Abstract
The oncogenic JAK2V617F mutation is found in myeloproliferative neoplasms (MPNs) and is believed to be critical for leukemogenesis. Here we show that JAK2V617F requires an intact SH2 domain for constitutive activation of downstream signaling pathways. In addition, there is a strict requirement of cytokine receptor expression for the activation of this oncogene. Further analysis showed that the SH2 domain mutation did not interfere with JAK2 membrane distribution. However, coimmunoprecipitated experiments revealed a role for the SH2 domain in the aggregation and cross-phosphorylation of JAK2V617F at the cell membrane. Forced overexpression of cytokine receptors could rescue the JAK2V617F SH2 mutant supporting a critical role of JAK2V617F abundance for constitutive activation. However, under physiologic cytokine receptor expression the SH2 domain is absolutely necessary for oncogenic JAK2V617F activation. This is demonstrated in a bone marrow transplantation model, in which an intact SH2 domain in JAK2V617F is required for the induction of an MPN-like disease. Thus, our results points to an indispensable role of the SH2 domain in JAK2V617F-induced MPNs.
Introduction
The V617F mutation within the pseudokinase domain of the nonreceptor tyrosine kinase JAK2 (janus kinase 2) has frequently been identified in patients with myeloproliferative neoplasms (MPNs) such as polycythemia vera (PV; 95%), idiopathic myelofibrosis (IMF; 50%), and essential thrombocythemia (ET; 20%-40%).1–4 In addition to MPNs, JAK2V617F has also been observed at lower frequencies in chronic myelomonocytic leukemia (3%-8%), myelodysplastic syndrome (4%), and rarely in systemic mastocytosis.5,6 A subset of JAK2V617F-negative PV patients showed gain-of-function mutations affecting exon 12 of JAK2.7 The high abundance of JAK2 mutations led to the revision of the World Health Organization (WHO) diagnostic criteria for PV, ET, and IMF.8 In mice, transplantation of JAK2V617F-infected bone marrow (BM) to irradiated recipients led to a PV-like disease.9–12 Recently, Xing et al demonstrated that JAK2V617F-transgenic mice develop a MPN with ET-, PV-, and (post-PV) IMF-like phenotypes.13 Tiedt et al showed that the ratio of mutant JAK2V617F to wild-type JAK2 determines the MPN phenotype (ET vs PV) in transgenic mice.14
Numerous cytokine type I receptors do not have an intrinsic tyrosine kinase domain and require JAK2 for signal transduction.15 In Epo receptor (EpoR)–expressing cells, it has been proposed that ligand binding leads to a conformational change of the receptor, which promotes JAK2 activation through reciprocal interaction and phosphorylation of 2 juxtapositioned JAK2 molecules.16 Subsequently, activated JAK2 phosphorylates tyrosine residues within the cytoplasmic tail of the cytokine receptor, which then serve as docking sites for signal transducers and activators of transcription (STATs), in particular STAT5.17–19 Because JAK2 is essential for EpoR signaling, JAK2−/− mice are embryonic lethal due to severe anemia.20,21
It has been demonstrated that JAK2V617F-mediated transformation of Ba/F3 cells is facilitated by the expression of homodimeric type I cytokine receptor such as the EpoR.22 Recently Lu et al showed that dimerization of the EpoR is necessary for the constitutive activation of JAK2V617F.23 However, parental Ba/F3 cells expressing JAK2V617F but lacking the EpoR are also able to proliferate interleukin-3 (IL-3) independently.2,24 These results indicate that the interaction between cytokine receptors and JAK2V617F may be crucial for oncogenic transformation, even though the exact mechanism and receptor requirements remain largely unclear.
The 4 mammalian JAK family members (JAK1, JAK2, JAK3, and Tyk2) contain 7 conserved domains, which are designated as JAK homology (JH) domains JH1-JH7. JH1 is a functional tyrosine kinase domain that becomes activated after cytokine stimulation. Phosphorylation of Y1007 in the activation loop of the kinase domain is required for JAK2 kinase activity.25 The pseudokinase domain (JH2) is adjacent to the kinase domain (JH1) and is thought to play a role in autoinhibition of the kinase activity of JAK2. Homology-based molecular modeling of JH2 showed 3 inhibitory regions (IR1-3), which mediate intermolecular interaction with the JH1 domain and regulate JAK2 kinase activity.26 The proximity of the recently discovered V617F mutation to the IR1 of the JH2 domain may explain the constitutive activity of JAK2V617F. However, there is no conclusive explanation for the inhibitory function of the JH1 domain, because the complete 3-dimensional structure of any JAK member has not been reported yet. The N-terminal FERM domain mediates binding of JAK2 to membrane proximal regions of cytokine receptors as well as processing and surface expression of EpoR.27–30 Consistent with these reports, mutation of the FERM domain impairs the JAK2V617F-induced transformation in both Ba/F3 cell lines and in mice.31 The presence of a SH2-like domain located between the FERM and the pseudokinase domain has been identified using structure prediction tools, but the function of the JAK2 SH2 domain has not been determined yet.32–34 A mutation of the highly conserved arginine at position 426 within the SH2 domain of JAK2 did not have an impact on interferon-γ signaling.35 Radtke et al were able to show that the SH2 domain of JAK1 does not exert the function of a classical SH2 domain, but might mediate receptor binding and receptor surface expression.36
Because the SH2 domain is adjacent to the pseudokinase domain containing the V617F mutation, we hypothesized a possible role of the SH2 domain for the constitutive phosphorylation of JAK2V617F. Our goal was to determine the role of the SH2 domain in JAK2V617F-mediated transformation and myeloproliferation. Using both cell lines as well as a murine BM transplantation model, we were able to find evidence for a SH2-dependent mechanism required for oncogenic activation of JAK2.
Methods
Cell lines
Murine Ba/F3 were obtained from the DSMZ and were grown in RPMI 1640 medium containing 10% fetal calf serum in the presence of murine IL-3 (mIL-3; R&D Systems). 293T and γ2A cells were maintained in Dulbecco modified Eagle medium (Gibco) containing 10% fetal calf serum.
Proliferation and viability
Proliferation was measured using an MTS [3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium]-based method by absorption of formazan at 490 nm (CellTiter 96; Promega). Measures were taken as triplicates after 72 and 96 hours of culture without cytokines. Cell viability was determined by trypan blue exclusion method.
Immunoblotting
Immunoblotting was performed as previously described.37 Antibodies to phospho-JAK2 (21 870-R), HA (F-7), STAT5 (G-2), EpoR (M-20), IL-3Rα (V-18), and βchain (T-20) were obtained from Santa Cruz Biotechnology. Antibodies to phosphotyrosine (PY20), flag, and myc were purchased from Upstate Biotechnology. Monoclonal antibody against phospho-STAT5 was kindly provided by Tom Wheelers and Henry B. Sadowski (Hamilton, New Zealand).
In vitro kinase assay
In vitro kinase assays were performed as described previously.39 In brief, purified JAK2 kinase domain (KD) was obtained from Cell Signaling Technology, JAK2V617F and JAK2V617F mSH2 proteins were immunoprecipitated (IP) from HEK293T cells. The assay was performed in 50 μL of kinase buffer containing 60mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.9, 5mM MgCl2, 5mM MnCl2, 3μM Na3VO4, 125mM dithiothreitol, 20μM ATP, 32P-γ-ATP. Histone-3 (Roche) was used as a substrate.
Expression vectors
Human WT JAK2 cDNA was purchased from RZPD. WT JAK2 was cloned into the EcoR1 site of the MigRI retroviral vector coexpressing enhanced green fluorescent protein (eGFP). The Flag tag sequence was generated at the N terminus of JAK2 by site-directed mutagenesis (Quickchange; Stratagene). The V617F, R426K, and Y40A/L41A mutations were inserted by site-directed mutagenesis (Quickchange) and confirmed by DNA sequencing. Murine stem cell virus (MSCV)–eGFP-HA-EpoR cDNA was kindly provided by Harvey Lodish. EpoR-Ba/F3 cell lines were generated by retroviral transduction followed by Epo-selection. Myc-tagged wild-type (WT) and mutant JAK2 were generated by cloning WT JAK2 into the EcoR1 site of pCMV-Myc and subsequently subcloned into the retroviral MSCV-neo vector. Ba/F3 cells were retrovirally transduced and GFP-positive Ba/F3 cells were sorted by flow cytometry.
BM transduction and transplantation
For details, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All experiments were approved by the Technical University of Munich Institutional Review Board and Animal Care and Use Committee.
Results
JAK2V617F-mediated IL3-independent growth of Ba/F3 cells requires an intact FERM and SH2 domain
It has been previously demonstrated that JAK2V617F is able to render Ba/F3 cells IL-3–independent.2 However, the exact mechanism how the V617F mutation leads to activation of the JAK2 pathway remains largely unclear. The JAKs contain several conserved domains possibly involved in the constitutive activation of JAK2V617F. The C-terminal FERM domain has been shown to be required for receptor association of the JAKs, and deletion of this domain leads to cytosolic localization and loss of JAK-mediated signaling.30,31,40 In addition, the SH2 domain is conserved in all JAKs and is adjacent to the pseudokinase domain containing the V617F mutation. However, little is known about the function of this domain in both WT and oncogenic JAK activation. To investigate the role of the SH2 domain in oncogenic JAK2 activation, we generated a JAK2 FERM domain mutant (L40A/Y41A) and changed the highly conserved arginine at position 426 within the SH2 domain to a lysine (R426K). The corresponding mutations in JAK1 have been described earlier.36
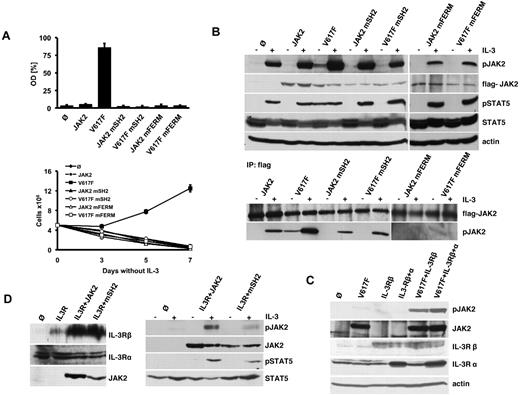
JAK2 constructs containing V617F, R426K or L40A/Y41A, or combinations of these mutations were stably introduced into IL-3–dependent Ba/F3 cells. As expected, JAK2 expression with or without mutations in the SH2 domain or FERM domain was not able to render Ba/F3 cells growth factor-independent, which was determined by both MTS assay and cell counting (Figure 1A). In contrast, expression of JAK2V617F in Ba/F3 cells led to factor-independent growth as described previously.2 JAK2V617F containing the FERM domain (JAK2 mFERM) mutation did not lead to this phenotype in Ba/F3 cells indicating that proper receptor scaffold is required for oncogenic JAK2-induced growth factor independence. Most strikingly, also cells expressing oncogenic JAK2V617F with the SH2 mutation (R426K) did not grow IL-3–independent (Figure 1A). These findings strongly indicate a critical role of both the FERM and the SH2 domain for the transforming function of oncogenic JAK2V617F.
JAK2V617F requires an intact FERM and SH2 domain for constitutive ligand-independent activation. (A) Proliferation of parental Ba/F3 cells (Ø) and Ba/F3 cells expressing WT-JAK2 (JAK2) or JAK2 containing the V617F (V617F), R426K (JAK2 mSH2), L40A/Y41A (JAK2 mFERM) single mutations, or the JAK2V617F+R426K (V617F mSH2) and JAK2V617F+L40A/Y41A (V617F mFERM) double mutations as indicated. Cell growth in the absence of IL-3 was quantified by the relative optical density (OD) after 96 hours using an MTS-based assay (top panel). Absolute cell numbers over time were measured in the absence of IL-3 by trypan blue exclusion (bottom panel). The figures represent 1 of 3 independent experiments. Values are expressed as mean of triplicates ± SEM. (B) Parental Ba/F3 and Ba/F3 cells expressing WT and mutant JAK2 as indicated and described in panel A were serum-starved for 12 hours and stimulated with IL-3 or vehicle for 5 minutes. Lysates were subjected to Western blotting with the indicated antibodies (top panel). Unstimulated and IL-3–stimulated cell lines expressing JAK2 constructs described in panel A were immunoprecipitated from whole-cell lysates using an anti-Flag antibody and immunoblotted with the indicated antibodies (bottom panel). Note that the detection of JAK2V617F mFERM required longer exposures compared with the other JAK2 constructs. (C) Cell lysates were prepared from γ2A cells stably expressing mock vector (Ø), JAK2V617F alone (V617F), IL-3Rβ chain (IL-3Rβ) alone, IL-3Rβ chain together with JAK2V617F (V617F+IL-3Rβ), and IL-3R β chain plus α chain (IL-3Rβ+α) alone and together with JAK2V617F (V617F+IL-3Rβ+α). Lysates were analyzed by Western blotting with the indicated antibodies. (D) Cell lysates of γ2A cell lines stably expressing control vector (Ø), WT-JAK2 (JAK2) and JAK2R426K (JAK2 mSH2) along with IL-3Rα and β chains (IL-3R) were immunoblotted with the indicated antibodies (left panel). γ2A cell lines stably expressing IL-3R, control vector (Ø), WT-JAK2 (JAK2), and JAK2R426K (JAK2 mSH2) were serum-starved for 12 hours followed by stimulation with IL-3 or vehicle. Cells were lysed and analyzed by immunoblotting with the indicated antibodies (right panel).
JAK2V617F requires an intact FERM and SH2 domain for constitutive ligand-independent activation. (A) Proliferation of parental Ba/F3 cells (Ø) and Ba/F3 cells expressing WT-JAK2 (JAK2) or JAK2 containing the V617F (V617F), R426K (JAK2 mSH2), L40A/Y41A (JAK2 mFERM) single mutations, or the JAK2V617F+R426K (V617F mSH2) and JAK2V617F+L40A/Y41A (V617F mFERM) double mutations as indicated. Cell growth in the absence of IL-3 was quantified by the relative optical density (OD) after 96 hours using an MTS-based assay (top panel). Absolute cell numbers over time were measured in the absence of IL-3 by trypan blue exclusion (bottom panel). The figures represent 1 of 3 independent experiments. Values are expressed as mean of triplicates ± SEM. (B) Parental Ba/F3 and Ba/F3 cells expressing WT and mutant JAK2 as indicated and described in panel A were serum-starved for 12 hours and stimulated with IL-3 or vehicle for 5 minutes. Lysates were subjected to Western blotting with the indicated antibodies (top panel). Unstimulated and IL-3–stimulated cell lines expressing JAK2 constructs described in panel A were immunoprecipitated from whole-cell lysates using an anti-Flag antibody and immunoblotted with the indicated antibodies (bottom panel). Note that the detection of JAK2V617F mFERM required longer exposures compared with the other JAK2 constructs. (C) Cell lysates were prepared from γ2A cells stably expressing mock vector (Ø), JAK2V617F alone (V617F), IL-3Rβ chain (IL-3Rβ) alone, IL-3Rβ chain together with JAK2V617F (V617F+IL-3Rβ), and IL-3R β chain plus α chain (IL-3Rβ+α) alone and together with JAK2V617F (V617F+IL-3Rβ+α). Lysates were analyzed by Western blotting with the indicated antibodies. (D) Cell lysates of γ2A cell lines stably expressing control vector (Ø), WT-JAK2 (JAK2) and JAK2R426K (JAK2 mSH2) along with IL-3Rα and β chains (IL-3R) were immunoblotted with the indicated antibodies (left panel). γ2A cell lines stably expressing IL-3R, control vector (Ø), WT-JAK2 (JAK2), and JAK2R426K (JAK2 mSH2) were serum-starved for 12 hours followed by stimulation with IL-3 or vehicle. Cells were lysed and analyzed by immunoblotting with the indicated antibodies (right panel).
JAK2V617F is constitutively phosphorylated in a ligand-independent manner resulting in cell growth in the absence of cytokines. We therefore determined the impact of the SH2 and FERM mutation on JAK2 and STAT5 phosphorylation (Figure 1B top panel). Consistent with cell proliferation data, WT-JAK2 expression did not reveal constitutive activation of JAK2-induced signaling in the absence of IL-3. In contrast, oncogenic JAK2V617F showed constitutive phosphorylation of JAK2 and STAT5. However, mutation of either the FERM or the SH2 domain abrogated growth factor-independent JAK2V617F signaling. Notably, IL-3 stimulation induced JAK2-dependent signaling in these cells (Figure 1B top panel). Because Ba/F3 cells express endogenous JAK2, which contributes to the pJAK2 and pSTAT5 signal detected in this experiment, we further delineated the phosphorylation status of overexpressed JAK2 harboring the R426K and L40A/Y41A mutation by Flag-IP (Figure 1B bottom panel). Constitutive phosphorylation of JAK2V617F was detectable and further enhanced by IL-3 stimulation. In contrast, phosphorylation of JAK2V617F mSH2, JAK2 mSH2, and WT-JAK2 was completely dependent on IL-3 stimulation. FERM domain-mutated JAK2 constructs were not phosphorylated even in the presence of IL-3. Thus, the FERM domain is absolutely required for oncogenic and cytokine-induced JAK phosphorylation, whereas the SH2 domain is required for the constitutive activation of oncogenic JAK2V617F only.
Because JAK2V617F could further be activated by the addition of IL-3, we were interested to determine whether cytokine receptor expression is a prerequisite for the constitutive activation of this oncogene. We therefore stably reconstituted JAK2-deficient γ2A cells with WT and oncogenic JAK2 (supplemental Figure 1). Even in the presence of IL-3, cells with WT-JAK2 expression did not show JAK2 or STAT5 activation. Strikingly, also oncogenic JAK2V617F did not display any activity in these cells. Thus, JAK2-deficient γ2A cells do not express functional common cytokine receptors required for constitutive JAK2 activation. Interestingly, these cells express the IL-3Rα chain but lack IL-3Rβ chain expression (Figure 1C). Ectopic expression of the IL-3Rβ chain was sufficient to restore oncogenic JAK2 activity indicating a strict requirement of cytokine receptor expression for its oncogenic activation (Figure 1C).
Finally, we aimed to investigate the role of the SH2 domain in cytokine-induced activation of WT-JAK2. To this end the phosphorylation of WT-JAK2 and JAK2 mSH2 in γ2A cells, ectopically expressing the human IL-3R with and without IL-3 stimulation was determined. JAK2 mSH2 showed a detectable but considerably decreased autophosphorylation and STAT5 phosphorylation compared with WT-JAK2 after IL-3 stimulation (Figure 1D). Thus, the JAK2 SH2 domain may be required for both oncogenic and cytokine-induced JAK2 activation.
High levels of EpoR can compensate for the loss of SH2 function in JAK2V617F
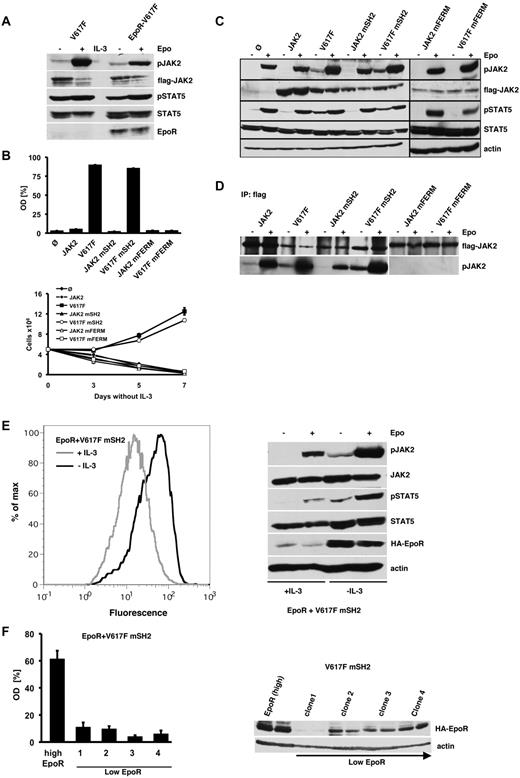
It has been reported that coexpression of homodimeric type I cytokine receptor is a prerequisite for JAK2V617F-mediated transformation.22 We therefore determined the activation of JAK2 and STAT5 in parental and EpoR-transduced Ba/F3 cells (Figure 2A). Interestingly, parental as well as EpoR-transduced Ba/F3 cells showed equal activation of JAK2V617F and STAT5, suggesting that endogenous IL-3R expression alone is sufficient for full JAK2V617F-mediated constitutive signaling. EpoR-Ba/F3 cells expressing JAK2V617F proliferated in the absence of Epo, whereas WT-JAK2, JAK2 mSH2, and FERM domain-deleted JAK2 were unable to render the cells Epo-independent (Figure 2B). In contrast to parental Ba/F3 cells, EpoR-expressing Ba/F3 cells could be transformed by JAK2V617F mSH2. These results as a start indicated that an intact SH2 domain might not be required for transformation of EpoR-Ba/F3 cells by JAK2V617F.
SH2 domain-mutated JAK2V617F can be rescued by forced overexpression of EpoR. (A) JAK2V617F-expressing Ba/F3 (V617F) and EpoR-Ba/F3 (EpoR) cells were selected by IL-3 withdrawal, serum-starved for 12 hours and stimulated with IL-3, Epo, or vehicle. Cell lysates were subjected to immunoblotting using the indicated antibodies. (B) EpoR-Ba/F3 cells expressing WT-JAK2 and the indicated JAK2 mutants described in Figure 1A were grown in the absence of Epo. Cell growth was measured by MTS-based assay and by trypan blue exclusion as in Figure 1A. The figures represent 1 of 3 independent experiments. Values are expressed as mean of triplicates ± SEM. (C) EpoR-Ba/F3 cells expressing WT or mutant JAK2 as indicated and selected by IL-3 withdrawal as described in panel B were serum-starved for 12 hours and stimulated with Epo or vehicle for 5 minutes. Lysates were subjected to Western blotting with the indicated antibodies. Note that the detection of JAK2V617F mFERM required longer exposures compared with the other JAK2 constructs. (D) EpoR-Ba/F3 cells containing WT-JAK2 or the JAK2 mutants as indicated were serum-starved and stimulated with Epo or vehicle. Flag-tagged JAK2 was IP from whole-cell lysates by an anti-Flag antibody and immuoblotted with the indicated antibodies. (E) Ba/F3 JAK2V617F mSH2 cells were transduced with MSCV-eGFP-EpoR (EpoR+V617F mSH2) and grown in the presence or absence of IL-3 over a period of 3 days. FACS analysis was used to determine surface expression levels of EpoR (left panel). Analysis of JAK2 and STAT5 activation in these 2 EpoR-expressing Ba/F3 cell populations in the absence and presence of Epo was determined by Western blot (right panel). (F) Proliferation of isolated SH2-mutated JAK2V617F (V617F mSH2) Ba/F3 single-cell clones expressing high or low levels of EpoR as quantified by the relative optical density (OD) using MTS assay. The figure represents 1 of 2 independent experiments. Values are expressed as mean of triplicates ± SEM (left panel). EpoR expression levels were determined by Western blot analysis (right panel).
SH2 domain-mutated JAK2V617F can be rescued by forced overexpression of EpoR. (A) JAK2V617F-expressing Ba/F3 (V617F) and EpoR-Ba/F3 (EpoR) cells were selected by IL-3 withdrawal, serum-starved for 12 hours and stimulated with IL-3, Epo, or vehicle. Cell lysates were subjected to immunoblotting using the indicated antibodies. (B) EpoR-Ba/F3 cells expressing WT-JAK2 and the indicated JAK2 mutants described in Figure 1A were grown in the absence of Epo. Cell growth was measured by MTS-based assay and by trypan blue exclusion as in Figure 1A. The figures represent 1 of 3 independent experiments. Values are expressed as mean of triplicates ± SEM. (C) EpoR-Ba/F3 cells expressing WT or mutant JAK2 as indicated and selected by IL-3 withdrawal as described in panel B were serum-starved for 12 hours and stimulated with Epo or vehicle for 5 minutes. Lysates were subjected to Western blotting with the indicated antibodies. Note that the detection of JAK2V617F mFERM required longer exposures compared with the other JAK2 constructs. (D) EpoR-Ba/F3 cells containing WT-JAK2 or the JAK2 mutants as indicated were serum-starved and stimulated with Epo or vehicle. Flag-tagged JAK2 was IP from whole-cell lysates by an anti-Flag antibody and immuoblotted with the indicated antibodies. (E) Ba/F3 JAK2V617F mSH2 cells were transduced with MSCV-eGFP-EpoR (EpoR+V617F mSH2) and grown in the presence or absence of IL-3 over a period of 3 days. FACS analysis was used to determine surface expression levels of EpoR (left panel). Analysis of JAK2 and STAT5 activation in these 2 EpoR-expressing Ba/F3 cell populations in the absence and presence of Epo was determined by Western blot (right panel). (F) Proliferation of isolated SH2-mutated JAK2V617F (V617F mSH2) Ba/F3 single-cell clones expressing high or low levels of EpoR as quantified by the relative optical density (OD) using MTS assay. The figure represents 1 of 2 independent experiments. Values are expressed as mean of triplicates ± SEM (left panel). EpoR expression levels were determined by Western blot analysis (right panel).
Accordingly, we observed constitutive phosphorylation of JAK2 and the downstream signaling molecule STAT5 in EpoR-Ba/F3 cells expressing JAK2V617F or JAK2V617F mSH2, which were selected by cytokine withdrawal (Figure 2C). In contrast, ectopic expression of WT-JAK2, JAK2 mSH2, or FERM domain deleted JAK2 in EpoR-Ba/F3 cells did not result in constitutive activation of JAK2 and STAT5. To specifically determine the phosphorylation of the transduced JAK2 proteins, we performed Flag-IPs and found constitutive activation of JAK2V617F and JAK2V617F mSH2 in EpoR-Ba/F3 cells (Figure 2D). In contrast, WT-JAK2 and JAK2 mSH2 were only activated in the presence of Epo. Notably, FERM domain-mutant JAK2 proteins remained unphosphorylated even after Epo stimulation again indicating the requirement of receptor binding for both constitutive and Epo-induced JAK2 phosphorylation.
EpoR-Ba/F3 cells were selected by cytokine withdrawal, which might result in cells with very high EpoR expression levels. To determine whether the level of EpoR overexpression is crucial for the rescue of the SH2-mutated JAK2V617F, we examined the level of EpoR expression before and after IL-3 withdrawal. As shown in Figure 2E left panel, JAK2V617F mSH2-positive cells expressing high EpoR levels are strongly selected. This result suggested that only high EpoR expression permits IL-3–independent growth induced by SH2-deficient JAK2V617F. Accordingly, constitutive activation of the JAK2-STAT5 axis could only be demonstrated in those JAK2V617F mSH2 cells, which were previously selected for high EpoR expression by IL-3 withdrawal and not in cells, which were permanently maintained in the presence of IL-3 (Figure 2E right panel). To confirm that only high-density EpoR expression allows transformation by the JAK2V617F SH2 mutant, we established single-cell clones expressing JAK2V617F mSH2 together with high or low EpoR by limited dilution. Indeed, cell lines expressing low-level EpoR and SH2-mutated JAK2V617F did not reveal cytokine-independent growth (Figure 2F). Thus, high-level EpoR expression is able to overcome the SH2 mutation of oncogenic JAK2. This might be due to the high abundance of the unstimulated receptor and the resulting high density of recruited JAK2 molecules at the membrane, facilitating the constitutive activation of SH2-mutated JAK2V617F.
Mutation of the SH2 domain does not affect membrane and receptor association of JAK2
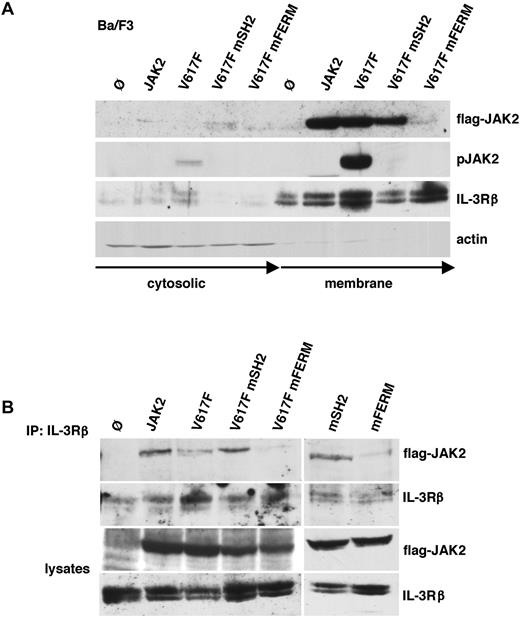
Because the assembly of cytokine receptors and JAK2 is important for JAK2 phosphorylation30,31,41 and JAK2V617F abundance at the receptor and the cell membrane may be critical for constitutive activation, we first studied the membrane localization of the FERM and SH2-mutated JAK2V617F protein (Figure 3A). Biotinylated surface proteins of Ba/F3 cells were immunoprecipitated using streptavidin beads. Subsequently, JAK2 expression and activity was determined within the membrane and cytosolic fraction. WT-JAK2, JAK2V617F, and JAK2V617F mSH2 as well as receptor expression was detectable within the membrane fraction. In contrast, FERM domain-mutated JAK2V617F was absent in the membrane fraction (Figure 3A). This indicates that the FERM domain but not the SH2 domain mediates membrane localization. FERM domain-mutated JAK2V617F was also absent in the cytosolic fraction, indicating that detachment from the cell membrane may lead to the degradation of the protein.42 Instability of FERM domain-mutated JAK2 was also noted when expressed in Ba/F3 cells, as longer film exposures were required to detect the protein (Figures 1B,2C). In accordance with our previous results, JAK2V617F was constitutively activated, whereas JAK2V617F mSH2 showed no autophosphorylation. In addition, complex formation of WT and mutated JAK2 with the IL-3R was determined by co-IP (Figure 3B). In line with the fractionation experiment, the FERM domain, but not the SH2 domain, was required for complex formation of JAK2 and JAK2V617F with the IL-3R. We also analyzed membrane distribution of JAK2 in EpoR-Ba/F3 cells. Similar to parental Ba/F3 cells, EpoR-Ba/F3 cells showed strong membrane recruitment of WT-JAK2, JAK2V617F, and JAK2V617F mSH2, whereas FERM domain-mutated JAK2 abrogates membrane localization (supplemental Figure 2). To rule out that the SH2 domain plays a role in surface expression of the EpoR, we determined EpoR surface expression by fluorescence-activated cell sorting (FACS) analysis in these cells. WT-JAK2, JAK2V617F, WT-JAK2 mSH2, and JAK2V617F mSH2 showed equal levels of EpoR surface expression, indicating that the SH2 domain is not required for EpoR membrane distribution (supplemental Figure 3A). EpoR-Ba/F3 cells transfected with empty vector also showed comparable levels of EpoR surface expression suggesting that endogenous JAK2 protein levels are sufficient for proper EpoR maturation in Ba/F3 cells. Using JAK2-deficient γ2A cells, Huang et al showed that JAK2 is required for EpoR membrane expression and EpoR maturation through the interaction of the receptor and the FERM domain of JAK2.30 In accordance with these data, we demonstrated that EpoR maturation was impaired in the absence of JAK2 and restored after WT-JAK2 reconstitution using a FACS-based method (supplemental Figure 3B). Similarly, JAK2 mSH2 was able to induce maturation of the EpoR, again confirming that the SH2 domain is dispensable for receptor maturation. Interestingly, also oncogenic JAK2V617F and JAK2V617F mSH2 induced EpoR maturation to the same extent than WT-JAK2 (supplemental Figure 3C). Maturation of EpoR in γ2A cells was also confirmed by treatment with Endo H (supplemental Figure 3D).
The SH2 domain mutation does not impair membrane and IL-3 receptor association of JAK2. (A) Parental Ba/F3 (Ø) and Ba/F3 cells expressing WT-JAK2 (JAK2), JAK2V617F (V617F), JAK2V617F+R426K (V617 mSH2), and JAK2V617F+L40A/Y41A (V617F mFERM) were surface-biotinylated followed by streptavidin IP. The bound (membrane) and unbound (cytosolic) fractions were subjected to immunoblotting with antibodies to Flag and phospho-JAK2. IL-3Rβ and actin were used as loading controls. (B) IL-3Rβ chain was immunoprecipitated from parental Ba/F3 (Ø) and Ba/F3 cells expressing WT-JAK2 (JAK2), JAK2V617F (V617F), JAK2V617F+R426K (V617F mSH2), JAK2V617F+L40A/Y41A (V617F mFERM), JAK2R426K (mSH2), and JAK2L40A/Y41A (mFERM). Coprecipitated JAK2 and precipitated IL-3Rβ were determined by Western blot as indicated (top panels). Western blot analysis of lysates used is shown in the 2 bottom panels.
The SH2 domain mutation does not impair membrane and IL-3 receptor association of JAK2. (A) Parental Ba/F3 (Ø) and Ba/F3 cells expressing WT-JAK2 (JAK2), JAK2V617F (V617F), JAK2V617F+R426K (V617 mSH2), and JAK2V617F+L40A/Y41A (V617F mFERM) were surface-biotinylated followed by streptavidin IP. The bound (membrane) and unbound (cytosolic) fractions were subjected to immunoblotting with antibodies to Flag and phospho-JAK2. IL-3Rβ and actin were used as loading controls. (B) IL-3Rβ chain was immunoprecipitated from parental Ba/F3 (Ø) and Ba/F3 cells expressing WT-JAK2 (JAK2), JAK2V617F (V617F), JAK2V617F+R426K (V617F mSH2), JAK2V617F+L40A/Y41A (V617F mFERM), JAK2R426K (mSH2), and JAK2L40A/Y41A (mFERM). Coprecipitated JAK2 and precipitated IL-3Rβ were determined by Western blot as indicated (top panels). Western blot analysis of lysates used is shown in the 2 bottom panels.
Thus, these findings suggest that in contrast to FERM domain-mutated JAK2, the lack of constitutive activation of the SH2-mutated JAK2V617F cannot be ascribed to impaired membrane and receptor association. Moreover, EpoR maturation and surface expression was not dependent on an intact SH2 domain. We also excluded the possibility that the mutation on the SH2 domain leads to reduced stability of JAK2V617F (supplemental Figure 4A) or significantly compromised kinase activity (supplemental Figure 4B).
Protein-protein interaction and transphosphorylation of JAK2V617F is dependent on an intact SH2 domain
The function of the SH2 domain in JAK proteins is largely unclear and the binding of the JAK SH2 domain to specific phosphorylated tyrosine residues could not be demonstrated so far.36 Pull-down experiments with GST-JAK2 SH2 revealed no tyrosine-phosphorylated binding partners. However, GST-JAK2 SH2 is able to precipitate JAK2 itself (data not shown).
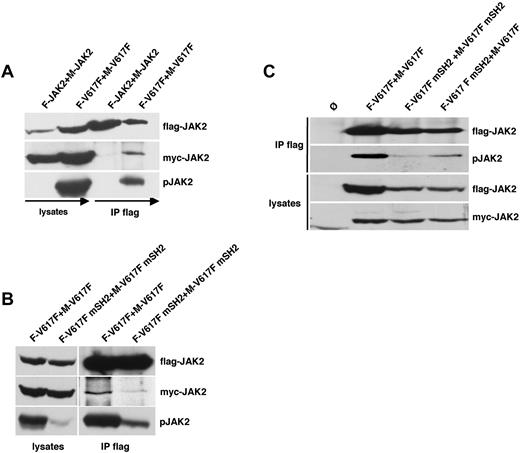
Because the SH2 domain therefore might be crucial for JAK2 self-association and subsequent transphosphorylation, we investigated the interaction between JAK2 molecules by Flag co-IP using both Flag-tagged and myc-tagged JAK2 expression constructs. Both constructs showed comparable pJAK2 signal, STAT5 signaling, and transforming ability in Ba/F3 cells (data not shown). As shown in Figure 4A, myc-tagged WT-JAK2 does not coimmunoprecipitate with Flag-tagged WT-JAK2. In contrast, IP of Flag-tagged JAK2V617F resulted in the co-IP of myc-tagged JAK2V617F. Constitutive phosphorylation of JAK2V617F could be observed in both the immunoprecipitated fraction and in the lysate. This result suggests that JAK2V617F, but not WT-JAK2, binds to each other in the absence of ligand.
The SH2 domain is required for the transphosphorylation of JAK2V617F. (A) 2 WT-JAK2 or JAK2V617F constructs containing either a myc (M-JAK2, M-V617F) or Flag tag (F-JAK2, F-V617F) were coexpressed in 293T cells. Flag-tagged JAK2 proteins were immunoprecipitated from cell lysates and analyzed for co-IP of myc-tagged JAK2 by anti-myc immunoblotting and phospho-JAK2. (B) 293T cells coexpressing either Flag- and myc-tagged JAK2V617F (F-V617F, M-V617F) or Flag- and myc-tagged JAK2V617F+R426K (F-V617F mSH2, M-V617F mSH2) were analyzed as described in panel A. (C) Ba/F3 cells stably expressing Flag- and myc-tagged JAK2V617F and JAK2V617F+R426K mutants as indicated were lysed analyzed by Flag-IP and subsequent phospho-JAK2 and flag immunoblot. Expression levels of the JAK2 constructs in the lysates were controlled by Flag and myc Western blot. The cell lines used in this experiment were mock-transduced Ba/F3 cells (Ø, lane 1), flag-JAK2V617F (F-V617F) with myc-JAK2V617F (M-V617F; lane 2), flag-JAK2V617F+R426K (F-V617F mSH2) with myc-JAK2V617F+R426K (M-V617F mSH2; lane 3), and Flag-JAK2V617F+R426K (F-V617F mSH2) with myc-JAK2V617F (M-V617F; lane 4).
The SH2 domain is required for the transphosphorylation of JAK2V617F. (A) 2 WT-JAK2 or JAK2V617F constructs containing either a myc (M-JAK2, M-V617F) or Flag tag (F-JAK2, F-V617F) were coexpressed in 293T cells. Flag-tagged JAK2 proteins were immunoprecipitated from cell lysates and analyzed for co-IP of myc-tagged JAK2 by anti-myc immunoblotting and phospho-JAK2. (B) 293T cells coexpressing either Flag- and myc-tagged JAK2V617F (F-V617F, M-V617F) or Flag- and myc-tagged JAK2V617F+R426K (F-V617F mSH2, M-V617F mSH2) were analyzed as described in panel A. (C) Ba/F3 cells stably expressing Flag- and myc-tagged JAK2V617F and JAK2V617F+R426K mutants as indicated were lysed analyzed by Flag-IP and subsequent phospho-JAK2 and flag immunoblot. Expression levels of the JAK2 constructs in the lysates were controlled by Flag and myc Western blot. The cell lines used in this experiment were mock-transduced Ba/F3 cells (Ø, lane 1), flag-JAK2V617F (F-V617F) with myc-JAK2V617F (M-V617F; lane 2), flag-JAK2V617F+R426K (F-V617F mSH2) with myc-JAK2V617F+R426K (M-V617F mSH2; lane 3), and Flag-JAK2V617F+R426K (F-V617F mSH2) with myc-JAK2V617F (M-V617F; lane 4).
To determine the role of the SH2 domain for JAK2V617F self-association, we performed co-IP experiments using Flag- and myc-tagged JAK2V617F mSH2 (Figure 4B). Again, JAK2V617F revealed reciprocal interactions as well as high levels of constitutive phosphorylation. In contrast, overexpressed Flag-tagged and myc-tagged JAK2V617F mSH2 could not be coimmunoprecipitated. This indicates that the SH2 domain seems to be crucial for the complex formation and subsequent transphosphorylation of JAK2V617F proteins. To further confirm these results, we performed additional co-IP experiments using Flag-tagged JAK2V617F and myc-tagged WT-JAK2 and vice versa (supplemental Figure 5). We could not detect co-IP of myc-tagged WT-JAK2 using Flag-tagged JAK2V617F or vice versa indicating that constitutively activated JAK2 is only able to interact with each other. Similarly, co-IP was carried out with the Flag-tagged JAK2V617F mSH2 and myc-tagged WT-JAK2 (supplemental Figure 5A). As expected, Flag-tagged JAK2V617F mSH2 could not coimmunoprecipitate myc-tagged WT-JAK2 and vice versa. We also performed co-IP experiments in γ2a cells lacking common cytokine receptors by stably expressing Flag-tagged and myc-tagged JAK2 mutant constructs with and without EpoR (supplemental Figure 6). Here, co-IP of myc-tagged JAK2V617F with Flag-tagged JAK2V617F could only be observed in EpoR-expressing γ2A cells, because JAK2V617F shows no activity in the absence of cytokine receptor expression. Therefore the results in γ2a cells support the notion that complex formation of JAK2V617F requires autophosphorylation. WT-JAK2 in accordance with the results in supplemental Figure 5 showed no complex formation even in the presence of cytokine receptor expression (supplemental Figure 6).
To further investigate a potential requirement of the SH2 domain for constitutive JAK2V617F-dependent phosphorylation, we co-infected Ba/F3 cells with Flag- and myc-tagged JAK2V617F constructs containing an intact SH2 (lane 2), mutated SH2 (lane 3), and both (lane 4) and performed Flag-IP (Figure 4C). As expected, control cells expressing Flag- and myc-tagged unmutated JAK2V617F showed high levels of constitutive Flag-JAK2V617F phosphorylation. Consistent with our previous data, phosphorylation of JAK2V617F was almost completely abrogated when the SH2 domain was mutated in both constructs. Interestingly, Flag-JAK2V617F mSH2 was partially phosphorylated when myc-JAK2V617F was coexpressed, indicating that SH2-mutated JAK2V617F can be phosphorylated in the presence of JAK2V617F containing an intact SH2. According to these results, the SH2 domain might be involved in the transphosphorylation and constitutive activation of JAK2V617F. Taken together, these results indicate that constitutive phosphorylation of JAK2V617F seems to require reciprocal binding via the SH2 domain.
JAK2V617F-mediated myeloproliferative disease in mice requires a functional SH2 domain
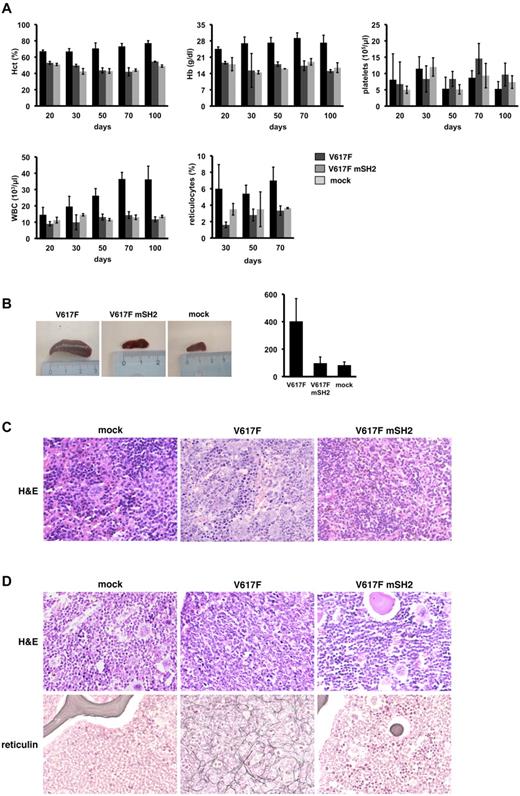
Retroviral transduction of JAK2V617F in BM cells leads to a MPN reminiscent of PV.9,10 To investigate the role of the SH2 domain in primary hematopoietic cells with physiologic cytokine receptor density, we retrovirally introduced JAK2V617F, JAK2V617F mSH2, and empty vector in BM cells derived from Balb/c mice and subsequently transplanted them into lethally irradiated recipient mice. Three independent transplantations with a total number of 12 mice for the JAK2V617F group and 10 mice for the JAK2V617F mSH2 group were performed. Details on all 3 transplantation experiments including viral titers used, efficacy of BM infection, number of transplanted cells and peripheral blood values at days 30 and 60, and spleen weights of all transplanted animals are listed in Table 1. Figure 5 exemplarily displays the blood values over time and spleen weight of the first transplantation experiment. As previously reported, transplantation of JAK2V617F-transduced BM leads to a marked leukocytosis, increased reticulocytes, blood hematocrit, and hemoglobin levels in transplanted animals. The platelet counts did not exceed normal levels. This phenotype became evident 3 weeks after transplantation and sustained for months (Figure 5A). Strikingly, Balb/c mice receiving BM cells ectopically expressing JAK2V617F mSH2 did not develop signs of a MPN and revealed blood counts comparable with control mice. The lack of phenotype in JAK2V617F mSH2-expressing animals cannot be ascribed to loss of oncogene expression. Analysis of JAK2V617F and JAK2V617F mSH2 expression in peripheral blood mononuclear cells by eEGP FACS (supplemental Figure 7A-B) and Western blot (supplemental Figure 7C) demonstrated sustained expression of both oncogenes at day 30 and day 60 post- transplantation.
Murine BM transplantation experiments
| . | Virus titer, CFU/mL . | BM-infection efficacy % . | n = mice . | EGFP+ cells transplanted . | Cells transplanted . | d30 WBC, G/L . | d30 HCT, % . | d30 Hb, g/dL . | d30 Retic., ‰ . | d30 Platelets, G/L . | d60 WBC, G/L . | d60 HCT, % . | d60 Hb, g/dL . | d60 Retic., ‰ . | d60 Platelets, G/L . | Spleen weight, mg . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st TX | ||||||||||||||||
| Mock | 5 × 105 | 23 | 2 | 50 000 | 215 000 | 10 ± 4 | 42 ± 2 | 16 ± 1.5 | 32 ± 2 | 1208 ± 270 | 13 ± 1.2 | 44 ± 2 | 19 ± 2 | 37 ± 2 | 938 ± 370 | 83.5 ± 23 |
| V617F | 4.3 × 105 | 17 | 4 | 50 000 | 295 000 | 20 ± 6 | 67 ± 3 | 27 ± 2.1 | 55 ± 10 | 1148 ± 366 | 38.5 ± 4 | 74 ± 2 | 32 ± 2 | 67.5 ± 10 | 871 ± 200 | 600 ± 40 |
| V617F | 4 × 105 | 16.5 | 3 | 50 000 | 300 000 | 9.9 ± 4 | 43 ± 2 | 18 ± 1.2 | 28 ± 6 | 816 ± 429 | 13 ± 2 | 42 ± 5 | 17.3 ± 1 | 37.5 ± 10 | 1438 ± 507 | 98 ± 45 |
| mSH2 | ||||||||||||||||
| 2nd TX | ||||||||||||||||
| Mock | 1 × 106 | 50 | 2 | 50 000 | 100 000 | 9.75 ± 1.5 | 56.5 ± 0.7 | 16.9 ± 0.7 | 45 ± 1.5 | 1068 ± 85 | 12.3 ± 3 | 59.2 ± 7 | 16.8 ± 2 | 35 | 780 ± 133 | 187 |
| V617F | 8 × 105 | 38.9 | 4 | 50 000 | 130 000 | 84.7 ± 16 | 90.5 ± 3.8 | 24 ± 1.2 | 99 ± 12.5 | 733 ± 110 | 157.5 ± 15 | 87.5 ± 11 | 25.3 ± 3.7 | 84.5 ± 8.3 | 468 ± 184 | 800 ± 5 |
| V617F | 7.8 × 105 | 38.8 | 3 | 50 000 | 130 000 | 10 ± 0.5 | 60.3 ± 0.5 | 18.3 ± 0.8 | 38.6 ± 3 | 806 ± 339 | 14.9 ± 1 | 63.3 ± 1.5 | 18 | 39.6 ± 3.2 | 994 ± 45 | 201 ± 10 |
| mSH2 | ||||||||||||||||
| 3rd TX | ||||||||||||||||
| V617F | 4 × 105 | 19 | 4 | 50 000 | 270 000 | 24.8 ± 2.5 | 89 ± 2 | 22.3 ± 1.4 | ND | 600 ± 89 | 41.6 ± 8 | 83.5 ± 13 | 24.7 ± 4 | ND | 604 ± 155 | ND |
| V617F | 4.5 × 105 | 18.3 | 4 | 50 000 | 270 000 | 9.6 ± 2 | 56 ± 1.5 | 16 ± 1.3 | ND | 815 ± 121 | 10.9 ± 2.6 | 54 ± 2.5 | 16 ± 1.3 | ND | 1145 ± 125 | ND |
| mSH2 |
| . | Virus titer, CFU/mL . | BM-infection efficacy % . | n = mice . | EGFP+ cells transplanted . | Cells transplanted . | d30 WBC, G/L . | d30 HCT, % . | d30 Hb, g/dL . | d30 Retic., ‰ . | d30 Platelets, G/L . | d60 WBC, G/L . | d60 HCT, % . | d60 Hb, g/dL . | d60 Retic., ‰ . | d60 Platelets, G/L . | Spleen weight, mg . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st TX | ||||||||||||||||
| Mock | 5 × 105 | 23 | 2 | 50 000 | 215 000 | 10 ± 4 | 42 ± 2 | 16 ± 1.5 | 32 ± 2 | 1208 ± 270 | 13 ± 1.2 | 44 ± 2 | 19 ± 2 | 37 ± 2 | 938 ± 370 | 83.5 ± 23 |
| V617F | 4.3 × 105 | 17 | 4 | 50 000 | 295 000 | 20 ± 6 | 67 ± 3 | 27 ± 2.1 | 55 ± 10 | 1148 ± 366 | 38.5 ± 4 | 74 ± 2 | 32 ± 2 | 67.5 ± 10 | 871 ± 200 | 600 ± 40 |
| V617F | 4 × 105 | 16.5 | 3 | 50 000 | 300 000 | 9.9 ± 4 | 43 ± 2 | 18 ± 1.2 | 28 ± 6 | 816 ± 429 | 13 ± 2 | 42 ± 5 | 17.3 ± 1 | 37.5 ± 10 | 1438 ± 507 | 98 ± 45 |
| mSH2 | ||||||||||||||||
| 2nd TX | ||||||||||||||||
| Mock | 1 × 106 | 50 | 2 | 50 000 | 100 000 | 9.75 ± 1.5 | 56.5 ± 0.7 | 16.9 ± 0.7 | 45 ± 1.5 | 1068 ± 85 | 12.3 ± 3 | 59.2 ± 7 | 16.8 ± 2 | 35 | 780 ± 133 | 187 |
| V617F | 8 × 105 | 38.9 | 4 | 50 000 | 130 000 | 84.7 ± 16 | 90.5 ± 3.8 | 24 ± 1.2 | 99 ± 12.5 | 733 ± 110 | 157.5 ± 15 | 87.5 ± 11 | 25.3 ± 3.7 | 84.5 ± 8.3 | 468 ± 184 | 800 ± 5 |
| V617F | 7.8 × 105 | 38.8 | 3 | 50 000 | 130 000 | 10 ± 0.5 | 60.3 ± 0.5 | 18.3 ± 0.8 | 38.6 ± 3 | 806 ± 339 | 14.9 ± 1 | 63.3 ± 1.5 | 18 | 39.6 ± 3.2 | 994 ± 45 | 201 ± 10 |
| mSH2 | ||||||||||||||||
| 3rd TX | ||||||||||||||||
| V617F | 4 × 105 | 19 | 4 | 50 000 | 270 000 | 24.8 ± 2.5 | 89 ± 2 | 22.3 ± 1.4 | ND | 600 ± 89 | 41.6 ± 8 | 83.5 ± 13 | 24.7 ± 4 | ND | 604 ± 155 | ND |
| V617F | 4.5 × 105 | 18.3 | 4 | 50 000 | 270 000 | 9.6 ± 2 | 56 ± 1.5 | 16 ± 1.3 | ND | 815 ± 121 | 10.9 ± 2.6 | 54 ± 2.5 | 16 ± 1.3 | ND | 1145 ± 125 | ND |
| mSH2 |
Details of 3 independent transplantation experiments are shown including viral titers used, infection efficiencies, numbers of transplanted cells, as well as blood counts of transplanted animals over time, and spleen weights. Retroviral titers were determined by transduction of 5 × 104 NIH3T3 cells with serial dilutions of retrovirus. Forty-eight hours after transduction, the percentage of infected cells was determined by flow cytometric analysis of eGFP expression. The titer (CFU/mL) was calculated by multiplication of the total number of eGFP-positive cells with the dilution factor of the retroviral supernatant.
TX indicates transplantation; CFU, colony-forming units; WBC, white blood cell count; HCT, hematocrit; Hb, hemoglobin; Retic., reticulocytes; d, day; and ND, not determined.
The SH2 domain is required for the induction of a JAK2V617F-driven MPN and myelofibrosis in a murine BM transplantation model. (A) Three independent transplantation experiments were performed. Results of one representative transplantation are depicted. Murine BM was retrovirally infected with JAK2V617F (V617F, n = 4), JAK2V617F+R426K (V617F mSH2, n = 3), or empty vector (mock, n = 2) and transplanted into lethally irradiated syngenic recipient mice. Hematocrit (%), hemoglobin (g/dL), platelets (G/L), WBC (G/L), and reticulocytes (‰) of transplanted mice at the indicated time points after transplantation are shown. (B) Spleens of representative transplanted animals are shown (left panel), and spleen size was quantified by weight (mg). Values are expressed as mean ± SEM of all animals transplanted. (C) The histopathologic analysis (hematoxylin and eosin staining, ×400) revealed hyperplastic, left-shifted myelopoiesis, and clusters of megakaryocytes in the spleens of JAK2V617F mice, whereas spleens of mock and JAK2V617F mSH2 mice showed normal cellularity. (D) Hematoxylin and eosin and reticulin stainings as labeled of representative tissue samples are shown (×400): left (mock) a normal cellular BM is shown. Note, that all hematopoietic cell lines are present, and the amount of BM fibers is normal. In the middle, BM from a JAK2V617F (V617F) mouse is shown, displaying a left-shifted increase of myeloid cells and a marked presence of collagen fibers, similar to the increase of reticulin fibers in human myeloproliferative disorders. BM obtained from JAK2V617 mSH2 (V617F mSH2) mice shows cellularity and fiber distribution nearly identical to the mock mice shown on the left. Slides were viewed with a Zeiss Axioplan 2 microscope (40×/0.75NA Plan-Neofluar air objective). Images were acquired using a Zeiss Axiocam MRc 5 camera and were processed with Axiovision Rel 4.6 scanning software.
The SH2 domain is required for the induction of a JAK2V617F-driven MPN and myelofibrosis in a murine BM transplantation model. (A) Three independent transplantation experiments were performed. Results of one representative transplantation are depicted. Murine BM was retrovirally infected with JAK2V617F (V617F, n = 4), JAK2V617F+R426K (V617F mSH2, n = 3), or empty vector (mock, n = 2) and transplanted into lethally irradiated syngenic recipient mice. Hematocrit (%), hemoglobin (g/dL), platelets (G/L), WBC (G/L), and reticulocytes (‰) of transplanted mice at the indicated time points after transplantation are shown. (B) Spleens of representative transplanted animals are shown (left panel), and spleen size was quantified by weight (mg). Values are expressed as mean ± SEM of all animals transplanted. (C) The histopathologic analysis (hematoxylin and eosin staining, ×400) revealed hyperplastic, left-shifted myelopoiesis, and clusters of megakaryocytes in the spleens of JAK2V617F mice, whereas spleens of mock and JAK2V617F mSH2 mice showed normal cellularity. (D) Hematoxylin and eosin and reticulin stainings as labeled of representative tissue samples are shown (×400): left (mock) a normal cellular BM is shown. Note, that all hematopoietic cell lines are present, and the amount of BM fibers is normal. In the middle, BM from a JAK2V617F (V617F) mouse is shown, displaying a left-shifted increase of myeloid cells and a marked presence of collagen fibers, similar to the increase of reticulin fibers in human myeloproliferative disorders. BM obtained from JAK2V617 mSH2 (V617F mSH2) mice shows cellularity and fiber distribution nearly identical to the mock mice shown on the left. Slides were viewed with a Zeiss Axioplan 2 microscope (40×/0.75NA Plan-Neofluar air objective). Images were acquired using a Zeiss Axiocam MRc 5 camera and were processed with Axiovision Rel 4.6 scanning software.
In addition to elevated blood counts, mice receiving JAK2V617F-infected BM developed a profound splenomegaly with a median spleen weight 4 times higher than that of control mice (Figure 5B). Microscopically, spleens derived from JAK2V617F mice showed a marked infiltration by hematopoietic cells with a left shifted granulopoesis, erythropoiesis, and clusters of mature megakaryocytes, comparable with changes seen in human myeloproliferative disorders (Figure 5C middle panel). Notably, BM transduced with JAK2V617F mSH2 did not give rise to a significant increase in spleen size and weight. On microscopy, these spleens showed a normal cellularity indistinguishable from control mice. (Figure 5C left and right panels). Analysis of the BM from JAK2V617F mice revealed an increased and left-shifted myelopoiesis reminiscent of a human MPN, whereas the BM of JAK2V617F mSH2 mice was normal and virtually identical to the BM of mock mice (Figure 5D top panel). In addition, JAK2V617F induced a marked increase of collagen fibers resembling secondary myelofibrosis (Figure 5D bottom panel). In contrast, myelofibrosis was absent in control and JAK2V617F mSH2 transplanted animals. Therefore, in murine hematopoietic cells with physiologic cytokine expression, deficiency of the SH2 function in JAK2V617F is associated with the complete loss of its oncogenic potential. Thus, a functional SH2 domain is indispensable for JAK2V617F to induce both a MPN and myelofibrosis in vivo.
Discussion
JAKs are necessary to activate signaling downstream of numerous cytokine receptors in hematopoietic progenitor cells. The identification of the V617F mutation within the pseudokinase domain of JAK2 in patients with MPNs elucidated a molecular mechanism for the pathogenesis of myeloproliferative disorders.1–4,43,44 Consequently, JAK inhibitors efficiently induced apoptosis in JAK2V617F-expressing Ba/F3 and HEL cells and marked attenuation of JAK2V617F-driven MPNs in mice.45 Therefore, JAK2 inhibition might be an attractive pharmacologic approach for the treatment of MPN patients, which led to the initiation of several phase 1 and 2 studies.46
Although JAK2V617F is of major clinical interest, the mechanism leading to the constitutive activation of JAK2V617F is largely unclear.
In general, JAK2 is able to bind to numerous cytokine receptors, but the receptor type required for the constitutive activation of JAK2V617F is still a matter of debate. Whereas some groups including ourselves showed transformation of IL-3R–postive Ba/F3 cells, Lu and coworkers found that a homodimeric type I cytokine receptor such as the EpoR is necessary for the oncogenic activation of JAK2V617F.2,22,24 As recently reported by the same group however, higher JAK2V617F expression levels circumvent the requirement of a homodimeric type I cytokine receptor for transformation.23 Moreover, in an earlier paper, Constantinescu et al proposed that EpoR exists in an oligomerized state even in the absence of ligand.47 It is possible that oligomerized EpoR might facilitate the transphosphorylation of JAK2V617F in the absence of ligand stimulation, which might account for these discrepant findings.
Here we show that expression of JAK2V617F in γ2A cells, which contain the IL-3Rα, but not the β chain, does not lead to JAK2V617F activation. Expression of a functional cytokine receptor such as the IL-3R in these cells restored JAK2V617F activation in the absence of ligand. Thus, oncogenic JAK2V617F requires complex formation and expression of either heterodimeric (IL-3R) or homodimeric (EpoR) cytokine receptors. To some degree, these results are in contrast to previous published results where expression of JAK2V617F led to activation of STAT5 in γ2A cells.2 However, in this study, the authors expressed JAK2 constructs by transient transfection, which leads to high expression levels and might enable binding of JAK2 to low-affinity receptors.
Unlike the kinase, pseudokinase, and FERM domain, the function of the JAK2 SH2-like domain in cytokine-induced and constitutive JAK2 activation is poorly understood. In this paper we provide evidence that the oncogenic and, to a lesser extent, cytokine-dependent JAK2 activation relies on a functional SH2 domain in the presence of the IL-3R. In accordance with previously published results, ectopic expression of JAK2V617F in parental Ba/F3 cells was able to induce IL-3–independent proliferation.2 Notably, a point mutation within the SH2 domain (R426K) abrogated the transforming potential of JAK2V617F as efficiently as the FERM domain mutation. In accordance with these cell biologic findings, both SH2- as well as FERM domain-mutated JAK2V617F lacked constitutive phosphorylation of JAK2 and STAT5. IP experiments showed that in contrast to FERM domain-mutated JAK2V617F, IL-3–induced phosphorylation, membrane and IL-3R association of SH2-mutated JAK2V617F in Ba/F3 cells was not impaired. In addition, protein stability as well as kinase activity was not affected by mutation of the SH2 domain. In γ2A cells, IL-3–induced phosphorylation of SH2-mutated JAK2 and STAT5 was intact but considerably reduced compared with WT-JAK2. These results indicate that both the FERM domain and the SH2 domain are necessary for JAK2V617F-induced transformation, although they seem to execute distinct molecular functions. The role of the SH2 domain in both WT-JAK2 and JAK2V617F has not been completely delineated yet. Mutation of arginine 426 in JAK2 did not seem to have a significant effect on interferon-γ signaling.35 Radtke et al demonstrated that the corresponding SH2 mutation in JAK1 (R466K) did not impair JAK1 receptor binding but led to a down-regulation of oncostatin M receptor surface expression.36 In our studies, IL-3Rβ and EpoR surface expression was unaffected by SH2-mutated JAK2V617F in different cell types, thus not explaining the profound loss of function of SH2 mutated JAK2V617F.
To further study the molecular mechanism regulated by the SH2 domain, we determined cytokine-independent growth and signaling in Ba/F3 cells expressing the various JAK2V617F mutants together with high and low levels of cytokine receptors. In contrast to Ba/F3 cells with endogenous IL-3R expression or Ba/F3 cells with low-level EpoR expression, SH2-mutated JAK2V617F induced proliferation in EpoR-Ba/F3 cells selected for high EpoR expression. These results show that the loss of the SH2 domain in JAK2V617F can be compensated by high-density cytokine receptor expression. Because receptor density modulates constitutive activation and JAK2V617F is coupled to cytokine receptors independently from the SH2 domain, we reasoned that the supplementary function of the SH2 domain might be involved in crossphosphorylation and constitutive activation of JAK2V617F. Indeed oncogenic JAK2V617F coprecipitated with itself and underwent crossphosphorylation. Both coprecipitation and crossphosphorylation required an intact SH2 domain.
Reciprocal JAK phosphorylation is thought to occur as the first phosphorylation step upon ligand binding and receptor dimerization.17,48 It has been demonstrated that the phosphorylation of several tyrosine residues including Y1007 within the activation loop are essential for the regulation of JAK2 activity.25,49,50 However, structural requirements for JAK2 transphosphorylation, especially in the context of the V617F mutation, have not extensively been studied yet. Although cytokine-induced JAK2 phosphorylation was markedly reduced but still detectable in JAK2 harboring the SH2 mutation, the SH2 domain seems to be indispensable for constitutive JAK2V617F activation. An explanation for this discrepancy might be because the SH2 domain mediates transphosphorylation when oncogenic JAKV617F is associated with a cytokine receptor in the absence of ligands. In the presence of ligands leading to the annealing of the receptor chains, the SH2 domain seems to be important for full activation, but not absolutely required. The notion that close proximity of receptor chains might overcome the strict requirement for the SH2 domain is further supported by the fact that a high density of EpoR on the cell surface is able to rescue the SH2 mutation. This observation might be explained by a higher density of receptor-bound JAK2 molecules and altered receptor assembly on the cell surface, enabling these JAK2 molecules to self-aggregate and transphosphorylate even in the absence of an intact SH2 domain. However, the exact role of the JAK2 SH2 domain remains to be determined. It has been demonstrated that the JAK SH2 domain does not function as a classical phosphotyrosine-binding SH2 domain.36 However, the SH2 domain seems to be required for self-aggregation and transphosphorylation of JAK2V617F. Funakoshi-Tago et al found that JAK2V613E, but not JAK2V617F, requires ectopic expression of EpoR for constitutive activation.41 This phenotype is reminiscent of the JAK2V617F+R426K double-mutant described in this study. The authors speculate that enhanced binding of the Y613E-mutated JH1 domain to the FERM domain might lead to increased self-inhibition. According to their model, ectopic expression of a homodimeric cytokine receptor could then result in enhanced FERM domain-receptor binding and a release of self-inhibition. Although this is an attractive model, we did not detect any difference in kinase activity between SH2-unmutated and -mutated JAK2V617F. Instead, we raised 2 hypotheses that might explain our findings. First, JAK2V617F self-aggregation is dependent on the binding between the SH2 domain and a region within the JAK2 molecule, which has not been defined yet. Second, the SH2 domain may be important for intrastructural integrity of the JAK2V617F protein, which is a prerequisite for proper complex formation, transphosphorylation, and signal transduction. To address these questions, the crystal structure of JAK2V617F would be of great interest.
Finally, we wished to determine the functional importance of the SH2 domain for oncogenic activation of JAK2V617F under physiologic cytokine receptor density in primary hematopoietic cells in an in vivo system. In accordance with other groups we found that retroviral infection of murine BM cells with JAK2V617F resulted in a MPN reminiscent of PV.9,10 Importantly, mice transplanted with BM cells expressing SH2-mutated JAK2V617F did not develop a MPN-like disease as determined by hematocrit, reticulocytes, leukocytes spleen weight, and histopathology and showed a phenotype indistinguishable from mock-infected control animals. In addition, myelofibrosis was only detectable in JAK2V617F and not in JAK2V617F mSH2 or control-infected mice. These results strongly indicate that JAK2V617F-induced MPNs and myelofibrosis require an intact SH2 domain in vivo. Together with our in vitro data, these results corroborate the critical role of the SH2 domain for JAK2V617F signaling under physiologic cytokine receptor expression and underscores the need for careful interpretation of data using cell lines overexpressing cytokine receptors at high densities.
The need for proper localization and cytokine receptor expression distinguishes JAK2V617F from other oncogenes associated with MPNs such as BCR-ABL or FIP1L1-PDGFRA. These oncogenic fusion proteins are activated in a very different way compared with their normal cellular counterparts. In contrast, JAK2V617F and endogenous ligand-activated JAK2 seem to have very similar requirements for activation. This similarity may make it difficult to develop JAK2V617F-specific inhibitors, which do not interfere with normal cytokine signaling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr H. Lodish for providing human EpoR cDNA and γ2A cells and Dr A. D. Whetton for providing cDNA for human IL-3R. Dr Wibke Leibig helped with FACS analysis.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft, SFB 684 to J.D.
Authorship
Contribution: S.P.G. and T.N.D. performed experiments and wrote the manuscript; R.G. and A.L.I. performed experiments; C.M.z.B. and C.P. designed experiments; M.K. examined the mice; and J.D. designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Justus Duyster, Department of Internal Medicine III, Technical University Munich, Ismaningerstr 22, 81675 Munich, Germany; e-mail: justus.duyster@lrz.tum.de.
References
Author notes
S.P.G. and T.N.D. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal