase in Mac1+Gr1+ cells in BM and spleen (supplemental Figure 6A).
JAK2V617F mice develop PV and myelofibrosis. (A) Blood parameters of mice with a PV-like phenotype displaying a marked increase in hematocrit and a fall in their platelet counts. (B) Mice with a PV-like phenotype show splenomegaly; BM hematoxylin and eosin (BM) showing erythroid and megakaryocytic hyperplasia with clustering and highly pleomorphic morphology; spleen hematoxylin and eosin (Sp) showing megakaryocytic and erythroid hyperplasia; reticulin stain showing no fibrosis in BM (BM retic) and spleen (not shown). (C) Blood parameters of a mouse with BM fibrosis displaying a gradual decline of blood count parameters, including hematocrit and white cells, and an increase in its platelet counts. (D) Splenomegaly in mouse with BM fibrosis; BM hematoxylin and eosin (BM) showing granulocytic hyperplasia with reduced megakaryocytic and erythroid cells; spleen hematoxylin and eosin (Sp) showing megakaryocytic, erythroid, and granulocytic expansion; reticulin stain showing fibrosis in BM (BM retic) but not in spleen (not shown).
JAK2V617F mice develop PV and myelofibrosis. (A) Blood parameters of mice with a PV-like phenotype displaying a marked increase in hematocrit and a fall in their platelet counts. (B) Mice with a PV-like phenotype show splenomegaly; BM hematoxylin and eosin (BM) showing erythroid and megakaryocytic hyperplasia with clustering and highly pleomorphic morphology; spleen hematoxylin and eosin (Sp) showing megakaryocytic and erythroid hyperplasia; reticulin stain showing no fibrosis in BM (BM retic) and spleen (not shown). (C) Blood parameters of a mouse with BM fibrosis displaying a gradual decline of blood count parameters, including hematocrit and white cells, and an increase in its platelet counts. (D) Splenomegaly in mouse with BM fibrosis; BM hematoxylin and eosin (BM) showing granulocytic hyperplasia with reduced megakaryocytic and erythroid cells; spleen hematoxylin and eosin (Sp) showing megakaryocytic, erythroid, and granulocytic expansion; reticulin stain showing fibrosis in BM (BM retic) but not in spleen (not shown).
One mouse developed marked BM fibrosis accompanied by splenomegaly together with the development of anemia and leukopenia (Figure 3C-D). BM histology showed granulocytic hyperplasia, patchy accumulation of immature cells, and a marked reduction of erythroid and megakaryocytic cells together with the development of collagen fibrosis (Figure 3D). FACS analysis demonstrated a decrease in Ter119+ erythroid cells in BM, an increase in CD71+Ter119+ erythroid cells in the spleen, and an increase in Mac1+Gr1+ cells in BM and spleen (supplemental Figure 6B). JAK2 transcript levels were not increased in BM mononuclear cells from mice with PV-like or fibrotic transformation (supplemental Figure 6C).
Expression of mutant JAK2 V617F is associated with increased terminal erythroid and megakaryocytic differentiation together with increased numbers of lineage-restricted progenitors
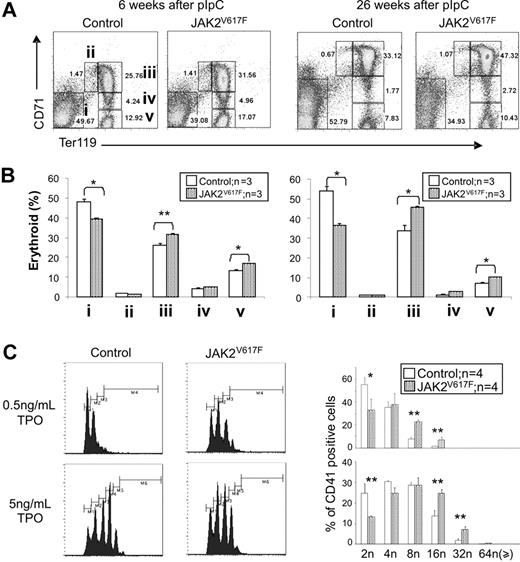
FACS analysis was used to look for abnormalities of erythroid and myeloid differentiation in JAK2V617F mice. In BM the proportion of pro-erythroblasts (CD71+Ter119+) and terminally differentiated erythroblasts (CD71−Ter119+) was increased at both 6 weeks and at 26 weeks after pIpC (Figure 4A-B). The proportion of Gr1+Mac1+ cells was also modestly increased 26 weeks after pIpC (supplemental Figure 7). There was no alteration in the total number of nucleated cells recovered from JAK2V617F BM at either time point (supplemental Figure 8), and FACS analysis of splenic erythroid and myeloid differentiation did not reveal any abnormalities (supplemental Figure 9).
JAK2V617F mice exhibit enhanced erythroid and megakaryocyte differentiation. (A) Representative FACS profiles stained with CD71 and Ter119 antibodies are shown; i through v correspond to progressive stages of erythroid differentiation. (B) Histograms show increased terminal erythroid differentiation in JAK2V617F mice BM at 6 and 26 weeks after pIpC. *P < .05. **P < .01. Data are mean ± SEM. (C) Increased megakaryocyte ploidy in JAK2V617F mice. FACS profiles for megakaryocytes grown in liquid culture are shown. Histograms represent results from 2 independent experiments. *P < .05. **P < .01. Data are mean ± SEM.
JAK2V617F mice exhibit enhanced erythroid and megakaryocyte differentiation. (A) Representative FACS profiles stained with CD71 and Ter119 antibodies are shown; i through v correspond to progressive stages of erythroid differentiation. (B) Histograms show increased terminal erythroid differentiation in JAK2V617F mice BM at 6 and 26 weeks after pIpC. *P < .05. **P < .01. Data are mean ± SEM. (C) Increased megakaryocyte ploidy in JAK2V617F mice. FACS profiles for megakaryocytes grown in liquid culture are shown. Histograms represent results from 2 independent experiments. *P < .05. **P < .01. Data are mean ± SEM.
To investigate megakaryocyte differentiation, BM cells were obtained from JAK2V617F and control mice 10 weeks after pIpC and were grown for 5 days in various concentrations of TPO. The proportion of CD41+cells and their ploidy was then assessed. In the absence of TPO, there were no differences in the proportion of CD41+ cells or their ploidy between JAK2V617F mice and controls. However, after culture in both 0.5 ng/mL and 5.0 ng/mL TPO, the ploidy of CD41+ cells was increased in JAK2V617F mice compared with controls (Figure 4C; and data not shown).
Because Bcl-xL (a transcriptional target of JAK/STAT signaling) modulates platelet survival,27 we examined whether expression of JAK2 V617F affects survival of platelets or erythrocytes. Mice were injected with NHS-biotin, and peripheral blood was analyzed at various time points by flow cytometry. There was no detectable difference in survival of platelets or erythrocytes between JAK2V617F and their littermates controls (supplemental Figure 10).
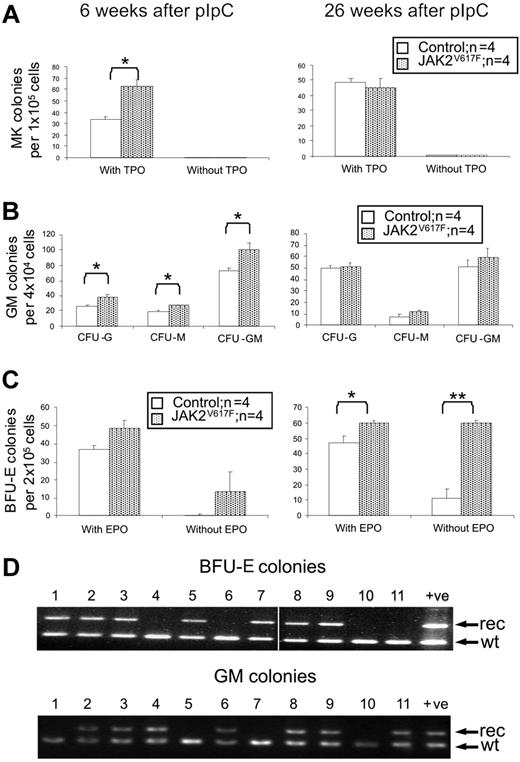
We next assessed whether JAK2 V617F was associated with alterations of lineage-restricted progenitors. The number of megakaryocytic colonies obtained from JAK2V617F BM was significantly increased at 6 weeks, but not at 26 weeks, after pIpC (Figure 5A). Furthermore, no TPO-independent colonies were detected at either time point. The numbers of BM-derived colony-forming units-granulocyte, colony-forming units-macrophage, and colony forming units granulocyte-macrophage (CFU-GM) were all increased at 6 weeks, but not 26 weeks, after pIpC (Figure 5B). This return to normal levels of myeloid and megakaryocytic colonies at 26 weeks does not reflect selection for cells lacking expression of JAK2 V617F because high levels of recombination within the targeted allele were still evident in HSCs, progenitors, and total BM at 26 weeks (Figure 1B; and data not shown). Erythroid progenitor numbers were also perturbed but demonstrated different kinetics. BM obtained from JAK2V617F mice at 6 weeks after pIpC gave a modest increase in BFU-E numbers (both in the presence or absence of EPO), but by 26 weeks there was a significant increase in the number of BFU-E grown with EPO with an even more striking increase in EPO-independent BFU-E (Figure 5C). The presence of small numbers of EPO-independent BFU-E in wild-type mice probably reflects variability in growth media and/or serum batch and may be related to observations that erythroid colony formation can occur in the absence of detectable EPO.28,29
Increased clonogenic progenitors in JAK2V617F mice. Colony assays were performed 6 and 26 weeks after pIpC. (A) Megakaryocyte colonies (CFU-MK). (B) Granulocyte-macrophage colonies (CFU-GM). (C) Erythroid colonies (BFU-E). *P < .05. **P < .01. Data are mean ± SEM. (D) PCR analysis of individual BFU-E and CFU-GM colonies from mice 26 weeks after pIpC using primers P1 + P2 + P5 (see Figure 1A). No colonies were homozygous for the recombined allele. Vertical line has been inserted to indicate removal of a gel lane. Lanes 1 to 11 indicate individual colonies; +ve, ES cell clone (JAK2R/+); rec, recombined allele (P1 + P2); and wt, wild type allele (P1 + P5).
Increased clonogenic progenitors in JAK2V617F mice. Colony assays were performed 6 and 26 weeks after pIpC. (A) Megakaryocyte colonies (CFU-MK). (B) Granulocyte-macrophage colonies (CFU-GM). (C) Erythroid colonies (BFU-E). *P < .05. **P < .01. Data are mean ± SEM. (D) PCR analysis of individual BFU-E and CFU-GM colonies from mice 26 weeks after pIpC using primers P1 + P2 + P5 (see Figure 1A). No colonies were homozygous for the recombined allele. Vertical line has been inserted to indicate removal of a gel lane. Lanes 1 to 11 indicate individual colonies; +ve, ES cell clone (JAK2R/+); rec, recombined allele (P1 + P2); and wt, wild type allele (P1 + P5).
Most patients with PV carry erythroid colonies homozygous for the JAK2 V617F mutation (as a consequence of mitotic recombination), whereas such colonies are rare in patients with JAK2 V617F–positive ET.12 We therefore genotyped individual BM-derived colonies from 3 JAK2V617F mice at 26 weeks after pIpC, looking for evidence of mitotic recombination. Of the individual colonies analyzed, 30 of 92 CFU-GM and 13 of 92 BFU-E were unrecombined, but none was found to be homozygous for the recombined allele (Figure 5D). Most mice showed more than 90% recombination in BM, but there was some mouse to mouse variability.
Taken together, these data indicate that JAK2V617F mice display increased terminal differentiation of both erythroid and megakaryocytic lineages together with an increase in megakaryocytic and myeloid progenitors early after pIpC treatment, and a sustained increase in erythroid progenitors not associated with JAK2 V617F homozygosity.
JAK2V617F mice develop reduced numbers of LSK cells, which exhibit increased DNA damage, reduced cell cycling, and reduced apoptosis
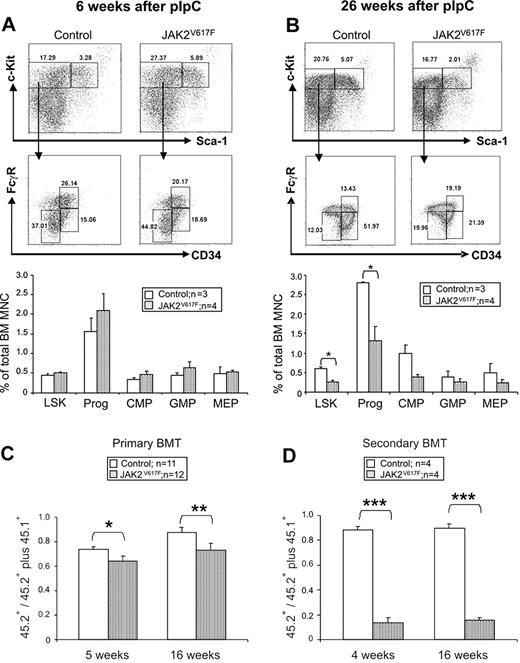
The JAK2 V617F mutation is thought to arise in the HSC compartment and confer a subtle selective advantage. It was therefore surprising to observe a progressive reduction in donor cell contribution to peripheral blood with prolonged follow-up of BM transplantation recipients (supplemental Figure 11). This observation raised the possibility that JAK2 V617F might impair HSC number and/or function. We therefore examined the LSK compartment (which contains LT-HSCs, ST-HSCs, and multipotent progenitors) together with late myeloid progenitor compartments. At 6 weeks after pIpC, there were no significant differences in the frequency of LSK cells, common myeloid progenitors, granulocyte-monocyte progenitors, and megakaryocyte-erythroid progenitors (Figure 6A). By contrast, at 26 weeks, the sizes of the LSK fraction and myeloid progenitor compartments were both significantly reduced compared with littermate controls (Figure 6B).
Reduced numbers and impaired function of hematopoietic stem and progenitor cells in JAK2V617F mice. (A-B) Representative FACS plots and histograms summarizing the frequencies of LSK and progenitor cells (lineage−Sca-1−c-Kit+), common myeloid progenitors (lineage−c-Kit+CD34+FcγR−), megakaryocyte-erythroid progenitor (lineage−c-Kit+CD34−FcγR−), and granulocyte-monocyte progenitor (lineage−c-Kit+CD34+FcγR+) at 6 and 26 weeks after pIpC. (C) BM cells from JAK2V617F mice display reduced repopulating capacity in competitive BM transplantation analysis. BM cells from 2 individual donors (6 weeks after pIpC) for each test genotype (ie, JAK2V617F or JAK2wt littermate controls) were injected into recipients F1 C57Bl6/129SvEvBrd (CD45.1/CD45.2). Histograms show the proportion of peripheral blood-nucleated cells derived from test cells (CD45.2+) compared with repopulation from total donor cells (ie, test plus competitor; CD45.2+ plus CD45.1+). (D) Marked HSC repopulation defect in secondary transplantation recipients. BM cells from primary transplantation recipients were injected into secondary recipients F1 C57Bl6/129SvEvBrd (CD45.1/CD45.2), which were analyzed 4 and 16 weeks after transplantation. Histograms represent the proportion of peripheral blood nucleated cells derived from the test cells (CD45.2+) compared with repopulation from the test plus competitor cells (CD45.2+ plus CD45.1+). *P < .05. **P < .01. ***P < .001.
Reduced numbers and impaired function of hematopoietic stem and progenitor cells in JAK2V617F mice. (A-B) Representative FACS plots and histograms summarizing the frequencies of LSK and progenitor cells (lineage−Sca-1−c-Kit+), common myeloid progenitors (lineage−c-Kit+CD34+FcγR−), megakaryocyte-erythroid progenitor (lineage−c-Kit+CD34−FcγR−), and granulocyte-monocyte progenitor (lineage−c-Kit+CD34+FcγR+) at 6 and 26 weeks after pIpC. (C) BM cells from JAK2V617F mice display reduced repopulating capacity in competitive BM transplantation analysis. BM cells from 2 individual donors (6 weeks after pIpC) for each test genotype (ie, JAK2V617F or JAK2wt littermate controls) were injected into recipients F1 C57Bl6/129SvEvBrd (CD45.1/CD45.2). Histograms show the proportion of peripheral blood-nucleated cells derived from test cells (CD45.2+) compared with repopulation from total donor cells (ie, test plus competitor; CD45.2+ plus CD45.1+). (D) Marked HSC repopulation defect in secondary transplantation recipients. BM cells from primary transplantation recipients were injected into secondary recipients F1 C57Bl6/129SvEvBrd (CD45.1/CD45.2), which were analyzed 4 and 16 weeks after transplantation. Histograms represent the proportion of peripheral blood nucleated cells derived from the test cells (CD45.2+) compared with repopulation from the test plus competitor cells (CD45.2+ plus CD45.1+). *P < .05. **P < .01. ***P < .001.
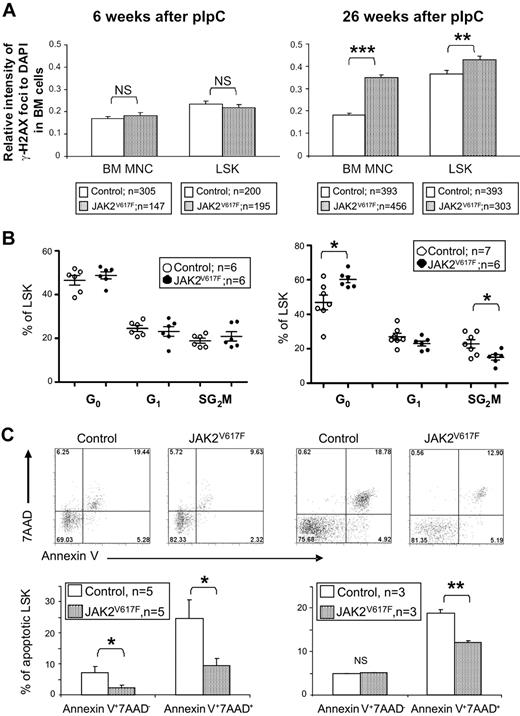
To begin to investigate the mechanisms responsible for the altered LSK numbers, we assessed DNA damage, cell-cycle status, and apoptosis in BM cells and/or LSK cells. JAK2 V617F has been reported to induce DNA damage30 and TEL-JAK2 was shown to promote formation of double-strand breaks.31 We therefore measured γ-H2AX levels as an indicator of the presence of double-strand DNA breaks (Figure 7A). Levels of γ-H2AX foci were normal in BM cells and LSK cells derived from JAK2V617F mice 6 weeks after pIpC. By contrast, significantly elevated numbers of γ-H2AX foci were detected in both cell populations when derived from JAK2V617F mice 26 weeks after pIpC.
LSK cells from JAK2V617F mice exhibit increased DNA damage, reduced cell cycling, and reduced apoptosis. (A) Intensities of γ-H2AX foci relative to DAPI obtained for BM cells (70-138 nuclei per mouse) and LSK cells (33-112 nuclei per mouse) from JAK2V617F mice at both 6 and 26 weeks after pIpC. Histograms show significantly higher relative intensities of γ-H2AX foci in cells from JAK2V617F mice compared with the cells from the control mice at 26 weeks, but not at 6 weeks after pIpC. (B) LSK cell-cycle analysis showing increased quiescence in JAK2V617F 26 weeks after pIpC. LSK cells were subjected to 2-parameter analysis with DNA content versus Ki-67 expression, and the percentages of LSK cells in each of the cell-cycle phases were obtained (G0, Ki-67lowPIlow; G1, Ki-67hiPIlow; S/G2/M, Ki-67hiPIhi). *P < .05. (C) Representative FACS plots and histograms showing reduced apoptosis in LSK cells from JAK2V617F mice. Annexin V and 7AAD staining was analyzed on gated LSK cell populations from JAK2V617F mice at both 6 and 26 weeks after pIpC. *P < .05. **P < .01. ***P < .001. NS indicates not significant.
LSK cells from JAK2V617F mice exhibit increased DNA damage, reduced cell cycling, and reduced apoptosis. (A) Intensities of γ-H2AX foci relative to DAPI obtained for BM cells (70-138 nuclei per mouse) and LSK cells (33-112 nuclei per mouse) from JAK2V617F mice at both 6 and 26 weeks after pIpC. Histograms show significantly higher relative intensities of γ-H2AX foci in cells from JAK2V617F mice compared with the cells from the control mice at 26 weeks, but not at 6 weeks after pIpC. (B) LSK cell-cycle analysis showing increased quiescence in JAK2V617F 26 weeks after pIpC. LSK cells were subjected to 2-parameter analysis with DNA content versus Ki-67 expression, and the percentages of LSK cells in each of the cell-cycle phases were obtained (G0, Ki-67lowPIlow; G1, Ki-67hiPIlow; S/G2/M, Ki-67hiPIhi). *P < .05. (C) Representative FACS plots and histograms showing reduced apoptosis in LSK cells from JAK2V617F mice. Annexin V and 7AAD staining was analyzed on gated LSK cell populations from JAK2V617F mice at both 6 and 26 weeks after pIpC. *P < .05. **P < .01. ***P < .001. NS indicates not significant.
Mice with defects in DNA repair pathways show reduced HSC numbers associated with an altered proportion of cycling HSCs, reduced self-renewal, and HSC exhaustion.32,33 The cell-cycle status of LSK cells from JAK2V617F mice and littermate controls was therefore compared (Figure 7B). At 6 weeks after pIpC, no difference was detected; but at 26 weeks, JAK2V617F mice had an increased percentage of LSK cells in G0. These results suggested that, rather than increasing LSK cell cycling, expression of JAK2 V617F may be inducing quiescence or senescence. Consistent with the latter concept, mutant Jak2 induced increased levels of senescence-associated βGal (SA-βGal) staining relative to wild-type Jak2, when introduced into mouse embryonic fibroblasts (supplemental Figure 12).
We have previously demonstrated that JAK2 V617F and BCR-ABL both impair the normal apoptotic response to DNA damage,34 thus providing a mechanism for the accumulation of genetic lesions thought to underlie disease evolution. In JAK2V617F mice, the percentage of apoptotic LSK cells was reduced at both 6 and 26 weeks after pIpC (Figure 7C). Taken together, our data are therefore consistent with a model in which JAK2V617F induces accumulation of DNA damage, increased senescence, and reduced apoptosis.
HSC function is impaired in JAK2V617F mice
To assess the function of long-term repopulating HSCs from JAK2V617F mice, competitive BM transplantation was performed using BM cells from mice 6 weeks after pIpC, a time point at which there was no significant difference in LSK numbers (Figure 6A). BM-nucleated cells from JAK2V617F or littermate control mice (both CD45.2) were mixed with equal numbers of CD45.1 competitor BM cells and transplanted into irradiated F1 C57Bl6/129SvEvBrd (CD45.1/CD45.2) recipients. FACS analysis of peripheral blood at 5 and 16 weeks after transplantation demonstrated a significant decrease in the short-term and long-term repopulating capacity of BM cells from JAK2V617F mice compared with controls (Figure 6C), with a reduced contribution to both B-cell and granulocyte populations (supplemental Figure 13). Recipients did not develop an overt myeloproliferative phenotype. This probably reflects a combination of impaired function of mutant HSCs together with the fact that mutant cells would be diluted not only by cotransplanted competitor cells but also by endogenous recovering hematopoiesis (recipients were sublethally irradiated). Secondary transplantation further emphasized the repopulation defect indicating reduced self-renewal of the HSC compartment in JAK2V617F mice (Figure 6D). The impaired repopulating capacity of BM at 6 weeks after pIpC was not a consequence of reduced numbers of LT-HSCs (lineage−c-Kit+Sca-1+CD34−Flt3−), ST-HSCs (lineage−c-Kit+Sca-1+CD34+Flt3−), or multipotent progenitor (lineage−c-Kit+Sca-1+CD34+Flt3+; supplemental Figure 14). Taken together, our results demonstrate that expression of JAK2 V617F impairs LT-HSC function.
Discussion
In this paper, we describe the generation of a conditional knock-in mouse, which expresses human JAK2 V617F under the control of an otherwise intact murine Jak2 locus. JAK2V617F mice develop a phenotype that shares many features with human JAK2 V617F-positive ET. In particular, the mice exhibit a chronic disorder with a modest thrombocytosis and elevated hematocrit but without splenomegaly or the extreme erythrocytosis associated with PV. Moreover, with prolonged follow-up, approximately 10% of mice develop marked erythrocytosis and/or myelofibrosis. Together, these features suggest that JAK2V617F mice are likely useful for dissecting the pathogenesis of chronic phase disease, for mutagenesis studies designed to identify lesions responsible for disease evolution, and for testing therapeutic agents.
It is informative to compare the phenotype of JAK2V617F mice with other mouse models for the human MPNs. Retroviral BM transduction and transplantation studies have predominantly generated recipient mice with marked increases in hematocrit but not in platelets.1,15,17,18,35 Substantial increases in white cell counts were also reported in some genetic backgrounds, especially Balb/C. Relative to wild-type JAK2, mutant JAK2 is overexpressed in these models, a feature that was thought to account for the pronounced erythroid phenotype and lack of thrombocytosis. Several other observations are consistent with the concept that increased signaling through mutant JAK2 results in a more PV-like phenotype. In particular, JAK2 V617F–homozygous clones are common in patients with PV but not ET,12 and JAK2 exon 12 mutations, found in a variant of PV but not in ET, are associated with an increased level of downstream signaling.14
Transgenic mice have also been generated in which mutant JAK2 is expressed under the control of H2Kb21 or Vav20 promoters. Both resulted in a myeloproliferative phenotype, but interpretation is difficult given the use of exogenous regulatory elements, multiple transgene copies, and/or integration site-related position effects. In a third study, transgenic mice were generated using a human BAC containing JAK2 5′ flanking sequence and retrofitted with a 3′ cDNA fragment. When crossed with Mx1Cre mice, multiple copies of the transgene were retained, mutant JAK2 expression was relatively high, and the mice developed increased levels of hemoglobin, white cells, and platelets. By contrast, when crossed with VavCre mice, relatively few transgene copies were retained, mutant JAK2 expression was relatively low, and mice developed thrombocytosis but no erythrocytosis.19 The latter mice therefore provide a model for ET, and it is worth emphasizing several differences with the JAK2V617F mice described here. In particular, the VavCre-induced mice developed much higher platelet counts (mean, ∼ 3500 × 109/L at 10-12 weeks of age), which varied between individual mice, but hemoglobin levels were normal and EPO-independent BFU-E were not observed. The mechanisms responsible for these differences are not clear but might include integration site-related position effects, clonal variation in transgene copy number, or loss of 3′ Jak2 cis-regulatory elements.
Recently, Akada et al have reported a knock-in mouse using a different strategy.36 A point mutation was introduced into the mouse gene together with a Dra1 site and a floxed partial cDNA. The resultant mice were bred with Mx1Cre mice and, after pIpC induction, developed a PV-like phenotype. Of particular note, hemoglobin levels were markedly elevated and splenomegaly was present even in heterozygous knock-in mice. In homozygous mice, hemoglobin levels were similar to, or even lower than, those of heterozygous mice, but the platelet counts of homozygous mice were considerably increased. These observations contrast with the fact that, in patients, JAK2 V617F homozygosity is associated with PV and not ET.12 The reason for the different phenotype compared with our knock-in mouse is currently unclear and could relate to technical issues associated with the different targeting strategies or to inherent differences in mutant human and mouse proteins. To validate our construct, we have therefore extensively characterized our targeted allele, demonstrated appropriate expression levels of the human transcript and protein, sequenced the entire mutant cDNA obtained from erythoid cells, obtained the same phenotype using 3 independently targeted ES cell clones, shown increased phosphorylation of Stat5 in response to EPO, and demonstrated quantitatively appropriate up-regulation of both Stat5 and Erk1/2 target genes in erythroid cells. Our data therefore raise the possibility that the V617F mutation may dysregulate kinase function to a different extent when introduced into the human compared with the murine Jak2 protein.
Importantly, the relatively mild phenotype reported here is consistent with many aspects of human ET. In patients with ET, splenomegaly is rare and the JAK2 mutation is associated with a mild but highly significant increase in hemoglobin levels that still lie within the normal range.11,37,38 The median platelet count for JAK2 V617F–positive ET patients is 846,11 and this value is an overestimate because it is based on a clinical trial that excluded all patients with platelet counts less than 600. Indeed, when looked for, JAK2 V617F–positive patients with platelet counts in the 400s are readily identified.39 Furthermore, patients with ET can undergo polycythemic or myelofibrotic transformation.
The results presented here demonstrate that JAK2 V617F influences hematopoiesis at multiple levels. JAK2V617F mice exhibited increased terminal erythroid and megakaryocytic differentiation, increases in clonogenic progenitors, and also an unexpected defect in HSC function. We considered the possibility that the HSC defect might reflect histoincompatibility and graft rejection. However, the AB2.2 ES cells used were from the identical 129 substrain (129SvEvBrd) as the Bl6/129 F1 recipients. Moreover, littermate controls were used for all transplantation experiments, and JAK2V617F donor cells showed a significant disadvantage in both noncompetitive and competitive transplantation experiments. As a consequence, we do not think that our data can be attributed to histocompatibility differences. LSK cells from JAK2V617F mice contained increased levels of DNA damage, consistent with reports that oncogenic JAK2 induces DNA damage30 and reduces DNA damage-induced apoptosis,34 thus providing a mechanism for accumulation of DNA damage in JAK2 mutant cells. Consistent with this model, LSK cells from JAK2V617F mice displayed reduced apoptosis despite increased levels of DNA damage. Accumulation of DNA damage is recognized to trigger senescence,40 and recent evidence suggests that oncogene-induced senescence provides a barrier to neoplastic progression of multiple malignancies in vivo.41,,–44 LSK cells from JAK2V617F mice displayed reduced cell cycling compared with littermate controls by 26 weeks after pIpC, and mutant Jak2 induced expression of the senescence-associated marker SA-βGal in mouse embryonic fibroblasts. Together, these observations suggest that mutant JAK2 promotes accumulation of DNA damage and consequent HSC senescence, thus resulting in HSC exhaustion after transplantation.
Our data are consistent with previous studies of MPN patients, which concluded that the JAK2 V617F–positive clone composes only a minority of the CD34+CD38− compartment in many ET and PV patients7,8 and that JAK2 V617F–positive CD34+ cells do not have a proliferative advantage relative to wild-type cells in immunodeficient recipient mice.8 Our results demonstrate that JAK2 V617F–positive HSCs have a subtle competitive disadvantage relative to normal HSCs that is revealed by the proliferative stress associated with transplantation. This insight provides an explanation for the remarkable lack of expansion of the neoplastic clone in many patients with chronic-phase ET or PV45 and in the rare unwitting recipient of a JAK2 V617F–positive allograft.46 Disease chronicity may therefore reflect a balance between opposing effects of JAK2 V617F, with impaired HSC function resulting in clonal restraint, whereas accumulation of DNA damage promotes clonal expansion and disease progression.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Allan Bradley for helpful advice; Tina Hamilton, Michelle Hammett, Frances Law, Polly Chan, and Siqin Bao for help in generating the mice; Jacinta Carter, Dean Pask, Yvonne Silber, and Rebecca Kelley for technical assistance; David Kent and Mark Dawson for constructive comments on the manuscript; Mark Arends and Wanfeng Zhao for help with histology; and Anna Petrunkina for FACS sorting.
This work is supported by the Leukemia Research Fund, the Wellcome Trust, the Medical Research Council, British Heart Foundation, the Kay Kendall Leukaemia Fund, the Cambridge NIHR Biomedical Research Center, and the Leukemia & Lymphoma Society of America. C.G. is a British Heart Foundation Intermediate Fellow (FS09039).
Wellcome Trust
Authorship
Contribution: J.L. designed and performed experiments, analyzed the data, and wrote the paper; D.S. performed LSK cell-cycle analysis and BFU-E colony assay; J.S.A. performed DNA damage analysis; S.A. performed LSK and progenitor FACS analysis; P.A.B. performed MK colony assay and blood cell survival analysis; C.G. performed ploidy analysis; E.C. performed immunoblot analysis; E.C. and J.S.A. performed SA-βGal staining; A.F. performed ES cell in vitro differentiation assay; L.M.S. provided helpful advice and critical reading of the manuscript; R.F. performed MK colony assay; P.J.C. performed statistical analysis of peripheral blood phenotypes; S.P.W. and B.J.P.H. provided constructive advice; P.L. provided collaboration in design and establishment of the model; W.N.E. examined and summarized the histology data; K.O. designed and performed BMT experiments; and A.R.G designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anthony R. Green, Cambridge Institute for Medical Research, Hills Rd, Cambridge, CB2 0XY, United Kingdom; e-mail: arg1000@cam.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal