Hypoxia is associated with increased metastatic potential and poor prognosis in solid tumors. In this study, we demonstrated in the murine 5T33MM model that multiple myeloma (MM) cells localize in an extensively hypoxic niche compared with the naive bone marrow. Next, we investigated whether hypoxia could be used as a treatment target for MM by evaluating the effects of a new hypoxia-activated prodrug TH-302 in vitro and in vivo. In severely hypoxic conditions, TH-302 induces G0/G1 cell-cycle arrest by down-regulating cyclinD1/2/3, CDK4/6, p21cip-1, p27kip-1, and pRb expression, and triggers apoptosis in MM cells by up-regulating the cleaved proapoptotic caspase-3, -8, and -9 and poly ADP-ribose polymerase while having no significant effects under normoxic conditions. In vivo treatment of 5T33MM mice induces apoptosis of the MM cells within the bone marrow microenvironment and decreases paraprotein secretion. Our data support that hypoxia-activated treatment with TH-302 provides a potential new treatment option for MM.
Introduction
Multiple myeloma (MM) is an incurable clonal B-cell malignancy characterized by the accumulation of neoplastic plasma cells in the bone marrow (BM).1 Studies have shown that the intimate reciprocal relationship between tumor cells and the cellular and noncellular microenvironment plays a pivotal role in MM growth and survival.2,3 Hypoxia, one of the important microenvironmental factors, is well known to be highly associated with increased angiogenesis and metastatic potential as well as poor prognosis in solid tumors. More recently, hypoxia has been demonstrated to be crucial for normal marrow hematopoiesis.4,–6 However, the role of hypoxia in the etiology, pathogenesis, and possible treatment of hematologic malignancies, such as MM, is still unknown.
Given very low oxygen levels, as found in tumors, are rarely observed in normal tissues, the presence of hypoxic tumor cells is therefore regarded not only as an adverse prognostic factor but also as a potential target for tumor-specific treatment. Currently, several hypoxia-targeted therapeutics are under development.7,,,,–12 TH-302 is a new hypoxia-activated prodrug that is being evaluated in phase 1/2 clinical trials for the treatment of solid tumors as a monotherapy and in combination with 4 chemotherapeutic agents (gemcitabine, pemetrexed, doxorubicin, and docetaxel). TH-302 is a 2-nitroimidazole prodrug of the cytotoxin bromo-isophosphoramide mustard, with a favorable physicochemical, metabolic, and pharmacokinetic profile and exhibits hypoxia-selective cytotoxicity across a broad spectrum of human cancer cell lines in vitro and in vivo efficacy in a large panel of human tumor xenografts.13,14 The doses used in the clinical studies are in the same range as the doses demonstrating efficacy in both in vitro and in vivo preclinical models.
In this study, we investigated the hypoxic nature of MM by staining the BM of naive and 5T33MM mice with the exogenous hypoxia marker pimonidazole and endogenous hypoxia marker hypoxia-inducible factor 1α (HIF-1α) and demonstrated that MM cells reside in a more hypoxic BM environment. Furthermore, we evaluated the effects of TH-302 on MM cell lines in vitro, focusing on apoptosis and cell cycle as well as associated signaling pathways in MM, and evaluated the potential therapeutic effects in the 5T33vv mouse MM model.
Methods
All experiments were approved by the Ethical Committee for Animal Experiments at Vrije Universiteit Brussels (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results and discussion
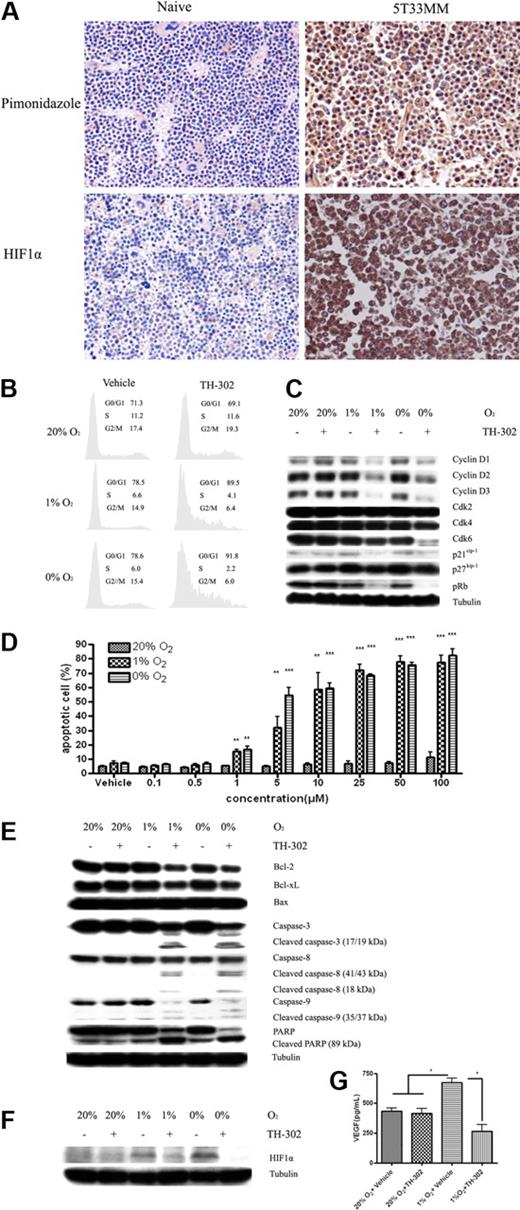
Despite major therapeutic advances, MM still remains incurable.1 Compelling evidence suggests that the BM microenvironment favors MM progression by promoting MM cell growth and drug resistance. In solid tumors, hypoxia is one of the microenvironmental factors that drives tumor progression and treatment resistance. MM develops in a specialized BM microenvironment, which has been shown to be low in oxygen tension; however, our knowledge in this field is still in its infancy and many details are unknown.15,,–18 In contrast to solid tumors and other tissues or organs, the inaccessibility of the BM to direct noninvasive oxygen measurements is a major hurdle for formal experimental investigation. Moreover, the invasive needle oxygen electrode techniques for detecting tissue pO2 measurement cannot show the heterogeneity of oxygen tension in the BM, the gradients of O2 from hypoxic niches to the higher O2 tension in the sinusoidal cavity. Considering the potential role of hypoxia in hematopoiesis and MM progression in the BM, we investigated the oxygen level in the BM of naive and 5T33MM mice, which closely mimics the human disease,19 by assessing the exogenous and endogenous hypoxia markers pimonidazole and HIF-1α. As shown in Figure 1A, our immunohistochemical staining results clearly delineate the hypoxic regions within the normal and MM BM. Both the exogenous marker pimonidazole and endogenous hypoxia marker HIF-1α were dramatically increased in the BM of 5T33MM mice in contrast to the sporadic and weak positivity of hypoxia markers in the naive mice, strongly suggesting that the majority of MM cells localize in an extensively hypoxic niche. Our results provide tangible evidence and more detailed information regarding the hypoxic nature of normal and MM BM and confirm the results recently reported by the group of Giuliani et al.20 A role for hypoxic bone marrow in other hematologic malignancies (lymphoma and leukemia) is also supported by the results of other recent studies.21,22
The hypoxia status of the BM and the in vitro effects of TH-302 on MM cells. (A) Immunohistochemistry staining of exogenous hypoxia marker and endogenous hypoxia marker in BM sections of naive and 5T33MM mice. Hypoxia was determined by the accumulation of pimonidazole and HIF-1α as described in supplemental Methods. Original magnification, × 40. Representative pictures, n = 6/group. (B) TH-302 induces G0/G1 cell-cycle arrest in 5T33vt cells in a hypoxia-selective manner. Similar results were also found in RPMI-8226, LP-1, MMS1, and Karpas-707 MM cells (data not shown). (C) Effects of TH-302 on components of the 5T33vt MM cell-cycle machinery. TH-302-induced G0/G1 cell-cycle arrest depends on down-regulating cyclin D1/2/3, CDK4/6, p21, p27, and pRb expression. Similar results were also found in RPMI-8226, LP-1, MMS1, and Karpas-707 MM cells (data not shown). (D) TH-302 triggers specific apoptosis in a dose-dependent manner in LP-1 cells under hypoxia. *P < .05, **P < .01, ***P < .001, compared with 20% O2 (n = 3). Similar results were also seen with RPMI-8226, 5T33vt, MMS1, and Karpas-707 cells (data not shown). (E) The mechanism of TH-302-induced apoptosis in LP-1 cells. Similar results were also found in RPMI-8226, 5T33vt, MMS1, and Karpas-707 cells. TH-302 = 5μM. (F) TH-302 decreases the accumulation of HIF-1α in hypoxic RPMI-8226 cells. Similar results were also found in 5T33vt, LP-1, MMS1, and Karpas-707 cells (data not shown). TH-302 = 5μM. (G) VEGFa secretion was reduced by TH-302 in 5T33vt cells. *P < .05. n = 3.
The hypoxia status of the BM and the in vitro effects of TH-302 on MM cells. (A) Immunohistochemistry staining of exogenous hypoxia marker and endogenous hypoxia marker in BM sections of naive and 5T33MM mice. Hypoxia was determined by the accumulation of pimonidazole and HIF-1α as described in supplemental Methods. Original magnification, × 40. Representative pictures, n = 6/group. (B) TH-302 induces G0/G1 cell-cycle arrest in 5T33vt cells in a hypoxia-selective manner. Similar results were also found in RPMI-8226, LP-1, MMS1, and Karpas-707 MM cells (data not shown). (C) Effects of TH-302 on components of the 5T33vt MM cell-cycle machinery. TH-302-induced G0/G1 cell-cycle arrest depends on down-regulating cyclin D1/2/3, CDK4/6, p21, p27, and pRb expression. Similar results were also found in RPMI-8226, LP-1, MMS1, and Karpas-707 MM cells (data not shown). (D) TH-302 triggers specific apoptosis in a dose-dependent manner in LP-1 cells under hypoxia. *P < .05, **P < .01, ***P < .001, compared with 20% O2 (n = 3). Similar results were also seen with RPMI-8226, 5T33vt, MMS1, and Karpas-707 cells (data not shown). (E) The mechanism of TH-302-induced apoptosis in LP-1 cells. Similar results were also found in RPMI-8226, 5T33vt, MMS1, and Karpas-707 cells. TH-302 = 5μM. (F) TH-302 decreases the accumulation of HIF-1α in hypoxic RPMI-8226 cells. Similar results were also found in 5T33vt, LP-1, MMS1, and Karpas-707 cells (data not shown). TH-302 = 5μM. (G) VEGFa secretion was reduced by TH-302 in 5T33vt cells. *P < .05. n = 3.
The strategy of microenvironment-targeted treatment is gaining increased attention in hemato-oncology, and we evaluated whether the hypoxic niche of MM could also serve as a treatment target. Our data demonstrate that the hypoxia activated prodrug TH-302 exhibits potent in vitro cytotoxicity to MM cells with hypoxic selectivity and dose dependency. To study the growth inhibitory effects of TH-302 on MM cells, we analyzed the cell-cycle phase distribution and apoptosis after drug treatment. Cell-cycle analysis showed that TH-302 can induce G0/G1 cell-cycle arrest under hypoxic conditions. Western blotting further revealed that the effect of TH-302 on cell-cycle machinery was mediated by down-regulating cyclin D1/2/3, CDK4/6, p21cip-1, p27kip-1, and pRb expression, whereas CDK2 expression remained undisturbed (Figure 1B-C). Similar results were also found in RPMI-8226, LP-1, MMS1, and Karpas-707 MM cells (supplemental Figures 1, 2). Furthermore, flow cytometric analysis demonstrated that TH-302 can induce dose-dependent apoptosis in both human and murine MM cells in hypoxic conditions (Figure 1D). Similar results were also seen in RPMI-8226, 5T33vt, MMS1, and Karpas-707 cells (supplemental Figure 3). Western blotting further demonstrated that TH-302-activated apoptosis was mediated through down-regulating the antiapoptotic proteins BCL-2 and BCL-xL, as well as up-regulating the expression of cleaved proapoptotic protein caspase-3, -8, and -9 and poly ADP-ribose polymerase. In contrast to the hypoxia-specific toxicity, TH-302 shows very low toxicity in normoxic condition, even at high concentrations (Figure 1E). Similar results were also found in RPMI-8226, 5T33vt, MMS1, and Karpas-707 cells (supplemental Figure 4). In addition, we demonstrated that the production of HIF-1α, the central regulator of the hypoxic response,23 is decreased with the treatment of TH-302. The expression of HIF-1α in a hypoxic condition was reduced after exposure to TH-302 (Figure 1F, similar results were also found in 5T33vt, LP-1, Karpas-707 cells, supplemental Figure 5). Accordingly, the secretion of VEGFa, which is a downstream target gene of HIF-1α, was also significantly decreased (Figure 1G).
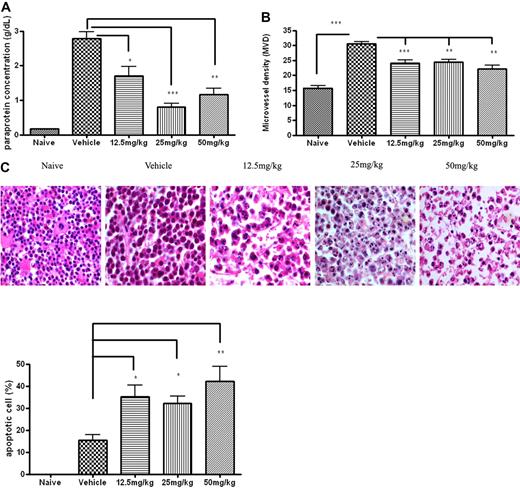
Furthermore, studies conducted in the 5T33MMvv mouse model demonstrated that in vivo treatment with TH-302 showed impressive improvements in multiple disease parameters. TH-302 induced significant MM cell apoptosis (12.5 mg/kg, 2.5-fold; 25 mg/kg, 2.1-fold; 50 mg/kg, 3.1-fold), decreased paraprotein secretion (12.5 mg/kg, 32% decrease; 25 mg/kg, 77% decrease; 50 mg/kg, 54% decrease), and significantly decreased microvessel density (MVD) (12.5 mg/kg, 19% decrease; 25 mg/kg, 20% decrease; 50 mg/kg, 26% decrease) in the BM of treated 5T33MMvv mouse, compared with vehicle-treated 5T33MMvv mice (Figure 2).
In vivo therapeutic effects of TH-302 on 5T33MM model. 5T33MMvv mice were treated prophylactically with TH-302 for 3 weeks from day 1. (A) Serum paraprotein level (as determined by serum electrophoresis) was decreased after treatment with TH-302. (B) Histomorphometric analysis of MVD. MVD was determined by CD31 staining as described in “Methods.” In the area with the highest blood vessel density (hot spot), the number of blood vessels was counted per 0.22 mm2. (C) Hematoxylin and eosin staining of bone marrow section. Nuclei from apoptotic cells show condensed, fragmented morphology. Original magnification, × 63; n = 10/group. Detailed image information available in supplemental Methods. Quantitative data are the frequency of apoptotic MM cells in bone marrow sections. *P < .05, **P < .01, significantly different from vehicle. n = 10/group.
In vivo therapeutic effects of TH-302 on 5T33MM model. 5T33MMvv mice were treated prophylactically with TH-302 for 3 weeks from day 1. (A) Serum paraprotein level (as determined by serum electrophoresis) was decreased after treatment with TH-302. (B) Histomorphometric analysis of MVD. MVD was determined by CD31 staining as described in “Methods.” In the area with the highest blood vessel density (hot spot), the number of blood vessels was counted per 0.22 mm2. (C) Hematoxylin and eosin staining of bone marrow section. Nuclei from apoptotic cells show condensed, fragmented morphology. Original magnification, × 63; n = 10/group. Detailed image information available in supplemental Methods. Quantitative data are the frequency of apoptotic MM cells in bone marrow sections. *P < .05, **P < .01, significantly different from vehicle. n = 10/group.
Using a set of defined gas mixtures (0%, 1%, 1.25%, 1.5%, 2%, 3%, 20% O2) for drug treatment of the MM cells, we evaluated the oxygen concentration-dependent activation of TH-302. Our results suggest that the threshold of activating prodrug TH-302 is lower than 1.5% O2 (supplemental Figure 6). 2-Nitroimidazole (the oxygen concentration sensing trigger for both pimonidazole and TH-302) has been shown to become activated in cells whose oxygen concentration is less than 10 mmHg pO2.24,25 It is reasonable to accept that the cytotoxic effect of TH-302 is tightly associated with the hypoxic nature of MM cells in the BM. In addition, the data from TH-302-treated naive mice show no significant toxicity in body weight, hemoglobin, red blood cell count, white blood cell count, hematocrit, and MVD compared with the vehicle-treated naive mice, further indicating the specific hypoxia-activated effect of TH-302 and the limited hypoxia in the normal BM (supplemental Figure 7).
Taken together, our results confirm that MM cells reside in an extensively hypoxic BM microenvironment. Hypoxia-activated treatment with TH-302 as a monotherapy shows efficacy in treatment of MM both in vitro and in vivo. The findings in this study indicate that targeting the hypoxic BM niche provides a potentially useful and novel treatment strategy for MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Willems and C. Seynaeve for expert technical assistance, Professor F. Gorus (Universitair Ziekenhuis van de Vrije Universiteit, Vrije Universiteit Brussel, Brussels) for serum paraprotein analysis, and Dr John Curd (Threshold Pharmaceuticals) for his contributions to the development of TH-302 in MM.
Authorship
Contribution: J.H. designed research, wrote the paper, and participated in most of the experiments; D.R.H., Q.L., J.D.S., and C.P.H. performed immunohistochemistry staining of hypoxia markers and wrote the paper; H.D.R. performed hematoxylin and eosin staining and MVD analysis; E.V.V., E.M., and I.V.B. performed 5T33MM treatment experiments and analyzed the data; B.V.C. contributed to the paper in discussion; and K.V. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: D.R.H., Q.L., J.D.S., and C.P.H. are employed by Threshold Pharmaceuticals. K.V. received research funding from Threshold Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Karin Vanderkerken, Department of Hematology and Immunology-Myeloma Center Brussels, Vrije Universiteit Brussel, Laarbeeklaan 103, 1090 Brussels, Belgium; e-mail: Karin.Vanderkerken@vub.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal