Abstract
Trimodal or bimodal age-specific incidence rates for Burkitt lymphoma (BL) were observed in the United States general population, but the role of immunosuppression could not be excluded. Incidence rates, rate ratios, and 95% confidence intervals for BL and other non-Hodgkin lymphoma (NHL), by age and CD4 lymphocyte count categories, were estimated using Poisson regression models using data from the United States HIV/AIDS Cancer Match study (1980-2005). BL incidence was 22 cases per 100 000 person-years and 586 for non-BL NHL. Adjusted BL incidence rate ratio among males was 1.6× that among females and among non-Hispanic blacks, 0.4× that among non-Hispanic whites, but unrelated to HIV-transmission category. Non-BL NHL incidence increased from childhood to adulthood; in contrast, 2 age-specific incidence peaks during the pediatric and adult/geriatric years were observed for BL. Non-BL NHL incidence rose steadily with decreasing CD4 lymphocyte counts; in contrast, BL incidence was lowest among people with ≤ 50 CD4 lymphocytes/μL versus those with ≥ 250 CD4 lymphocytes/μL (incidence rate ratio 0.3 [95% confidence interval = 0.2-0.6]). The bimodal peaks for BL, in contrast to non-BL NHL, suggest effects of noncumulative risk factors at different ages. Underascertainment or biological reasons may account for BL deficit at low CD4 lymphocyte counts.
Introduction
Burkitt lymphoma (BL) is an aggressive B-cell non-Hodgkin lymphoma (NHL) with 3 clinical variants: endemic (eBL), sporadic (sBL), and acquired immunodeficiency-associated BL (aBL).1 These clinical variants, which are defined in part by where they occur geographically, are histologically indistinguishable1 and their etiology is incompletely understood.2 eBL occurs in children mostly as extranodal jaw or orbital masses in equatorial Africa and Papua New Guinea.3 sBL occurs anywhere in the world at any age mostly with abdominal or nodal involvement.4,5 Immunodeficiency-associated BL is diagnosed in people with HIV,1 among whom it is often the first indication of AIDS onset at least in the West. Risk for both eBL and sBL appears to be highest at ages 5-9 years and sBL rates are also elevated at the oldest ages.4,6 Because BL is a rapidly growing tumor, doubling its cell mass approximately every 1-2 days,7 the interval from trigger to diagnosis may be relatively short, so study of age-specific risk may provide etiologic information.
In an assessment of age-specific risk for BL in the United States, using data from the National Cancer Institute Surveillance, Epidemiology, and End Results Program (1973-2005),8 we observed 3 incidence peaks near ages 10, 40, and 70 years among males and 2 peaks near ages 10 and 70 years among females for both whites and blacks. However, the role of AIDS-related immunosuppression could not be excluded9,10 because we were not able to separately analyze AIDS and non-AIDS BL. To address this limitation, we investigated age-specific BL incidence among persons with AIDS (PWA) in the United States Because age is a surrogate for cumulative exposure to deleterious infections a linear increase in risk for BL with age in PWA would suggest cumulative impact of deleterious infections given immunosuppression, whereas a nonlinear risk increase would suggest that age may be a surrogate for differences in the etiology or biology of BL diagnosed at different ages that occur independent of immunosuppression.8
Methods
Data were obtained from the National Cancer Institute US population-based HIV/AIDS Cancer Match (HACM) study.11-14 The study links registry records of a cohort of persons with HIV/AIDS in 15 United States state and metropolitan areas to their corresponding cancer registry record to ascertain cancer outcomes, based on a probabilistic matching algorithm.15 Analysis was performed using de-identified data for 1980-2005. Institutional review boards at all participating sites gave ethical approval to conduct the study.
The primary outcome was BL morphology code 9687, based on the International Classification of Diseases for Oncology (ICD-O-3, 3rd ed)16 obtained from the cancer registry. Data for other NHLs (all other NHL morphology codes excluding BL) were obtained for comparative analyses. Person-years were calculated from the date of AIDS diagnosis to the earliest of date of death, 60 months from AIDS diagnosis, or end of cancer registry coverage. Because ascertainment of mortality of the AIDS cohort may be incomplete, we truncated follow-up at 60, which includes the period when we expected ascertainment of mortality to be most complete, to minimize overestimating person-years of follow-up. The date of AIDS diagnosis was set to the date of BL diagnosis when the date of BL diagnosis given by the cancer registry preceded the date of AIDS diagnosis given by the AIDS registry because BL is an AIDS-defining condition.9 Cases diagnosed during 0 to 3 months from AIDS diagnosis were categorized as prevalent; cases diagnosed from 4 to 60 months after AIDS diagnosis were considered incident. Prevalent cases were excluded from analyses because they may be biased by characteristics related to diagnosis of AIDS and/or survival up to AIDS. Incident cases were used in analyses because they are less biased and they allow inferences about the temporal relationship between risk factors associated with disease to be made.
Mean age for prevalent versus incident BL and for prevalent versus incident other NHLs were compared using the Student t test. Crude and adjusted incidence rates, rate ratios (IRR), and 95% confidence intervals (95% CIs) were calculated using Poisson methods by patient characteristics: sex, race/ethnicity (non-Hispanic white, non-Hispanic black, and Hispanic), HIV transmission risk categories (men who had sex with men [MSM], intravenous drug users [IDU], heterosexual, and other), CD4 lymphocyte count categories at AIDS diagnosis (< 50, 50-149, 150-249, ≥ 250 cells/μL), age at AIDS diagnosis (0-19, 20-31, 32-39, 40-51, 52-59, ≥ 60 years), and calendar-year of AIDS diagnosis (1980-1989, 1990-1995, and 1996-2005). We used the most recent value for CD4 lymphocyte count at AIDS during 6 months before to 3 months after AIDS diagnosis. Calendar-year period reflected progressive improvement in antiretroviral therapies: no or monotherapy, mono or dual therapy, and highly active antiretroviral therapy (HAART). Rates were only presented for categories with 10 or more cases to avoid presenting unstable rates, consistent with the practice of the International Agency for Research on Cancer. Statistical tests were 2-tailed, and P values < .05 were considered statistically significant.
Because we previously observed trimodal peaks in BL from the Surveillance, Epidemiology, and End Results Program,8 we plotted age-specific incidence rates for BL by 6 age groups on a log (y) and linear (x) scale, such that a slope of 10 degrees equaled a 1% per year change in rates,17 to assess whether trimodal age-specific patterns were present in immunosuppressed persons. We also plotted log BL incidence by 4 CD4 lymphocyte groups to explore the risk patterns with immunosuppression, given that BL occurs over a wide range of CD4 lymphocyte counts. Log incidence rates for other NHLs were plotted by age and CD4 lymphocytes for comparative purposes. Analyses were performed using SAS Version 9.1 (SAS Institute Inc).
Results
We analyzed 1103 cases of BL, including 797 that were prevalent and 306 that were incident, among 567 865 PWA followed for an average of 2.4 years. By comparison, we identified 15 730 other NHLs, including 7580 prevalent and 8150 incident cases, during the same follow-up period. The mean ages for prevalent and incident BL were similar (38.3 vs 38.5 years, P = .48). In contrast, the mean age for prevalent other NHLs was on average 3 years younger than the mean age for incident other NHLs (38.1 years vs 41.1 years, P < .001). Table 1 shows the demographic characteristics of PWA studied, the numbers of incident BL, the crude incidence rates, and the crude and multivariate-adjusted incidence rate ratios (aIRR). As expected, most BL (89.2%) were aged 20 to 51 years, and only 3.3% were aged < 20 years and 7.5% were aged 52 years or older.
Crude incidence and incidence rate ratios for BL among persons with AIDS in the United States, 1980-2005
| Characteristics . | No. of subjects (%) . | No. of incident BL cases . | Crude incidence rate, per 100 000 person-years . | Crude incidence rate ratios (95% CI) . | Adjusted incidence rate ratios (95% CI)* . |
|---|---|---|---|---|---|
| Overall | 567 865 (100) | 306 | 22.0 | ||
| Sex | |||||
| Male | 456 635 (80.4) | 273 | 24.4 | 2.0 (1.4-2.9) | 1.6 (1.1-2.3) |
| Female | 111 230 (19.6) | 33 | 12.2 | 1 | 1 |
| Race/ethnicity | |||||
| Non-Hispanic white | 218 917 (38.5) | 168 | 30.1 | 1 | 1 |
| Non-Hispanic black | 235 578 (41.5) | 54 | 9.6 | 0.3 (0.2-0.4) | 0.4 (0.3-0.5) |
| Hispanic | 113 370 (20.0) | 84 | 30.9 | 1.0 (0.8-1.3) | 1.2 (0.9-1.5) |
| Mode of HIV transmission | |||||
| MSM | 281 365 (49.6) | 190 | 26.4 | 1 | 1 |
| IDU | 137 533 (24.2) | 49 | 15.5 | 0.6 (0.4-0.8) | 0.7 (0.5-1.0) |
| Heterosexual | 70 283 (12.4) | 23 | 12.7 | 0.5 (0.3- 0.7) | 0.7 (0.5-1.1) |
| Other/unknown | 78 633 (13.9) | 44 | 25.1 | 1.0 (0.7-1.3) | 1.2 (0.8-1.8) |
| Age at AIDS-onset, y | |||||
| 0-19 | 9339 (1.6) | 10 | 39.5 | 1.6 (0.8-3.0) | 2.3 (1.2-4.4) |
| 20-31 | 129 139 (22.7) | 49 | 14.2 | 0.6 (0.4-0.8) | 0.6 (0.4-0.8) |
| 32-39 | 200 765 (35.4) | 125 | 24.7 | 1 | 1 |
| 40-51 | 175 019 (30.8) | 99 | 24.2 | 1.0 (0.8-1.3) | 1.0 (0.8-1.3) |
| 52-59 | 35 450 (6.2) | 13 | 17.9 | 0.7 (0.4-1.3) | 0.8 (0.4-1.4) |
| 60 or older | 18 153 (3.2) | 10 | 31.7 | 1.3 (0.7-2.4) | 1.4 (0.7-2.7) |
| CD4 lymphocyte count/μL† | |||||
| 0-49 | 108 512 (31.4) | 23 | 9.6 | 0.3 (0.2-0.5) | 0.3 (0.2-0.6) |
| 50-149 | 111 304 (32.2) | 90 | 29.8 | 1.0 (0.7-1.5) | 1.0 (0.7-1.5) |
| 150-249 | 91 315 (24.4) | 67 | 23.9 | 0.8 (0.5-1.2) | 0.8 (0.5-1.2) |
| 250+ | 34 183 (9.8) | 33 | 30.7 | 1 | 1 |
| Missing‡ | 120 902 (25.9) | 36 | 13.6 | ||
| Calendar year of AIDS onset | |||||
| 1980-1989 | 101 649 (17.9) | 57 | 29.4 | 1.3 (1.0-1.8) | 1.6 (1.1-2.4) |
| 1990-1995 | 247 922 (43.7) | 146 | 22.5 | 1 | 1 |
| 1996-2005 | 218 294 (38.4) | 103 | 18.8 | 0.8 (0.6-1.1) | 0.9 (0.7-1.1) |
| Characteristics . | No. of subjects (%) . | No. of incident BL cases . | Crude incidence rate, per 100 000 person-years . | Crude incidence rate ratios (95% CI) . | Adjusted incidence rate ratios (95% CI)* . |
|---|---|---|---|---|---|
| Overall | 567 865 (100) | 306 | 22.0 | ||
| Sex | |||||
| Male | 456 635 (80.4) | 273 | 24.4 | 2.0 (1.4-2.9) | 1.6 (1.1-2.3) |
| Female | 111 230 (19.6) | 33 | 12.2 | 1 | 1 |
| Race/ethnicity | |||||
| Non-Hispanic white | 218 917 (38.5) | 168 | 30.1 | 1 | 1 |
| Non-Hispanic black | 235 578 (41.5) | 54 | 9.6 | 0.3 (0.2-0.4) | 0.4 (0.3-0.5) |
| Hispanic | 113 370 (20.0) | 84 | 30.9 | 1.0 (0.8-1.3) | 1.2 (0.9-1.5) |
| Mode of HIV transmission | |||||
| MSM | 281 365 (49.6) | 190 | 26.4 | 1 | 1 |
| IDU | 137 533 (24.2) | 49 | 15.5 | 0.6 (0.4-0.8) | 0.7 (0.5-1.0) |
| Heterosexual | 70 283 (12.4) | 23 | 12.7 | 0.5 (0.3- 0.7) | 0.7 (0.5-1.1) |
| Other/unknown | 78 633 (13.9) | 44 | 25.1 | 1.0 (0.7-1.3) | 1.2 (0.8-1.8) |
| Age at AIDS-onset, y | |||||
| 0-19 | 9339 (1.6) | 10 | 39.5 | 1.6 (0.8-3.0) | 2.3 (1.2-4.4) |
| 20-31 | 129 139 (22.7) | 49 | 14.2 | 0.6 (0.4-0.8) | 0.6 (0.4-0.8) |
| 32-39 | 200 765 (35.4) | 125 | 24.7 | 1 | 1 |
| 40-51 | 175 019 (30.8) | 99 | 24.2 | 1.0 (0.8-1.3) | 1.0 (0.8-1.3) |
| 52-59 | 35 450 (6.2) | 13 | 17.9 | 0.7 (0.4-1.3) | 0.8 (0.4-1.4) |
| 60 or older | 18 153 (3.2) | 10 | 31.7 | 1.3 (0.7-2.4) | 1.4 (0.7-2.7) |
| CD4 lymphocyte count/μL† | |||||
| 0-49 | 108 512 (31.4) | 23 | 9.6 | 0.3 (0.2-0.5) | 0.3 (0.2-0.6) |
| 50-149 | 111 304 (32.2) | 90 | 29.8 | 1.0 (0.7-1.5) | 1.0 (0.7-1.5) |
| 150-249 | 91 315 (24.4) | 67 | 23.9 | 0.8 (0.5-1.2) | 0.8 (0.5-1.2) |
| 250+ | 34 183 (9.8) | 33 | 30.7 | 1 | 1 |
| Missing‡ | 120 902 (25.9) | 36 | 13.6 | ||
| Calendar year of AIDS onset | |||||
| 1980-1989 | 101 649 (17.9) | 57 | 29.4 | 1.3 (1.0-1.8) | 1.6 (1.1-2.4) |
| 1990-1995 | 247 922 (43.7) | 146 | 22.5 | 1 | 1 |
| 1996-2005 | 218 294 (38.4) | 103 | 18.8 | 0.8 (0.6-1.1) | 0.9 (0.7-1.1) |
BL indicates Burkitt lymphoma; CI, confidence interval; MSM, men who have sex with men; and IDU, intravenous drug users.
Incidence rate ratio for BL for each factor is adjusted for all other factors in the table; records with missing CD4 data were coded as missing and included in the model.
CD4 lymphocyte count analyses based on data from 1990-2005.
Percentage of the CD4 lymphocyte counts for “missing” are of the total; for known categories, the percentages are based on those with known CD4 lymphocyte counts.
Overall BL incidence was 22.0 per 100 000 persons (Table 1). The crude and adjusted incidence rate ratios were different for some variables, suggesting confounding was present, so both the crude and adjusted incidence rate ratios are reported (Table 1). The crude male to female IRR was reduced from 2 to 1.6 (95% CI 1.1-2.3) in the adjusted model, and the aIRR was lower in non-Hispanic black than non-Hispanic white PWA (0.4, 95% CI 0.3-0.5). Compared with MSM, the crude BL incidence rates were lower in PWA whose HIV transmission risk category was IDU or heterosexual, but the differences became nonsignificant in multivariate-adjusted analysis. Compared with PWA aged 32-39 years, the crude IRR for BL was nonsignificantly elevated among PWA aged 0-19 years (1.6, 95% CI 0.8-3.0), but it became significant in multivariate analysis (2.3, 95% CI 1.2-4.4). The crude and adjusted IRR for BL among PWA aged 20-31 years were significantly decreased (both 0.6, 95% CI 0.4-0.8), but the crude and adjusted IRR for BL among PWA aged 40-51 years, 52-59 years, and 60 years or older were not significantly different. The crude and adjusted IRR for BL were notably lower among PWA having < 50 CD4 lymphocyte cells/μL than those with ≥ 250 cells/μL at AIDS (aIRR 0.3, 95% CI 0.2-0.6). The BL rate among PWA declined over time from 1980-1989 to 1996-2005.
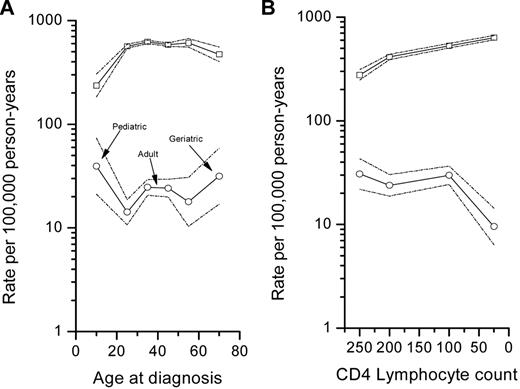
The age-specific incidence rates for BL suggested 3 peaks during the pediatric, adult, and geriatric years, although the adult confidence limits overlapped (Figure 1A). In contrast, age-specific incidence rates for other NHLs increased from childhood to adulthood and stabilized thereafter (Figure 1A). The risk for other NHLs was 6× that of BL among those aged 0-19 years, 24-40-fold greater among those aged 20-59 years, and 15-fold among those ≥ 60 years (Figure 1A).
Log incidence rates for Burkitt lymphoma (○) and for other non-Hodgkin lymphomas (other NHLs), excluding Burkitt lymphoma, (□). (A) By age group (0-19, 20-31, 32-39, 40-51, 52-59, and ≥ 60 years). (B) By 4 CD4 lymphocyte count groups (0-49, 50-149, 150-249, and ≥ 250 cells/μL). Dotted lines show the upper and lower 95% CI.
Log incidence rates for Burkitt lymphoma (○) and for other non-Hodgkin lymphomas (other NHLs), excluding Burkitt lymphoma, (□). (A) By age group (0-19, 20-31, 32-39, 40-51, 52-59, and ≥ 60 years). (B) By 4 CD4 lymphocyte count groups (0-49, 50-149, 150-249, and ≥ 250 cells/μL). Dotted lines show the upper and lower 95% CI.
The incidence of BL declined steeply with CD4 lymphocyte counts < 50 cells/mL at AIDS (Figure 1B). In contrast, the incidence for other NHLs increased consistently with decreasing CD4 lymphocyte counts (Figure 1B). The risk for other NHLs was 9× that of BL among those with ≥ 250 cells/μL, 17-fold among those with 50-259, and 66-fold among PWA with < 50 cells/μL.
Discussion
The major findings were bimodal and perhaps trimodal age-specific BL incidence peaks, significant decline in BL risk at low CD4 lymphocyte counts at AIDS, and lack of association between risk for BL and HIV transmission risk categories, which are a surrogate for sexual and blood-borne infections that are highly prevalent in the United States HIV/AIDS population. Our finding of bimodal or trimodal BL incidence peaks in PWA replicates our results in the United States general population,8 and suggests that bi/trimodality of BL may occur independently of severe immunosuppression.8 The notion that BL may be multimodal is controversial. Several malignancies, including lymphoblastic leukemia, Hodgkin lymphoma, kidney and renal pelvis cancer, and brain cancer are bimodal.18 However, BL is considered isomorphic and mostly a pediatric lymphoma, with occasional cases diagnosed at other ages.19 Multimodality in cancer suggests a mixture of 2 or more types of cancer that resemble and are coded as one.20 For BL, the types may include the well accepted pediatric BL as one type and a mixture of the adult and/or geriatric BL as the other type(s).8 If so, the pediatric and adult/geriatric types might have different risk factors, different pathogenesis pathways, and different molecular characteristics. Whether the subtle variation in morphologic, cytogenetic, and molecular patterns in BL19,21-23 is related to the age-specific incidence patterns we observed is unknown, but worth investigating.
Our finding that risk for BL declined steeply at very low CD4 lymphocyte counts is surprising, given that risk for BL as for others lymphomas is attributed to deleterious effects of HIV or oncogenic infections2 such as EBV10 and/or growth factor expression in altered immunostates,24 that are normally most expressed at low CD4 lymphocyte counts. Although some pioneer studies have noted a link between CD4 lymphocyte counts and BL,25-28 our study represents the largest study of AIDS related BL and was able to look at age-specific and CD4 count-specific BL risk, and therefore represents a major extension of the findings from these previous studies. Our results and historical data10 indicate that the risk for BL peaks at CD4 lymphocyte counts well above the laboratory cutoff for AIDS onset (200 cells/μL), provocatively suggesting that its expression may require functional CD4 lymphocytes. The higher risk for BL during 1980 to 1989 than later may reflect a larger portion of recent HIV infections with relatively higher CD4 lymphocyte counts in that period than later, a shift in the demographic profile of the United States AIDS epidemic from predominantly non-Hispanic white men, whose risk for BL is higher, to predominantly non-Hispanic black men, Hispanics, and women,29 whose risk for BL is lower, or to access to HAART after 1996. In the HACM study, the proportion of blacks, who have a lower risk for BL compared with whites, increased from 30% in 1980-1989, to 39% in 1990-1995, and to 50% in 1996-2005. Conversely, the proportion of whites, whose risk for BL is higher than blacks, decreased from 54% in 1980-1989, to 41% in 1990-1995, and to 29% in 1996-2005. Possibly, the paradoxical relationship between risk for BL and CD4 lymphocyte counts may be a clue to a physiological role of functional CD4 lymphocytes in BL lymphomagenesis.
Burkitt lymphoma is a germinal center (GC) tumor.2,30-32 The seminal event in BL, the c-myc immunoglobulin (Ig) translocation,33 is thought to occur as a result of an error in activation-induced cytidine deaminase (AID)–mediated Ig class switch recombination in GCs,34 an event driven in B cells activated by exposure to antigen and CD4 lymphocyte help in the GC.35 High expression of AID in peripheral blood mononuclear cells has been shown to precede the development of BL by up to 8 years in HIV-infected patients,36 consistent with AID-mediated c-myc/Ig abnormalities preceding the development of BL. However, frequent and persistent detection of single or multiple clones of c-myc/Ig translocation-bearing B cells, although elevated with HIV infection,37 did not predict NHL or BL, suggesting that presence of c-myc/Ig-clonotypic cells, per se, is insufficient to cause rapidly growing lymphomas. The relationship between AID expression and/or c-myc/Ig–translocation-positive B-cell clones and CD4 lymphocyte counts is unknown. CD4 lymphocytes may be required to rescue c-myc/Ig translocation-positive clonotypic B cells from programmed cell death. Chronic HIV infection disrupts the GC reaction,38 leads to depletion of GC- and peripheral blood-CD4 lymphocytes,39 and without a cognate CD4 lymphocyte cell help, B cells are shunted into programmed cell death. In the absence of cognate help from CD4 lymphocytes, GC B-cell activation would decrease, and therefore, the rate of generating c-myc/Ig translocation positive B cells would decrease, which would account for the deficit of BL at low CD4 lymphocyte counts. The low CD4 lymphocyte counts, however, could be a marker for other exposures, which could also reduce the rate of generation of translocations and lower risk for BL, such as substantial nonspecific destruction of lymph nodes and atrophic GC reaction in advanced HIV disease.40 Nonspecific disruption of B-cell production, however, is unlikely to be the explanation, given the continuing risk of other B-cell NHLs at low CD4 lymphocyte counts.
Our study is the largest to assess age-specific incidence of BL among persons with AIDS. Even so, the small number of cases in some categories, such as age groups 52-59 years and ≥ 60 years, prevented us from obtaining stable estimates of fluctuating risk with age to definitively show trimodality. Lack of centralized tumor pathology review is a limitation; however, misclassification of BL diagnosis by age or CD4 lymphocyte count groups is likely to be random and unlikely to explain the results. We lacked CD4 lymphocyte counts after AIDS; thus, patterns of CD4 lymphocyte counts at BL, which may be more relevant to etiology, could not be assessed.
To summarize, BL among PWA in the United States was characterized by at least 2 and perhaps 3 age-specific incidence peaks, suggestive of bimodality and perhaps trimodality, and BL incidence declined at low CD4 lymphocyte counts, suggesting functional CD4 lymphocytes may be required for BL to develop.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the staff at the HIV/AIDS and cancer registries at the following locations: Colorado; Connecticut; Florida; Illinois; Georgia; Massachusetts; Michigan; New Jersey; New York City, NY; Los Angeles, San Diego, and San Francisco, CA; Seattle, WA; Texas; and Washington, DC. We thank Mr Tim McNeel of Information Management Services Inc (Silver Spring, MD) for creating the dataset for analysis. We thank Dr Otoniel Martinez-Maza (University of California, Los Angeles, CA) for comments on the manuscript.
This work was supported by the Intramural Research Program of the National Cancer Institute (contracts N02-CP-31003 and N01-CO-12400), National Institutes of Health, Department of Health and Human Services.
National Institutes of Health
Authorship
Contribution: M.G.-O. analyzed and interpreted data and drafted the manuscript; E.S. and W.F.A. analyzed and interpreted data; K.B. interpreted data; E.A.E. was the Principal Investigator of the National Cancer Institute HACM study, contributed to analysis, and interpreted data; S.S.D. interpreted data and critically edited the paper; S.M.M. conceived the idea, guided data analysis, interpreted data, and edited the paper; and all authors had access to data and commented on and contributed to the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mercy Guech-Ongey, Infections and Immunoepidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, 6120 Executive Blvd, Executive Plaza South, Rm 7078, Rockville, MD 20852; e-mail: guechome@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal