The aberrant overexpression of Wilms tumor 1 (WT1) in myeloid leukemia plays an important role in blast cell survival and resistance to chemotherapy. High expression of WT1 is also associated with relapse and shortened disease-free survival in patients. However, the mechanisms by which WT1 expression is regulated in leukemia remain unclear. Here, we report that heat shock protein 90 (Hsp90), which plays a critical role in the folding and maturation of several oncogenic proteins, associates with WT1 protein and stabilizes its expression. Pharmacologic inhibition of Hsp90 resulted in ubiquitination and subsequent proteasome-dependant degradation of WT1. RNAi-mediated silencing of WT1 reduced the survival of leukemia cells and increased the sensitivity of these cells to chemotherapy and Hsp90 inhibition. Furthermore, Hsp90 inhibitors 17-AAG [17-(allylamino)-17-demethoxygeldanamycin] and STA-9090 significantly reduced the growth of myeloid leukemia xenografts in vivo and effectively down-regulated the expression of WT1 and its downstream target proteins, c-Myc and Bcl-2. Collectively, our studies identify WT1 as a novel Hsp90 client and support the crucial role for the WT1–Hsp90 interaction in maintaining leukemia cell survival. These findings have significant implications for developing effective therapies for myeloid leukemias and offer a strategy to inhibit the oncogenic func-tions of WT1 by clinically available Hsp90 inhibitors.
Introduction
The Wilms tumor 1 (WT1) gene encodes a zinc finger transcription factor that is important for normal urogenital development and cancer pathogenesis.1,–3 Overexpression of WT1 has been observed in a wide range of solid tumors and hematopoietic malignancies, including acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) in blastic phase, as well as in myelodysplastic syndromes (MDSs).4,–6 Several studies have suggested that WT1 expression plays an important role in myelopoiesis, cell proliferation, and differentiation arrest.7,8 In addition, overexpression of WT1 has been proposed to sustain survival of leukemia blast cells.9 Coexpression of WT1 and the fusion protein AML1-ETO in transgenic mice rapidly induces AML, further emphasizing the proto-oncogenic function of WT1.8 Furthermore, high levels of WT1 expression in acute leukemias have been associated with lower complete remission rates and reduced overall and disease-free survival.10,11 WT1 has also shown to be a repressor or activator for several important genes, such as the antiapoptotic gene Bcl-2 and the oncogene c-Myc.12 This overexpression of WT1 not only makes it an attractive prognostic marker for minimal residual disease but also a promising target for immunotherapy.13,14 However, despite these findings, little is known about the molecular mechanisms of WT1 regulation in leukemia.
Heat shock protein 90 (Hsp90) is an important molecular chaperone that plays a key role in the conformational maturation and stabilization of signaling proteins involved in cell growth and survival.15,16 Hsp90 is considered a promising therapeutic target, as its inhibition simultaneously affects the activity of multiple oncogenic proteins.17 The first-generation Hsp90 inhibitor 17-AAG [17-(allylamino)-17-demethoxygeldanamycin], which preferentially binds to the active form of Hsp90 in tumor cells,18 has shown promising antitumor activity in preclinical models and is currently in clinical trials.19,20 More potent second-generation Hsp90 inhibitors that are structurally unrelated to 17-AAG, such as STA-9090, a novel resorcinol-containing compound,21 are also in clinical development. Hsp90 also cooperates with the chaperone protein Hsp70 to properly fold its protein substrates, and this functional cooperation is mediated by additional cochaperones.22 Although Hsp70 has been shown to chaperone WT1 and plays a crucial role in its proper functioning during normal kidney development,23 the role of Hsp90 in regulating WT1 expression has not been determined. Here, we demonstrate that WT1 directly associates with and is regulated by Hsp90 and that the small molecule Hsp90 inhibitors 17-AAG and STA-9090 target WT1 for degradation via the proteasome pathway. Furthermore, we show that 17-AAG and STA-9090 inhibit tumor growth in myeloid leukemia xenograft models and that this correlates with decreased expression of WT1 and its downstream targets c-Myc and Bcl-2.
Methods
Plasmids, antibodies, and reagents
A human WT1 cDNA (encoding isoform A, which contains exon 5 [17AA] and the exon 9-amino acid KTS) was obtained from RIKEN BioResource Center (Japan) and subcloned in the pCR3.1 vector (Invitrogen) using the EcoRI and NotI sites. An Hsp90 IMAGE cDNA clone was obtained from OriGene Technologies, and a full-length Hsp90 sequence was polymerase chain reaction (PCR) amplified and subcloned into pCR3.1 at the NotI and XbaI sites. Different N- and C-terminal deletion mutants of WT1 and Hsp90 were created by PCR and subcloned into pCR3.1. GST-WT1 and GST-Hsp90 plasmids were generated by subcloning WT1 (EcoRI-NotI fragment) and Hsp90 (SmaI-NotI fragment) in-frame into the pGEX-4T2 plasmid (GE Healthcare). All expression vectors were sequence verified and purified using the EndoFree Plasmid Maxi Kit (QIAGEN). Antibodies against WT1 (C-19), c-Myc, Bcl-2, and Hsp90, and agarose-conjugated anti-Hsp90 (Hsp90-AC) were purchased from Santa Cruz Biotechnology. These Hsp90 antibodies can recognize both the Hsp90α and Hsp90β isoforms. Anti-WT1 and anti–β-actin mouse monoclonal antibodies were obtained from Dako and Sigma-Aldrich, respectively. Etoposide, 17-AAG and lactacystin were obtained from Sigma-Aldrich. STA-9090 was synthesized by Synta Pharmaceuticals Corporation.
Expression and purification of fusion proteins and GST pull-down assays
GST-fusion proteins were expressed in Escherichia coli BL21 (DE3) pLysS cells (Promega) with 0.2mM IPTG (isopropyl β-D-1-thiogalactopyranoside) induction for 3 hours at 30°C. Bacterial cells were lysed by sonication in NENT buffer (100mM NaCl, 1mM EDTA [ethylenediaminetetraacetic acid], 0.5% Nonidet P-40, 20mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 8.0) containing a protease inhibitor cocktail (Roche Diagnostics). Glutathione s-transferase (GST) fusion proteins were immobilized onto glutathione-Sepharose 4B beads (GE Healthcare) according to the manufacturer's protocol. GST-pull down assays were performed as described previously.24
Cell culture and patient samples
The human CML cell line K562 and AML cell lines KG-1, MV4-11 and Kasumi-1 were purchased from ATCC and grown in RPMI 1640 media supplemented with 10% fetal bovine serum and penicillin/streptomycin in a humidified incubator at 37°C with 5% CO2. Peripheral blood mononuclear blasts were isolated from deidentified AML patient samples obtained from the Institution Review Board–approved biorepository at the Cancer Therapy and Research Center, purified using Ficoll-Hypaque density gradient, and cultured in RPMI 1640 as above but without antibiotics.
Transfections and establishment of stable cell lines
K562 cells were transfected with non-specific (NS) or WT1 shRNA plasmids (Open Biosystems) using an Amaxa Nucleofector (Lonza) according to the manufacturer's protocol, and 48 hours later cells were grown and expanded in medium containing 2 μg/mL puromycin for 2 weeks. Subsequently, puromycin-resistant cells were isolated and cloned by limiting dilution.
Confocal microscopy
Confocal microscopy was performed after immobilizing K562 cells on Alcian blue–treated cover slips. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 for 30 minutes at room temperature (RT), then washed, and blocked with 10% goat serum in phosphate-buffered saline for 45 minutes at RT. Cells were incubated with anti-Hsp90 (rabbit polyclonal) or anti-WT1 (mouse monoclonal) diluted in phosphate-buffered saline containing 1% bovine serum albumin for 1.5 hours at RT. Cells were washed 3× before incubation for 1 hour at RT with 1:500 Alexa Fluor 647– and Alexa Fluor 488–conjugated secondary antibodies (Molecular Probes). After 3 washes, cells were mounted on glass slides in Aqua-Poly/Mount medium containing DAPI (4,6 diamidino-2-phenylindole; Polysciences). Confocal imaging was performed using a Fluoview FX1000 confocal microscope (Olympus).
Measurement of cell proliferation, apoptosis, and colony formation
Cell proliferation was measured using the alamarBlue assay (Invitrogen) according to the manufacturer's protocol with a SpectraMax microplate reader (Molecular Devices). Apoptosis was measured using the annexin V–fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (BioVision) according to the manufacturer's protocol with a FACScan flow cytometer (BD Biosciences) and CellQuest software Version 3.1. For colony formation, K562 cells stably expressing nonspecific control and WT1 shRNAs were plated in triplicate in 6-well plates with 2 mL of complete Methocult medium (StemCell Technologies) and cultured for 10 days at 37°C with 5% CO2. Colonies were defined as aggregates of greater than 50 cells and expressed as a percentage of control colony growth.
Coimmunoprecipitation and Western blot analyses
For coimmunoprecipitation assays, cells were lysed in NENT buffer (100mM NaCl, 1mM EDTA, 0.5% Nonidet P-40, 20mM Tris-HCl, pH 8.0) on ice for 30 minutes, and then centrifuged for 25 minutes at 12 000g. The protein concentration was determined using the DC Protein Assay (Bio-Rad). Cell extracts were precleared with agarose-conjugated mouse immunoglobulin G (IgG) for 1 hour at 4°C and immunoprecipitated with anti–Hsp90-AC overnight at 4°C. The agarose beads were washed 3× with lysis buffer and boiled in sodium dodecyl sulfate (SDS) sample buffer, and immunoprecipitates were probed with anti-WT1 (C-19) antibody. For Western blot analysis, cell extracts or immunoprecipitates were resolved on SDS–polyacrylamide gel electrophoresis (PAGE) gels and transferred to polyvinylidene difluoride membranes (Millipore). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween-20 and immunoblotted with various antibodies. The antigen–antibody complex was visualized using enhanced chemiluminescence (ECL) Western blotting detection reagents (GE Healthcare). For Western blotting of tumor lysates, K562 and MV4-11 xenograft tumors with average volumes of 100-200 mm3 were excised, cut in half, and flash frozen in liquid nitrogen. Each tumor fragment was lysed in 1 mL of lysis buffer (150mM NaCl, 1mM EDTA, 1mM EGTA [ethyleneglycoltetraacetic acid], 1% Nonidet P-40, 10% glycerol, 20mM Tris-HCl, pH 7.4) containing 2× concentrations of protease and phosphatase inhibitor cocktails (Calbiochem), using a FastPrep-24 homogenizer and Lysing Matrix A (MP Biomedicals) with a speed setting of 4.5 m/s for 40 seconds. Lysates were cleared by centrifugation at 21 500 g for 15 minutes at 4°C and then stored at −80°C prior to Western blotting.
Xenograft tumor models
Female immunodeficient Crl: CD1-Foxn1nu (Nude) and CB-17/Icr-Prkdcscid/Crl (severe combined immunodeficiency [SCID]) mice (Charles River Laboratories) were maintained in a specific pathogen-free environment, and all in vivo procedures were approved by the Synta Pharmaceuticals Corporation Institutional Animal Care and Use Committee. K562, Kasumi-1 and MV4-11 cells were cultured, washed and resuspended at 5-10 × 107 cells/mL in 1:1 nonsupplemented RPMI 1640 media and Matrigel (BD Biosciences). Aliquots of 0.1 mL (5 × 106-1 × 107 cells/mouse) were subcutaneously injected with a 27-gauge needle into the flanks of 7- to 8-week-old female Nude (MV4-11) and SCID (K562, Kasumi-1) mice. Tumor volumes (V) were calculated by caliper measurement of the width (W), length (L), and thickness (T) of each tumor using the formula: V = 0.5236(LWT). Tumors were permitted to develop until the majority had reached 100-200 mm3 in tumor volume, and were then randomized into treatment groups so that the average tumor volumes in each group were approximately 150 mm3.
Animals were treated by intravenous bolus injection into the tail vein using a 30-gauge needle at 10 mL/kg with 17-AAG or STA-9090 formulated in 10/18 DRD. To formulate compounds in 10/18 DRD, stock solutions were prepared by dissolving the appropriate amount of powdered compound in dimethyl sulfoxide (DMSO) by sonication in an ultrasonic water bath. Stock solutions were prepared weekly, stored at −20°C and diluted fresh each day for dosing. A solution of 20% Cremophor RH 40 (polyoxyl 40 hydrogenated castor oil; BASF Corporation) in 5% dextrose in water (Abbott Laboratories) was also prepared by first heating 100% Cremophor RH 40 at 50-60°C until liquefied and clear, diluting 1:5 with 5% dextrose in water, reheating again until clear and then mixing well. This solution could be stored at room temperature for up to 3 months prior to use. To prepare compounds for daily dosing, the DMSO stock solutions were diluted 1:10 with 20% Cremophor RH 40.
Xenograft studies were conducted at the highest nonseverely toxic doses (HNSTDs), which can be considered to be similar to a maximum tolerated dose, except that only morbidity and body weight changes were monitored. The HNSTD for each agent was based on prior tolerability studies conducted in nontumor bearing animals of the same mouse strains (data not shown) and was defined as the dose and schedule at which no morbidity was observed, and at which no individual animal lost > 20% of its body weight and no treatment group lost > 10% average body weight over the course of the study.
Tumor volumes were measured 2-3 times per week and body weights were monitored daily. As a measurement of in vivo efficacy, the %T/C value was determined from the change in average tumor volumes of each treated group relative to the vehicle-treated group or itself in the case of tumor regression. A %T/C ≤ 42 is considered to represent significant efficacy in a xenograft tumor model,25 and −100 indicates complete responses observed in every animal in a group. Statistical analyses of significance, where relevant, were performed using a Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks. Due to a nonnormal distribution in the tumor volume datasets, the ANOVA test was followed by the Tukey test multiple comparison procedure (additional treatment groups for which results were not presented in Figure 6, were also included in the analysis). Values of P < .05 were considered statistically significant.
Results
WT1 interacts with Hsp90 in leukemia cells
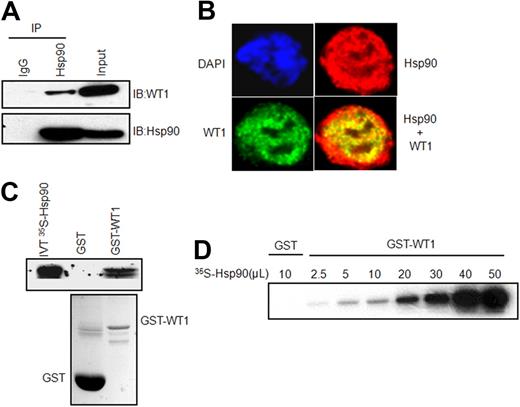
The molecular chaperone Hsp70 plays an essential role in regulating the activity of WT1 protein. Because Hsp70 is an important component of the Hsp90 multichaperone complex, we hypothesized that WT1 would also associate with Hsp90. To evaluate a potential WT1–Hsp90 interaction, we performed immunoprecipitation experiments in K562 leukemia cells with an anti-Hsp90 antibody and analyzed the precipitates for the presence of WT1. Endogenous WT1 was coimmunoprecipitated by the Hsp90 antibody but not by a control antibody (Figure 1A). We also established the intracellular localization of both WT1 and Hsp90 by immunofluorescence confocal microscopy in K562 leukemia cells. When the WT1 and Hsp90 staining patterns were merged, WT1 was seen to colocalize with Hsp90 in the nucleus (Figure 1B). We further examined the interaction of WT1 and Hsp90 by a GST pull-down assay. As shown in Figure 1C, Hsp90 showed strong binding to the recombinant GST-WT1 fusion protein but not to GST alone. Next, we performed a saturation-binding experiment where increasing concentrations of 35S-methionine–labeled Hsp90 protein were added to a constant amount of GST-WT1 protein. The observed dose-dependent increase in Hsp90 binding to WT1 further confirmed the direct association between Hsp90 and WT1 proteins (Figure 1D). Taken together, these results identify WT1 as a novel Hsp90-binding protein.
Direct interaction between WT1 and Hsp90. (A) Equal amounts of K562 protein extracts were immunoprecipitated (IP) with agarose-conjugated mouse immunoglobulin G (IgG) or anti-Hsp90 antibodies, and immunoprecipitates were subjected to SDS-PAGE and immunoblotted (IB) for WT1 (top panel) and Hsp90 (bottom panel). Input represents ∼5% of the total protein extract used for immunoprecipitation. (B) Subcellular colocalization of WT1 and Hsp90. K562 cells were stained with DAPI (blue, nuclear stain) and antibodies to WT1 (green) or Hsp90 (red), and confocal images were acquired at 100× magnification. (C) GST pull-down assay. In vitro–translated and 35S-methionine–labeled full-length Hsp90 was incubated with GST or GST-WT1 protein immobilized on glutathione-sepharose beads, and bound WT1 was detected by fluorography (top panel). 20% of the in vitro–translated protein was used for pull-downs. The bottom panel shows the purity of GST-fused proteins on a Coomassie blue-stained SDS-PAGE gel. (D) Dose-dependent binding of Hsp90 to WT1. Increasing amounts of 35S-methionine–labeled Hsp90 were added to GST-WT1, and binding was analyzed by autoradiography.
Direct interaction between WT1 and Hsp90. (A) Equal amounts of K562 protein extracts were immunoprecipitated (IP) with agarose-conjugated mouse immunoglobulin G (IgG) or anti-Hsp90 antibodies, and immunoprecipitates were subjected to SDS-PAGE and immunoblotted (IB) for WT1 (top panel) and Hsp90 (bottom panel). Input represents ∼5% of the total protein extract used for immunoprecipitation. (B) Subcellular colocalization of WT1 and Hsp90. K562 cells were stained with DAPI (blue, nuclear stain) and antibodies to WT1 (green) or Hsp90 (red), and confocal images were acquired at 100× magnification. (C) GST pull-down assay. In vitro–translated and 35S-methionine–labeled full-length Hsp90 was incubated with GST or GST-WT1 protein immobilized on glutathione-sepharose beads, and bound WT1 was detected by fluorography (top panel). 20% of the in vitro–translated protein was used for pull-downs. The bottom panel shows the purity of GST-fused proteins on a Coomassie blue-stained SDS-PAGE gel. (D) Dose-dependent binding of Hsp90 to WT1. Increasing amounts of 35S-methionine–labeled Hsp90 were added to GST-WT1, and binding was analyzed by autoradiography.
Identification of domains involved in WT1–Hsp90 interaction
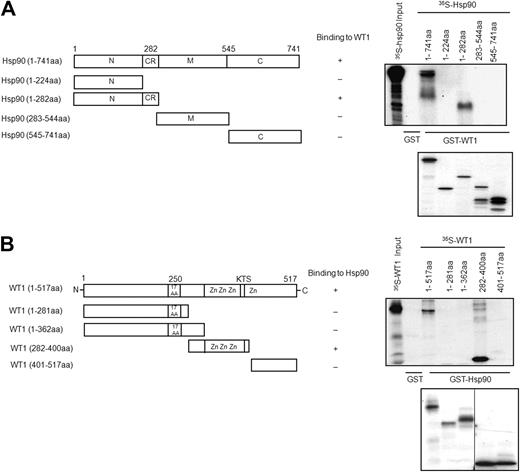
Because the above data indicated that Hsp90 formed a complex with WT1, we next determined the critical domains of Hsp90 and WT1 required for this interaction. A series of truncation mutants of Hsp90 were generated, and we tested the ability of these in vitro–translated 35S-methionine–labeled proteins to bind GST-WT1 (1-517 amino acids [aa]) by pull-down assay. As shown in Figure 2A, full-length Hsp90 (1-741 aa) and a N-terminal mutant containing the charged linker region of Hsp90 (1-282 aa) both strongly interacted with WT1. However, the Hsp90 truncation mutants 1-224 aa, 283-544 aa, and 545-741 aa failed to show any binding to WT1. This suggests that amino acid residues 225-282 aa in the N-terminal domain of Hsp90, which contains a charged region that has been shown to enhance the chaperone activity of the N-terminal domain,26 is involved in binding with WT1. We next mapped the Hsp90-binding domain of WT1. Hsp90 (1-282 aa) was expressed as a GST-fusion protein, and its association was tested with different in vitro–translated truncation mutants of WT1. Only the full-length WT1 and WT1 (282-400 aa) bound Hsp90 (Figure 2B), while other deletion mutants did not show any detectable binding. This suggests that the zinc finger region of WT1, which is involved in DNA binding and protein–protein interactions,27 is required for binding with Hsp90. Taken together, these data indicate that WT1 interacts with the N-terminal domain and charged region of Hsp90 (1-282 aa), whereas Hsp90 interacts with zinc finger regions 1, 2, and 3 (282-400 aa) of WT1.
Mapping of Hsp90–WT1 interacting domains by GST pull-down assay. (A) Schematic diagram of Hsp90 domains on the left, with the expression constructs and their binding to WT1 on the right. GST pull-down assays were performed using bacterially expressed GST-WT1 (1-517 aa) and GST (negative control) and in vitro–translated and 35S-methionine–labeled full-length and deletion mutants of Hsp90. Bound proteins were separated by SDS-PAGE and visualized by fluorography. Bottom panel shows the in vitro–translated Hsp90 proteins. (B) Schematic diagram of WT1 domains on the left, with the expression constructs and their binding to Hsp90 on the right. GST pull-down assays were performed using bacterially expressed GST-Hsp90 (1-282 aa), GST (negative control), and in vitro–translated and 35S-methionine–labeled full-length and deletion mutants of WT1 and analyzed in panel A. A vertical line has been inserted to indicate a repositioned gel lane in the bottom panel.
Mapping of Hsp90–WT1 interacting domains by GST pull-down assay. (A) Schematic diagram of Hsp90 domains on the left, with the expression constructs and their binding to WT1 on the right. GST pull-down assays were performed using bacterially expressed GST-WT1 (1-517 aa) and GST (negative control) and in vitro–translated and 35S-methionine–labeled full-length and deletion mutants of Hsp90. Bound proteins were separated by SDS-PAGE and visualized by fluorography. Bottom panel shows the in vitro–translated Hsp90 proteins. (B) Schematic diagram of WT1 domains on the left, with the expression constructs and their binding to Hsp90 on the right. GST pull-down assays were performed using bacterially expressed GST-Hsp90 (1-282 aa), GST (negative control), and in vitro–translated and 35S-methionine–labeled full-length and deletion mutants of WT1 and analyzed in panel A. A vertical line has been inserted to indicate a repositioned gel lane in the bottom panel.
Inhibition of Hsp90 function reduces WT1 expression
The chaperoning activity of Hsp90 regulates the stability and functions of multiple oncogenic client proteins. Because the experiments described in Figures 1 and 2 demonstrated association between WT1 and Hsp90, we analyzed whether the chaperone activity of Hsp90 was also essential for WT1 protein stability. To address this, we treated K562 and KG-1 leukemia cells with increasing concentrations of the Hsp90 inhibitor 17-AAG, as well as the much more potent next-generation Hsp90 inhibitor STA-9090.21 The dose-dependent reduction of WT1 protein levels observed in cells treated with 17-AAG (Figure 3A-B) and STA-9090 (Figure 3C-D) suggested that the molecular-chaperoning activity of Hsp90 plays a crucial role in WT1 stability in leukemia cells. These effects were observed at drug concentrations and time points at which Hsp90 inhibition had only a minimal effect on K562 cell viability (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In contrast, 17-AAG and STA-9090 treatment had little or no effect on WT1 mRNA levels in K562 cells (supplemental Figure 2). As expected, inhibition of Hsp90 by 17-AAG resulted in the induction of Hsp70 expression (Figure 3A-B). WT1 has also been implicated in the control of cell proliferation and apoptosis through transcriptional activation of the c-Myc and Bcl-2 genes.28,29 Consistent with this, WT1 down-regulation by Hsp90 inhibition resulted in reduced expression of c-Myc protein (Figure 3A). Hsp90 inhibitor STA-9090 also reduced the WT1 protein levels in 2 other leukemia cell lines, Kasumi-1 (Figure 3E) and MV4-11 (Figure 3F). Similar results were obtained in the K562 cell line using radicicol, another Hsp90 inhibitor structurally unrelated to 17-AAG (data not shown). Next, we examined the effect of STA-9090 on WT1 expression in primary leukemia blasts derived from 5 different AML patients (supplemental Table 1). Similar to results obtained from myeloid leukemia cell lines, WT1 levels were reduced in primary leukemia blasts after STA-9090 treatment (Figure 3G). Finally, WT1 degradation was also observed more broadly in nonmyeloid leukemia and breast cancer cell lines treated with STA-9090 (supplemental Figure 3). Together, these results indicate that in human myeloid leukemias, WT1 stability is dependent upon Hsp90 activity and that WT1 is then susceptible to specific degradation following treatment with Hsp90 inhibitors.
Pharmacologic inhibition of Hsp90 down-regulates WT1 protein. (A) K562 and (B) KG-1 leukemia cells were treated with the Hsp90 inhibitor 17-AAG for 24 hours. The cells were lysed, and protein extracts were subjected to SDS-PAGE and analyzed by Western blotting for WT1, Hsp90, and/or the WT1-regulated protein c-Myc. β-actin was used as loading control. (C) K562, (D) KG1, (E) Kasumi-1, and (F) MV4-11 leukemia cells were treated with the Hsp90 inhibitor STA-9090 for 24 hours and analyzed for WT1 expression by Western blotting. (G) Primary myeloid leukemia blasts from 5 AML patients (PS#1-5) were isolated, treated with STA-9090 for 24 hours, and analyzed for WT1 and β-actin by Western blotting.
Pharmacologic inhibition of Hsp90 down-regulates WT1 protein. (A) K562 and (B) KG-1 leukemia cells were treated with the Hsp90 inhibitor 17-AAG for 24 hours. The cells were lysed, and protein extracts were subjected to SDS-PAGE and analyzed by Western blotting for WT1, Hsp90, and/or the WT1-regulated protein c-Myc. β-actin was used as loading control. (C) K562, (D) KG1, (E) Kasumi-1, and (F) MV4-11 leukemia cells were treated with the Hsp90 inhibitor STA-9090 for 24 hours and analyzed for WT1 expression by Western blotting. (G) Primary myeloid leukemia blasts from 5 AML patients (PS#1-5) were isolated, treated with STA-9090 for 24 hours, and analyzed for WT1 and β-actin by Western blotting.
Hsp90 inhibition induces proteasome-dependent degradation of WT1
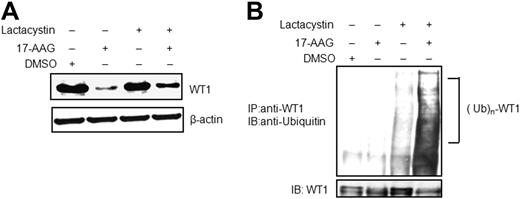
Hsp90 inhibitors cause degradation of Hsp90 client proteins via a proteasome-dependent pathway. Therefore, we examined whether proteasomal degradation mediates the loss of WT1 protein after treatment with 17-AAG. As shown in Figure 4A, lactacystin (a highly specific inhibitor of the 20S proteasome subunit) antagonized degradation of WT1 protein induced by treatment with 17-AAG. Previous reports have demonstrated that 17-AAG–mediated proteasomal degradation of Hsp90 client proteins was preceded by their ubiquitination30 ; therefore, we then tested whether WT1 was ubiquitinated prior to its degradation in 17-AAG–treated cells. Immunoprecipitation of WT1 followed by Western blot analysis with an anti-ubiquitin antibody detected significantly higher levels of ubiquitinated WT1 in the presence of the combination of lactacystin and 17-AAG, compared with either agent alone (Figure 4B). Low-level ubiquitination of WT1 was also visible after treatment with 17-AAG alone (supplemental Figure 3). These data demonstrated that 17-AAG promoted ubiquitination followed by proteasome-dependent degradation of WT1 protein. Thus WT1 shares the same protein degradation pathway following Hsp90 inhibition as other well-established Hsp90 client proteins.
17-AAG promotes ubiquitination and proteasome-dependent degradation of WT1. (A) K562 cells were treated with 10μM 17-AAG alone, 10μM lactacystin alone or with a combination of both agents for 24 hours, and cell lysates were subjected to Western blot analysis using anti-WT1 and anti–β-actin. (B) K562 cells were treated as above for 8 hours, and proteins extracts were immunoprecipitated (IP) with anti-WT1. The ubiquitination of WT1 was analyzed by Western blotting (IB) with anti-ubiquitin (top panel), and WT1 protein levels were assessed by anti-WT1 (bottom panel).
17-AAG promotes ubiquitination and proteasome-dependent degradation of WT1. (A) K562 cells were treated with 10μM 17-AAG alone, 10μM lactacystin alone or with a combination of both agents for 24 hours, and cell lysates were subjected to Western blot analysis using anti-WT1 and anti–β-actin. (B) K562 cells were treated as above for 8 hours, and proteins extracts were immunoprecipitated (IP) with anti-WT1. The ubiquitination of WT1 was analyzed by Western blotting (IB) with anti-ubiquitin (top panel), and WT1 protein levels were assessed by anti-WT1 (bottom panel).
Silencing WT1 with shRNA enhances etoposide and 17-AAG–induced apoptosis
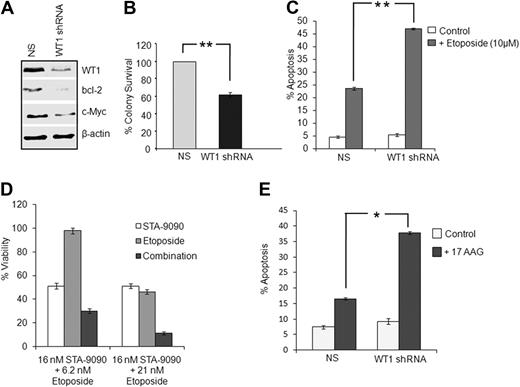
We observed that treatment with 17-AAG or STA-9090 for 72 hours inhibited the in vitro proliferation and induced apoptosis of K562 leukemia cells with IC50 values of 105nM and 18nM, respectively (data not shown), whereas 24-hour treatment had only a minor effect. However, it was possible that this was due to decreased expression of multiple Hsp90 client proteins in addition to WT1. To assess the role of WT1 in leukemia cells, we silenced its expression in K562 cells using shRNA. As shown in Figure 5A, transient expression of shRNA targeting WT1 effectively inhibited the expression of WT1 and its downstream-regulated proteins c-Myc and Bcl-2. Stable WT1 shRNA expression also significantly decreased the in vitro clonogenic growth of K562 leukemia cells (Figure 5B). Next we assessed the effect of stable WT1 shRNA expression on chemotherapy-induced apoptosis in K562 cells by annexin V staining. The combination of WT1 silencing and treatment with the topoisomerase II inhibitor etoposide treatment significantly enhanced cell death (Figure 5C). Similarly, the combination of STA-9090 (or 17-AAG; data not shown) and etoposide also displayed enhanced cytotoxicity relative to either agent alone (Figure 5D). However, it is still likely that additional Hsp90 client proteins other than WT1 also play a role in the cytoxicity of Hsp90 inhibitors toward leukemia cells. To test whether WT1 plays a role in determining the in vitro response to Hsp90 inhibitors, we examined the sensitivity of WT1-silenced K562 cells toward 17-AAG. As seen in Figure 5E, 17-AAG in vitro cytotoxicity was enhanced toward K562 cells stably transfected with WT1 shRNA compared with control cells. Collectively, these data show that WT1 plays an important antiapoptotic role and that WT1 depletion further sensitized leukemia cells to etoposide and 17-AAG treatment.
Silencing WT1 expression decreases colony formation capacity and enhances the sensitivity of leukemia cells to etoposide and 17-AAG. (A) K562 cells were transiently transfected with nonspecific (NS) and WT1 shRNA plasmids. After 48 hours, silencing of WT1 protein and the downstream WT1-regulated proteins Bcl-2 and c-Myc were analyzed by Western blotting. (B) K562 cells stably expressing NS or WT1 shRNA were plated in triplicate in semisolid media, and colony formation was scored after 10 days. Colonies were defined as aggregates of greater than 50 cells. Data represent means ±SDs of triplicates (**P < .005). (C) K562 cells stably expressing NS or WT1 shRNA as in panel B were left untreated or treated with 10μM etoposide for 24 hours. Apoptosis was assessed by annexin V staining and flow cytometry. Data represent means ± SDs of 2 independent experiments (*P < .05). (D) K562 cells were treated with either STA-9090 or etoposide alone or with a combination of both for 72 hours. Viability was assessed by alamarBlue assay and fluorometry. (E) K562 cells stably transfected with NS or WT1 shRNA were left untreated or treated with 5μM 17-AAG for 48 hours. Apoptosis was measured as described in panel C. Data represent means of ± SDs of triplicates (*P < .05).
Silencing WT1 expression decreases colony formation capacity and enhances the sensitivity of leukemia cells to etoposide and 17-AAG. (A) K562 cells were transiently transfected with nonspecific (NS) and WT1 shRNA plasmids. After 48 hours, silencing of WT1 protein and the downstream WT1-regulated proteins Bcl-2 and c-Myc were analyzed by Western blotting. (B) K562 cells stably expressing NS or WT1 shRNA were plated in triplicate in semisolid media, and colony formation was scored after 10 days. Colonies were defined as aggregates of greater than 50 cells. Data represent means ±SDs of triplicates (**P < .005). (C) K562 cells stably expressing NS or WT1 shRNA as in panel B were left untreated or treated with 10μM etoposide for 24 hours. Apoptosis was assessed by annexin V staining and flow cytometry. Data represent means ± SDs of 2 independent experiments (*P < .05). (D) K562 cells were treated with either STA-9090 or etoposide alone or with a combination of both for 72 hours. Viability was assessed by alamarBlue assay and fluorometry. (E) K562 cells stably transfected with NS or WT1 shRNA were left untreated or treated with 5μM 17-AAG for 48 hours. Apoptosis was measured as described in panel C. Data represent means of ± SDs of triplicates (*P < .05).
Inhibition of Hsp90 blocks in vivo tumor growth in leukemia models
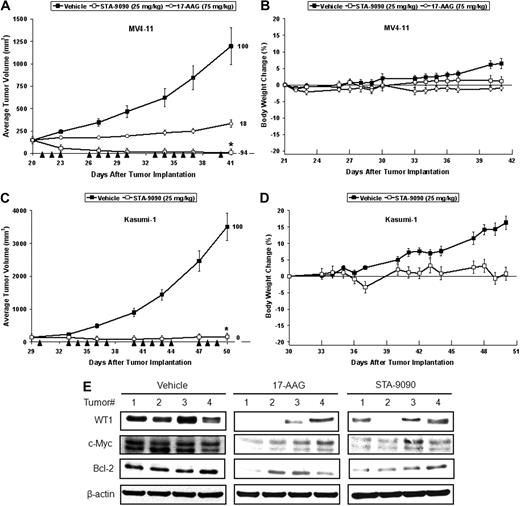
To translate these in vitro results to an in vivo context, we conducted xenograft tumor studies using the MV4-11, Kasumi-1, and K562 leukemia cell lines subcutaneously implanted into the flanks of Nude or SCID mice. As shown in Figure 6A, both 17-AAG and STA-9090 dramatically inhibited tumor growth in the MV4-11 model when intravenously dosed 5× per week at their respective HNSTDs, with %T/C values of 18 and −94, respectively. A %T/C value of < 42 is considered to represent significant activity.25 Consistent with our observations in vitro, STA-9090 displayed significantly greater anticancer activity in vivo than 17-AAG. Infrequent dosing of STA-9090 at its HNSTD on a 1× per week schedule gave an identical %T/C value of −94 in this model (data not shown). Significant efficacy was also observed for STA-9090 in the Kasumi-1 (Figure 6B) and K562 (supplemental Figure 5A) xenograft models. Both Hsp90 inhibitors were well tolerated, with minimal cumulative average body weight changes for each treatment group over the course of these studies (Figure 6C-D and data not shown). In addition, Western blotting demonstrated that both 17-AAG and STA-9090 significantly reduced the expression of WT1 and its downstream targets c-Myc and Bcl-2 in MV4-11 tumors 6 hours after single doses of each drug (Figure 6E). Similar results were observed in the K562 model (supplemental Figure 5B). We also observed reduced expression of Akt, a known Hsp90 client protein, and concomitant up-regulation of Hsp70 (data not shown).
Hsp90 inhibitors decrease tumor growth and WT1 expression in leukemia tumor models. (A-B) 17-AAG and STA-9090 treatment at their respective HNSTDs significantly inhibited tumor growth in the MV4-11 (A) and the Kasumi-1 (B) xenograft models. In both studies, intravenous dosing was conducted 5× per week (arrowheads). STA-9090 treatment significantly inhibited tumor growth relative to vehicle in both models (*P < .05), and there was a strong trend toward STA-9090 being superior to 17-AAG in the MV4-11 model, although this difference or the difference between the 17-AAG and vehicle-treated groups did not reach statistical significance. Error bars represent ± SEM (n = 8/group). (C-D) Both compounds were well tolerated, as indicated by minimal affects on body weight loss in the MV4-11 (C) and Kasumi-1 (D) xenograft models over the course of the study. The differences in body weight changes between the vehicle- and Hsp90 inhibitor–treated groups were largely due to increasing tumor mass in the vehicle-treated animals over the course of these studies. (E) MV4-11 tumor-bearing animals were given single doses of vehicle, STA-9090 and 17-AAG, and after 6 hours, tumors were removed for Western blot analysis using anti-WT1, anti–Bcl-2, anti–c-Myc, and anti–β-actin. The expression levels of WT1, c-Myc, and Bcl-2 were significantly decreased after treatment with both Hsp90 inhibitors.
Hsp90 inhibitors decrease tumor growth and WT1 expression in leukemia tumor models. (A-B) 17-AAG and STA-9090 treatment at their respective HNSTDs significantly inhibited tumor growth in the MV4-11 (A) and the Kasumi-1 (B) xenograft models. In both studies, intravenous dosing was conducted 5× per week (arrowheads). STA-9090 treatment significantly inhibited tumor growth relative to vehicle in both models (*P < .05), and there was a strong trend toward STA-9090 being superior to 17-AAG in the MV4-11 model, although this difference or the difference between the 17-AAG and vehicle-treated groups did not reach statistical significance. Error bars represent ± SEM (n = 8/group). (C-D) Both compounds were well tolerated, as indicated by minimal affects on body weight loss in the MV4-11 (C) and Kasumi-1 (D) xenograft models over the course of the study. The differences in body weight changes between the vehicle- and Hsp90 inhibitor–treated groups were largely due to increasing tumor mass in the vehicle-treated animals over the course of these studies. (E) MV4-11 tumor-bearing animals were given single doses of vehicle, STA-9090 and 17-AAG, and after 6 hours, tumors were removed for Western blot analysis using anti-WT1, anti–Bcl-2, anti–c-Myc, and anti–β-actin. The expression levels of WT1, c-Myc, and Bcl-2 were significantly decreased after treatment with both Hsp90 inhibitors.
Discussion
WT1 has emerged as an important factor in normal and malignant hematopoiesis.12 Previous studies have demonstrated that the WT1 gene is highly expressed in myeloid leukemias, and this correlates with poor prognosis.10,11 Given its role as an oncoprotein necessary for tumor growth and leukemogenesis, controlling the expression of WT1 represents a potentially attractive approach to cancer therapy. Hsp90 is also overexpressed in many cancers, including AML16 , and is involved in the proper folding and stabilization of oncoproteins critical to cancer progression.15 We show here that Hsp90 associates with WT1 and stabilizes its expression in leukemia cells. Furthermore, we show that inhibition of Hsp90 chaperone function dramatically reduces the expression of WT1 in cell lines and primary myeloid leukemia blasts via a ubiquitin-dependent proteasome degradation pathway. This is the first report demonstrating WT1 as a Hsp90 client protein that can be targeted for degradation by Hsp90 inhibitors.
We first demonstrated interaction between WT1 and Hsp90 by in vitro GST pull-down assays, as well as by coimmunoprecipitation experiments in leukemia cells. Hsp90 consists of 3 functional and structural domains: an N-terminal ATP-binding domain, a middle domain that regulates the ATPase activity of the N-terminal domain, and a C-terminal dimerization domain.31 Specific domains of Hsp90 involved in protein binding have been identified. For example, the N-terminal domain of Hsp90 binds to survivin, and selective targeting of the survivin–Hsp90 complex promotes apoptosis and mitotic defects.32 Our study demonstrated that amino acid residues 224-282 aa in the N-terminal domain of Hsp90 are required to mediate its binding to WT1. The Hsp90 (1-282 aa) region contains the ATP-binding domain and a highly charged domain, both of which play important roles in regulating Hsp90 chaperone function.26,33 Our observation is consistent with the previous reports that both the N-terminal and middle domains of Hsp90 are necessary for interaction between Hsp90 and its client proteins.34 On further analysis, we found that the C-terminal (282-400 aa) region of WT1 is responsible for its binding to Hsp90. The C-terminal DNA-binding domain of WT1 contains 4 Cys2His2 zinc fingers, each encoded by a discrete exon (numbers 7-10).35,36 The 282-400 aa region also contains the KTS insert, which appears to modulate the strength of DNA binding and interaction with mRNAs, thus affecting target gene expression and translation.37,38
Next, we established the biochemical importance of the WT1–Hsp90 interaction by demonstrating that treatment with 2 structurally unrelated Hsp90 inhibitors, 17-AAG (a derivative of the natural fungal product geldanamycin) and STA-9090 (a novel resorcinol-containing compound), resulted in down-regulation of WT1 protein in different myeloid leukemia cell lines. In these studies, STA-9090 was found to be substantially more potent than 17-AAG. In addition, down-regulation of WT1 was associated with a decrease in the downstream WT1 targets, Bcl-2 and c-Myc. Previous work in primary human samples from leukemia patients has demonstrated a statistically significant elevation of WT1 mRNA expression.39 Our studies clearly indicate that the overexpression of WT1 in AML can be targeted by pharmacologic inhibition of Hsp90 leading to beneficial biologic consequences.
Several reports have shown that Hsp90 inhibitors induce the degradation of client proteins by targeting them for ubiquitination and proteasomal degradation, and this process can be inhibited by proteasome inhibitors.40 The present study shows that 17-AAG induced the degradation of WT1, which was mediated by the proteasome and antagonized by lactacystin, a proteasome inhibitor. We observed that lactacystin caused an accumulation of ubiquitinated WT1. These results strongly suggest that the observed down-regulation of WT1 protein was a direct effect of Hsp90 inhibition and also indicate that Hsp90 is necessary for the stability of WT1. Recently, Makki et al reported down-regulation of WT1 expression by the histone deacetylase (HDAC) inhibitor trichostatin A.41 Galimberti et al observed synergistic effects between the proteasome inhibitor bortezomib and the HDAC inhibitor vorinostat on WT1 down-regulation, leading to inhibition of leukemic cell proliferation and reduced viability.42 HDAC inhibitors have been reported to cause reversible acetylation of Hsp90, which was further associated with inhibition of ATP and client protein binding, leading to proteasomal degradation of Hsp90 client proteins.43,44 The down-regulation of WT1 by HDAC inhibitors appears to be the result of disruption of Hsp90 chaperone function and further supports WT1 as a client protein of Hsp90. Furthermore, this also lays the foundation to develop specific inhibitors of the WT1–Hsp90 interaction, similar to shepherdin, a recently described cell-permeable peptidomimetic inhibitor of the survivin-Hsp90 complex with antileukemic activity.45
WT1 has been shown to have antiapoptotic function in leukemia cells, and siRNA-mediated down-regulation of WT1 results in cell death46 through the activation of the intrinsic apoptotic pathway.47 We also observed apoptosis after shRNA-mediated down-regulation of WT1, and this down-regulation reduced the survival of leukemic blasts and enhanced etoposide-induced apoptosis in leukemia cells. Consistent with this, treatment with Hsp90 inhibitors also enhanced etoposide activity. Interestingly, there are reports of high levels of Bcl-2 expression in AML patients who exhibit poor response to chemotherapy.46 Moreover, Karakas et al reported that coexpression of WT1 and Bcl-2 contributes to an unfavorable prognosis in adult AML.46,48 Thus shRNA-mediated reduction in WT1 and its target gene Bcl-2 may be one of the mechanisms underlying the sensitization of leukemia cells to chemotherapy-induced apoptosis in our studies.
We also showed that 17-AAG treatment is more effective in leukemic cells stably transfected with WT1 shRNA. Because Hsp90 inhibition also targets other signaling molecules that may synergize with inhibition of the WT1 pathway, the observed synergy may be due to the effective down-regulation of multiple Hsp90 client proteins that may be involved in leukemogenesis. For example, Elmaagacli et al have shown that inhibiting Bcr-Abl (a known Hsp90 client protein) and WT1 together using specific small interfering RNAs reduced the proliferation and induced apoptosis of K562 leukemia cells.49 Furthermore, Hawkins et al observed that 17-AAG enhanced the activity of the Bcr-Abl inhibitor imatinib in pediatric acute lymphoblastic leukemia (ALL) cell lines by decreasing WT1 in addition to other Hsp90 client proteins.50 However, this study did not establish the direct association between WT1 and Hsp90.
Finally, we found that both Hsp90 inhibitors, 17-AAG and STA-9090, were effective in blocking MV4-11, Kasumi-1, and K562 in vivo tumor growth. Furthermore, we showed that a single dose of 17-AAG or STA-9090 could significantly down-regulate expression of WT1 protein in xenograft tumors. This was accompanied by the reduction of downstream WT1 target proteins, c-Myc and Bcl-2. We therefore conclude that the efficacy of Hsp90 inhibitors in our xenograft studies may be attributable at least in part to down-regulating WT1 and its downstream influence on cell proliferation mediated by c-Myc and antiapoptosis mediated by Bcl-2. Several other Hsp90 clients, including c-Kit, Flt3, Akt, and Bcr-Abl, play important roles in hematopoiesis and leukemogenesis. Taken together, our findings support WT1 as one of the central targets in leukemia, in addition to the other leukemia-associated Hsp90 client proteins, the inhibition of which provides the basis for treatment of leukemias and the enhancement of other chemotherapeutic agents.
In conclusion, WT1 expression, in addition to being a prognostic marker in leukemia, thus far has been found to have clinical significance in the evaluation of response and minimal residual disease and has lent itself to use as a panleukemic marker. With this report, WT1 joins the list of known Hsp90 substrates as a pivotal player in leukemia that can be used as a valid drug target. Furthermore, our results establish the grounds for investigating the response to WT1 inhibition in patients with AML. As there is no established therapy that durably inhibits WT1 oncogenic functions, targeting WT1 expression by clinically available Hsp90 inhibitors may offer new strategies to limit the survival-promoting effects of WT1 in myeloid leukemias.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Elizabeth Repasky, Dr Gokul Das, Dr Emre Dayanc, and Dr Naveen Bangia (Roswell Park Cancer Institute, Buffalo, NY) for their help. We thank Dr Donald G. McEwen (Greehey Children's Cancer Research Institute) for assistance with confocal microscopy. We acknowledge the Roswell Park Cancer Institute's shRNA and Flow Cytometry core facilities. We also thank Kim Wright, Michael Karol, Tim Korbut, Don Smith, Chaohua Zhang, and Theresea A. Seifert for their technical assistance.
This work was supported by an American Cancer Society Institutional Grant (S.P., Roswell Park), the AT&T Foundation (F.J.G.), and the National Cancer Institute (3P30CA054174-17S109; H.B., S.B., F.J.G and S.P.).
National Institutes of Health
Authorship
Contribution: H.B., S.B., and S.P. designed and performed research, collected, analyzed, and interpreted data, and wrote the paper; J.S., W.Y., and D.A.P. performed experiments and analyzed the data; and M.R., K.P.F., R.K.B., J.B., M.R.B., K.K., R.S., G.E.T., M.B., F.J.G., and K.P.L. analyzed data and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: K.P.F., J.S., D.A.P., R.A.B., W.Y., and J.B. are employees and shareholders of Synta Pharmaceuticals Corp, which is developing STA-9090. The remaining authors declare no competing financial interests.
Correspondence: Swaminathan Padmanabhan, Institute for Drug Development, Cancer Therapy and Research Center at the UT Health Science Center at San Antonio, 7979 Wurzbach Rd, Mail Code no. 8232, San Antonio, TX 78229; e-mail: padmanabhans@uthscsa.edu; or Sanjay Bansal, Cancer Therapy and Research Center at the UT Health Science Center at San Antonio, 7979 Wurzbach Rd, Mail Code no. 8232, San Antonio, TX 78229; e-mail: bansals@uthscsa.edu.