Abstract
Polycythemia vera (PV) treatment with interferon α (IFNα) is frequently limited by dose-related toxicity. PV CD34+ cells are characterized by overexpression of Bcl-xL, which can be antagonized by ABT-737 leading to apoptosis. We explored the effects of ABT-737 and IFNα on PV hematopoiesis. Both IFNα and ABT-737 alone or in combination had a modest effect on normal hematopoiesis but each individually were able to markedly induce PV CD34+ cell apoptosis and suppress hematopoietic colony formation. The inhibitory activities of these agents in combination were greater against PV hematopoiesis than either agent alone. The exposure of PV CD34+ cells to low doses of IFNα and ABT-737 in combination resulted in the reduction of the proportion of JAK2V617F+ colonies similar to that observed with higher doses of IFNα. These data provide the rationale for combination therapy with low doses of IFNα and a BH3 mimetic for patients with PV.
Introduction
Therapeutic options in polycythemia vera (PV) include phlebotomy, low-dose aspirin hydroxyurea, and interferon α (IFNα).1 IFNα effectively controls erythrocytosis and thrombocytosis2 and can correct the marrow morphology, induce cytogenetic remissions, reduce the JAK2V617F allele burden, and revert monoclonal to polyclonal hematopoiesis.3-8 Our laboratory has reported that IFNα selectively eliminates JAK2V617F hematopoietic progenitor cells (HPC).9 IFNα use is limited by lack of accessibility and concern over therapy-associated toxicity.10 Because its toxicity is related to dose, we hypothesize that combinations of drugs to enhance the therapeutic effectiveness of IFNα might permit the administration of lower doses of IFNα.
Silva and colleagues reported that Bcl-xl was dysregulated in PV erythroblasts which might contribute to the development of erythrocytosis.11 Similarly megakaryocytes (MK) are also characterized by overexpression of Bcl-xL.12 This overexpression of Bcl-xL is likely a downstream consequence of JAK2V617F.13 ABT-737 is a small-molecule inhibitor that binds the antiapoptotic proteins, Bcl-2, and Bcl-xL, and promotes apoptosis.14 Zeuner and coworkers and Will et al15,16 have each provided evidence that ABT-737 preferentially induces apoptosis of JAK2V617F-positive PV erythroblasts and CD34+ cells, respectively. We hypothesize that a combination of Peg-IFNα 2a and ABT-737 might prove to be an effective treatment strategy for PV patients.
Methods
Isolation and purification of CD34+ cells
The CD34+ cells were isolated using a human CD34+ cell selection kit (StemCell Technologies) as previously described.17 Peripheral blood (PB) was obtained from 14 different PV patients after informed consent was obtained according to the guidelines established, in accordance with the Declaration of Helsinki, and with the approval of the Institutional Review Board of the Mount Sinai School of Medicine, New York, NY.
Western blot analysis
The cord blood (CB) or PV CD34+ cells were lysed and the equivalent protein from each sample was separated on SDS-PAGE, and Bcl-xL, cleaved caspapse-3, and α-tubulin levels were visualized using specific antibodies.
Flow cytometric analysis
Primary CB and PV CD34+ cells were stained with a CD34+ monoclonal antibody and annexin V (BD Biosciences). The data were analyzed using a FACSCalibur.
Mitochondria membrane potential assay
The mitochondrial membrane potential was determined with a Mitochondrial Membrane Potential kit (Stratagene). This used a fluorescent cationic dye JC-1 to signal the loss of mitochondrial membrane potential, which leads to the dye appearing green rather than red.
Hematopoietic progenitor cell assays
CD34+ cells (5 × 102) were cultured in the presence of various doses of Peg-IFNα 2a (Hoffman-La Roche Inc) and ABT-737 (kindly provided by Abbott Laboratories). Individual hematopoietic colonies were plucked from the cultures, and JAK2V617F was detected using a nested allele-specific PCR as previously described.17
Results and discussion
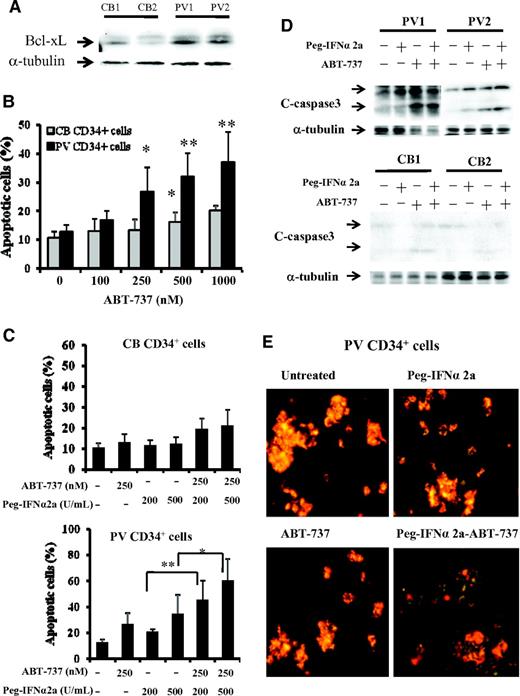
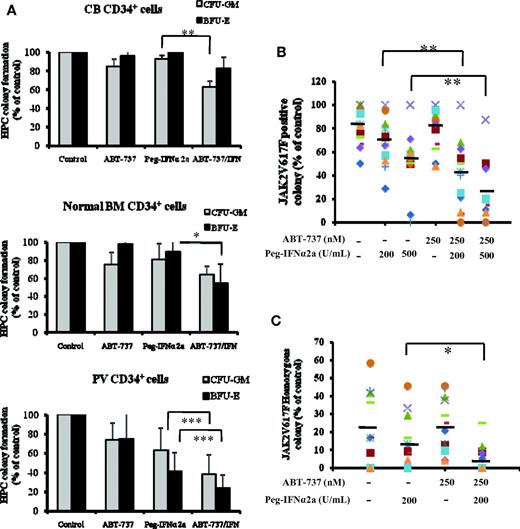
As can be seen in Figure 1A, the expression of Bcl-xL was greater in PV CD34+ cells than that observed in CB CD34+ cells. After 3 days of incubation in vitro with 500nM or greater doses of ABT-737, a significant degree of apoptosis by CB CD34+ cells was observed (P < .05), whereas 250nM or greater doses of ABT-737 induced a far more dramatic degree of apoptosis in PV CD34+ cells (P < .02), indicating that PV CD34+ cells are more sensitive to the actions of ABT-737 (Figure 1B). Several groups including our own have previously reported that IFNα is capable of eliminating PV HPC.3,7-9 We therefore compared the effects of treatment with a fixed dose ABT-737 (250nM) and Peg-IFNα 2a (200 and 500 U/mL) alone and in combination on the degree of apoptosis by CB and PV CD34+ cells. At the doses tested ABT-737 or IFNα alone or in combination did not increase the degree of CB CD34+ cell apoptosis. However, Peg-IFNα 2a at a dose of 500 U/mL and ABT-737 (250nM) were each alone able to increase the degree of PV CD34+ cell apoptosis. Furthermore, a low dose of IFNα (200 U/mL), which was inactive against PV CD34+ cells when administered in combination with ABT-737, was able to induce a similar degree of CD34+ cell apoptosis as 500 U/mL Peg-IFNα 2a administered alone (Figure 1C). A possible explanation for this synergistic effect observed with ABT-737 and IFNα on CD34+ cell apoptosis is shown in Figure 1D. At the doses tested treatment of PV CD34+ cells but not CB CD34+ cells with both drugs in combination led to the generation of greater amounts of the cleaved form of caspase 3, a mediator of apoptosis, than either agent alone. To further explore the mechanism by IFNα and ABT-737 on CD34+ cell apoptosis, the mitochondrial membrane potential (MMP) of PV and CB CD34+ cell were evaluated. As can be seen in Figure 1E, after 2 days of treatment with 200 U/mL Peg-IFNα 2a and 250nM of ABT-737 in combination, the MMP of PV CD34+ cells was decreased (green color). This reduction in MMP was not observed in PV CD34+ cells treated with each reagent alone or in CB CD34+ cells treated with 2 agents in combination (data not shown). The effects of such a combination of drugs on the ability of CB, normal marrow, or PV CD34+ cells to form hematopoietic colonies in vitro was next examined (Figure 2A). Treatment with a combination of these 2 agents was able to inhibit colony formation by both normal marrow and CB CD34+ cells. The same doses of these drugs in combinations were, however, able to inhibit hematopoietic colony formation by PV CD34+ cells to a statistically significantly greater degree (P < .001) than that observed with normal HPC.
PV CD34+ cells are more sensitive to treatment with ABT-737 than CB and normal bone marrow CD34+ cells. (A) Western blotting demonstrates greater expression of Bcl-xL in PV CD34+ cells than CB CD34+ cells (CB1, CB2, PV1, and PV2). (B) The flow-cytometric analysis results represent the percentage of apoptotic-positive cells observed after the treatment of CB and PV CD34+ cells with ABT-737 at various doses for 3 days. Cells were stained with annexin V. The data were analyzed by the Student t test (*P < .05, **P < .01; n = 6). (C) Effects of 200 and 500 U/mL Peg-IFNα 2a and 250 nM ABT-737 alone and in combination on CB and PV CD34+ cell apoptosis. The numbers of apoptotic cells were also determined flow cytometrically after 3 days of incubation. The data were analyzed by the Student t test (*P < .05, **P < .01; n = 6). (D) Western blotting showed that the cleaved forms of caspase-3 were increased in PV CD34+ cells after treatment with 500 U/mL Peg-IFNα 2a and 250 nM ABT-737 in combination compared with that observed with Peg-IFNα 2a and ABT-737 alone. The α-tubulin serves as the loading control in the Western blot analysis. (E) Effect of Peg-IFNα 2a and ABT-737 alone or in combination on mitochondrial membrane potential in progenitor cells of PV patients. Cells were stained with the JC-1 mitochondrial membrane potential dye after 2 days of treatment with 200 U/mL Peg-IFNα 2a alone, 250 nM of ABT-737 alone, or a combination of 2 drugs. The cells were observed immediately with a fluoroscope (Nikon Eclipse-E600; 10 × .45 objective lens) using a dual-bandpass filter designed to simultaneously detect fluorescein and Texas Red dyes. In live nonapoptotic cells, JC-1 accumulates as aggregates in the mitochondrial membrane which stain red. In apoptotic cells, JC-1 exists in the monomeric form because of the low mitochondrial membrane potential, staining the cytosol green.
PV CD34+ cells are more sensitive to treatment with ABT-737 than CB and normal bone marrow CD34+ cells. (A) Western blotting demonstrates greater expression of Bcl-xL in PV CD34+ cells than CB CD34+ cells (CB1, CB2, PV1, and PV2). (B) The flow-cytometric analysis results represent the percentage of apoptotic-positive cells observed after the treatment of CB and PV CD34+ cells with ABT-737 at various doses for 3 days. Cells were stained with annexin V. The data were analyzed by the Student t test (*P < .05, **P < .01; n = 6). (C) Effects of 200 and 500 U/mL Peg-IFNα 2a and 250 nM ABT-737 alone and in combination on CB and PV CD34+ cell apoptosis. The numbers of apoptotic cells were also determined flow cytometrically after 3 days of incubation. The data were analyzed by the Student t test (*P < .05, **P < .01; n = 6). (D) Western blotting showed that the cleaved forms of caspase-3 were increased in PV CD34+ cells after treatment with 500 U/mL Peg-IFNα 2a and 250 nM ABT-737 in combination compared with that observed with Peg-IFNα 2a and ABT-737 alone. The α-tubulin serves as the loading control in the Western blot analysis. (E) Effect of Peg-IFNα 2a and ABT-737 alone or in combination on mitochondrial membrane potential in progenitor cells of PV patients. Cells were stained with the JC-1 mitochondrial membrane potential dye after 2 days of treatment with 200 U/mL Peg-IFNα 2a alone, 250 nM of ABT-737 alone, or a combination of 2 drugs. The cells were observed immediately with a fluoroscope (Nikon Eclipse-E600; 10 × .45 objective lens) using a dual-bandpass filter designed to simultaneously detect fluorescein and Texas Red dyes. In live nonapoptotic cells, JC-1 accumulates as aggregates in the mitochondrial membrane which stain red. In apoptotic cells, JC-1 exists in the monomeric form because of the low mitochondrial membrane potential, staining the cytosol green.
The effects of Peg-IFNα 2a and ABT-737 alone and in combination on hematopoietic colony formation by JAK2V617F-positive PV CD34+ cells. (A) CFU-GM and BFU-E derived colony formation by CD34+ cells isolated from 6 different CB, 6 different bone marrow samples, and peripheral blood from 14 different patients with PV in the presence of 200 U/mL Peg-IFNα 2a and 250 nM of ABT-737 alone or in combination; hematopoietic colony numbers were enumerated after 14 days of incubation. Data were analyzed by the Student t test (*P < .05, **P < .01; ***P < .001; n = 14). (B) Effect of 200 and 500 U/mL Peg-IFNα 2a and 250 nM ABT-737 alone or combination treatment on the JAK2 V617F-positive colony formation by PV HPC. Individual hematopoietic colonies were randomly plucked from the cultures; JAK2V617F was detected using a nested allele-specific PCR. At least 50% of colonies were examined if the number of colonies exceeded 50 in an individual dish. If the number of colonies in an individual dish were less than 50, all colonies were plucked and examined. In these experiments, more than 78% of the total number of colonies were plucked and analyzed. Data were analyzed by the Student t test (**P < .01; n = 14). (C) Treatment with 200 U/mL Peg-IFNα 2a combined with 250 nM of ABT-737 significantly decreased the number of JAK2V617F homozygous hematopoietic colonies compared with treatment with Peg-IFNα 2a alone (*P < .05; n = 9). In panels B and C, different symbols stand for the different samples.
The effects of Peg-IFNα 2a and ABT-737 alone and in combination on hematopoietic colony formation by JAK2V617F-positive PV CD34+ cells. (A) CFU-GM and BFU-E derived colony formation by CD34+ cells isolated from 6 different CB, 6 different bone marrow samples, and peripheral blood from 14 different patients with PV in the presence of 200 U/mL Peg-IFNα 2a and 250 nM of ABT-737 alone or in combination; hematopoietic colony numbers were enumerated after 14 days of incubation. Data were analyzed by the Student t test (*P < .05, **P < .01; ***P < .001; n = 14). (B) Effect of 200 and 500 U/mL Peg-IFNα 2a and 250 nM ABT-737 alone or combination treatment on the JAK2 V617F-positive colony formation by PV HPC. Individual hematopoietic colonies were randomly plucked from the cultures; JAK2V617F was detected using a nested allele-specific PCR. At least 50% of colonies were examined if the number of colonies exceeded 50 in an individual dish. If the number of colonies in an individual dish were less than 50, all colonies were plucked and examined. In these experiments, more than 78% of the total number of colonies were plucked and analyzed. Data were analyzed by the Student t test (**P < .01; n = 14). (C) Treatment with 200 U/mL Peg-IFNα 2a combined with 250 nM of ABT-737 significantly decreased the number of JAK2V617F homozygous hematopoietic colonies compared with treatment with Peg-IFNα 2a alone (*P < .05; n = 9). In panels B and C, different symbols stand for the different samples.
To explore the effects of Peg-IFNα 2a and/or ABT-737 on malignant PV HPC, individual hematopoietic colonies were randomly plucked and genomic DNA was assayed. Exposure of PV CD34+ cells to 500 U/mL Peg-IFNα 2a but not 200 U/mL alone decreased the proportion of JAKV617F-positive HPC whereas exposure to 250nM ABT-737 did not affect the proportion of malignant HPC (Figure 1B). The effect of 250nM ABT-737 in combination with 200 U/mL Peg-IFNα 2a, however, was equivalent to that of 500 U/mL IFN alone in eliminating JAK2V617F HPC. Furthermore, the addition of ABT-737 was also capable of potentiating the ability of 500 U/mL IFN to further reduce the proportion of JAK2V617F HPC. We have previously reported that the JAK2V617F homozygous HPC are more sensitive than JAK2V617F heterozygous HPCs to the in vitro actions of IFNα.9 As can be seen in Figure 2C, ABT-737 was not effective in eliminating JAK2V617F homozygous HPC but did augment the activity of a low dose of IFNα to dramatically reducing the proportion of JAK2V617F homozygous HPC. In the 9 cases where JAK2V617F homozygous colonies were observed the addition of 200 U/mL Peg-IFNα 2a plus 250nM ABT-737 led to either the total elimination of such homozygous colonies or a marked reduction in their numbers.
IFNα therapy is interrupted frequently by dose-related toxicity.2,7,8,10 Cessation of IFNα therapy ultimately leads to relapse of the underlying disease.18 The successful treatment of most hematologic malignancies requires the use of combinations of drugs. Our data suggest that combinations of low doses of IFNα and ABT-737 might serve as an effective means of treating PV patients. IFNα treatment activates caspase through p38 MAP kinase which activates Bax.8,19 The activity of a combination of IFNα and ABT-737 observed in these studies, therefore, appears likely to be the consequence of the activation of caspase by 2 independent pathways. The results we observed with the combination of ABT-737 and Peg-IFNα 2a strongly suggest that this strategy might be an even more optimal approach for the treatment of MPN and merits further testing in the clinic.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Myeloproliferative Disorders Foundation (R.H.) and the National Cancer Institute (1P01CA108671 to R.H.).
National Institutes of Health
Authorship
Contribution: M.L. designed and performed the research, collected and analyzed data, and wrote the manuscript; J.W., Y.L., D.B., X.W., and J.M. assisted in performing the experiments; M.X. reviewed the manuscript; and R.H. designed the research, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Ronald Hoffman, Division of Hematology/Oncology, Tisch Cancer Institute, Department of Medicine, Mount Sinai School of Medicine, One Gustave L. Levy Pl, Box 1079, New York, NY 10029; e-mail: ronald.hoffman@mssm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal