Abstract
We analyzed a cohort of 26 patients with chronic myeloid leukemia who had failed imatinib and a second tyrosine kinase inhibitor but were still in first chronic phase and identified prognostic factors for response and outcomes. The achievement of a prior cytogenetic response on imatinib or on second-line therapy were the only independent predictors for the achievement of complete cytogenetic responses on third-line therapy. Younger age and the achievement of a cytogenetic response on second line were the only independent predictors for overall survival (OS). At 3 months, the 9 patients who had achieved a cytogenetic response had better 30-month probabilities of complete cytogenetic responses and OS than the patients who had failed to do so. Factors measurable before starting treatment with third line therapy and cytogenetic responses at 3 months can accurately predict subsequent outcome and thus guide clinical decisions.
Introduction
Dasatinib and nilotinib are effective second-line therapies for chronic phase (CP) chronic myeloid leukemia (CML) patients who are resistant or intolerant to imatinib,1-6 although fewer than 50% of patients actually obtain durable complete cytogenetic responses (CCyR). Currently patients who fail to respond to second-line dasatinib or nilotinib may either undergo allogeneic stem cell transplantation or receive third-line therapy with a different tyrosine kinase inhibitor (TKI). It has been shown that the efficacy of third line dasatinib or nilotinib is limited,7 so it is of paramount importance to identify those patients most likely to benefit from a third-line TKI. In this study we present our experience managing with a third TKI patients still in CP who have failed both imatinib and second-line dasatinib or nilotinib. We identify prognostic factors for response to third-line therapy that can be used to guide clinical decisions.
Methods
Patients
Between March 2005 and January 2008, we evaluated 26 consecutive patients with CML in CP who had been treated with dasatinib (n = 20) or nilotinib (n = 6) after failing imatinib (Table 1) in various phase II clinical studies. Patients gave informed consent in accordance with the Declaration of Helsinki, with approval from the institutional review board of Imperial College London. Failure to second line was defined as previously described by others.7 Patients harboring a T315I mutation in the BCR-ABL kinase domain (KD) were not included in this study. All patients consented to the use of their data. The median follow up after starting third-line therapy for the surviving patients was 21.5 months (range, 6-46.5 months). Dasatinib and nilotinib were administered at standard doses as described by others.4,5,8-10 CP, complete hematologic responses (CHR), CCyR major cytogenetic response (MCyR), and major molecular response (MMR) were defined by conventional criteria.11
Responses and outcome on third-line therapy according to the baseline characteristics of the patients
| Variable . | n . | Response to third-line therapy . | |||
|---|---|---|---|---|---|
| MCyR (%) . | CCyR (%) . | OS (%) . | EFS (%) . | ||
| Age* | P = .3 | P = .5 | P = .03 | P = .7 | |
| ≤ 64 y | 13 | 52.0 | 41.0 | 60.6 | 49.9 |
| > 64 y | 13 | 42.3 | 53.8 | 24.9 | 39.8 |
| Sex | P = .9 | P = .9 | P = .9 | P = .8 | |
| Female | 12 | 44.4 | 45.3 | 45.0 | 51.3 |
| Male | 14 | 53.1 | 55.1 | 47.6 | 41.4 |
| Status at the onset of imatinib therapy | P = .3 | P = .3 | P = .4 | P = .8 | |
| Early CP | 19 | 57.1 | 64.3 | 80.0 | 53.6 |
| Late CP† | 7 | 47.8 | 52.6 | 40.4 | 43.5 |
| Sokal risk group | P = .9 | P = .6 | P = .9 | P = .8 | |
| Low + intermediate‡ | 14 | 44.9 | 38.6 | 39.0 | 33.3 |
| High | 11 | 60.7 | 49.3 | 50.0 | 56.6 |
| Best cytogenetic response on imatinib | P = .004 | P = .01 | P = .4 | P = .1 | |
| No response | 16 | 28.6 | 13.5 | 42.4 | 32.7 |
| At least MiCyR | 10 | 85.0 | 62.5 | 57.1 | 72.0 |
| Best cytogenetic response on second-line therapy | P < .001 | P < .001 | P = .03 | P = .04 | |
| No response | 14 | 16.7 | 0 | 25.6 | 23.4 |
| At least MiCyR | 12 | 83.3 | 70.0 | 88.9 | 83.3 |
| Prior history of clonal evolution | P = .1 | P = .3 | P = .9 | P = .9 | |
| No | 18 | 49.5 | 27.8 | 57.0 | 46.1 |
| Yes | 8 | 0 | 0 | 50.0 | 50.0 |
| Prior history of KD mutation | P = .9 | P = .4 | P = .2 | P = .3 | |
| No | 14 | 45.5 | 53.2 | 50.0 | 36.9 |
| Yes | 12 | 53.1 | 50.6 | 53.9 | 54.0 |
| Prior history of hematologic resistance to TKI therapy§ | P = .007 | P = .04 | P = .4 | P = .04 | |
| No | 19 | 67.8 | 63.9 | 61.9 | 64.7 |
| Yes | 7 | 0 | 0 | 44.4 | 28.6 |
| Prior history of intolerance to TKI therapy | P = .5 | P = .5 | P = .6 | P = .3 | |
| No | 9 | 52.6 | 46.6 | 50.0 | 40.0 |
| Yes | 17 | 59.7 | 52.9 | 46.5 | 45.4 |
| Percentage of Philadelphia chromosome–positive at start of third-line therapy | P = .04 | P = .03 | P = .1 | P = .2 | |
| ≥ 95% | 22 | 48.5 | 39.1 | 50.9 | 55.9 |
| < 95% | 4 | 100 | 100 | 100 | 100 |
| Time from diagnosis to third-line therapy¶ | P = .9 | P = .7 | P = .9 | P = .9 | |
| ≤ 63 months | 11 | 46.7 | 66.7 | 45.0 | 47.0 |
| > 63 months | 12 | 50.7 | 53.5 | 51.6 | 49.0 |
| Variable . | n . | Response to third-line therapy . | |||
|---|---|---|---|---|---|
| MCyR (%) . | CCyR (%) . | OS (%) . | EFS (%) . | ||
| Age* | P = .3 | P = .5 | P = .03 | P = .7 | |
| ≤ 64 y | 13 | 52.0 | 41.0 | 60.6 | 49.9 |
| > 64 y | 13 | 42.3 | 53.8 | 24.9 | 39.8 |
| Sex | P = .9 | P = .9 | P = .9 | P = .8 | |
| Female | 12 | 44.4 | 45.3 | 45.0 | 51.3 |
| Male | 14 | 53.1 | 55.1 | 47.6 | 41.4 |
| Status at the onset of imatinib therapy | P = .3 | P = .3 | P = .4 | P = .8 | |
| Early CP | 19 | 57.1 | 64.3 | 80.0 | 53.6 |
| Late CP† | 7 | 47.8 | 52.6 | 40.4 | 43.5 |
| Sokal risk group | P = .9 | P = .6 | P = .9 | P = .8 | |
| Low + intermediate‡ | 14 | 44.9 | 38.6 | 39.0 | 33.3 |
| High | 11 | 60.7 | 49.3 | 50.0 | 56.6 |
| Best cytogenetic response on imatinib | P = .004 | P = .01 | P = .4 | P = .1 | |
| No response | 16 | 28.6 | 13.5 | 42.4 | 32.7 |
| At least MiCyR | 10 | 85.0 | 62.5 | 57.1 | 72.0 |
| Best cytogenetic response on second-line therapy | P < .001 | P < .001 | P = .03 | P = .04 | |
| No response | 14 | 16.7 | 0 | 25.6 | 23.4 |
| At least MiCyR | 12 | 83.3 | 70.0 | 88.9 | 83.3 |
| Prior history of clonal evolution | P = .1 | P = .3 | P = .9 | P = .9 | |
| No | 18 | 49.5 | 27.8 | 57.0 | 46.1 |
| Yes | 8 | 0 | 0 | 50.0 | 50.0 |
| Prior history of KD mutation | P = .9 | P = .4 | P = .2 | P = .3 | |
| No | 14 | 45.5 | 53.2 | 50.0 | 36.9 |
| Yes | 12 | 53.1 | 50.6 | 53.9 | 54.0 |
| Prior history of hematologic resistance to TKI therapy§ | P = .007 | P = .04 | P = .4 | P = .04 | |
| No | 19 | 67.8 | 63.9 | 61.9 | 64.7 |
| Yes | 7 | 0 | 0 | 44.4 | 28.6 |
| Prior history of intolerance to TKI therapy | P = .5 | P = .5 | P = .6 | P = .3 | |
| No | 9 | 52.6 | 46.6 | 50.0 | 40.0 |
| Yes | 17 | 59.7 | 52.9 | 46.5 | 45.4 |
| Percentage of Philadelphia chromosome–positive at start of third-line therapy | P = .04 | P = .03 | P = .1 | P = .2 | |
| ≥ 95% | 22 | 48.5 | 39.1 | 50.9 | 55.9 |
| < 95% | 4 | 100 | 100 | 100 | 100 |
| Time from diagnosis to third-line therapy¶ | P = .9 | P = .7 | P = .9 | P = .9 | |
| ≤ 63 months | 11 | 46.7 | 66.7 | 45.0 | 47.0 |
| > 63 months | 12 | 50.7 | 53.5 | 51.6 | 49.0 |
The table shows the characteristics of the patients at the moment of starting third-line therapy and the 30-month probabilities of MCyR, CCyR, OS and EFS.
Median age at the onset of third-line therapy was 64 years.
Patients were considered to be in late CP at the moment of starting imatinib if they had commenced the imatinib > 6 months after diagnosis or had received prior interferon-α therapy.
One patient was classified as low risk and 13 as intermediate risk. The Sokal score could not be calculated in one patient.
Hematologic resistance was defined as either failure to achieve a CHR or loss of a previously achieved CHR.
Sixty-three months was the median time from diagnosis of CML to the start of third-line therapy.
Statistical analysis
Probabilities of overall survival (OS), progression-free survival (PFS), and event-free survival (EFS), all as defined previously,10 were calculated using the Kaplan-Meier method. The probabilities of cytogenetic and molecular responses were calculated as previously described.11 However, data from the 6 patients who underwent allogeneic stem cell transplantation were censored at the moment of transplantation for the analysis of cytogenetic responses but not for EFS, PFS, and OS. Univariate and multivariate analyses were carried out as described.11
Results and discussion
Responses to third-line TKI
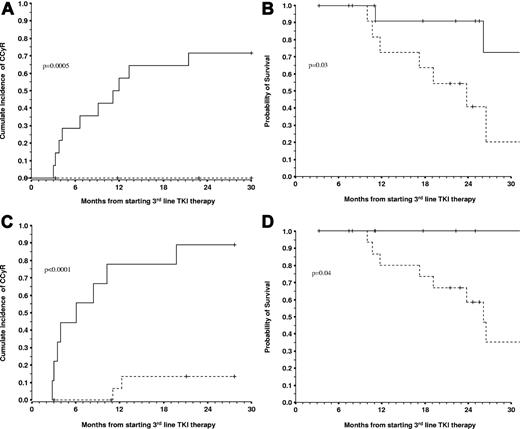
During the follow-up, 13 (50.0%), 9 (34.6%), and 5 (19.2%) patients achieved a MCyR, CCyR, and MMR, respectively. The 2.5-year (30-month) cumulative incidences of MCyR, CCyR, and MMR were 48.2%, 32.4%, and 21.1%, respectively. Univariate and multivariate analyses were performed to identify factors that predict for cytogenetic responses to third-line therapy (Table 1). Patients who achieved a cytogenetic response either on imatinib or a second-line therapy had a higher probability of also achieving cytogenetic response on the third line. Patients who had developed hematologic resistance during prior TKI therapy were less likely to achieve cytogenetic responses (Table 1). The presence of a KD mutation at the onset of the third-line therapy did not affect the response. Multivariate analysis showed that the achievement of a cytogenetic response on imatinib (relative risk [RR] = 5.6, P = .03) or on second-line therapy (RR = 11.8, P = .006) were the only independent predictors for the achievement of CCyR. The achievement of cytogenetic response on second line was the only independent predictor for MCyR (RR = 15.4, P < .001). When we combined both variables we found that the patients who had achieved cytogenetic response on one of the 2 prior therapies had a significantly higher probability of achieving cytogenetic response on third-line therapy (ie, the 30-month probability of MCyR and CCyR were 100% vs 12.5% [P < .0001] and 71.4% vs 0% [P = .0005], respectively; Figure 1).
Outcome of patients treated with third-line TKI according to the cytogenetic response obtained with the prior TKI therapy and to the cyto-genetic response obtained after 3 months on third-line therapy. The 14 patients who achieved at least MiCyR on imatinib or on second-line therapy (solid line) had a significantly better probability of achieving CCyR (A) and a better OS (B) on third-line nilotinib or dasatinib than the 12 patients who had primary cytogenetic resistance to the prior 2 lines of TKI therapy (broken line). The 30-month cumulative incidence of CCyR for the 2 groups was of 71.4% vs 0% (P = .0005), and the 30-month OS was 72.7% vs 20.4% (P = .03). When we excluded the only patient who died of nonleukemia-related reasons and in CCyR the OS were 90.1% vs 20.4% (P = .01). The 14 patients who achieved at least MiCyR on one of the prior TKI therapies also had a better EFS that the 14 patients with primary cytogenetic resistance, specifically 70.5% vs 16.2% (P = .02). (C-D) Results of the landmark analyses for the achievement of CCyR and OS (excluding the nonleukemia-related death) according to the cytogenetic response at 3 months on nilotinib or dasatinib as third-line therapy. At 3 months, 9 patients had achieved at least MiCyR (solid line). These patients had higher probabilities of achieving CCyR (C) and OS (D) than the 17 patients who had failed to do so (broken line), specifically 88.9% vs 13.3% (P < .0001), and 100% vs 35.0% (P = .04; see text).
Outcome of patients treated with third-line TKI according to the cytogenetic response obtained with the prior TKI therapy and to the cyto-genetic response obtained after 3 months on third-line therapy. The 14 patients who achieved at least MiCyR on imatinib or on second-line therapy (solid line) had a significantly better probability of achieving CCyR (A) and a better OS (B) on third-line nilotinib or dasatinib than the 12 patients who had primary cytogenetic resistance to the prior 2 lines of TKI therapy (broken line). The 30-month cumulative incidence of CCyR for the 2 groups was of 71.4% vs 0% (P = .0005), and the 30-month OS was 72.7% vs 20.4% (P = .03). When we excluded the only patient who died of nonleukemia-related reasons and in CCyR the OS were 90.1% vs 20.4% (P = .01). The 14 patients who achieved at least MiCyR on one of the prior TKI therapies also had a better EFS that the 14 patients with primary cytogenetic resistance, specifically 70.5% vs 16.2% (P = .02). (C-D) Results of the landmark analyses for the achievement of CCyR and OS (excluding the nonleukemia-related death) according to the cytogenetic response at 3 months on nilotinib or dasatinib as third-line therapy. At 3 months, 9 patients had achieved at least MiCyR (solid line). These patients had higher probabilities of achieving CCyR (C) and OS (D) than the 17 patients who had failed to do so (broken line), specifically 88.9% vs 13.3% (P < .0001), and 100% vs 35.0% (P = .04; see text).
Intolerant patients
All patients were classified according to the degree of cytogenetic response achieved on imatinib or second-line therapy (Table 1), but patients were also classified according their tolerance to the previous therapy. For example, a patient who took imatinib for 2 years and required several dose reductions because of thrombocytopenia but never achieved a cytogenetic response would have been classified as failing to achieve MCyR on imatinib and also as intolerant (Table 1). We classified as having a history of intolerance to TKI those patients who required dose reduction during prior TKI therapy on account of recurrent toxicity or who permanently discontinued the imatinib or second-line therapy because of grade III-IV nonhematologic side effects. Seventeen patients (65%) met this criterion (Table 1). Intolerant patients had a probability of responding to the third line therapy similar to that of the resistant patients, but when we subdivided this cohort according to the type of intolerance we found that the 11 patients who had hematologic toxicity with either the imatinib or the second-line therapy had a probability of 30-month CCyR lower than that of the remaining 15 patients (11.1% vs 47.5%, P = .03), while the 8 patients with nonhematologic intolerance to the imatinib or to the second line had a probability of 30-month CCyR greater than that of the remaining 18 patients (87.5% vs 5.6%, P < .001).
EFS and OS
During follow-up, 11 (42.3%) patients failed third-line therapy and 9 (34.6%) died. One patient died of nonleukemia causes (myocardial infarction) while the remaining 8 died after progression to blastic phase. The 30-month probabilities of EFS and OS were 45.7% and 46.7%, respectively. Patients with primary cytogenetic resistance to the second-line therapy had a significantly worse EFS and OS (Table 1). Multivariate analysis showed that the development of hematologic resistance was the only independent predictor for EFS (RR = 0.43, P = .02). The achievement of a cytogenetic response on second line and age younger than 64 years (possibly reflecting eligibility for transplantation) were the only independent predictors for OS (RR = 6.5, P = .02 and RR = 0.13, P = .02).
Influence of response on outcome
At 3 months, 26 patients remained on follow-up, of whom 9 had achieved at least MiCyR (< 95% Philadelphia chromosome–positive metaphases). These 9 patients has better 30-month probabilities of CCyR, EFS, and OS than the patients who had failed to achieve MiCyR, specifically 88.9% vs 13.3% (P < .0001), 87.5% vs 28.0% (P = .007), and 87.5% vs 35.0% (P = .1). When we excluded the only patient who died of nonleukemia-related causes while in CCyR, the probabilities of EFS and OS were 100% vs 28% (P = .007) and 100% vs 35.0% (P = .04), respectively (Figure 1).
The cytogenetic response obtained on prior therapy proved highly informative. Patients who had achieved some degree of cytogenetic response during the first- or second-line therapy had a much higher probability of achieving MCyR and CCyR, and had better EFS and OS than the patients who had primary cytogenetic resistance to both first- and second-line therapy (Figure 1). In our hands, none of the patients with primary cytogenetic resistance to both initial lines achieved CCyR on the third line, clearly indicating that third-line TKI is not useful for these patients. Patients who had had nonhematologic side effects as the primary reason for change of therapy fared well, whereas patients with a history of hematologic toxicity to one of the prior TKIs fared worse. We have previously reported the association between hematologic toxicity and lack of cytogenetic response in CP patients treated with imatinib as first-line therapy,11 in patients treated with imatinib after interferon-α failure,12 and in patients treated with nilotinib or dasatinib after imatinib failure.10 In these cases, the poor prognosis associated with hematologic toxicity may in part explained by the lack of an expandable population of Philadelphia chromosome–negative cells.12
We report here the largest series of CML patients in CP treated with a third-line TKI after failing both imatinib and another TKI. Our rather disappointing results stress the need to select more carefully the patients who may benefit from a third line TKI, as for many patients allogeneic stem cell transplantation or an alternative experimental therapy may be a more appropriate.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful for support from the NIHR Biomedical Research Center Funding Scheme. We also thank the patients who participated in this study.
Authorship
Contribution: A.R.I. collected patient data, performed statistical analysis, and wrote the manuscript; C.P. collected patient data and commented on the manuscript; M.B., D.M., H.d.L., K.R., F.D., M.B., and J.F.A. provided patient care and commented on the manuscript; R.S. reviewed the statistical analysis and commented on the manuscript; J.S.K. performed the molecular studies, assembled the molecular data, and commented on the manuscript; L.F. supervised the day-to-day running of the Minimal Residual Disease laboratory and commented on the manuscript; A.R. performed the cytogenetics and commented on the manuscript; J.M.G. wrote the manuscript; and D.M. designed the study, performed the statistical analysis, supervised patient care, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Marin, Department of Haematology, Imperial College London, Du Cane Road, London W12 0NN, United Kingdom; e-mail: d.marin@imperial.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal