Abstract
Few studies have examined the outcome of large numbers of patients with the microgranular variant (M3V) of acute promyelocytic leukemia (APL) in the all-trans retinoic acid era. Here, the outcome of 155 patients treated with all-trans retinoic acid–based therapy on 3 clinical trials, North American Intergroup protocol I0129 and Programa para el Estudio de la Terapéutica en Hemopatía Maligna protocols LPA96 and LPA99, are reported. The complete remission rate for all 155 patients was 82%, compared with 89% for 748 patients with classical M3 disease. The incidence of the APL differentiation syndrome was 26%, compared with 25% for classical M3 patients, and the early death rate was 13.6% compared with 8.4% for patients with classical M3 morphology. With a median follow-up time among survivors of 7.6 years (range 3.6-14.5), the 5-year overall survival, disease-free survival, and cumulative incidence of relapse for patients with M3V were 70%, 73%, and 24%, respectively. With a median follow-up time among survivors of 7.6 years (range 0.6-14.3), the 5-year overall survival, disease-free survival, and cumulative incidence of relapse among patients with classical M3 morphology were 80% (P = .006 compared with M3V), 81% (P = .07), and 15% (P = .005), respectively. When outcomes were adjusted for the white blood cell count or the relapse risk score, none of these outcomes were significantly different between patients with M3V and classical M3 APL.
Introduction
Approximately 15%-25% of adults and perhaps a somewhat higher incidence of children with acute promyelocytic leukemia (APL) have the microgranular variant (M3V) characterized by leukemia promyelocytes that are generally devoid of or have only sparse fine granules1-6 and infrequent Auer rods.7 In addition to the distinctive morphologic features, this variant form of the disease is associated with unique biological characteristics including a higher white blood cell count (WBC) at presentation8 and frequent expression of CD2,9-11 the stem cell marker CD34,10,11 and FLT3 internal tandem duplication (ITD) mutations.12,13 Several series9,14 but not all15,16 reported an association of the S-isoform of promyelocyte (PML) with M3V. Classical hypergranular APL and the M3V have distinct gene expression signatures.17 Historically, when treated with conventional chemotherapy, the M3V has been associated with a higher incidence of early death,2 but not necessarily with an inferior outcome compared with that associated with classical APL.18-24 However, few studies in the all-trans retinoic acid (ATRA) era have reported the outcome of a large number of patients with M3V.
Therefore, we sought to determine the outcome of patients with M3V when treated with ATRA-based strategies. In the present study, we undertook an analysis of 3 large series of patients treated with ATRA plus anthracycline-based regimens, North American Intergroup protocol I0129 and Programa de Estudio Tratamiento de las Hemopatias Malignas (PETHEMA) protocols LPA96 and LPA99, to have sufficient numbers of patients to definitively determine the outcome.
Methods
Patients with M3V registered on either North American Intergroup Protocol I0129 or PETHEMA Protocols LPA96 or LPA99 with a confirmed diagnosis of APL by either cytogenetics or molecular genetics were analyzed. The diagnosis of M3V was established when most of the leukemic cells were devoid of granules or had only sparse granules.25,26 The abnormal promyelocytes had bilobed nucleoli with basophilic cytoplasm that varied from faint to strong. Rare cells with multiple Auer rods were found almost invariably. Myeloperoxidase and granulocyte esterase were strongly positive as in classical APL. The morphology establishing the diagnosis of M3V among patients treated on the North American Intergroup Protocol I0129 was centrally reviewed by a single author (J.M.B.). The morphology from the bone marrow of M3V patients treated on the PETHEMA protocols was not centrally reviewed. The diagnoses were confirmed either cytogenetically or molecularly in all 3 studies.
North American Intergroup Protocol I0129
The results of the North American Intergroup Protocol I0129 have been previously reported.23,24 Briefly, patients registered to North American Protocol I0129 were randomly assigned for induction to receive either ATRA or chemotherapy, which included daunorubicin plus cytarabine. Patients assigned to ATRA were to receive 45 mg/m2/d orally in 2 divided doses given every 12 hours. Patients assigned to chemotherapy were to receive daunorubicin 45 mg/m2/d by intravenous bolus on days 1-3 plus cytarabine 100 mg/m2/d by continuous intravenous infusion on days 1-7 (DA). All patients achieving a complete remission (CR) with either ATRA or chemotherapy received 2 courses of consolidation. The first was identical to the first induction chemotherapy regimen, and the second included high-dose cytarabine 2 gm/m2 as a 1-hour intravenous infusion every 12 hours for 4 consecutive days with daunorubicin 45 mg/m2/d by intravenous infusion on days 1 and 2. For patients less than 3 years of age, the second cycle included cytarabine 67 mg/kg as a 1-hour intravenous infusion every 12 hours for 4 consecutive days with daunorubicin 1.5 mg/kg/d by intravenous infusion on days 1 and 2. Patients randomized to ATRA were to continue the drug until CR occurred or a maximum of 90 days. Patients continuing in CR after consolidation were randomized to either 1 year of daily maintenance ATRA or observation. Patients randomized to chemotherapy (DA) only for induction and not ATRA were excluded from all analyses.
PETHEMA protocols LPA96 and LPA99
Results of the PETHEMA protocols LPA96 and LPA99 have been previously reported.27-29 Briefly, the induction regimen consisted of oral ATRA 45 mg/m2/d until CR and intravenous idarubicin (12 mg/m2/d) on days 2, 4, 6, and 8 (all-trans retinoic acid and idarubicin [AIDA] regimen). From November 1999, the idarubicin on day 8 was omitted for patients older than 70 years. Patients in CR received 3 monthly consolidation courses. The first course consisted of idarubicin 5 mg/m2/d for 4 days, the second of mitoxantrone 10 mg/m2/d for 5 days, and the third of idarubicin 12 mg/m2/d for 1 day. From November 1, 1999 (LPA99 study), intermediate- and high-risk patients, as previously defined,29 received ATRA 45 mg/m2/d for 15 days combined with the reinforced single-agent chemotherapy courses.28 Risk of relapse was established at diagnosis according to a predictive model based on patient leukocyte and platelet counts at diagnosis, as reported elsewhere.30 Low-risk patients had a WBC less than or equal to 10 × 109/L and a platelet count more than 40 × 109/L; intermediate-risk patients had a WBC less than or equal to 10 × 109/L and a platelet count less than or equal to 40 × 109/L; and high-risk patients had a WBC more than 10 × 109/L.
Patients who tested negative for PML/RARA fusion transcript at the end of consolidation were started on maintenance therapy with oral mercaptopurine 50 mg/m2/d, intramuscular methotrexate 15 mg/m2/wk, and oral ATRA 45 mg/m2/d for 15 days every 3 months over 2 years. Details of the supportive therapy have been described elsewhere.27,28
Definition of the APL differentiation syndrome
For patients on I0129, APL differentiation syndrome (DS) was defined as grade 2 or higher pulmonary or cardiac toxicity with unexplained fever, weight gain, respiratory distress, interstitial pulmonary infiltrates, and/or pleural or pericardial effusions.23,24 The definition of the APL DS for patients treated on the 2 PETHEMA protocols matched that used for patients on I0129 except that renal failure, itself defined as a creatinine above the upper limit of normal, was also considered a criterion for the DS.31
Statistical analysis
Descriptive statistical analysis was performed to assess patient baseline characteristics. The Cochran-Mantel-Haenszel test was used for comparison of categorical variables and a stratified Wilcoxon rank-sum test was used for comparison of continuous variables. All tests were stratified by the source of patients: I0129, LPA96, LPA99.
Overall survival (OS) and disease-free survival (DFS) were calculated using the Kaplan-Meier method. The stratified log-rank test was used for comparison of Kaplan-Meier curves, stratifying by the source of patients. OS was defined as the time between start of induction and death from any cause. DFS was defined as the time between documented date of CR and relapse or death from any cause. Cumulative incidence curves for relapse with or without death were constructed reflecting time to nonrelapse death as a competing risk. Time to relapse and time to nonrelapse death were measured from the documented date of CR to relapse or nonrelapse death. Patients who were alive without relapse were censored at the time last seen alive and relapse-free. The difference between cumulative incidence curves in the presence of a competing risk was tested using the Gray method32 stratified by the source of patients. The impact of the APL morphology subtype (M3 vs. M3V) on the outcomes was also examined in multivariable proportional hazards model for OS and DFS and multivariable competing risks regression model33 for relapse and nonrelapse death. In multivariable models, age, sex, WBC, platelet, and hemoglobin were included along with the APL morphology subtype. All interaction terms between the APL morphology subtype and prognostic factors were examined.
Results
Patient characteristics
Patient characteristics are shown in Table 1. A total of 155 patients with M3V accrued to the 3 clinical trials were analyzed. The median age was 39 years (range 3-79). The median WBC was 15.8 × 109/L (range 0.60--550 × 109). Among 748 patients with classical M3 APL, the median age was 40 years (range 1-83) and the median WBC was 1.8 × 109/L (range 0.2-460 × 109/L). There was a significant difference in WBC at baseline between patients with M3V and classical M3 (P < .0001). The proportion of the relapse risk score at diagnosis, according to PETHEMA–Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) criteria, for patients with M3V was 15% low, 25% intermediate, and 61% high risk. Among patients with classical M3 APL, the proportions of patients among the 3 risk groups were 24%, 60%, and 16%, respectively. Thus, the difference in distribution of the relapse risk score between M3V and classical M3 was significant (P < .0001). Patients with M3V were more likely to have high-risk disease at diagnosis because of the higher WBC in these patients.
Baseline patient characteristics
| . | M3 . | M3V . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| I0129 (n = 150) . | LPA96 (n = 145) . | LPA99 (n = 453) . | All (n = 748) . | I0129 (n = 24) . | LPA 96 (n = 30) . | LPA 99 (n = 101) . | All (n = 155) . | ||
| Median age, y (range) | 37 (1-81) | 42 (2-78) | 40 (2-83) | 40 (1-83) | 36 (5-76) | 40 (12-71) | 39 (3-79) | 39 (3-79) | .51 |
| Male, n (%) | 71 (47) | 87 (60) | 216 (48) | 374 (51) | 11 (46) | 16 (53) | 50 (53) | 77 (50) | .93 |
| WBC, median (range) | 1.8 (0.3-95.2) | 1.5 (0.3-148.0) | 1.8 (0.2-460) | 1.8 (0.2-460) | 5.8 (0.6-550) | 19.8 (1.4-210) | 15.9 (0.7-188.5) | 15.8 (0.6-550) | < .0001 |
| Platelets, median (range) | 36.5 (5-246) | 20 (1-161) | 22 (1-207) | 24 (1-246) | 36 (6-123) | 19.5 (4-146) | 22 (1.8-207) | 23 (1.8-207) | .52 |
| Hemoglobin, median (range) | 9.5 (2.9-16.4) | 9.5 (4.4-12.8) | 8.9 (3.0-15.3) | 9.2 (2.9-16.4) | 9.6 (2.1-13.2) | 9.1 (4.3-15.2) | 10.0 (4.4-16.9) | 9.8 (2.1-16.9) | .0005 |
| Relapse risk, n (%) | < .0001 | ||||||||
| Low | 56 (37) | 31 (21) | 94 (21) | 181 (24) | 8 (33) | 3 (10) | 12 (12) | 23 (15) | |
| Intermediate | 72 (48) | 91 (63) | 284 (63) | 447 (60) | 7 (29) | 6 (20) | 25 (25) | 38 (25) | |
| High | 22 (15) | 23 (16) | 75 (17) | 120 (16) | 9 (38) | 21 (70) | 64 (64) | 94 (61) | |
| RAS, n (%) | 43 (29) | 41 (28) | 102 (23) | 186 (25) | 1 (4.2) | 40 (31) | 40 (31) | 41 (26) | .66 |
| CR rate, % | 80* | 92 | 92 | 89* | 79* | 77 | 84 | 82* | .004 (.64)† |
| Follow-up time among survivors, median (range) | 11.9 (0.6-14.3) | 10.6 (9.4-12) | 6.4 (3.5-9) | 7.6 (0.6-14.3) | 10.9 (4.3-14.5) | 10.6 (9.7-12.1) | 6.9 (3.6-9.3) | 7.6 (3.6-14.5) | .34 |
| . | M3 . | M3V . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| I0129 (n = 150) . | LPA96 (n = 145) . | LPA99 (n = 453) . | All (n = 748) . | I0129 (n = 24) . | LPA 96 (n = 30) . | LPA 99 (n = 101) . | All (n = 155) . | ||
| Median age, y (range) | 37 (1-81) | 42 (2-78) | 40 (2-83) | 40 (1-83) | 36 (5-76) | 40 (12-71) | 39 (3-79) | 39 (3-79) | .51 |
| Male, n (%) | 71 (47) | 87 (60) | 216 (48) | 374 (51) | 11 (46) | 16 (53) | 50 (53) | 77 (50) | .93 |
| WBC, median (range) | 1.8 (0.3-95.2) | 1.5 (0.3-148.0) | 1.8 (0.2-460) | 1.8 (0.2-460) | 5.8 (0.6-550) | 19.8 (1.4-210) | 15.9 (0.7-188.5) | 15.8 (0.6-550) | < .0001 |
| Platelets, median (range) | 36.5 (5-246) | 20 (1-161) | 22 (1-207) | 24 (1-246) | 36 (6-123) | 19.5 (4-146) | 22 (1.8-207) | 23 (1.8-207) | .52 |
| Hemoglobin, median (range) | 9.5 (2.9-16.4) | 9.5 (4.4-12.8) | 8.9 (3.0-15.3) | 9.2 (2.9-16.4) | 9.6 (2.1-13.2) | 9.1 (4.3-15.2) | 10.0 (4.4-16.9) | 9.8 (2.1-16.9) | .0005 |
| Relapse risk, n (%) | < .0001 | ||||||||
| Low | 56 (37) | 31 (21) | 94 (21) | 181 (24) | 8 (33) | 3 (10) | 12 (12) | 23 (15) | |
| Intermediate | 72 (48) | 91 (63) | 284 (63) | 447 (60) | 7 (29) | 6 (20) | 25 (25) | 38 (25) | |
| High | 22 (15) | 23 (16) | 75 (17) | 120 (16) | 9 (38) | 21 (70) | 64 (64) | 94 (61) | |
| RAS, n (%) | 43 (29) | 41 (28) | 102 (23) | 186 (25) | 1 (4.2) | 40 (31) | 40 (31) | 41 (26) | .66 |
| CR rate, % | 80* | 92 | 92 | 89* | 79* | 77 | 84 | 82* | .004 (.64)† |
| Follow-up time among survivors, median (range) | 11.9 (0.6-14.3) | 10.6 (9.4-12) | 6.4 (3.5-9) | 7.6 (0.6-14.3) | 10.9 (4.3-14.5) | 10.6 (9.7-12.1) | 6.9 (3.6-9.3) | 7.6 (3.6-14.5) | .34 |
P value: comparison between M3 and M3V stratified by study (I0129, LPA96, LPA99).
Accounting for ATRA patients who achieved CR after crossing over to induction chemotherapy.
After controlling for WBC.
As to the difference in baseline characteristics among 3 studies with M3V, WBC was higher in LPA96 and LPA99 (19.8 × 109/L, 15.9 × 109/L, respectively) compared with I0129 (5.8 × 109/L) (P = .01), but platelet was lower (19.5 × 109/L, 22 × 109/L, respectively) compared with I0129 (36 × 109/L, P = .005).
The median follow-up of all surviving patients with M3 and M3V combined was 7.6 years (range 0.6-14.5): 11.9 and 10.9 years for M3 and M3V, respectively, on I0129 (range 0.6-14.5) and 10.6 and 10.7 years, respectively, (range 9.4-12.1) on LPA96 and 6.4 and 6.9 years, respectively (range 3.5-9.3) on LPA99. The median follow-up time among survivors was similar between M3V and M3 (7.6 years) (Table 1).
Induction therapy
Complete remission.
The CR rate among all patients with M3V treated with ATRA-based regimens was 82%, 79% for patients treated with ATRA alone on I0129, 77% on LPA96, and 84% among patients treated with AIDA on LPA99. Among patients with classical M3 treated with ATRA-based regimens, the CR rate was 89%, 80% among those treated with ATRA alone on I0129 and 92% among those treated with AIDA on LPA96 and LPA99 (P = .004 compared with M3V). However, when the CR rate was controlled for WBC, the difference is no longer significant. Among patients with M3V, the CR rates for those who presented with a WBC less than 5 × 109/L, 5-10 × 109/L, and greater than or equal 10 × 109/L were 89%, 92%, and 77%, respectively, compared with 92%, 80%, and 82%, respectively, for patients with classical M3 APL (P = .64). The CR rate did not differ among the 3 protocols in patients with the M3V.
APL differentiation syndrome.
Among all M3V patients treated with ATRA-based regimens for induction, the incidence of the APL DS was 26%, 4% among patients treated on I0129 and 31% among patients treated on LPA96 and LPA99 combined. Among patients with classical M3 APL, the APL DS developed in 25% of the 748 patients, 29% of patients on I0129 and 24% of patients treated on the 2 PETHEMA protocols (P = .66) (Table 1).
Early death rate.
The death rate within 30 days of the induction therapy for all M3V patients was 13.6%: 8.3% on I0129 and 20% on LPA96 and 12.9% on LPA99 (Table 2). Among patients with classical M3, the induction death rate within 30 days was 8.4%: 10.7% on I0129 and 6.9% on LPA96 and 7.5% on LPA99 (P = .02). This difference was no longer significant when the early death rate accounted for WBC (P = .87). Cause of 30-day induction death is listed in Table 3. There appears to be no apparent correlation between hemorrhage and the morphology subtype. Among M3V patients, hemorrhage was the main cause of early death in all 3 protocols. The same is true for patients with classical M3 morphology.
Early death rate (within 30 days of induction)
| . | M3, % . | M3V, % . | P . |
|---|---|---|---|
| I0129 | 10.7 (2.5) | 8.3 (5.6) | — |
| LPA96 | 6.9 (2.1) | 20 (7.3) | — |
| LPA99 | 7.5 (1.2) | 12.9 (3.3) | — |
| All | 8.4 (1.0) | 13.6 (2.8) | .02* |
| . | M3, % . | M3V, % . | P . |
|---|---|---|---|
| I0129 | 10.7 (2.5) | 8.3 (5.6) | — |
| LPA96 | 6.9 (2.1) | 20 (7.3) | — |
| LPA99 | 7.5 (1.2) | 12.9 (3.3) | — |
| All | 8.4 (1.0) | 13.6 (2.8) | .02* |
Standard error given in parentheses.
Stratified by study (I0129, LPA96, LPA99).
Cause of death (within 30 days of induction)
| . | M3, % . | M3V, % . |
|---|---|---|
| I0129 | ||
| Hemorrhage | 10 (62.5) | 1 (50) |
| DS | 2 (12.5) | 0 (0) |
| Other | 4* (25) | 1† (50) |
| LPA96 | ||
| Infection | 2 (20) | 3 (50) |
| Hemorrhage | 6 (60) | 3 (50) |
| DS | 2 (20) | 0 (0) |
| LPA99 | — | — |
| Infection | 8 (23.5) | 3 (23) |
| Hemorrhage | 20 (58.8) | 7 (53.8) |
| DS | 6 (17.6) | 2 (15.4) |
| Other | 0 (0) | 1‡ (7.7) |
| . | M3, % . | M3V, % . |
|---|---|---|
| I0129 | ||
| Hemorrhage | 10 (62.5) | 1 (50) |
| DS | 2 (12.5) | 0 (0) |
| Other | 4* (25) | 1† (50) |
| LPA96 | ||
| Infection | 2 (20) | 3 (50) |
| Hemorrhage | 6 (60) | 3 (50) |
| DS | 2 (20) | 0 (0) |
| LPA99 | — | — |
| Infection | 8 (23.5) | 3 (23) |
| Hemorrhage | 20 (58.8) | 7 (53.8) |
| DS | 6 (17.6) | 2 (15.4) |
| Other | 0 (0) | 1‡ (7.7) |
DS indicates APL differentiation syndrome.
One respiratory arrest, 1 myocardial infarction, 1 multiorgan thrombosis, 1 liver failure.
One myocardial infarction.
One myocardial infarction.
Outcome
Overall survival.
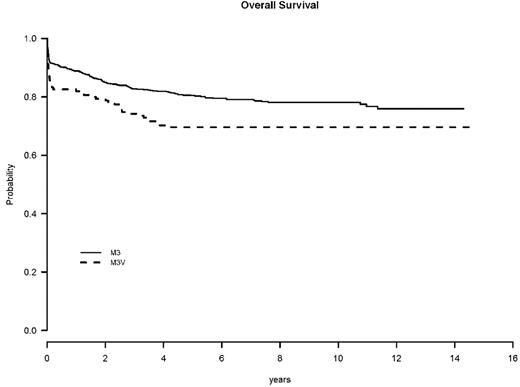
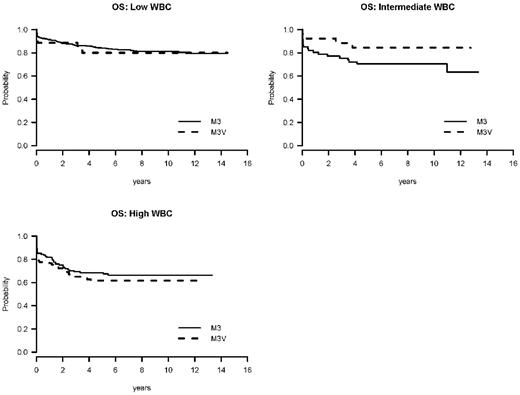
The OS at 5 years was 70% for M3V patients (63% for I0129, 57% for LPA96, and 75% for LPA99, P = .007) and 80% for M3 patients (71% for I0129, 80% for LPA96, and 84% for LPA99, P = .006) (Table 4 and Figure 1). However, when the OS was calculated accounting for the WBC, the difference is no longer present. Among patients with M3V, the 5-year OS rates for those who presented with a WBC less than 5 × 109/L, 5-10 × 109/L, and greater than or equal to 10 × 109/L were 80%, 84%, and 62%, respectively, compared with patients with classical M3 APL: 84%, 70%, and 68%, respectively (P = .70, .15, .47 for WBC less than 5 × 109/L, 5-10 × 109/L, and greater than or equal to 10 × 109/L, respectively, and P = .87 for overall WBC adjusted) (Table 5). The same result was seen in a multivariable Cox model. When age, male sex, WBC, platelet, hemoglobin, and APL morphology subtype were included in the stratified Cox regression model, the APL morphology subtype was not significant in OS (HR = 1.04 for M3V compared with M3, P = .84) (Table 6). In this model, the unfavorable prognostic factors for OS were age greater than or equal to 60 (HR = 3.13, P < .0001), male sex (HR = 1.55, P = .003), and high WBC (HR = 2.38, P < .0001) (Figure 2).
Overall survival and disease-free survival
| . | . | M3 . | M3V . | P . | ||||
|---|---|---|---|---|---|---|---|---|
| n . | 5-year, % (SE) . | 10-year, % (SE) . | n . | 5-year, % (SE) . | 10-year, % (SE) . | |||
| OS | I0129 | 150 | 71 (3.7) | 67 (3.9) | 24 | 63 (9.9) | 63 (9.9) | .7 |
| LPA96 | 145 | 80 (3.3) | 78 (3.4) | 30 | 57 (9.1) | 57 (9.1) | .007 | |
| LPA99 | 453 | 84 (1.8) | — | 101 | 75 (4.3) | — | .06 | |
| All | 748 | 80 (1.5) | — | 155 | 70 (3.7) | — | .006 (.87*) | |
| DFS | I0129 | 120 | 65 (4.4) | 61 (4.5) | 19 | 63 (11.1) | 63 (11.1) | .79 |
| LPA96 | 133 | 80 (3.4) | 79 (3.5) | 23 | 57 (10.3) | 57 (10.3) | .01 | |
| LPA99 | 416 | 86 (1.7) | — | 85 | 80 (4.3) | — | .24 | |
| All | 669 | 81 (1.5) | — | 127 | 73 (3.9) | — | .07 (0.5*) | |
| . | . | M3 . | M3V . | P . | ||||
|---|---|---|---|---|---|---|---|---|
| n . | 5-year, % (SE) . | 10-year, % (SE) . | n . | 5-year, % (SE) . | 10-year, % (SE) . | |||
| OS | I0129 | 150 | 71 (3.7) | 67 (3.9) | 24 | 63 (9.9) | 63 (9.9) | .7 |
| LPA96 | 145 | 80 (3.3) | 78 (3.4) | 30 | 57 (9.1) | 57 (9.1) | .007 | |
| LPA99 | 453 | 84 (1.8) | — | 101 | 75 (4.3) | — | .06 | |
| All | 748 | 80 (1.5) | — | 155 | 70 (3.7) | — | .006 (.87*) | |
| DFS | I0129 | 120 | 65 (4.4) | 61 (4.5) | 19 | 63 (11.1) | 63 (11.1) | .79 |
| LPA96 | 133 | 80 (3.4) | 79 (3.5) | 23 | 57 (10.3) | 57 (10.3) | .01 | |
| LPA99 | 416 | 86 (1.7) | — | 85 | 80 (4.3) | — | .24 | |
| All | 669 | 81 (1.5) | — | 127 | 73 (3.9) | — | .07 (0.5*) | |
Comparison between M3 and M3V, stratified by WBC.
Overall survival and disease-free survival by WBC count
| . | WBC . | M3 . | M3V . | P . | ||||
|---|---|---|---|---|---|---|---|---|
| n . | 5-year, % (SE) . | 10-year, % (SE) . | n . | 5-year, % (SE) . | 10-year, % (SE) . | |||
| OS | .87* | |||||||
| 0-< 5 | 566 | 84 (1.6) | 81 (1.7) | 35 | 80 (6.8) | 80 (6.8) | .7 | |
| ≤ 5-< 10 | 61 | 70 (5.8) | 70 (5.8) | 26 | 84 (7.2) | 84 (7.2) | .15 | |
| ≥ 10 | 121 | 68 (4.2) | 66 (4.4) | 94 | 62 (5.0) | 62 (5.0) | .47 | |
| DFS | .5* | |||||||
| 0-< 5 | 521 | 85 (1.6) | 82 (1.8) | 31 | 84 (6.6) | 84 (6.6) | .87 | |
| ≤ 5-< 10 | 49 | 80 (5.8) | 80 (5.8) | 24 | 79 (8.3) | 79 (8.3) | .96 | |
| ≥ 10 | 99 | 63 (4.9) | 62 (5.0) | 72 | 67 (5.6) | 67 (5.6) | .45 | |
| . | WBC . | M3 . | M3V . | P . | ||||
|---|---|---|---|---|---|---|---|---|
| n . | 5-year, % (SE) . | 10-year, % (SE) . | n . | 5-year, % (SE) . | 10-year, % (SE) . | |||
| OS | .87* | |||||||
| 0-< 5 | 566 | 84 (1.6) | 81 (1.7) | 35 | 80 (6.8) | 80 (6.8) | .7 | |
| ≤ 5-< 10 | 61 | 70 (5.8) | 70 (5.8) | 26 | 84 (7.2) | 84 (7.2) | .15 | |
| ≥ 10 | 121 | 68 (4.2) | 66 (4.4) | 94 | 62 (5.0) | 62 (5.0) | .47 | |
| DFS | .5* | |||||||
| 0-< 5 | 521 | 85 (1.6) | 82 (1.8) | 31 | 84 (6.6) | 84 (6.6) | .87 | |
| ≤ 5-< 10 | 49 | 80 (5.8) | 80 (5.8) | 24 | 79 (8.3) | 79 (8.3) | .96 | |
| ≥ 10 | 99 | 63 (4.9) | 62 (5.0) | 72 | 67 (5.6) | 67 (5.6) | .45 | |
Comparing overall M3 versus M3V, adjusted for by WBC category.
Stratified Cox regression model
| . | OS . | DFS . | ||
|---|---|---|---|---|
| Hazard ratio (95 confidence interval) . | P . | Hazard ratio (95 confidence interval) . | P . | |
| Age ≥ 60 y vs age < 60 y | 3.13 (2.32–4.21) | < .0001 | 2.13 (1.48–3.07) | < .001 |
| Male vs female | 1.55 (1.17–2.05) | .003 | 1.57 (1.15–2.15) | .005 |
| Intermediate vs low WBC | 1.50 (0.95–2.39) | .08 | 1.16 (0.67–2.03) | .59 |
| High vs low WBC | 2.38 (1.71–3.32) | < .0001 | 2.70 (1.88–3.88) | < .0001 |
| Platelet (≤ 40 vs < 40) | 0.78 (0.56–1.08) | .14 | 0.77 (0.53–1.11) | .16 |
| Hemoglobin | 0.99 (0.93–1.05) | .70 | 1.00 (0.94–1.07) | .93 |
| M3V vs M3 | 1.04 (0.72–1.50) | .84 | 0.91 (0.60–1.38) | .67 |
| . | OS . | DFS . | ||
|---|---|---|---|---|
| Hazard ratio (95 confidence interval) . | P . | Hazard ratio (95 confidence interval) . | P . | |
| Age ≥ 60 y vs age < 60 y | 3.13 (2.32–4.21) | < .0001 | 2.13 (1.48–3.07) | < .001 |
| Male vs female | 1.55 (1.17–2.05) | .003 | 1.57 (1.15–2.15) | .005 |
| Intermediate vs low WBC | 1.50 (0.95–2.39) | .08 | 1.16 (0.67–2.03) | .59 |
| High vs low WBC | 2.38 (1.71–3.32) | < .0001 | 2.70 (1.88–3.88) | < .0001 |
| Platelet (≤ 40 vs < 40) | 0.78 (0.56–1.08) | .14 | 0.77 (0.53–1.11) | .16 |
| Hemoglobin | 0.99 (0.93–1.05) | .70 | 1.00 (0.94–1.07) | .93 |
| M3V vs M3 | 1.04 (0.72–1.50) | .84 | 0.91 (0.60–1.38) | .67 |
WBC: Low if WBC <5 × 109/L; intermediate if 5 to <10 × 109/L; high if ≥ 10 × 109/L.
Disease-free survival.
The DFS at 5 years was 73% for M3V patients and 81% for M3 patients (P = .07) (Table 3A and Figure 3). As in OS, when the DFS was adjusted for the WBC, the difference in DFS is no longer seen. Among patients with M3V, the 5-year DFS rates for those who presented with a WBC less than 5 × 109/L, 5-10 × 109/L, and greater than or equal to 10 × 109/L were 84%, 79%, and 67%, respectively, compared with 85%, 80%, and 63%, respectively, for patients with classical M3 APL (P = .87, .96, .45 for WBC less than 5 × 109/L, 5-10 × 109/L, and greater than or equal to 10 × 109/L, respectively, and P = .50 for overall, WBC adjusted) (Table 5). A similar result was seen in a multivariable model. When age, male sex, WBC, platelet hemoglobin, and APL morphology subtype were included in the stratified Cox regression model, the APL morphology subtype was not significant in DFS (HR = 0.91, P = .67) (Table 4). In this model, the unfavorable prognostic factors for DFS were age greater than or equal to 60 (HR = 2.31, P < .001), male sex (HR = 1.57, P = .005), and high WBC (HR = 2.70, P < .0001) (Figure 4). The model was repeated with relapse risk score instead of WBC and platelet counts. The APL morphology subtype was virtually unchanged (HR = 0.95, P = .82), and high relapse risk was an unfavorable prognostic factor (HR = 3.27, P < .0001)
Cumulative incidence of relapse.
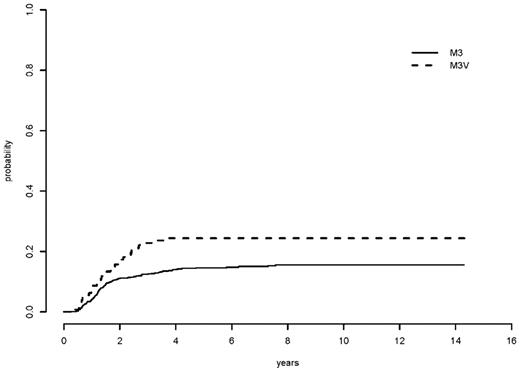
The 5-year cumulative incidence rate of relapse (CIR) was 24% for all M3V patients and 15% for all classical M3 patients (relapse risk unadjusted P = .005) (Table 7 and Figure 5). However, the 5-year CIR for low-, intermediate-, and high-relapse risk groups were 15%, 14%, and 32%, respectively, among M3V patients and 9.5%, 12%, and 35%, respectively, for classical M3 patients (P = .64, .71, .62 for low, intermediate, and high risk group, respectively, and P = .84 for overall, relapse risk adjusted) (Table 7 and Figure 6). When age, sex, relapse risk, hemoglobin, and morphology were included in a multivariable competing risks regression analysis, the APL morphology subtype was again not significant in CIR (HR = 0.94, P = .81). The unfavorable prognostic factors for relapse were male sex (HR = 1.78, P = .003) and high relapse risk score (HR = 4.06, P < .0001).
Cumulative incidence of relapse*
| . | M3, n (%) . | M3V, n (%) . | P . | ||||
|---|---|---|---|---|---|---|---|
| n . | 5-year . | 10-year . | n . | 5-year . | 10-year . | ||
| I0129 | 120 | 33 (4.3) | 35 (4.4) | 19 | 37 (11.4) | 37 (11.4) | .9 |
| LPA96 | 133 | 15 (3.1) | 15 (3.1) | 23 | 39 (10.5) | 39 (10.5) | .005 |
| LPA99 | 416 | 9 (1.4) | — | 85 | 18 (4.2) | — | .03 |
| ALL | 669 | 15 (1.4) | — | 127 | 24 (3.8) | — | .005† |
| Relapse risk | .84‡ | ||||||
| Low | 169 | 9.5 (2.3) | 9.5 (2.3) | 20 | 15 (8.2) | 15 (8.2) | .64† |
| Intermediate | 402 | 12 (1.6) | 13 (1.8) | 35 | 14 (6) | 14 (6) | .71† |
| High | 98 | 35 (4.9) | — | 72 | 32 (5.5) | — | .62† |
| . | M3, n (%) . | M3V, n (%) . | P . | ||||
|---|---|---|---|---|---|---|---|
| n . | 5-year . | 10-year . | n . | 5-year . | 10-year . | ||
| I0129 | 120 | 33 (4.3) | 35 (4.4) | 19 | 37 (11.4) | 37 (11.4) | .9 |
| LPA96 | 133 | 15 (3.1) | 15 (3.1) | 23 | 39 (10.5) | 39 (10.5) | .005 |
| LPA99 | 416 | 9 (1.4) | — | 85 | 18 (4.2) | — | .03 |
| ALL | 669 | 15 (1.4) | — | 127 | 24 (3.8) | — | .005† |
| Relapse risk | .84‡ | ||||||
| Low | 169 | 9.5 (2.3) | 9.5 (2.3) | 20 | 15 (8.2) | 15 (8.2) | .64† |
| Intermediate | 402 | 12 (1.6) | 13 (1.8) | 35 | 14 (6) | 14 (6) | .71† |
| High | 98 | 35 (4.9) | — | 72 | 32 (5.5) | — | .62† |
Standard error given in parentheses.
Taking nonrelapse death as a competing risk.
Stratified by study (I0129, LPA96, LPA99).
Stratified by relapse risk.
FLT3 ITD mutations.
Of 903 patients, only 332 (37%) patients had FLT3 ITD status available. Among patients with FLT3 ITD status available, WBC was higher in patients with FLT3 ITD mutation (median 15.4, range 0.6-550) compared with patients without mutation (median 1.9, range 0.2--133, P < .0001), and the incidence rate of FLT3 ITD mutation was 17% in M3 and 52% in M3V (P < .001).
Among 155 patients with M3V, 62 (40%) had FLT3 ITD status available. The 5-year OS was 72% for patients with a FLT3 ITD mutation and 63% for patients without a mutation (P = .56). The 5-year DFS was 78% for patients with FLT3 ITD mutation and 68% for patients without mutation (P = .46). The 5-year CIR was 22% for patients with FLT3 ITD mutation and 28% for patients without mutation (P = .64). Among 748 patients with M3, 270 (36%) had FLT3 ITD status available. The 5-year OS was 78% for patients with a FLT3 ITD mutation and 85% for patients without a mutation (P = .44). The 5-year DFS was 74% for patients with FLT3 ITD mutation and 84% for patients without mutation (P = .33). The 5-year CIR was 26% for patients with FLT3 ITD mutation and 12% for patients without mutation (P = .03).
To investigate whether the patient cohort with FLT3 ITD information represents a random subset, OS was compared between the patients without FLT3 ITD mutation information available (n = 571) and those with FLT3 ITD available (n = 332). The 5-year OS for the patients without FLT3 ITD information was 77% and 81% for patients with FLT3 ITD information available (P = .46). Similarly, the 5-year DFS was 79% for the patients without the information and 81% for those with (P = .80); the 5-year CIR was 16% for the patients with the information and 16% for those without (P = .87). When the analysis was repeated by APL morphology subtype, a similar result was found (data not shown).
PML isoform.
Of 174 I0129 pts, 108 (62%) patients had the isoform information. Of 729 PETHEMA patients, 651 (89%) had the isoform information. The detailed distribution of PML isoform is presented in Table 8. Based on these 759 patients with isoform data available, WBC was higher in patients with S-isoform (median 3.5 × 109/L, range 0.3-210 × 109/L) compared with patients with other (L/V/VL) isoforms (median 1.8 × 109/L, range 0.2-550 × 109/L, P = .002). The incidence of S-isoform is higher in M3V compared with M3 (58% in M3V vs 35% in M3, P < .001). However, there was no difference between S-isoform and other isoforms in CR rate (89% in S-isoform vs 90% in other isoforms, P = .59), OS (HR = 1.13, P = .45 in univariable; HR = 0.91, P = .55 in multivariable Cox model), and DFS (HR = 1.34, P = .08 in univariable, HR = 1.15, P = .42 in multivariable Cox model).
Distribution of isoform type
| Isoform . | M3, n (%) . | M3V, n (%) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| I0129 (n = 91) . | LPA96 (n = 137) . | LPA99 (n = 395) . | All (n = 623) . | I0129 (n = 17) . | LPA96 (n = 30) . | LPA99 (n = 89) . | All (n = 136) . | ||
| L | 51 (56) | 72 (53) | 201 (51) | 324 (52) | 9 (53) | 10 (33) | 36 (40) | 55 (40) | — |
| S | 32 (35) | 53 (39) | 148 (37) | 233 (37) | 8 (47) | 20 (67) | 51 (57) | 79 (58) | < .001* |
| V | 8 (9) | 9 (7) | 10 (3) | 27 (4) | 0 | 0 | 1 (1) | 1 (1) | — |
| VL | 0 | 3 (2) | 36 (9) | 39 (6) | 0 | 0 | 1 (1) | 1 (1) | — |
| Isoform . | M3, n (%) . | M3V, n (%) . | P . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| I0129 (n = 91) . | LPA96 (n = 137) . | LPA99 (n = 395) . | All (n = 623) . | I0129 (n = 17) . | LPA96 (n = 30) . | LPA99 (n = 89) . | All (n = 136) . | ||
| L | 51 (56) | 72 (53) | 201 (51) | 324 (52) | 9 (53) | 10 (33) | 36 (40) | 55 (40) | — |
| S | 32 (35) | 53 (39) | 148 (37) | 233 (37) | 8 (47) | 20 (67) | 51 (57) | 79 (58) | < .001* |
| V | 8 (9) | 9 (7) | 10 (3) | 27 (4) | 0 | 0 | 1 (1) | 1 (1) | — |
| VL | 0 | 3 (2) | 36 (9) | 39 (6) | 0 | 0 | 1 (1) | 1 (1) | — |
S-isoform type vs other, stratified by study (I0129, LPA96, LPA99).
Discussion
This study of a large number of patients with long-term follow-up showed that the outcome of patients with the M3V was not different from that of patients with classical morphology when treated with ATRA plus anthracycline-based regimens when adjusted for WBC or relapse risk score. A potential limitation of the data reported herein is the fact that precise quantitative criteria to establish a definitive diagnosis of M3V is lacking, and not all patients had the diagnosis of M3V established centrally. Indeed, there are several recognized microgranular variants with subtleties in establishing the diagnosis.1 Some patients may have had the M3V but may not have been recognized and not included in the analysis. Furthermore, patients were not treated identically, though this issue was handled by performing stratified analysis. Patients treated on the PETHEMA protocols received idarubicin whereas those on the North American protocol were given daunorubicin. In addition, the number of cycles and intensity of consolidation differed. Nevertheless, the patients reported here represent the largest series of M3V patients treated with ATRA plus anthracycline-based therapy.
Historically, before the introduction of ATRA, the early mortality among patients with M3V APL was reported to be higher than that among patients with classical morphology, but the CR rate, except for one series,21 and OS were not clearly inferior.2,18-20 Early mortality among patients with M3V may be attributable to extensive hemostatic abnormalities and fatal bleeding, particularly intracerebral hemorrhage.8 Patients with M3V morphology often have hyperleukocytosis.4,6 The outcome of such patients appears to be influenced more by the WBC than the specific morphology of M3V. Other factors may also influence outcome among patients with M3V. It is possible that expression of CD2, associated in some reports with M3V3,6,8,10,11,15,34,35 may be related to the hyperleukocytosis observed. This may be attributable to interaction with its ligand CD58 or lymphocyte function-associated antigen 3, a cell surface glycoprotein, which induces proliferation of T cells to which it mediates adhesion.35 In our study, CD2 expression was not determined. It is also possible that the hyperleukocytosis associated with the M3V is attributable in part to expression of the FLT3 gene mutation.12,13 Some,12 but not all, reports13,36,37 have demonstrated a relationship between the presence of the FLT3 gene mutation and induction death in patients with APL. Furthermore, a recent study suggested that increased ITD mutant/wild-type ratio or longer ITD size was associated with a shorter 5-year relapse-free survival.38 We did not examine the correlation of patients with high allelic ratio of the FLT3 gene mutation with M3V, which may be important in unraveling a potential association of FLT3 mutations with M3V and/or hyperleukocytosis in APL. However, we did find that the mutation rate appears to be higher in M3V compared with M3, although the sample size that resulted from missing data is a limiting factor. Telomerase activity and telomere length appear to correlate with disease progression and relapse among arsenic trioxide–treated patients.39 In our analysis, there was a correlation between the S-isoform subtype of PML and M3V morphology There may well be other as yet unidentified factors, likely molecular and currently elusive, for which WBC serves as a surrogate, which determine the prognosis of patients with the M3V.
The significantly higher induction death rate, attributable to hemorrhage in 50% or more of patients, observed in patients with M3V compared with those with classical M3 was influenced by the association of morphologic subtype with WBC. This finding was reported in a previous PETHEMA study, in which morphologic subtype had a prognostic impact on induction death rate in univariable, but not in multivariable, analysis.40 Regarding other outcomes, such as DFS, CIR, and OS, a similar finding of the impact of morphology on prognosis was observed in univariable analysis, but again it was a result of its association with WBC and relapse risk score, as previously reported.27,28
The difference in the incidence of the APL DS between the I0129 and LPA trials requires comment. The incidence of the APL DS on the I0129 trial was 29% (43 of 150) among all patients with APL receiving ATRA during induction.23,24 Of these 43 patients, 41 had classical M3, and only 2 had M3V. One of these 2 patients with M3V was erroneously identified as having APL DS and thus subsequently excluded from further analysis.41 Few patients with the APL DS in this early trial were identified as having the M3V despite central morphologic review by one person. It does not appear that the difference can be explained by a higher incidence of pediatric patients, suggested as having a higher incidence of M3V morphology, in the LPA trials because the incidence of the APL DS was 6.2% on the PETHEMA trials, and on the I0129 trial it was 13%. In addition, the expanded definition of the APL DS in the PETHEMA trials does not provide an explanation because there were no patients diagnosed with DS based solely on the presence of renal failure. A contributing factor may be the fact that there was central pathology review for patients entered in the North American Trial and not for the PETHEMA patients.
ATRA remains the mainstay for all subtypes of APL, including M3V. Despite the higher risk of complications reported among patients with M3V, the addition of ATRA markedly improves outcome as demonstrated in the large series reported here. At the present time, patients with the M3V do not require treatment modification based on the morphology alone. Given the apparent high incidence of the FLT3 gene mutation in M3V and the less favorable prognosis among patients with a high WBC, it is of interest to speculate as to a possible role for FLT3 inhibitors in treatment of patients with the M3V. However, the availability of several very effective agents likely makes this possibility premature. Interestingly, coexpression of the PML-RAR-alpha with the FLT3 ITD in myeloid progenitors in a mouse model leads to a disease with morphologic features resembling M3V.42 Nevertheless, the M3V itself does not independently predict for a less favorable outcome compared with classical M3 APL.
Presented in part at the 46th annual meeting of the American Society of Hematology, December 4-7, 2004, San Diego, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, MD, Chair) and supported in part by Public Health Service Grants ECOG CA23318, CA66636, CA21115, CA17145, CALGB CA31936 and CA33601, SWOG Grant CA32102, CA38926, CA20319, CA12213, CA14028, CA56771, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: M.S.T. and M.A.S. were the principal investigators of the clinical trial; M.S.T. and M.A.S. participated in the conception and design of the trial; J.d.l.S., G.D., J.G., J.H.F., M.G., J.D.G., G.M., J.M.R., C. Rayon, L.S., C. Rivas, and E.V. provided study materials and/or patients for the trial; M.S.T., H.T.K., R.G., E.P., and P.M. participated in the analysis and interpretation of data; M.S.T., H.T.K., and M.A.S. wrote the manuscript; and P.M., F.R.A., C.A.S., J.d.l.S., J.M.B., G.D., C.D.B., J.G., J.H.F., M.G., R.A.L., J.D.G., G.M., J.M.R., C. Rayon, L.S., C. Rivas, E.V., P.H.W., R.G., E.P., J.S., and C.L.W. participated in manuscript review and approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin S. Tallman, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Box 380, New York, NY 10065; e-mail: tallmanm@mskcc.org.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal