Abstract
Adult T-cell leukemia (ATL), a heterogeneous disease, can be divided into smoldering, chronic, lymphoma, and acute types clinically. In addition to different clinical manifestations, different stages of ATL have different molecular signatures. Here, we demonstrated that smoldering/chronic ATL peripheral blood mononuclear cells spontaneously proliferated ex vivo in a cytokine (interleukin -12 [IL-12]/IL-9/IL-15)–dependent manner, while acute-type ATL peripheral blood mononuclear cells did not proliferate or proliferated independent of cytokines. Smoldering/chronic ATL cells produced IL-2 and IL-9 in 6-day ex vivo cultures. Interestingly, the addition of an anti–IL-2R-α monoclonal antibody profoundly inhibited IL-9 expression, suggesting optimal expression of IL-9 was dependent on IL-2 signaling in these patients. To determine whether there would be autonomous proliferation of ATL leukemic cells, we purified leukemic cells from patients with smoldering/chronic ATL. Purified leukemic cells cultured alone produced IL-2/IL-9, and the downstream Janus kinase/signal transducer and activator of transcription pathway was activated. However, the leukemic cells did not proliferate independently, but required coculture with autologous monocytes to induce proliferation. Moreover, interaction between leukemic cells and monocytes was contact dependent, and major histocompatibility complex class II expression may have contributed to this interaction. In conclusion, our data provide evidence that there is autocrine/paracrine cytokine stimulation of leukemic cell proliferation in patients with smoldering/chronic ATL that could be targeted for treatment.
Introduction
Adult T-cell leukemia (ATL) that is caused by human T-cell lymphotropic virus I (HTLV-1) is an aggressive malignancy of CD4- and CD25-expressing leukemia, and lymphoma cells. ATL is a heterogeneous disease that can be divided broadly into 4 stages: smoldering, chronic, lymphoma, and acute-type ATL. The common clinical manifestations of ATL are skin lesions, hypercalcemia, immunologic anergy to antigen stimulation, and cells with “flower-like” nuclei in the circulation. Smoldering/chronic ATL patients have normal or mildly increased white blood cell counts with a variable number of leukemic cells in the circulation and are generally associated with a better prognosis. Patients with acute-type ATL have organ dysfunction associated with circulating leukemic cells and are generally associated with a poor prognosis. The mechanisms underlying the progression from smoldering/chronic stage to the acute stage are unknown; however, the accumulation of molecular mutations is thought to play a role in this progression.
Although the pathogenesis of ATL is unknown, the virally encoded regulatory protein, HTLV-1 Tax, seems to play a central role in the initial leukemogenesis of ATL. Hasegawa et al demonstrated that overexpression of Tax in immature thymocytes induced leukemia/lymphoma in mice with clinical, pathologic, and immunologic features characteristic of ATL after a long latency.1 Subsequently, Ohsugi et al2 showed that Tax is able to promote oncogenesis not only with immature T cells, but also with mature T cells. Both experiments highlighted the importance of Tax in the initial development of ATL.
Beyond the in vivo mouse models, numerous in vitro studies have demonstrated the essential role of Tax in ATL initiation and shed light on the mechanism of Tax-mediated cellular transformation.3 Tax deregulates the expression of genes involved in cellular proliferation, cell-cycle control, and apoptosis through physical interaction with cellular elements, including transcription factors such as nuclear factor (NF)-κB and nuclear factor of activated T cell.4 In particular, activation of NF-κB by Tax up-regulates the expression of several cytokines and their corresponding receptor genes.5-7 The up-regulation of cytokine and cytokine-receptor expression is thought to play an important role in promoting proliferation/survival of ATL cells and resistance to apoptosis, thereby maintaining the leukemic cells in the body for a long time before they acquire additional molecular mutations. One such cytokine/cytokine receptor pair is interleukin (IL)–2/IL-2R-α. The observation that IL-2R-α expression is increased on the surface of ATL cells suggests that IL-2 production by such cells may play an important role in their autocrine/paracrine growth in the early phase of the disease.
However, although the concept of an autocrine IL-2 loop has been widely accepted in another HTLV-1–induced disease termed HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP),8,9 the literature on an autocrine IL-2 loop in ATL is inconsistent. Some investigators have reported the expansion of primary ATL cells by exogenous IL-2,10,11 and therefore concluded that IL-2–dependent autonomous growth exists in acute-type ATL because of the observed constitutively activated Janus kinase/signal transducer and activator of transcription (Jak/STAT) and NF-κB pathways.12 Others have opposed this idea due to the lack of IL-2 secretion and IL-2 mRNA in HTLV-1–infected T-cell lines or T-cell clones.9,13,14 Furthermore, the addition of the IL-2R-α–blocking monoclonal antibody, anti-Tac, had no effect on the spontaneous proliferation of those cells.9 Similarly, our previous data with peripheral blood mononuclear cells (PBMCs) from 10 patients with acute ATL showed no spontaneous proliferation ex vivo, implying that there was no autocrine cytokine stimulation in those patients.15 However, our clinical use of the murine anti-Tac monoclonal antibody directed to IL-2R-α was associated with the development of a complete or partial remission in 6 of 19 patients with ATL.16 This suggested that the IL-2/IL-2R-α signaling pathway is important for the survival of some primary ATL cells in vivo. In an attempt to predict the clinical response of ATL patients to daclizumab (humanized anti-Tac, anti–IL-2R-α) treatment, we have routinely set up a 6-day spontaneous proliferation assay of ATL PBMCs cultured ex vivo alone in media as well as with neutralizing antibodies to cytokines and cytokine receptors, including those to IL-2 and IL-2R-α. Our ex vivo proliferation data suggested that there was an IL-2–dependent proliferation in several patients with chronic/smoldering ATL, but not in the patients with acute-type ATL. To further dissect the autocrine/paracrine cytokine stimulation in ATL leukemic cells, we took advantage of magnetic-activated cell sorting (MACS), which allowed us to evaluate selected, enriched leukemic cell populations in the ex vivo culture systems. The specific questions we asked were: (1) is there an autocrine IL-2/IL-2R-α loop in ATL leukemic cells; (2) other than IL-2, do other cytokine/cytokine receptors contribute to the ex vivo ATL proliferation; (3) is there a hierarchy in the expression of the cytokines; (4) is there any difference in the proliferation/cytokine production pattern in patients with different stages of disease (ie, chronic/smoldering vs. acute type); and (5) in addition to the leukemic cells, are other cells required for the spontaneous proliferation of the ATL cells?
Methods
Patient materials
All patient samples were obtained from patients under the care of the clinical trials team of Metabolism Branch, National Cancer Institute (NCI). ATL subtypes classified according to the diagnostic criteria proposed by Shimoyama et al17 were: (1) smoldering type: 5% or more abnormal lymphocytes of T-cell nature in peripheral blood, normal lymphocyte levels (< 4 × 109/L), no hypercalcemia (corrected calcium level < 2.74mM), lactate dehydrogenase (LDH) value of up to 1.5 times the normal upper limit; (2) chronic type: absolute lymphocytosis (4 × 109/L or more) with T lymphocytosis more than 3.5 × 109/L, LDH value up to twice the normal upper limit, no hypercalcemia; (3) lymphoma type: no lymphocytosis, 1% or less circulating abnormal T lymphocytes, and histologically proven lymphadenopathy with or without extranodal lesions; and (4) acute type: remaining ATL patients who have usual leukaemic manifestations and tumor lesions. The clonality of ATL cells was analyzed by polymerase chain reaction (PCR) to look at T-cell receptor (TCR) rearrangement in the patients. Of the 11 smoldering and chronic ATL patients studied in this article, 6 patients had a monoclonal ATL population, while the other 5 patients appeared to have polyclonal population at the time the ex vivo proliferation was performed when the patients were in partial remission with too few circulating malignant T cells to yield the detection of the monoclonal population. The study protocol was approved by the Institutional Review Board of the NCI.
Cell culture, antibodies, and reagents
All cells were cultured in RPMI 1640 plus 10% fetal bovine serum (FBS). NK-92, a cytokine-dependent natural killer (NK) cell line, was maintained with the addition of 60 U/mL of recombinant human IL-2 (rhIL-2).18 The IL-2– and IL-15–neutralizing monoclonal antibodies were obtained from R&D Systems, and the IL-9– and IL-9R-α–neutralizing antibodies were purchased from Biolegend. Daclizumab (humanized anti–IL-2R-α) was purchased from Hoffmann-La Roche. HuMik-β1 (humanized anti–IL-2/IL-15R-β) was produced by the Biopharmaceutical Development Program of the NCI.
Six-day spontaneous proliferation assay
Human PBMCs were isolated by Ficoll-Hypaque density centrifugation. The PBMCs were washed with phosphate-buffered saline (PBS) and then resuspended in RPMI 1640 media supplemented with 10% FBS at a concentration 1 × 106 cells/mL. Then, 100-μL aliquots of the cell suspensions were seeded into 96-well microtiter plates in duplicates and cultured for 6 days at 37°C in 5% CO2. Antibodies were added at the initiation of the culture period at 10 μg/mL. If 2 antibodies were combined, the concentration of each antibody was 5 μg/mL. Cultures were pulsed with 1 μCi 3H thymidine 6 hours before harvesting.
Taqman real-time quantitative RT-PCR
Total RNA was extracted from PBMCs using the RNeay Mini Kit (QIAGEN) according to the manufacturer's instructions. Reverse transcription (RT) reactions were carried out for each sample (250 ng) using the Superscript First-Strand Synthesis System (Invitrogen). The Taqman Universal PCR Master Mix, the human IL-2, IL-9 primer/probe, HTLV-I Tax primer/probe, and the HPRT1 primer/probe sets were purchased from Applied Biosystems. The HTLV-I Tax primer/probe has been described previously.5 The detection of human IL-2, IL-9, HTLV-1 Tax, and HPRT1 were performed using an ABI prism 7500 sequence detection system (Applied Biosystems) according to the manufacturer's instructions. The copy numbers of IL-2, IL-9 mRNA, and HTLV-1 Tax mRNA were normalized to the copy number of human HPRT1 mRNA.
IL-2, IL-15, and IL-9 ELISA
IL-2 and IL-15 were measured using the human IL-2 and IL-15 quantikine enzyme-linked immunosorbent assay (ELISA) kit (D2050/D1500; R&D Systems). IL-9 was measured using the LEGEND MAX Human IL-9 ELISA kit (BioLegend).
NK-92 proliferation assay
Supernatants from ATL PBMC cultures were collected at day 6. NK-92 cells were washed 4 times in PBS, then resuspended in RPMI 1640 media plus 10% FBS and kept at 37°C to release surface-bound IL-2. Four hours later, the cells were washed 4× in PBS, then resuspended in RPMI 1640 media plus 10% FBS and starved overnight at 37°C in 5% CO2. The rested NK-92 cells were then cultured with ATL PBMC culture supernatants for 48 hours. Antibodies were added at the initiation of the culture period at 10 μg/mL. Cultures were pulsed with 1 μCi 3H-thymidine 6 hours before harvesting.
ATL leukemic cell and monocyte separation
ATL leukemic cell and monocyte separations were performed using the human regulatory T-cell (Treg) separation kit and human monocyte isolation kit II (Miltenyi Biotec) respectively. ATL leukemic cells were separated by a 2-step purification procedure. First, non-CD4+ cells were labeled by a cocktail of biotin-conjugated antibodies against CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCR-γ/δ, and CD235a. After incubation with anti-biotin microbeads, the non-CD4+ cells were depleted by separation over a MACS column. In the second step, CD4+CD25bright cells were directly labeled with CD25 MicroBeads and isolated by positive selection from the pre-enriched CD4+ T-cell fraction. All the cell separations were performed using an AutoMACS cell separator (Miltenyi Biotec) according to the manufacturer's instruction.
FACS and intracellular staining
anti-CD4FITC, anti-CD25PE, anti-CD3APC, and isotype controls were purchased from BD Biosciences. Phospho-STAT5 Alexa 488 was obtained from Cell Signaling Technology. To stain cell-surface markers, the cells were blocked by a human FcγR binding inhibitor (eBioscience) at 4°C for 20 minutes, the cells were then stained with the fluorescence-activated cell sorting (FACS) developing antibody at 4°C for 30 minutes. After the wash step, the cells were analyzed using a FACSCaliber (BD Biosciences). The intracellular staining of p-STAT5 followed the method of Ilangumaran et al.19
Results
Cytokine-dependent spontaneous proliferation of smoldering/chronic ATL PBMCs in 6-day ex vivo culture
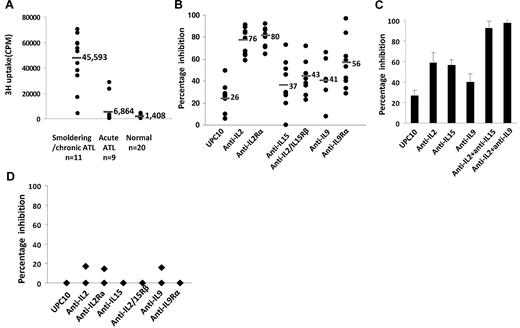
Adult T-cell leukemia can be divided into smoldering, chronic, lymphoma type, and acute type clinically. We analyzed the spontaneous proliferation of PBMCs from 20 patients with ATL without the addition of mitogens or antigens and compared their proliferation with that of normal healthy donors (n = 20). Among the 20 patients with ATL, 11 patients had smoldering/chronic type ATL (9 smoldering ATL, 2 chronic ATL) and 9 patients had acute-type ATL. The 11 patients with smoldering/chronic ATL studied were in the very early phase of their leukemia, as only 6 patients showed monoclonal leukemic population by PCR. PBMCs from all 11 patients with smoldering/chronic ATL proliferated ex vivo in RPMI 1640 media/10% FBS without the addition of mitogens or antigens ( > 3000 cpm; mean, 45 593 cpm) (Figure 1A). In contrast, the PBMCs from most of the patients with acute-type ATL did not proliferate ( ≤ 3000 cpm; 7 of 9 patients), with the exception of 2 patients whose PBMCs proliferated at 23 262 and 28 569 cpm, respectively. PBMCs from normal donors did not proliferate ex vivo (< 3000 cpm; n = 20). Furthermore, the proliferation of PBMCs from smoldering/chronic-type ATL was profoundly inhibited by the addition of antibodies to IL-2 or IL-2R-α (76%, P < .001; 80%, P < .001), modestly by anti–IL-15 or anti–IL-2/15R-β (37%, P = .03; 43%, P < .03) and anti–IL-9 or anti–IL-9R-α (41%, P = .06; 56%, P < .01), compared with that with a nonspecific control antibody, UPC10 (26%; Figure 1B). More interestingly, in 3 patients, the inhibitions observed with a combination of antibodies to IL-2 and IL-15, or the combination of antibodies to IL-2 and IL-9, were greater than those observed with single antibodies to the individual cytokines, IL-2, IL-15, or IL-9 alone (patient 4, Figure 1C). In contrast, the proliferations of the PBMCs from the 2 patients with acute-type ATL that proliferated ex vivo (23 262 and 28 569 cpm) were not inhibited by the addition of antibodies to IL-2/IL-2R-α, IL-15/IL-15R-α, or IL-9/IL-9R-α (Figure 1D). These observations support the view that autocrine/paracrine IL-2, IL-9, and IL-15 stimulations are more likely to be meaningfully involved in the spontaneous proliferation of PBMCs from patients with smoldering and chronic ATL than from patients with acute ATL.
PBMCs from patients with smoldering/chronic ATL-manifested autocrine/paracrine cytokine-dependent spontaneous proliferation. (A) The 6-day spontaneous proliferation of ex vivo PBMCs was assayed on cells from smoldering/chronic ATL (n = 11, 9 smoldering ATL, 2 chronic ATL), acute-type ATL (n = 9), and normal donors (n = 20). (B) Spontaneous proliferation of smoldering/chronic ATL PBMCs (n = 11) was assayed in the presence of 10 μg/mL of monoclonal antibodies to IL-2 and IL-2R-α, IL-15 and IL-2/15R-β, IL-9 and IL-9R-α, and of a nonspecific control antibody (UPC10). (C) The spontaneous proliferation of PBMCs from a patient (patient 4) with smoldering/chronic ATL was assayed in the presence of 10 μg/mL monoclonal antibodies to IL-2, IL-9, IL-15, the combinations of anti-IL-2 with anti-IL-15, anti-IL-2 and anti-IL-9, and with a nonspecific control antibody (UPC10). This represents the data from 3 patients. (D) Spontaneous proliferation of PBMCs from 2 patients with acute ATL in the presence of 10 μg/mL monoclonal antibodies to IL-2 and IL-2R-α, IL-15 and IL-2/15R-β, IL-9 and IL-9R-α, and of a nonspecific control antibody (UPC10). The PBMCs proliferated at 23 262 and 28 569 cpm, respectively, without the addition of any antibody. The percentage inhibition was calculated by comparing the proliferation to that without any antibody.
PBMCs from patients with smoldering/chronic ATL-manifested autocrine/paracrine cytokine-dependent spontaneous proliferation. (A) The 6-day spontaneous proliferation of ex vivo PBMCs was assayed on cells from smoldering/chronic ATL (n = 11, 9 smoldering ATL, 2 chronic ATL), acute-type ATL (n = 9), and normal donors (n = 20). (B) Spontaneous proliferation of smoldering/chronic ATL PBMCs (n = 11) was assayed in the presence of 10 μg/mL of monoclonal antibodies to IL-2 and IL-2R-α, IL-15 and IL-2/15R-β, IL-9 and IL-9R-α, and of a nonspecific control antibody (UPC10). (C) The spontaneous proliferation of PBMCs from a patient (patient 4) with smoldering/chronic ATL was assayed in the presence of 10 μg/mL monoclonal antibodies to IL-2, IL-9, IL-15, the combinations of anti-IL-2 with anti-IL-15, anti-IL-2 and anti-IL-9, and with a nonspecific control antibody (UPC10). This represents the data from 3 patients. (D) Spontaneous proliferation of PBMCs from 2 patients with acute ATL in the presence of 10 μg/mL monoclonal antibodies to IL-2 and IL-2R-α, IL-15 and IL-2/15R-β, IL-9 and IL-9R-α, and of a nonspecific control antibody (UPC10). The PBMCs proliferated at 23 262 and 28 569 cpm, respectively, without the addition of any antibody. The percentage inhibition was calculated by comparing the proliferation to that without any antibody.
Smoldering/chronic ATL PBMCs produced IL-2 and IL-9 in the ex vivo cultures
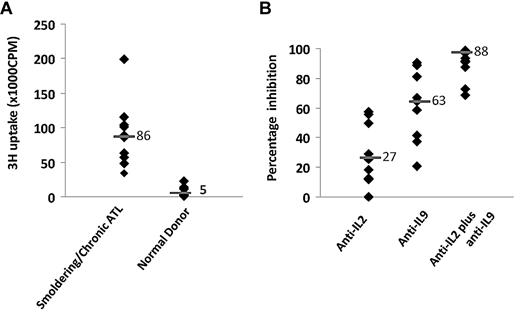
We then restricted our focus to patients with smoldering/chronic ATL for further characterization. We analyzed the supernatants of the ex vivo cultures of smoldering/chronic ATL PBMCs (n = 11) for their stimulatory activity on the cytokine-dependent cell line, NK-92.18 The supernatants from the smoldering/chronic ATL PBMC cultures stimulated vigorous NK-92 proliferation (11 of 11; mean, 86 000 cpm), whereas the supernatants from most normal donors did not stimulate NK-92 proliferation (17 of 20; ≤ 5000 cpm) (Figure 2A). Typically, the proliferations were partially inhibited by the addition of an antibody to IL-2 or an antibody to IL-9; while the combination of antibodies to both IL-2 and IL-9 added simultaneously profoundly inhibited the activity on the NK-92 cells (Figure 2B). This suggests that both IL-2 and IL-9 were produced by smoldering/chronic ATL PBMCs. To further determine how much IL-2 and/or IL-9 were made by the smodering/chronic ATL PBMCs ex vivo, we performed an ELISA assay to measure the concentrations of IL-2 and IL-9 in the 6-day culture supernatants. As shown in Table 1, the smoldering/chronic ATL PBMCs secreted meaningful quantities of IL-2 and IL-9 into the ex vivo culture supernatants, whereas IL-2 and IL-9 could not be detected in the 6-day culture supernatants of normal donors (data not shown). IL-15 was not detected in the ex vivo culture supernatants of ATL cells, as assessed both by ELISA and by NK-92 stimulation and proliferation.
IL-2 and IL-9 were secreted by smoldering/chronic ATL PBMCs. (A) NK-92 cell-line assay of 6-day culture supernatants of smoldering/chronic ATL PBMCs (n = 11) and normal donor PBMCs (n = 20). (B) NK-92 cell-line assay of supernatants from 6-day cultures of smoldering/chronic ATL PBMCs in the presence of 10 μg/mL anti–IL-2, anti–IL-9, and the combination of anti–IL-2 and anti–IL-9. Percentage inhibition of NK-92 cell proliferation was calculated by comparing the proliferation with antibodies to that without any antibody.
IL-2 and IL-9 were secreted by smoldering/chronic ATL PBMCs. (A) NK-92 cell-line assay of 6-day culture supernatants of smoldering/chronic ATL PBMCs (n = 11) and normal donor PBMCs (n = 20). (B) NK-92 cell-line assay of supernatants from 6-day cultures of smoldering/chronic ATL PBMCs in the presence of 10 μg/mL anti–IL-2, anti–IL-9, and the combination of anti–IL-2 and anti–IL-9. Percentage inhibition of NK-92 cell proliferation was calculated by comparing the proliferation with antibodies to that without any antibody.
IL-2 and IL-9 protein was secreted in most of the PBMC cultures of patients with smoldering/chronic ATL
| Patient no. . | IL-2 (pg/mL) . | IL-9 (pg/mL) . |
|---|---|---|
| ATL1 | Not detected* | 9975 |
| ATL2 | 10 | 296 |
| ATL3 | 391 | 1374 |
| ATL4 | 1196 | 532 |
| ATL5 | 358 | 161 |
| ATL6 | Not detected* | 310 |
| ATL7 | 11 | 359 |
| ATL8 | 1639 | 945 |
| ATL9 | 47 | Not done |
| ATL10 | Not detected* | 2214 |
| ATL11 | Not detected* | 10 |
| Patient no. . | IL-2 (pg/mL) . | IL-9 (pg/mL) . |
|---|---|---|
| ATL1 | Not detected* | 9975 |
| ATL2 | 10 | 296 |
| ATL3 | 391 | 1374 |
| ATL4 | 1196 | 532 |
| ATL5 | 358 | 161 |
| ATL6 | Not detected* | 310 |
| ATL7 | 11 | 359 |
| ATL8 | 1639 | 945 |
| ATL9 | 47 | Not done |
| ATL10 | Not detected* | 2214 |
| ATL11 | Not detected* | 10 |
The level of IL-2 was < 7 pg/mL.
IL-2 was required for IL-9 expression in smoldering/chronic ATL PBMCs
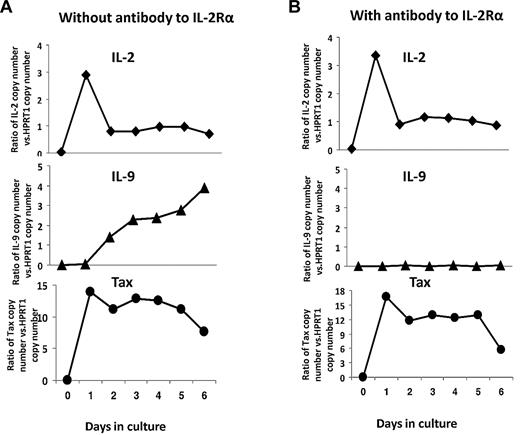
To better understand the kinetics of cytokine production in the ex vivo cultures of PBMCs from patients with smoldering/chronic ATL, we used Taqman real-time RT-PCR to detect the production of message for IL-2, IL-9, and Tax at different time points during the 6-day culture. As shown in Figure 3A, Tax mRNA expression was not detectable at day 0, but was induced at day 1 and then stabilized from days 2 through 6. The IL-2 mRNA expression was dramatically induced at day 1 and then decreased during the culture period. Interestingly, IL-9 message was not induced until day 2 and increased over the culture period (Figure 3A). This delayed expression of IL-9 prompted us to examine the relationship between IL-2 and IL-9 expression. To determine whether IL-2 signaling would be needed for IL-9 production, we added a monoclonal antibody to IL-2R-α at the initiation of the ATL PBMC culture period and analyzed the cells for IL-9 message expression at subsequent time points. As shown in Figure 3B, the IL-9 expression by smoldering/chronic ATL PBMCs was dramatically reduced by the addition of the anti–IL-2R-α antibody, while the expression of IL-2 and Tax was not changed by the addition of anti–IL-2R-α. ELISA analysis for IL-2 and IL-9 in the supernatants confirmed the decrease in IL-9 protein production that was associated with the addition of an anti–IL-2R-α antibody (Table 2).
IL-2 signaling was required for optimal IL-9 expression in ex vivo cultures of PBMCs from patients with smoldering/chronic ATL. (A) IL-2, IL-9, and Tax mRNA levels were determined every day in ex vivo cultures by TaqMan real-time RT-PCR. The copy numbers of IL-2, IL-9, and Tax mRNA were normalized to the copy number of hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1) mRNA. The data are representative of data from 4 different patients. (B) IL-2, IL-9, and Tax mRNA levels in the presence of 10 μg/mL anti–IL-2R-α every day in ex vivo cultures by TaqMan real-time RT-PCR. The data are representative of data from 2 different patients.
IL-2 signaling was required for optimal IL-9 expression in ex vivo cultures of PBMCs from patients with smoldering/chronic ATL. (A) IL-2, IL-9, and Tax mRNA levels were determined every day in ex vivo cultures by TaqMan real-time RT-PCR. The copy numbers of IL-2, IL-9, and Tax mRNA were normalized to the copy number of hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1) mRNA. The data are representative of data from 4 different patients. (B) IL-2, IL-9, and Tax mRNA levels in the presence of 10 μg/mL anti–IL-2R-α every day in ex vivo cultures by TaqMan real-time RT-PCR. The data are representative of data from 2 different patients.
The production of IL-9 was decreased by a monoclonal anti–IL-2R-α antibody in ex vivo cultures of PBMCs from a patient with smoldering/chronic ATL
| PBMC culture . | IL-2 (pg/mL) . | IL-9 (pg/mL) . | ||
|---|---|---|---|---|
| Without anti–IL-2R-α . | With anti–IL-2R-α . | Without anti–IL-2R-α . | With anti–IL-2R-α . | |
| Day 1 | 585 | 528 | Not detected* | Not detected* |
| Day 2 | 954 | 957 | 46 | Not detected* |
| Day 3 | 1048 | 1170 | 146 | Not detected* |
| Day 4 | 1138 | 1424 | 196 | Not detected* |
| Day 5 | 1156 | 1633 | 287 | Not detected* |
| Day 6 | 1196 | 1704 | 532 | Not detected* |
| PBMC culture . | IL-2 (pg/mL) . | IL-9 (pg/mL) . | ||
|---|---|---|---|---|
| Without anti–IL-2R-α . | With anti–IL-2R-α . | Without anti–IL-2R-α . | With anti–IL-2R-α . | |
| Day 1 | 585 | 528 | Not detected* | Not detected* |
| Day 2 | 954 | 957 | 46 | Not detected* |
| Day 3 | 1048 | 1170 | 146 | Not detected* |
| Day 4 | 1138 | 1424 | 196 | Not detected* |
| Day 5 | 1156 | 1633 | 287 | Not detected* |
| Day 6 | 1196 | 1704 | 532 | Not detected* |
The data represent 2 independent experiments.
The level of IL-9 was < 1 pg/mL.
Autocrine IL-2 stimulation of leukemic cells from patients with smoldering/chronic ATL
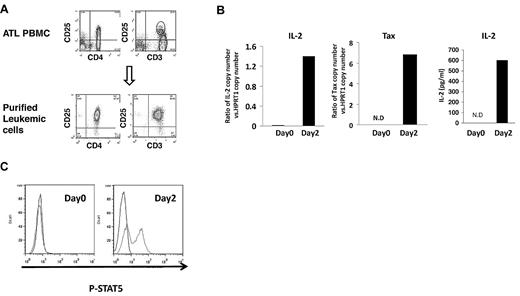
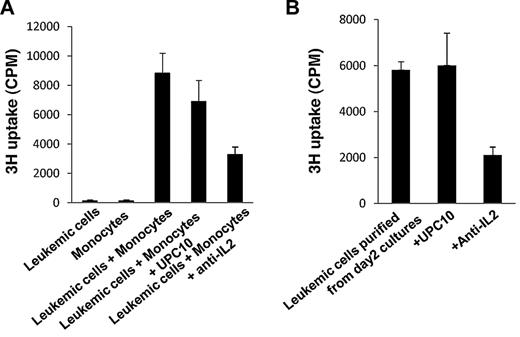
The analysis of the ex vivo cultures of smoldering/chronic ATL PBMCs clearly showed that IL-2 and IL-9 were produced, and that the 2 cytokines were important for the ex vivo proliferation of PBMCs observed. Because the paracrine IL-9 loop in ATL has been described,5 we focused on IL-2 in this study. To determine whether there is an autocrine IL-2–mediated stimulation of ATL leukemic cells, we purified ATL leukemic cells from patients with smoldering/chronic ATL and examined the IL-2 production by the purified leukemic cells. ATL leukemic cells are usually phenotypically characterized as CD4+CD25brightCD3dim cells.20,21 We selected for leukemic cells based on their surface expression of CD4 and their high level of expression of CD25. FACS analysis of these purified leukemic cells confirmed that they also express decreased levels of CD3, compared with the nonleukemic CD3-expressing population (Figure 4A). The purity of the selected leukemic cell population was approximately 92%-97% (Figure 4A). When placed into culture, the purified leukemic cells alone expressed the message for IL-2 at day 2, as assessed by Taqman analysis (Figure 4B). Furthermore, the supernatants of the purified leukemic cell cultures contained 598 pg/mL of IL-2 protein (Figure 4B). To investigate whether IL-2 activates downstream signaling pathways in purified leukemic cells, since they all expressed high levels of IL-2R-α (CD25) on their surface, we analyzed the activation status of STAT5. As shown in Figure 4C, STAT5 was phosphorylated in purified leukemic cells cultured alone for 2 days. This suggested that ATL leukemic cells were able to make IL-2 in the ex vivo cultures, and that the cytokine could signal through the high-affinity IL-2 receptor on the cell surfaces of the leukemic cells.
Autocrine IL-2 stimulation in the leukemic cells from smoldering/chronic ATL PBMCs. (A) FACS analysis of CD4, CD25, and CD3 expression of purified leukemic cells and PBMCs from patients with smoldering/chronic ATL. The data are representative of data from 4 independent experiments. (B) IL-2 and Tax mRNA expression by purified leukemic cells cultured alone for 2 days by TaqMan real-time RT-PCR. The copy numbers of IL-2 and Tax mRNA were normalized to the copy number of hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1) mRNA. The IL-2 levels in the supernatants of 2-day purified leukemic cell cultures were also measured by ELISA. N.D: not detected. (C) FACS analysis of phospho-STAT5 in the purified leukemic cells at day 0 or cultured alone for 2 days in the ex vivo culture.
Autocrine IL-2 stimulation in the leukemic cells from smoldering/chronic ATL PBMCs. (A) FACS analysis of CD4, CD25, and CD3 expression of purified leukemic cells and PBMCs from patients with smoldering/chronic ATL. The data are representative of data from 4 independent experiments. (B) IL-2 and Tax mRNA expression by purified leukemic cells cultured alone for 2 days by TaqMan real-time RT-PCR. The copy numbers of IL-2 and Tax mRNA were normalized to the copy number of hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1) mRNA. The IL-2 levels in the supernatants of 2-day purified leukemic cell cultures were also measured by ELISA. N.D: not detected. (C) FACS analysis of phospho-STAT5 in the purified leukemic cells at day 0 or cultured alone for 2 days in the ex vivo culture.
Monocytes were required to initiate the spontaneous proliferation of purified ATL leukemic cells
We next examined whether the purified ATL leukemic cells could proliferate spontaneously ex vivo. Surprisingly, the purified ATL leukemic cells, when cultured alone, did not proliferate, although they produced IL-2 and activated the Jak-STAT pathway in the ex vivo cultures (Figure 5A). Previously, we showed that purified ATL T cells proliferated when cocultured with autologous monocytes.5 We then tested, in the present study, if coculture with autologous monocytes could induce the proliferation of purified ATL leukemic cells. When cultured with autologous monocytes, the purified ATL leukemic cells proliferated, and this proliferation was partially inhibited by the addition of an antibody to IL-2 (Figure 5A). These data suggested that although autocrine IL-2 stimulation was important for the spontaneous proliferation of ATL leukemic cells, that it was not sufficient to induce the proliferation of ATL leukemic cells. Stimulation from autologous monocytes was also required for proliferation. More interestingly, when we purified CD4+CD25brightCD3dim leukemic cells from 2-day ATL PBMC cultures, the purified leukemic cells alone proliferated ex vivo (Figure 5B). This proliferation was also partially inhibited by the addition of an antibody to IL-2 (Figure 5B). These data suggested that IL-2 signaling was required throughout the ex vivo culture, whereas monocytes were required to be present only early in the ex vivo cultures to initiate the proliferation.
Monocytes were needed early in the ex vivo cultures to initiate the spontaneous proliferation of purified leukemic cells. (A) Six-day spontaneous proliferation of purified leukemic cells, monocytes, and mixtures of purified leukemic cells and monocytes (leukemic cells:monocytes = 1:1). 3H-thymidine was added to the cultures during the last 6 hours of culture. Anti–IL-2 or the control antibody, UPC10, was added to the cultures at day 0. Cells were harvested and analyzed for 3H incorporation. (B) Four-day spontaneous proliferation of purified leukemic cells from 2-day PBMC cultures. Anti–IL-2 and control antibody UPC10 were added at the beginning of the culture. 3H-thymidine was added during the last 6 hours of culture. The data are representative of data from 4 different patients.
Monocytes were needed early in the ex vivo cultures to initiate the spontaneous proliferation of purified leukemic cells. (A) Six-day spontaneous proliferation of purified leukemic cells, monocytes, and mixtures of purified leukemic cells and monocytes (leukemic cells:monocytes = 1:1). 3H-thymidine was added to the cultures during the last 6 hours of culture. Anti–IL-2 or the control antibody, UPC10, was added to the cultures at day 0. Cells were harvested and analyzed for 3H incorporation. (B) Four-day spontaneous proliferation of purified leukemic cells from 2-day PBMC cultures. Anti–IL-2 and control antibody UPC10 were added at the beginning of the culture. 3H-thymidine was added during the last 6 hours of culture. The data are representative of data from 4 different patients.
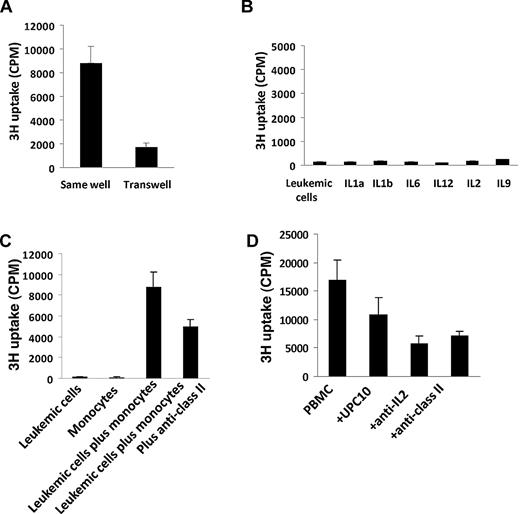
MHC class II expression contributed to the contact-dependent interaction between leukemic cells and monocytes
To better understand the interaction between ATL leukemic cells and autologous monocytes, we performed a 2-chamber T-cell proliferation assay. When the purified leukemic cells and purified monocytes were put into different chambers of the Transwell, which allows only soluble factors to pass through, the purified leukemic cells did not proliferate (Figure 6A). When the purified leukemic cells and purified monocytes were put into the same chamber, proliferation was induced (Figure 6A). This suggested that the contact between leukemic cells and monocytes was important for proliferation, whereas soluble factors made by monocytes were not sufficient. To further examine the latter point, we cultured purified leukemic cells with exogenously added recombinant cytokines that could be produced by monocytes (IL-1a, IL-1b, IL-6, and IL-12) and by leukemic cells (IL-2, IL-9). None of the exogenously added cytokines was able to induce the purified leukemic cells to proliferate (Figure 6B). Monocytes from ATL patients expressed high levels of major histocompatibility complex (MHC) class I and class II molecules.5 We then determined if the MHC molecules were involved in the interaction between leukemic cells and monocytes. The addition of a monoclonal antibody to MHC class II partially inhibited the spontaneous proliferation induced by the coculture of leukemic cells and monocytes (Figure 6C). Moreover, an anti–MHC class II antibody also partially inhibited the proliferation of smoldering/chronic ATL PBMC cultures (Figure 6D). These results suggest that MHC molecules might contribute to the contact-dependent interaction between leukemic cells and monocytes.
MHC class II contributed to the proliferation induced by the interaction between purified leukemic cells and monocytes. (A) Purified leukemic cells and monocytes were cultured in the same chamber or a different chamber of the Transwell (0.4-μm) for 6 days. 3H-thymidine was added to the culture during the last 6 hours of the culture. (B) Proliferation of purified leukemic cells cultured with or without 10 ng/mL recombinant human IL-1-α, IL-1b, IL-6, IL-12, IL-2, and IL-9. (C) Six-day spontaneous proliferation of purified leukemic cells, monocytes, and mixtures of purified leukemic cells and monocytes (leukemic cells: monocytes = 1:1). 3H-thymidine was added to the culture during the last 6 hours of culture. Anti-MHC class II or control antibody UPC10 were added to the culture at day 0. (D) Six-day spontaneous proliferation of smoldering/chronic ATL PBMCs with or without anti-MHC class II antibody and control antibody UPC10. 3H-thymidine was added to the cultures during the last 6 hours of culture. Anti-MHC class II or control antibody UPC10 were added to the cultures at day 0. The data are representative of data from 4 different patients.
MHC class II contributed to the proliferation induced by the interaction between purified leukemic cells and monocytes. (A) Purified leukemic cells and monocytes were cultured in the same chamber or a different chamber of the Transwell (0.4-μm) for 6 days. 3H-thymidine was added to the culture during the last 6 hours of the culture. (B) Proliferation of purified leukemic cells cultured with or without 10 ng/mL recombinant human IL-1-α, IL-1b, IL-6, IL-12, IL-2, and IL-9. (C) Six-day spontaneous proliferation of purified leukemic cells, monocytes, and mixtures of purified leukemic cells and monocytes (leukemic cells: monocytes = 1:1). 3H-thymidine was added to the culture during the last 6 hours of culture. Anti-MHC class II or control antibody UPC10 were added to the culture at day 0. (D) Six-day spontaneous proliferation of smoldering/chronic ATL PBMCs with or without anti-MHC class II antibody and control antibody UPC10. 3H-thymidine was added to the cultures during the last 6 hours of culture. Anti-MHC class II or control antibody UPC10 were added to the cultures at day 0. The data are representative of data from 4 different patients.
Discussion
ATL can be divided into smoldering, chronic, lymphoma, and acute types, based on their clinical manifestations. Other than clinical symptoms, different stages of ATL have different molecular signatures. Although the mechanisms underlying the progression from smoldering/chronic to the acute stage are unknown, various genetic events as well as epigentic events involved in the progression have been reported. These include the accumulation of gene mutations that break down cell-cycle regulation (eg, p53 and p16 mutations),22 down-regulation or silencing of negative regulators, such as gene down-regulated by V-Src,23 Src homology 2 domain–containing protein tyrosine phosphatase-1,24 and ASY/Nogo,25 expression of acute-phase–specific genes that are associated with cell growth26 and apoptosis resistance (eg, MET, NCOA3, HSPD1, GTF3A, and BIRC5),27 expression-pattern change of HTLV-1 Tax, increasing genomic instability and chromosome abnormalities,26 and epigenetic modifications including DNA hypermethylation in specific target genes28 and the presence of CIMP (CpG island methylator phenotype) that involves multigene methylation simultaneously.29
In this study, our data clearly demonstrated the cytokine (IL-2, IL-9, and IL-15)–dependent spontaneous proliferation in smoldering/chronic ATL PBMCs as an addition of monoclonal antibodies to cytokines or cytokine receptors inhibited the ex vivo spontaneous proliferations (anti–IL-2/anti–IL-2R-α (CD25), 76%/80%; anti–IL-15/anti–IL-2/15R-β (CD122), 37%/43%; anti–IL-9/anti–IL-9R-α (CD129), 41%/56% (Figure 1B). Furthermore, the smoldering/chronic PBMCs expressed IL-2 and IL-9 message, as assessed by Taqman analysis (Figure 3), and the supernatants from PBMC cultures from patients with smoldering/chronic ATL contained IL-2 and IL-9 (Tables 1–2). IL-15 was not detected in any of the 11 smoldering/chronic ATL supernatants, possibly due to high-affinity binding of IL-15 to its receptor on cell surfaces.30 The 11 smoldering/chronic ATL patients studied appeared to be in the very early phase of their leukemia, as only 6 patients showed monoclonal leukemic population by PCR at the time the ex vivo proliferation was performed. On the contrary, acute-type ATL either did not spontaneously proliferate (no proliferation in 7 of 9 cases; Figure 1A) or only proliferated independent of autologous cytokines (IL-2/IL-9/IL-15; Figure 1D). They did not generate and secret IL-2 and IL-9 into the ex vivo cultures either (data not shown). This suggested that they had gone past the cytokine (IL-2/IL-9/IL-15)–dependent phase of the disease. Consistent with this observation, in our phase 2 trial with daclizumab (anti–IL-2R-α), we observed partial or complete remissions in some patients with smoldering/chronic ATL, but this antibody appears to have little activity in the more aggressive acute subtype of ATL (J. Berkowitz, T.A.W., and J.C.M., unpublished data, October 2010).
To further determine whether there is an autocrine IL-2–mediated stimulation in the ATL leukemic cells, we purified ATL leukemic cells from the PBMCs of patients with smoldering/chronic ATL. The purified ATL leukemic cells alone produced IL-2 (Figure 4B) and IL-9 (data not shown) in ex vivo cultures and activated the Jak/STAT-signaling pathway. Because ATL leukemic cells express high levels of CD25, the IL-2 receptor alpha, but not the IL-9 receptor alpha,5 the activation of STAT5 was probably due to IL-2 signaling. Despite the expression of IL-2/IL-2R-α and the activation of the Jak/STAT pathway, the purified ATL leukemic cells alone did not proliferate spontaneously (Figure 5A). They needed help from autologous monocytes, as the coculture of purified leukemic cells with purified monocytes induced proliferation (Figure 5A). Interestingly, the monocytes appeared to be required only very early in the ex vivo cultures, as the leukemic cells isolated from PBMCs that were cultured for 2 days before the separation proliferated spontaneously without monocytes. Once the proliferation was induced, IL-2 signaling seemed to play a central role in maintaining the proliferation, as the addition of an anti-IL-2 antibody inhibited the proliferation of leukemic cell/monocyte cocultures as well as the cultures of ATL cells isolated from 2-day PBMC cultures. This suggested that the efficacy of daclizumab therapy in smoldering/chronic patients was probably due to its blockade of IL-2–dependent proliferation/survival of leukemic cells.
Adherent cells have been shown to be important elements in the spontaneous proliferation of HTLV-1–infected T cells.31,32 In patients with HAM/TSP, removal of adherent cells from ex vivo cultures led to a significant reduction in the proliferation manifested by the T cells.32 Similarly, we have reported previously that IL-9R-α–expressing monocytes contribute to ATL cell spontaneous proliferation in select patients with ATL.5 However, the requirement for monocytes for ATL cell ex vivo proliferation seems to be a more general phenomenon than implied by their IL-9R-α expression. Here, we demonstrated that purified leukemic cells need autologous monocytes to proliferate (Figure 5A). Furthermore, the interaction between purified leukemic cells and monocytes was contact dependent, as the leukemic cells did not proliferate when put into a different chamber of a Transwell from the monocytes (Figure 6A). Moreover, contrary to the observation of others,33 exogenously added cytokines, such as IL-1, IL-6, and IL-12, did not replace the requirement for monocytes, suggesting that monocytes provided signals other than cytokines to the leukemic cells (Figure 6B). Interestingly, a monoclonal antibody to MHC class II inhibited the proliferation induced by leukemic cell monocyte cocultures as well as ATL PBMC cultures (Figure 6C-D). This suggested that MHC class II was probably a participant in the interaction between leukemic cells and monocytes. CD14+ monocytes can be infected by HTLV-I virus34 ; moreover, in HAM/TSP, CD14+ monocytes provided a signal for virus-specific CD8 cell expansion and cytotoxic T-lymphocyte degranulation in a MHC class I–restricted manner in the ex vivo cultures.34 Although the virus-specific CD4 T-cell response in HAM/TSP has not been analyzed, it would be interesting to investigate virus-specific CD4 cell expansion in patients with ATL.
IL-9 is a Th2 cytokine. IL-9 was produced in more than 80% of smoldering/chronic ATL ex vivo PBMC cultures. We have previously shown that HTLV-1 Tax transactivates IL-9 expression in HTLV-1–infected T-cell lines.5 However, it appears that IL-2 signaling was also needed for the optimal expression of IL-9 in the ex vivo culture of PBMCs from patients with smoldering/chronic ATL. The addition of an antibody to IL-2R-α profoundly inhibited the production of IL-9, while having little effect on IL-2 and Tax expression. Consistent with our observation, Fung et al and Kajiyama et al showed that IL-2 induced IL-9 production in HTLV-1–infected cell lines and allergen-specific human helper T-cell clones.35,36 Further analysis of the IL-9 promoter would be helpful to identify transcriptional elements and epigenetic events that are responsible for IL-2–induced IL-9 expression.
Taken together, our studies demonstrated that PBMCs from patients with smoldering/chronic ATL proliferated spontaneously ex vivo in a cytokine-dependent manner. Both IL-2 and IL-9, through the interaction of IL-2 with IL-2R-α, are expressed by the ATL cells and are involved in the monocyte-dependent proliferation of ATL leukemic cells. IL-2R-α–directed therapy (anti-Tac, daclizumab) of patients with smoldering and chronic ATL has been conducted with partial or complete remission in a few patients. However, in light of the fact that both IL-2 and IL-9 are involved in the proliferation of ATL cells, it is difficult to completely inhibit the proliferation with a single cytokine-receptor–directed monoclonal antibody. IL-2, IL-9, and IL-15 share the use of the common γ-chain and the Jak3/STAT5-signaling pathway.37 Based on these findings, it would be interesting to test agents that target Jak3 in the treatment of patients with smoldering/chronic ATL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported by the intramural research program of the National Cancer Institute, National Institutes of Health.
National Institutes of Health
Authorship
Contribution: J.C. designed and performed research, collected, analyzed and interpreted data, and wrote the manuscript; M.P., B.R.B., V.P.N., and C.K.G. performed research; J.C.M. and J.E.J. provided patient care and collected patient samples; R.B. provided critical insights in research design; and T.A.W. designed research and revised the manuscript.
Conflict-of interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas A. Waldmann, Bldg 10, Rm 4N115, 10 Center Dr, National Institutes of Health, Bethesda, MD 20892-1374; e-mail: tawald@helix.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal