Abstract
Multiple myeloma (MM) is the most common hematologic malignancy in blacks. Some prior studies suggest inferior survival in blacks; others suggest similar survival. Using the original 9 Surveillance, Epidemiology, and End Results registries, we conducted a large-scale population-based study including 5798 black and 28 939 white MM patients diagnosed 1973-2005, followed through 2006. Age-adjusted incidence rates, disease-specific survival, and relative survival rates were calculated by race, age, and time period of diagnosis. Mean age at diagnosis was 65.8 and 69.8 years for blacks and whites, respectively (P < .001). Incidence among blacks was m twice that among whites; this disparity was greater among patients < 50 years (P = .002). Over the entire study period, disease-specific and relative survival rates were higher in blacks than whites (P < .001). For whites, 5-year relative survival rates increased significantly 1973-1993 to 1994-1998 (26.3% to 30.8%; P < .001) and 1994-1998 to 1999-2005 (30.8% to 35.0%; P = .004). Survival improvements among blacks were smaller and nonsignificant (1973-1993 to 1999-2005: 31.0% to 34.1%; P = .07). We found (1) a younger age of onset among blacks; (2) better survival in blacks 1973-2005; and (3) significant survival improvement among whites over time, with smaller, nonsignificant change seen among blacks, possibly due to unequal access to and/or disparate responsiveness to novel therapies.
Introduction
Multiple myeloma (MM) is the most common hematologic malignancy among blacks in the US and the second most common hematologic malignancy in the country.1,2 M 20 000 new cases are diagnosed annually.1,2 MM is characterized by clonal expansion of plasma cells. Classic clinical manifestations include hypercalcemia, renal failure, anemia, and lytic bone lesions as well as recurrent bacterial infections and extramedullary soft-tissue plasmacytomas.3,4 Recent data show that MM is consistently preceded by monoclonal gammopathy of undetermined significance (MGUS).5,6 Compared with whites, MGUS has been noted to occur twice as frequently in blacks, with similar transformation rates in blacks and whites.7-10 Although the etiology of MM remains unclear, observed racial disparity patterns and reported familial clustering in MGUS and MM suggest a role for susceptibility genes.11,12
There have been few published descriptive studies of MM incidence and survival by race. Prior data from the Statistics, Epidemiology, and End Results (SEER) program and the Multiple Risk Factor Intervention Trial have shown consistently higher incidence and mortality among blacks.13,14 However, mortality reflects the combined impact of cancer incidence and outcome, whereas survival is a measure of cancer outcome separate from incidence. A prior single-center study found poorer survival among 52 patients with MM at a predominantly black hospital compared with 92 patients at a predominantly white hospital; however, this difference did not persist when adjusted for socioeconomic status.15 Similarly, a single-institution review of records for 292 patients with MM found that neither race nor socioeconomic status independently related to overall survival.16 Retrospective data from the Southwest Oncology Group showed comparable outcomes among blacks and whites before the advent of autologous stem cell transplantation (ASCT).17 A recent study of 91 patients receiving ASCT in an equal access health system observed no difference in survival by race18 a registry study by the Center for International Bone Marrow Transplantation confirmed this finding.19
Four population-based studies20-23 have demonstrated improved survival in MM after the advent of novel therapies such as ASCT (1994),24-26 immunomodulatory drugs (IMiDs; 1999),27-30 and bortezomib (2003).31,32 None of these studies assessed the impact of new treatments on survival by race. To address racial disparities in MM incidence and survival patterns, we have conducted the first large-scale, population-based study to specifically assess differences in incidence and survival patterns in MM among blacks and whites in the US.
Methods
All data were obtained from the original 9 registries of the NCI SEER program (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, CA, Seattle-Puget Sound, WA, and Utah), based on the November 2009 submission.33 These 9 registries include approximately 10% of the US population. Cases were diagnosed from January 1973 through December 2005, with follow-up of vital status through 2006.
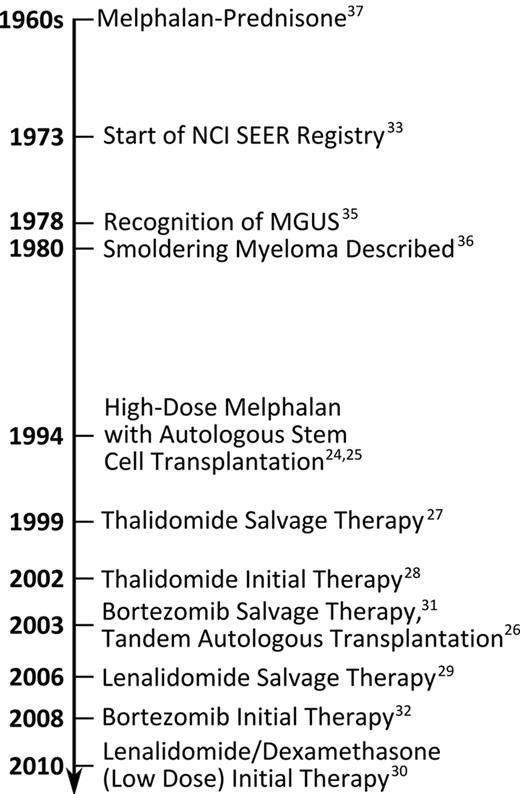
MM was defined using International Classification of Disease for Oncology, 3rd Edition (ICD-O-3) topographic (C42.1) and morphologic (9732/3) codes.34 Data on year of diagnosis, race, age, and sex were available for each case. Patients were divided into age groups and periods of diagnosis based on advances in MM diagnosis and treatment (Figure 1).4,24-33,35-37 Patients were stratified by age above/below 70 years, based on the fact that ASCT is not usually performed on patients above the age of 70.38
Statistical analysis
Incidence rates (age-adjusted using the 2000 US standard) and corresponding 95% confidence intervals (CIs) were calculated using SEER*Stat (version 6.6.2; 2010). Frequency distributions and age-specific incidence curves with log-linear scales were constructed and plotted as previously reported.39,40
The SEER data also were accessed in ASCII format and analyzed using the Kaplan-Meier method and log-rank test to assess for racial differences in cumulative disease-specific survival as obtained from death certificates reviewed by SEER abstracters.41 In parallel, to account for changing patterns of survival due to all causes of death in the general population over the study period, relative survival rates (RSRs) were computed using the actuarial method in SEER*Stat. All survival calculations included patients diagnosed from January 1973 to December 2005 and followed through December 2006. RSRs, defined as the ratio of the patients' observed survival to the expected survival of a similar group from the general population, measure the reduced survival associated with a diagnosis of MM. Importantly, accurate classification of cause of death is not required to determine RSRs and both direct and indirect causes of death are included. Comparisons of RSRs were based on the method of Brown.42 All statistical tests are 2-sided, with P < .05 considered statistically significant.
Results
Incidence
From 1973-2005, 5798 black and 28 939 white patients were diagnosed with MM (Table 1). The age-adjusted MM incidence rates were 11.0 and 4.9 per 100 000 person-years for blacks and whites, respectively (P < .001). This 2-3× higher incidence in blacks was consistent over time (Table 1). MM incidence rates rose slightly for both races from the 1970s to the 1990s before flattening in recent years. For both blacks and whites, incidence rates among males were approximately 50% higher than for females of each race; however, the black:white incidence rate ratio was somewhat higher among females (2.40) than males (2.16).
MM patient characteristics and incidence patterns
| . | Blacks . | Whites . | Black:white rate ratio . | ||
|---|---|---|---|---|---|
| n (%) . | Incidence (95% CI) . | n (%) . | Incidence (95% CI) . | ||
| Total | 5798 (100) | 11.0 (10.7-11.3) | 28 939 (100) | 4.9 (4.8-4.9) | 2.25 |
| Sex | |||||
| Male | 2883 (50) | 13.2 (12.7-13.7) | 15 255 (53) | 6.1 (6.0-6.2) | 2.16 |
| Female | 2915 (50) | 9.6 (9.2-9.9) | 13 684 (47) | 4.0 (3.9-4.1) | 2.40 |
| Year of MM diagnosis | |||||
| 1973-1979 | 744 (13) | 10.1 (9.3-10.9) | 4536 (15) | 4.5 (4.4-4.6) | 2.24 |
| 1980-1986 | 1048 (18) | 10.9 (10.3-11.7) | 5424 (19) | 4.7 (4.6-4.8) | 2.32 |
| 1987-1993 | 1244 (21) | 11.2 (10.5-11.8) | 6384 (22) | 5.0 (4.9-5.1) | 2.24 |
| 1994-1998 | 1097 (19) | 11.9 (11.2-12.7) | 5074 (18) | 5.1 (5.0-5.3) | 2.33 |
| 1999-2005 | 1665 (29) | 11.0 (10.5-11.6) | 7521 (26) | 5.0 (4.9-5.1) | 2.20 |
| Age, y | |||||
| < 50 | 633 (11) | 1.2 (1.1-1.3) | 1629 (5) | 0.4 (0.4-0.4) | 3.00 |
| 50-69 | 2787 (48) | 24.0 (23.1-24.8) | 11 490 (40) | 9.7 (9.5-9.9) | 2.47 |
| ≥ 70 | 2378 (41) | 62.2 (59.7-64.7) | 15 820 (55) | 30.4 (29.9-30.8) | 2.05 |
| Median age, y (interquartile range) | 66 (56-74) | 70 (61-78) | |||
| . | Blacks . | Whites . | Black:white rate ratio . | ||
|---|---|---|---|---|---|
| n (%) . | Incidence (95% CI) . | n (%) . | Incidence (95% CI) . | ||
| Total | 5798 (100) | 11.0 (10.7-11.3) | 28 939 (100) | 4.9 (4.8-4.9) | 2.25 |
| Sex | |||||
| Male | 2883 (50) | 13.2 (12.7-13.7) | 15 255 (53) | 6.1 (6.0-6.2) | 2.16 |
| Female | 2915 (50) | 9.6 (9.2-9.9) | 13 684 (47) | 4.0 (3.9-4.1) | 2.40 |
| Year of MM diagnosis | |||||
| 1973-1979 | 744 (13) | 10.1 (9.3-10.9) | 4536 (15) | 4.5 (4.4-4.6) | 2.24 |
| 1980-1986 | 1048 (18) | 10.9 (10.3-11.7) | 5424 (19) | 4.7 (4.6-4.8) | 2.32 |
| 1987-1993 | 1244 (21) | 11.2 (10.5-11.8) | 6384 (22) | 5.0 (4.9-5.1) | 2.24 |
| 1994-1998 | 1097 (19) | 11.9 (11.2-12.7) | 5074 (18) | 5.1 (5.0-5.3) | 2.33 |
| 1999-2005 | 1665 (29) | 11.0 (10.5-11.6) | 7521 (26) | 5.0 (4.9-5.1) | 2.20 |
| Age, y | |||||
| < 50 | 633 (11) | 1.2 (1.1-1.3) | 1629 (5) | 0.4 (0.4-0.4) | 3.00 |
| 50-69 | 2787 (48) | 24.0 (23.1-24.8) | 11 490 (40) | 9.7 (9.5-9.9) | 2.47 |
| ≥ 70 | 2378 (41) | 62.2 (59.7-64.7) | 15 820 (55) | 30.4 (29.9-30.8) | 2.05 |
| Median age, y (interquartile range) | 66 (56-74) | 70 (61-78) | |||
Patients diagnosed 1973-2005 (SEER-9). All incidence rates are per 100 000 person-years and adjusted using the 2000 US population standard. The present study defined multiple myeloma cases ICD-O-3 codes 9732/3, which includes only cases coded as MM. However, the annually published SEER Cancer Statistics Review uses the Site and Morphology code for “Myeloma,” which includes a biologically and clinically heterogeneous group of plasma cell disorders including MM, solitary plasmacytoma, and plasma cell leukemia.
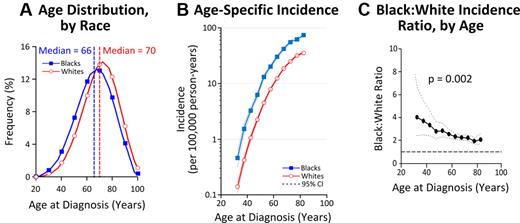
Age distributions and age-specific incidence rates varied by race (Figure 2A-C). The median age at diagnosis was 66 years in blacks and 70 years in whites (Figure 2A), and the mean age at diagnosis was 65.8 years in blacks and 69.8 years in whites (P < .001). To explore whether this difference was a reflection of shorter life-span in blacks, age-specific incidence curves were plotted (Figure 2B). Rates rose exponentially with age and were consistently higher among blacks than whites. Moreover, the black:white incidence rate-ratio decreased with age from > 3:1 among patients < 50 years old to approximately 2:1 in patients ≥ 70 years old (P = .002; Figure 2C).
Survival
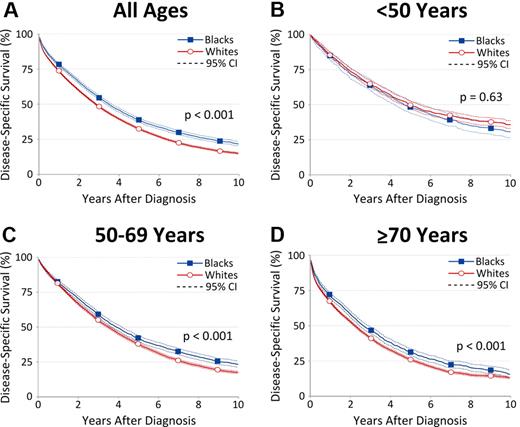
For patients diagnosed 1973-2005, survival rates were consistently higher for blacks than for whites (Figure 3A). Disease-specific survival decreased with age among patients of both races (Figure 3B-D). Notably, there was no racial survival disparity among blacks and whites ages < 50 years (P = .63; Figure 3B). In contrast, 5-year disease-specific survival was significantly greater among blacks than whites ages 50-69 years (41.6% vs 37.4%; P < .001) and ages ≥ 70 years (31.1% vs 25.9%; P < .001; Figure 3C-D). In parallel with the cumulative disease-specific survival analysis, we calculated RSR estimates. Both methods showed similar race-related survival disparities, though RSR differences were less pronounced between blacks and whites ages 50-69 years (34.7% vs 33.2%; P = .051) and ≥ 70 years (23.6% vs 21.3%; P = .004).
Changes in 5-year RSRs for blacks and whites before/after 1994 and 1999 (chosen based on the years that ASCT and thalidomide were introduced, respectively; see Figure 1) were compared (Figure 4). Among whites, 5-year RSR improved significantly over the 3 time periods 1973-1993, 1994-1998, and 1999-2005 for all ages (from 26.3% to 30.8%, and to 35.0%; P < .001 and P = .004, respectively), especially among patients aged < 70 years (from 31.2% to 37.3%, and to 44.6%; P < .001 for both changes). In addition, significant survival improvement was observed among whites ≥ 70 years old from 1973-1993 to 1994-1998 (19.8% to 23.3%; P < .001) but not later. Further analysis by age revealed that RSR significantly improved in whites up to 85 years of age (P < .001), with most improvement seen in whites 70-74 years (data not shown). In contrast, smaller improvements in 5-year RSR were suggested among blacks from 1973-1993, 1994-1998, and 1999-2005 (31.0%, 33.0%, and 34.1%, respectively), especially among younger patients, but they were less pronounced than those observed among whites and did not reach statistical significance. However, improvement among blacks < 70 years of age from 34.3% (1973-1993) to 40.4% (1999-2005) reached statistical significance (P = .007), the magnitude of which was less than 50% that for whites.
Five-year RSRs by race and age group (SEER-9). *P < .05; **P < .01; ***P < .001; and –, P ≥ .05.
Five-year RSRs by race and age group (SEER-9). *P < .05; **P < .01; ***P < .001; and –, P ≥ .05.
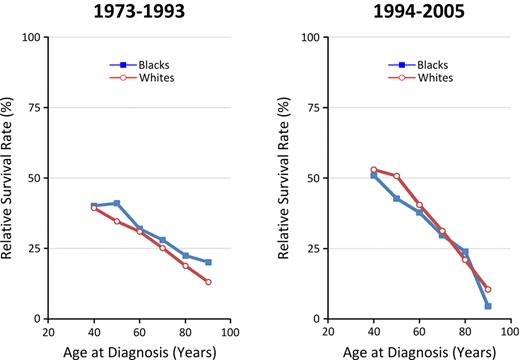
Survival rates varied markedly with age at diagnosis with changing racial patterns in recent years. Figure 5 illustrates higher 5-year RSR among blacks across all age groups for patients diagnosed 1973-1993. By 1994-2005, RSRs had improved dramatically among all but the oldest patients, with much more pronounced improvement in RSRs among whites compared with blacks at ages < 70 years.
Age-specific 5-year RSRs before and after 1994 (novel therapies), by race.
Discussion
We have conducted the largest population-based study specifically examining disparities in MM incidence and outcome by race, elucidating 3 novel findings: (1) blacks have a 4 year younger age of onset than whites; (2) over the entire study period, disease-specific survival was greater for blacks than whites; and (3) over time, survival improvement was much less pronounced among blacks than whites.
At this time, the underlying causes of MM remain elusive. Previous reports of familial clustering of MM and MGUS,11,12 substantially higher incidence of MM in blacks compared with whites even when accounting for socioeconomic status,14,43,44 an earlier increase in age-specific incidence rates among blacks,44 and our observed earlier age of MM onset in blacks may suggest a role for susceptibility genes in the process of myelomagenesis.
The better survival outcomes seen among black MM patients compared with whites is a novel finding rarely seen in malignancy,45 suggestive of disease heterogeneity by race. Concurrently, a mortality in blacks 2× higher than that in whites has been previously reported from the SEER registries.13,18 However, it is important to note that mortality, a measure of the frequency of deaths, is partially dependent on incidence, whereas survival is not; higher mortality is observed in blacks because MM is more common, regardless of better outcomes. Our finding of longer survival among blacks may suggest that biologically indolent subtypes of MM could be more common among blacks than whites. Molecular studies by race are needed to elucidate potential underlying molecular mechanisms of our findings. Ultimately, such studies may facilitate the development of personalized management and treatment for MM.
Four prior studies of predominantly white populations have found that MM patients, especially those < 70 years of age who are the best candidates for aggressive therapy, have experienced significantly improved survival after the introduction of ASCT and IMiDs in the 1990s.20-23 Strikingly, we have found that the magnitude of survival improvement among blacks was less than 50% that seen in whites. This suggests that blacks may not have had the same access to new therapies as whites, consistent with previous reports that blacks are 50% less likely than whites to undergo ASCT,46 while other studies have reported blacks and whites to have similar outcomes with ASCT.18,19 Likewise, a SEER Medicare-based study found that among MM patients ≥ 65 years of age, blacks were less likely than whites to receive chemotherapy.47 Based on small numbers, we attempted to separately assess changes in survival after the introduction of ASCT and novel drugs. We found improved survival among whites < 70 years old diagnosed before/after 1994 and 1999, suggesting that ASCT and novel drugs, respectively, may have had a role in improving survival. Meanwhile, no clear benefit was seen among blacks after the introduction of either modality. Although our study did not include information regarding treatment for individual patients, based on our results, we hypothesize that the previously reported lack of access to ASCT46 may extend to novel drugs. The consistent black:white incidence rate ratios over time does not indicate that this disparity in survival improvement resulted from earlier diagnosis in whites. As an alternative explanation, one might speculate that blacks perhaps have more indolent disease which in turn may have a poorer response to therapy, or there may be a combination of these effects.
In addition, we have, for the first time, found significantly improved survival among whites ≥ 70 years old from 1973-1993 to 1994-1998. Future studies are needed to better define the survival impact of novel therapies and gradual improvements in supportive care for the elderly. Regardless, comparable improved survival over time was not seen among blacks ≥ 70 years old.
The strengths of our study include the population-based design, very large sample size, geographic, socioeconomic, and racial diversity present in the SEER registries, and rigorously evaluated data, allowing for > 98% case ascertainment for hematologic malignancies.47 In addition, we demonstrated consistent racial disparity patterns using parallel statistical methods, strengthening our observations. We chose to present disease-specific survival curves because these are common in the clinical literature. As expected, racial disparities observed using RSR were less pronounced given that blacks have more comorbidities42 and RSRs provide the mathematical risk estimates without requiring determination of cause of death. Our study is limited by the lack of specific clinical data regarding diagnosis and treatment. In addition, the inaccessibility of tumor tissue and serum samples in patients included in our study precludes an analysis of prognostic markers or gene-expression derived disease subtypes by race.48 Lack of sufficient follow-up time after the introduction of IMiDs and bortezomib prevented complete assessment of the impact of these agents separately on survival in the population. Future studies including longer follow-up data in the era of novel drugs, especially those regimens tailored for the elderly,30 are needed.
In summary, our findings are consistent with a genetic basis for myelomagenesis, either at development of MM or earlier at the level of precursor disease conferring greater susceptibility to developing MM and may suggest that blacks have different disease biology than whites. Future research is needed to explore potential molecular underpinnings of observed racial differences. The realization that the previously reported improved survival after the advent of novel MM therapies primarily reflects improvements among whites raises serious concerns regarding a potential lack of access to newer treatments among blacks in the US. These facts further emphasize the need to effectively translate efficacious treatments to patients regardless of race.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by the Intramural Research Program of the NCI of the National Institutes of Health (NIH).
National Institutes of Health
Authorship
Contribution: A.J.W., S.S.D., W.F.A., K.A.M., and O.L. oversaw all aspects of the study and participated in the study conception and design; A.J.W., S.S.D., W.F.A., and K.A.M. were responsible for the statistical analysis and take responsibility for the accuracy of the data analysis; A.J.W., P.J.M., S.S.D., W.F.A., B.M.W., S.Y.K., K.A.M., and O.L. interpreted the data and made important intellectual contributions to the manuscript; A.J.W. and O.L. drafted the manuscript, managed all revisions to the manuscript, and obtained funding for the study; and all authors had full access to all the data in the study, had final responsibility for the decision to submit the article for publication, and read, gave comments, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests. A.J.W. is a member of the NIH Clinical Research Training Program, which is funded jointly by the NIH and the Foundation for NIH (in part by a grant from Pfizer Inc).
Correspondence: Ola Landgren, National Cancer Institute, National Institutes of Health, Center for Cancer Research, Medical Oncology Branch, 9000 Rockville Pike, Bldg 10/Rm 13N240, Bethesda, MD, 20892; e-mail: landgreo@mail.nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal