Abstract
In erythroid cells, ferrous iron is imported into the mitochondrion by mitoferrin-1 (Mfrn1). Previously, we showed that Mfrn1 interacts with Abcb10 to enhance mitochondrial iron importation. Herein we have derived stable Friend mouse erythroleukemia (MEL) cell clones expressing either Mfrn1-FLAG or Abcb10-FLAG and by affinity purification and mass spectrometry have identified ferrochelatase (Fech) as an interacting protein for both Mfrn1 and Abcb10. Fech is the terminal heme synthesis enzyme to catalyze the insertion of the imported iron into protoporphyrin IX to produce heme. The Mfrn1-Fech and Abcb10-Fech interactions were confirmed by immunoprecipitation/Western blot analysis with endogenous proteins in MEL cells and heterologous proteins expressed in HEK293 cells. Moreover, Fech protein is induced in parallel with Mfrn1 and Abcb10 during MEL cell erythroid differentiation. Our findings imply that Fech forms an oligomeric complex with Mfrn1 and Abcb10 to synergistically integrate mitochondrial iron importation and use for heme biosynthesis.
Introduction
The terminal step of heme biosynthesis in eukaryotic cells, the insertion of ferrous iron into protoporphyrin IX to produce protoheme IX (heme), is catalyzed by the enzyme ferrochelatase (Fech).1,2 Fech is broadly distributed in nature from bacteria to eukaryotes and is conserved in essential catalytic residues, although the prokaryotic and eukaryotic enzymes vary with regard to their cellular localization and the presence or absence of a [2Fe-2S] cluster.2
The mammalian Fech is nuclear encoded, cytoplasmically synthesized as a precursor form, and translocated into the mitochondrion where it is proteolytically processed to its mature size. Human Fech is an 86-kDa homodimeric protein with 2 [2Fe-2S] clusters, and it is associated with the inner mitochondrial membrane with its active site facing into the membrane.2,3 The Fech-catalyzed reaction involves binding of ferrous iron and protoporphyrin IX substrates to the enzyme, distortion of the protoporphyrin macrocycle followed by porphyrin deprotonation, iron insertion, and product release.2,4
In mammals, the pathways for iron trafficking and use involve the binding of ferric iron to transferrin for transportation in the circulation and extravascular fluid to cells bearing specific transferrin receptors. Transferrin receptor 1 (TfR1) on erythroid precursors selectively binds diferric transferrin and internalizes it through the receptor-mediated endocytosis of the transferrin-TfR1 complex.5 In erythroid cells, the iron sequestered by the tranferrin-TfR1 pathway is directed specifically to6 and imported by the mitochondrial solute carrier Mfrn1,7 which is stabilized during erythroid differentiation by Abcb10,8 into the mitochondrion to satisfy the high demand for iron during heme biosynthesis. Ultimately, the Mfrn1-imported iron must be delivered to Fech for heme synthesis. The mechanism of how iron is transferred into Fech in the mitochondrial matrix, however, remains unclear, although it has been suggested that the 2 proteins may form a transient complex4,9 to facilitate direct iron transfer. Herein we present data demonstrating that Fech forms an oligomeric complex with Mfrn1 and Abcb10 to facilitate mitochondrial ferrous iron transfer for erythroid heme biosynthesis.
Methods
Clones
Subclones of mouse Mfrn1 and mouse Abcb10 in pEF1α vector were generated as previously described.8 Full-length human Fech cDNA was purchased from Open Biosystems.
Tissue culture
Mouse erythroleukemia (MEL; DS19 clone) cells were maintained as previously described.8 For erythroid differentiation, MEL cells were chemically induced with 1.5% dimethyl sulfoxide for 3 days.
Procedures
All experiments were performed using protocols approved by the Institutional Animal Care and Use Committee at Children's Hospital Boston. Stable transfectants of MEL cell clones expressing Mfrn1-FLAG or Abcb10-FLAG were derived by electroporation. HEK293 cells were transfected with Mfrn1-FLAG, Abcb10-FLAG, Fech constructs using Lipofectamine 2000 (Invitrogen). Mitochondrial protein extraction, affinity purification, and mass spectrometry (MS) identification were performed as described previously.8 Western blot analysis was performed using anti-Fech, anti-FLAG, anti-Mfrn1, anti-Abcb10 antibodies. As a loading control, membranes were reprobed using anti-Hsp60 antibody. Fech and Hsp60 antibodies are from Santa Cruz Biotechnology.
Results and discussion
Stable MEL clones expressing Mfrn1-FLAG, Abcb10-FLAG, or empty vector were induced to undergo erythroid differentiation. Duplicate samples were affinity-purified and analyzed by MS analysis, respectively, for Mfrn1-FLAG or Abcb10-FLAG lysates. The data obtained were compared with triplicate runs of control FLAG-eluted lysates from differentiating MEL cells transformed with empty vector. Identified peptides of Fech, found to be unique in samples enriched by Mfrn1-FLAG or Abcb10-FLAG bait proteins, are summarized in supplemental Tables 1 and 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In addition to the bait Mfrn1 or Abcb10 proteins, Fech was consistently identified by MS analyses of 2 independent pulldowns, respectively.
The weak interactions between Mfrn1:Fech and Abcb10:Fech proteins were rigorously confirmed by serial immunoprecipitation (IP) and Western blot analysis using endogenous proteins in differentiated MEL cells and heterologous proteins in transiently cotransfected HEK293 cells (Figure 1). After MEL cell erythroid differentiation, protein complexes interacting with Mfrn1 or Abcb10 were affinity-purified and analyzed by Western blot analysis with anti-Fech antibody. The presence of the input bait Mfrn1-FLAG or Abcb10-FLAG protein was detected by reprobing with anti-FLAG antibody. The endogenous Fech was enriched as an oligomeric complex by either Mfrn1-FLAG or Abcb10-FLAG proteins in differentiated MEL cells (Figure 1A). The physical interactions of Fech with Mfrn1 and Abcb10 were further validated in transiently cotransfected HEK293 cells (Figure 1B).
Physical interactions between Fech with either Mfrn1 or Abcb10 proteins are confirmed in endogenous MEL cells and transfected heterologous cells. (A) IP/Western blot analysis of interactions of endogenous Fech with Mfrn1 and Abcb10 in differentiated MEL cells stably expressing empty vector, engineered Mfrn1-FLAG, or Abcb10-FLAG. (B) IP/Western blot analysis of interactions of heterologous Fech with Mfrn1 and Abcb10 from transiently cotransfected HEK293 cells with control vector, Mfrn1-FLAG, or Abcb10-FLAG. Protein input lysate is shown on the respective left columns. Fech is selectively copurified in the presence of Mfrn1 or Abcb10.
Physical interactions between Fech with either Mfrn1 or Abcb10 proteins are confirmed in endogenous MEL cells and transfected heterologous cells. (A) IP/Western blot analysis of interactions of endogenous Fech with Mfrn1 and Abcb10 in differentiated MEL cells stably expressing empty vector, engineered Mfrn1-FLAG, or Abcb10-FLAG. (B) IP/Western blot analysis of interactions of heterologous Fech with Mfrn1 and Abcb10 from transiently cotransfected HEK293 cells with control vector, Mfrn1-FLAG, or Abcb10-FLAG. Protein input lysate is shown on the respective left columns. Fech is selectively copurified in the presence of Mfrn1 or Abcb10.
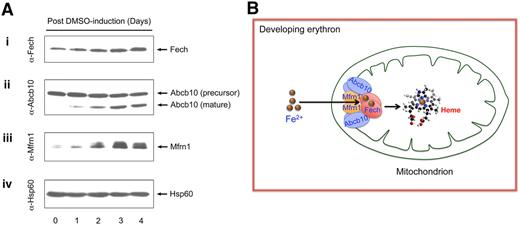
Consistent with their roles in erythroid heme biosynthesis, Fech protein is induced in differentiated MEL cells in parallel with Mfrn1 and Abcb10 (Figure 2A). We previously showed that Mfrn1 forms oligomeric complexes with other mitochondrial proteins during erythroid maturation.8 Similarly, Fech and Abcb10 each form higher-order complexes with other mitochondrial proteins in differentiated MEL cells (supplemental Figures 1-2). Combining our results in this study and previous data showing the Mfrn1-Abcb10 interaction,8 we propose that these 3 homodimeric proteins (Mfrn1, Abcb10, and Fech) form an oligomeric complex to integrate and coordinate mitochondrial iron importation with porphyrin metallation in erythroid cells, as summarized in our proposed model (Figure 2B). This model proposes that a transient, rather than a stable, complex forms between Fech and Mfrn1/Abcb10, and their interactions could be either a direct physical or indirect requiring additional bridging proteins, which may explain the lower levels of interactions observed in our study between Fech and Mfrn1/Abcb10 compared with the interaction between Mfrn1 and Abcb10.
Interacting Fech, Mfrn1, and Abcb10 proteins are coordinately induced during MEL cell erythroid differentiation. (A) Endogenous Fech, Mfrn1, and Abcb10 are coinduced in chemically differentiated MEL cells. Mitochondrial lysates collected from MEL cells exposed to 1.5% dimethyl sulfoxide (DMSO) for various lengths of days were serially probed with antisera against Fech (i), antisera against Mfrn1 (ii), antisera against Abcb10 (iii), and antisera against loading control Hsp60 (iv). Endogenous Fech and Mfrn1 proteins are induced (i,ii) and Abcb10 protein is proteolytically processed to a mature isoform in MEL cells during erythroid differentiation (iii). (B) A schematic model for Mfrn1, Abcb10, and Fech forming an oligomeric protein complex, which facilitates the importation of mitochondrial iron for heme biosynthesis in the developing red blood cells.
Interacting Fech, Mfrn1, and Abcb10 proteins are coordinately induced during MEL cell erythroid differentiation. (A) Endogenous Fech, Mfrn1, and Abcb10 are coinduced in chemically differentiated MEL cells. Mitochondrial lysates collected from MEL cells exposed to 1.5% dimethyl sulfoxide (DMSO) for various lengths of days were serially probed with antisera against Fech (i), antisera against Mfrn1 (ii), antisera against Abcb10 (iii), and antisera against loading control Hsp60 (iv). Endogenous Fech and Mfrn1 proteins are induced (i,ii) and Abcb10 protein is proteolytically processed to a mature isoform in MEL cells during erythroid differentiation (iii). (B) A schematic model for Mfrn1, Abcb10, and Fech forming an oligomeric protein complex, which facilitates the importation of mitochondrial iron for heme biosynthesis in the developing red blood cells.
The physiologic consequence of forming a Mfrn1-Abcb10-Fech protein complex during erythroid differentiation is to facilitate Mfrn1-imported iron transfer to Fech for heme biosynthesis with greater efficiency than would be anticipated if iron was freely transported into the matrix compartment.9 Evidence exists demonstrating that a defect in any component of the Mfrn1-Abcb10-Fech complex in mice results in a heme homeostasis defect with resultant anemia.10-12 Furthermore, the human inherited disorder erythropoietic protoporphyria, which results from decreased Fech activity,13 has been recently found in persons with misexpression of Mfrn1,14 thus providing clinical support for direct interactions between Fech and Mfrn1.
Fech protein stability depends on iron-sulfur (Fe-S) cluster availability.15 Mfrn1-imported iron may not only be a substrate for Fech but also may be used for Fe-S cluster synthesis.7 Abcb7 exports an as yet, uncharacterized molecule to the cytosol that is important for regulating mitochondrial iron homeostasis.16,17 In addition, Abcb7 may play a role in heme biosynthesis through its interaction with Fech as previously proposed.18 This Abcb7-Fech interaction was also observed in our study (supplemental Figure 3), although the interactions between Fech-Abcb7 and Fech-Abcb10 have some differential selectivity.
Abcb10 probably exports some metabolite because the ATPase site in Abcb10 molecule is localized in mitochondrial matrix.19 Because mitochondrial heme is biosynthesized by the enzyme Fech, our discovery of the Abcb10-Fech interaction raises the possibility that Abcb10 may function in heme export. An interaction between Fech and erythroid-induced Abcb10 and Abcb7 may also provide reversible inhibition of Fech to help regulate iron distribution between the synthesis of both Fe-S clusters and heme.20
There are several examples of existing mitochondrial multiprotein complexes. Mitochondrial β-oxidation is a complex pathway involving at least 16 proteins that are organized into 2 functional subdomains.21 Mammalian mitochondrial complex I (nicotinamide adenine dinucleotide plus hydrogen/ubiquinone oxidoreductase) is the largest protein assembly of the respiratory chain. It is embedded in the inner mitochondrial membrane and structurally consists of 45 subunits.22 Because Fech is present at the convergence of protoporphyrin IX and iron import pathways to produce heme,2 and Mfrn1 forms higher-order protein complexes8 that are much larger than predicted for an Mfrn1-Abcb10-Fech complex, it is rational to anticipate that Mfrn1-Abcb10-Fech could form larger complexes with other heme biosynthetic pathway enzymes. Indeed, a model proposing an interaction between the terminal 3 of heme biosynthetic enzymes has been forwarded,23 and data in support of a complex between PPO and Fech have been presented.4,23,24 Such a complex could coordinate the correct molar ratio and sufficient, but not excessive, supply of protoporphyrin IX and Fe2+ substrates for Fech to produce heme.6,25
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Mitchell Weiss and Arthur Skoultchi for the MEL DS19 clone, Dr Orian Shirihai for the anti-Abcb10 antibody, and Dr Alan Cantor for the pEF1α-BT expression vector.
This work was supported by the March of Dimes Foundation (B.H.P.) and the National Institutes of Health (grant R01 DK032303, H.A.D.; and grants R01 DK070838, P01 HL032262, and P30 DK072437, B.H.P.).
National Institutes of Health
Authorship
Contribution: W.C. designed the research, performed the experiments, analyzed data, and wrote the paper; H.A.D. commented on and critically reviewed the manuscript; and B.H.P. designed the research, analyzed data, and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barry H. Paw, Brigham & Women's Hospital, Hematology Division, Karp Bldg 06.213, 1 Blackfan Cir, Boston, MA 02115; e-mail: bpaw@rics.bwh.harvard.edu.