Abstract
Retroviral overexpression of NF-Ya, the regulatory subunit of the transcription factor NF-Y, activates the transcription of multiple genes implicated in hematopoietic stem cell (HSC) self-renewal and differentiation and directs HSCs toward self-renewal. We asked whether TAT-NF-Ya fusion protein could be used to transduce human CD34+ cells as a safer, more regulated alternative approach to gene therapy. Here we show that externally added recombinant protein was able to enter the cell nucleus and activate HOXB4, a target gene of NF-Ya, using real-time polymerase chain reaction RNA and luciferase-based protein assays. After TAT-NF-Ya transduction, the proliferation of human CD34+ cells in the presence of myeloid cytokines was increased 4-fold. Moreover, TAT-NF-Ya-treated human primary bone marrow cells showed a 4-fold increase in the percentage of huCD45+ cells recovered from the bone marrow of sublethally irradiated, transplanted NOD-Scid IL2Rγnull mice. These data demonstrate that TAT-peptide therapies are an alternative approach to retroviral stem cell therapies and suggest that NF-Ya peptide delivery should be further evaluated as a tool for HSC/progenitors ex vivo expansion and therapy.
Introduction
The ability of hematopoietic stem cells (HSCs) to sustain hematopoiesis throughout life involves the coordinated interaction of several genes and signaling pathways, including HoxB4, Notch1, Bmi-1, and Wnts.1-11 Manipulating the levels of expression of one or more of these genes offers the potential to artificially alter the balance of stem cell proliferation versus differentiation for analytic and therapeutic applications.
We previously identified the trimeric transcription factor Nuclear Factor Y (NF-Y) as a regulated activator of HoxB4 transcription, as well as several other genes involved in HSC proliferation, including HoxC4, HoxD4, Notch1, and Lef-1, and suggested that NF-Y is a candidate key regulator of HSC self-renewal.12 NF-Y is composed of 3 subunits: NF-Ya, NF-Yb, and NF-Yc. NF-Yb and NF-Yc are constitutively expressed in most cells and interact via a histone-fold motif to form heterodimers. When NF-Ya peptide is expressed, trimers form that bind to a subset of CCAAT consensus binding site.13-17 NF-Y regulates the expression of many genes important in diverse cell types, including the cell cycle control genes cyclin A2, cyclin B1, and cyclin B2,18 and some erythroid-specific genes19,20 MDR121 and GADD45 γ.22 Cellular, molecular, and developmental specificity for NF-Y activity is established through multiple mechanisms. In addition to the regulation of transcription, direct interaction of NF-Y with other transcription factors into larger-order transcription units plays a key role. Probably because of the importance of NF-Y in diverse cellular processes, constitutive deletion of NF-Ya in embryonic stem cells results in early embryonic lethality.23 NF-Y plays an essential role in hematopoiesis through the regulated expression of NF-Ya. Postnatal deletion of NF-Ya within HSCs leads to a G2M block in HSCs and hematopoieitic progenitor cells, resulting in complete hematopoietic failure.24 NF-Ya is preferentially expressed in HSCs and declines with their differentiation. Retroviral overexpression of NF-Ya in HSCs activates the transcription of genes implicated in self-renewal and differentiation, including the Hox4 paralogs HoxB4, HoxC4, and HoxD4, as well as Hes-1, LEF1, Notch1, p27, and telomerase, and biases HSCs toward self-renewal rather then differentiation, showing a prominent increase in their in vivo repopulating ability after bone marrow transplantation.12
Although retroviral expression of NF-Ya is efficient and powerful, its application to clinical therapeutics is problematic because of both the difficulty in controlling the level and duration of expression of the transgene and the potential for insertional leukemogenesis. Dogs and macaques transplanted with primary CD34+ cells transduced by retroviral vector overexpressing HoxB4, developed myeloid leukemia 2 years after transplantation.25 These results suggest that viral delivery of genes biasing cells to undifferentiated state poses a significant threat of complications.
Nonviral methods of gene delivery, such as microinjection, electroporation, liposomes are much less effective than viral because of technical difficulties, cell damage, or toxicity. These limitations prompted us to explore protein transduction using cell-penetrating peptides (CPPs). CPPs, usually shorter than 30 amino acids, are capable of penetrating the cell and nuclear membranes and carrying associated with them cargo, which may be represented by proteins, nucleic acids, liposomes, and even nanoparticles. CPPs were first discovered by Frankel and Pabo26 and Green and Loewenstein27 who independently showed that the transactivating (TAT) protein of HIV-1 virus could enter cells. A possibility of practical use of CPP was demonstrated by Schwarze et al,28 who delivered proteins fused to the 11-amino acid TAT protein transduction domain. Since then, the family of CPPs has grown to dozens (reviewed by Hietz et al29 ), and the technique has advanced to the development of automated delivery systems tested with cell cultures and suitable for clinical applications.30
To deliver NF-Ya, we constructed a fusion protein, including human NF-Ya and 11-amino-acid TAT protein transduction domain. TAT belongs to the family of polycationic, arginine-rich CPP whose translocation abilities are based mostly on electrostatic interactions with the cell membrane. The details of the mechanism of cellular uptake for TAT-associated macromolecules remain controversial. Early reports pointed to passive transport driven by concentration gradient, and independent of endocytosis and energy supply.31 Subsequently, the mechanisms of membrane penetration of many CPPs have been reported to be mediated by endocytosis.32-35 Although the actual mechanism by which TAT and other CPPs deliver their cargo is not yet certain, it is definitely established that TAT is able to translocate proteins into the cell nucleus36 and that translocated proteins retain their functional activity as demonstrated by ability of TAT-CRE to induce DNA recombination.37,38 Given these issues and opportunities, we asked whether TAT-mediated transduction of NF-Ya peptide to human HSCs can stimulate their proliferation. We chose to use transcriptional activation of HOXB4 as one marker for biochemically active TAT-NF-Ya, and used in vitro cell growth, clonogenic assay, and in vivo transplantation to measure functional progenitor cell proliferation.
Methods
Fusion protein construction and purification
TAT-NF-Ya constructs were prepared by cloning TAT and protein purification tags along with the short-form isomer of NF-Ya that is preferentially expressed in HSCs.39 All expression cassettes were prepared by inserting a polymerase chain reaction (PCR) fragment containing in frame a TAT transduction domain sequence (YGRKKRRQRRR), a hemagglutinin (HA) tag, and a NF-Ya coding sequence flanked by engineered restriction sites into the corresponding vectors. Selected transformants were checked for the correct insert by sequencing and fusion proteins were purified under native conditions. A His-TAT-HA-NF-Ya expression cassette was generated by cloning a human NF-Ya coding sequence into XhoI and EcoRI sites within the pTAT-HA vector. TAT-HA-NF-Ya-His expression vector was produced by subcloning the TAT-HA-NF-Ya sequence flanked by NheI and EcoRI sites in pET-21b(+). A GST-TAT-HA-NF-Ya construct was designed by introducing a SalI-TAT-HA-NF-Ya-NotI PCR fragment into pGEX-6p1 plasmid. PCR products were verified by sequencing. All fusion proteins were expressed using Escherichia coli strain Rosetta(DE3)pLysS (Novagen) and induced for 3 hours with 0.1mM isopropyl-β-D-thiogalactoside at 37°C. His-TAT-β-Gal protein was also cloned and purified for use in cell transduction visualization assays.
For purification of His-tagged proteins, the cell pellet was lysed in 50mM Tris-HCl, pH 7.5, 300mM NaCl, 10% glycerol, 0.2% sarcosyl, 10 mM imidazole buffer and sonicated. Fusion proteins were purified from clear lysates using batch/gravity-flow procedure with Talon Metal Affinity Resin (Clontech) according to the manufacturer's protocol, eluted from resin with lysis buffer containing 150mM imidazole, dialyzed against 1 times phosphate-buffered saline (PBS)-20% glycerol and stored at −80°C. GST-tagged fusion proteins were purified based on modification of a published protocol.40 Bacterial cells were lysed in STE (10mM Tris, pH 8.0, 150mM NaCl, 1mM ethylenediaminetetraacetic acid), and 1.4% sarcosyl. After sonication, Triton X-100 was added to 2% and lysate was incubated for 30 minutes at room temperature. Then batch/gravity-flow protein purification was performed with glutathione Sepharose 4B (Pharmacia Biotech) according to instructions. Pure protein was dialysed against 1 times PBS-20% glycerol and stored at −80°C. Protein concentration was determined by the Bradford method (Bio-Rad Protein Assay).
Experiments with recombinant DNA were performed according to the National Institutes of Health guidelines.
Western blotting
Cell lysates or 5 to 10 μg of pure proteins were boiled in Laemmli buffer, separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked with 5% nonfat dried milk, 0.1% Tween 20 in PBS (PBS-T). Primary goat anti–hemagglutinin-horseradish peroxidase (HRP) polyclonal antibody or mouse anti–GST-HRP monoclonal antibody (both from Santa Cruz Biotechnology) were used for 1 hour. After washing membranes in PBS-T, proteins were detected with ECL Plus reagents (GE Healthcare).
EMSA
Mixtures of proteins containing 50 ng of each were incubated for 30 minutes at room temperature with or without 2.5 μg of rabbit polyclonal anti–NF-Ya antibody (Santa Cruz Biotechnology). To identify specific DNA-protein interactions, the 99-bp HOXB4 core promoter probe,41 radiolabeled with [α-32P] deoxycytidine triphosphate and Klenow fragment, was incubated with the protein mixture for 30 minutes at 30°C. The reaction mixture then was subjected to electrophoresis under nondenaturing conditions on prerun 5% agarose gels in 0.5 times Tris borate–ethylenediaminetetraacetic acid at 200 V in cold room. Gels were dried, and specific bands were detected by autoradiography.
TAT-NF-Ya protein stability assay
A total of 3 × 104 cells/well in 96-well plates were incubated with 20nM TAT-NF-Ya peptide in RPMI 1640 with or without fetal bovine serum (FBS) at 37°C. After incubation, cells were centrifuged at 800g, supernatant was mixed with 4 times SDS sample buffer (Novagen) and loaded 5.2 on to 10% PAGE. After transfer on nitrocellulose membrane, blot was probed with mouse anti–GST-HRP monoclonal antibody (Santa Cruz Biotechnology) and detected with ECL plus reagents (GE Healthcare). Band density was measured by scanning densitometry (SI Molecular Dynamics) and quantified by ImageQuant Version 5.2 Software.
TAT-NF-Ya protein delivery to the cells
NIH/3T3 fibroblasts and human K562 cells, cultured routinely in RPMI 1640 with 10% FBS, were washed with RPMI without serum before protein delivery. Human CD34+ cells from peripheral blood or bone marrow were cultured in StemSpan H3000 (StemCell Technologies) supplemented with a cytokine cocktail of Fms-like tyrosine kinase 3 (Flt-3)/Flk-2L, SCF, and thrombopoietin 100 ng/mL each and IL-3, IL-6 20 ng/mL each (BD Biosciences PharmMingen). Cells were treated with aliquots of the fusion protein (60-70 nM with 250mM sucrose) added 6 times (every 15 minutes for 90 minutes) at 37°C in 5% CO2, then washed with PBS, and either assayed directly or continued in liquid culture. To verify intracellular protein uptake, cells were fixed for 10 minutes on ice in PBS–4% paraformaldehyde and treated with 100% EtOH for 10 minutes (K562 and CD34+ cells were cytospun on glass slides before ethanol treatment). The fixed cells were washed with cold PBS, blocked with PBS, 0.1% Triton X-100, 1% bovine serum albumin for 30 minutes, washed with PBS, 0.1% Triton X-100, and incubated with an anti-GST AlexaFluor 488 conjugate (Invitrogen) for 1 hour. After extensive washing with PBS, cells were covered with Vectashield mounting media (Vector Laboratories) and cell fluorescence was detected with an Olympus BX60 microscope using 40 × 0.60 Ph2 lense, Photometrics cool snap HQ camera and Perkin Elmer Openlab 5.5 software.
Luciferase assay
For transient transfection, 5 × 106/sample K562 cells were washed with RPMI 1640 to remove serum. The cell pellet was resuspended in 400 μL of RPMI containing 2 μg of each plasmid expressing HOXB4-luciferase, NF-Yb, NF-Yc, USF1, USF2, and Renilla luciferase and then electroporated at 240 V, 950 μFD (Gene Pulser, Bio-Rad) in a 0.4-cm electroporation cuvette (Bio-Rad). The electroporated cells were cultured in complete RPMI media for 48 hours at 37°C, then washed with RPMI to remove serum, and incubated with TAT-NF-Ya fusion peptide. After culture with TAT-NF-Ya, cells were washed twice with PBS, resuspended in passive lysis buffer of the Dual-Luciferase Reporter Assay System (Promega), and luciferase activity was measured using the Turner Designs Model TD-20/20 Luminometer. For stable HOXB4-luciferase K562 transformants, 2 × 106 K562 cells were washed with RPMI, seeded on 24-well plates, and incubated with fusion protein at 37°C under 5% CO2. After incubation, cells harvested and washed with PBS were counted and then lysed for luciferase assay.
Colony-forming cells assay
Progenitor (colony-forming cell) assays were performed using semisolid methylcellulose media MethoCult SF H4436 (StemCell Technologies) supplemented with human transferrin, recombinant human insulin, stem cell factor (SCF; 100 ng/mL), granulocyte-macrophage colony-stimulating factor (100 ng/mL), interleukin-3 (IL-3; 20 ng/mL), IL-6 (20 ng/mL), granulocyte colony-stimulating factor (100 ng/mL), and erythropoietin (2 U/mL). Cultured human peripheral blood CD34+ cells were seeded in triplicate at 250 and 500 cells/35-mm dish with 1 mL of methylcellulose at day 4 or 9 after TAT-NF-Ya protein delivery to the cells and incubated at 37°C in a humidified atmosphere of 5% CO2. Individual colonies were scored after 14 days for colony-forming unit granulocyte macrophage (CFU-GM), colony-forming unit-erythroid, and after 21 days, for colony-forming unit granulocyte, erythrocyte, macrophage, megakaryocyte (CFU-GEMM) progenitors. For liquid cultures, CD34+ cells were cultured in StemSpan H3000 (StemCell Technologies).
Real-time PCR
Total RNA was extracted from cells with RNeasy mini kit (QIAGEN) and reverse transcribed using TaqMan Reverse Transcription Reagents kit (Applied Biosystems) according to the manufacturer's protocols. PCR was performed on 7500 Real-Time PCR System (Applied Biosystems) in a 20-μL reaction volume containing cDNA, TaqMan Universal PCR master mix with TaqMan primers and probes for human HOXB4 and glyceraldehyde-3-phosphate dehydrogenase from Applied Biosystems. All samples were run in triplicate, and the data were normalized to glyceraldehyde-3-phosphate dehydrogenase expression. A comparative 2−ΔΔCt method was used to detect relative gene expression.
Mice
All animal experiments were approved by both the University of Pennsylvania and Haverford College Institutional Animal Care and Use Committees. NOD-Scid IL2Rγnull mice (stock no. 005557; The Jackson Laboratory) were bred and housed in the Animal Services Unit of the University of Pennsylvania under specific pathogen–free conditions. Mice of both sexes at 7 to 8 weeks of age were used for experiments. They were irradiated at 275 cGy (137Cs source) up to 24 hours before intravenous injection of cells.
Transplantation experiments
Bone marrow or peripheral blood CD34+ cells (received from Fred Hutchinson Cancer Center) were treated with 70 nM TAT-NF-Ya protein for 1.5 hours, then cultured for 3, 6, or 9 days in serum-free media StemSpan H3000 (StemCell Technologies) supplemented with a cytokine cocktail of Fms-like tyrosine kinase 3 (Flt-3)/Flk-2L, SCF, and thrombopoietin 100 ng/mL each and IL-3, IL-6 20 ng/mL each (BD Biosciences PharMingen), and then 3 × 105 CD34+ cells were transplanted by tail vein injection into sublethally irradiated immunodeficient NOD-Scid IL2Rγnull mice. For the secondary transplantation, immunodeficient mice were engrafted using bone marrow from mice after primary transplantation. Each inoculum contained 1.5 × 106 of huCD45+ cells. Nine weeks later, bone marrow from tibias, femurs, and hips of killed mice was flushed and treated with 6% ammonium chloride to remove erythrocytes. Aliquots of freshly isolated murine BM cells were analyzed for the presence of human common leukocyte antigen CD45 and multilineage engraftment by staining with conjugated anti–human monoclonal CD45-allophycocyanin, CD33-peridinin chlorophyll protein, CD19-phycoerythrin antibodies and with matching isotype controls (BD Biosciences). After staining, cells were washed with fluorescence-activated cell sorter solution (PBS/0.1% sodium azide/2% FBS) and analyzed using FACSCalibur and FlowJo Version 6.3.3 software. A total of 0.5 × 106 to 1 × 106 events per sample were collected.
Statistical analysis
Unless otherwise indicated, the results are expressed as mean plus or minus SD. The statistical significance was determined using Student t test to evaluate the difference between control and experimental groups. P values less than .05 were considered significant.
Results
Expression and purification of recombinant fusion proteins
Three fusion constructs encoding human NF-Ya and affinity tags for protein purification were cloned into a TAT protein expression vector42 : with an N-terminal 6-histidine leader, a C-terminal 6xHis tag, and a GST tag at the N-terminus of NF-Ya (Figure 1A). Recombinant protein bearing the N-terminal His-tag displayed a low level of expression and contamination with proteins from bacterial host under different purification conditions (Figure 1B-C). The problem of copurification of recombinant 6xHis-tagged proteins and histidine-rich proteins from E coli host using metal affinity chromatography was also reported by the other researchers.43,44 Moving the His-tag to the C-terminus of the fusion protein improved its purity but did not increase protein yield significantly (Figure 1D). On the other hand, substantially higher levels of protein yield and purity were obtained with the GST-tagged construct (Figure 1E-F), confirming observations by Hammarström et al.45 All further experiments were performed using the GST-construct, which we refer to as TAT-NF-Ya.
Cloning and purification of TAT-NF-Ya fusion proteins. (A) Design of human TAT-NF-Ya expression constructs. An N-terminal His-TAT-HA-NF-Ya expression construct was generated by cloning a human NF-Ya coding sequence into XhoI and EcoRI sites of the pTAT-HA vector. The C-terminally tagged TAT-HA-NF-Ya-His expression vector was produced by subcloning the TAT-HA-NF-Ya sequence into the pET-21b(+) vector. A GST-TAT-HA-NF-Ya was designed by introducing SalI-TAT-HA-NF-Ya-NotI PCR fragment into pGEX-6p1 plasmid. All PCR products were verified by sequencing. Fusion proteins were expressed using E coli strain Rosetta(DE3)pLysS (Novagen) and induced for 3 hours with 0.1mM isopropyl-β-D-thiogalactoside at 37°C. (B) Coomassie blue-stained SDS-PAGE showing affinity purification of His-TAT-NF-Ya. Western blots of His-TAT-HA-NF-Ya (C) and TAT-HA-NF-Ya-His (D) proteins probed with anti-HA antibody. (E-F) Purification of GST-TAT-HA-NF-Ya: Coomassie-stained gel (E) and immunoblot (F) with anti-GST antibody. Arrows indicate fusion proteins. NI indicates noninduced culture; CL, clear lysate; FT, flow through; W, wash; and E, eluate.
Cloning and purification of TAT-NF-Ya fusion proteins. (A) Design of human TAT-NF-Ya expression constructs. An N-terminal His-TAT-HA-NF-Ya expression construct was generated by cloning a human NF-Ya coding sequence into XhoI and EcoRI sites of the pTAT-HA vector. The C-terminally tagged TAT-HA-NF-Ya-His expression vector was produced by subcloning the TAT-HA-NF-Ya sequence into the pET-21b(+) vector. A GST-TAT-HA-NF-Ya was designed by introducing SalI-TAT-HA-NF-Ya-NotI PCR fragment into pGEX-6p1 plasmid. All PCR products were verified by sequencing. Fusion proteins were expressed using E coli strain Rosetta(DE3)pLysS (Novagen) and induced for 3 hours with 0.1mM isopropyl-β-D-thiogalactoside at 37°C. (B) Coomassie blue-stained SDS-PAGE showing affinity purification of His-TAT-NF-Ya. Western blots of His-TAT-HA-NF-Ya (C) and TAT-HA-NF-Ya-His (D) proteins probed with anti-HA antibody. (E-F) Purification of GST-TAT-HA-NF-Ya: Coomassie-stained gel (E) and immunoblot (F) with anti-GST antibody. Arrows indicate fusion proteins. NI indicates noninduced culture; CL, clear lysate; FT, flow through; W, wash; and E, eluate.
TAT-NF-Ya fusion protein binds the HOXB4 promoter in vitro
The role of NF-Y in activating the HOXB4 promoter was originally identified via mutational analysis of promoter expression constructs mapping in hematopoietic cells that identified 2 critical sites: HOX response element 1 (HxRE-1) and HxRE-2. NF-Y was found to bind to HxRE-1, forming a multimeric complex with USF-1/2 dimers that bind cooperatively to HxRE-2.39 To determine whether purified TAT-NF-Ya was able to bind the HOXB4 promoter, electrophoretic mobility shift assay (EMSA) was performed with equimolar concentrations of TAT-NF-Ya and purified NF-Yb, NF-Yc, USF 1, and USF 2 mixed with a 99-nucleotide HOXB4 promoter probe (Figure 2A), as well as anti–NF-Ya antibody (Figure 2B). NF-Ya antibody generated a supershifted band with lower mobility when the TAT-NF-Ya is in the complex. This confirmed that anti–NF-Ya antibody identified the specific protein in the DNA-protein complex (ie, the sequence-specific binding of TAT-NF-Ya to HOXB4 promoter sequences).
TAT-NF-Ya, NF-Yb, NF-Yc, USF1 complex binds to a HOXB4 promoter. (A) Coomassie-stained purified GST-tagged NF-Y subunits and USF-1, after SDS-PAGE. (B) EMSA detection of specific binding of TAT-NF-Ya protein to the HOXB4 promoter. A total of 50 ng each of NF-Ya, NF-Yb, NF-Yc, and USF1 was incubated for 30 minutes at room temperature with or without 2.5 μg of rabbit polyclonal anti–NF-Ya antibody, incubated with a radiolabeled 99-bp HOXB4 core promoter probe for 30 minutes at 30°C, and then subjected to electrophoresis under nondenaturing conditions on 5% agarose gels. Bands representing protein complexes retarded by complexing with transcription factors and/or antibody were detected by autoradiography. NF-Ya antibody retards a specific band only in the presence of TAT-NF-Ya protein within the reaction mixture.
TAT-NF-Ya, NF-Yb, NF-Yc, USF1 complex binds to a HOXB4 promoter. (A) Coomassie-stained purified GST-tagged NF-Y subunits and USF-1, after SDS-PAGE. (B) EMSA detection of specific binding of TAT-NF-Ya protein to the HOXB4 promoter. A total of 50 ng each of NF-Ya, NF-Yb, NF-Yc, and USF1 was incubated for 30 minutes at room temperature with or without 2.5 μg of rabbit polyclonal anti–NF-Ya antibody, incubated with a radiolabeled 99-bp HOXB4 core promoter probe for 30 minutes at 30°C, and then subjected to electrophoresis under nondenaturing conditions on 5% agarose gels. Bands representing protein complexes retarded by complexing with transcription factors and/or antibody were detected by autoradiography. NF-Ya antibody retards a specific band only in the presence of TAT-NF-Ya protein within the reaction mixture.
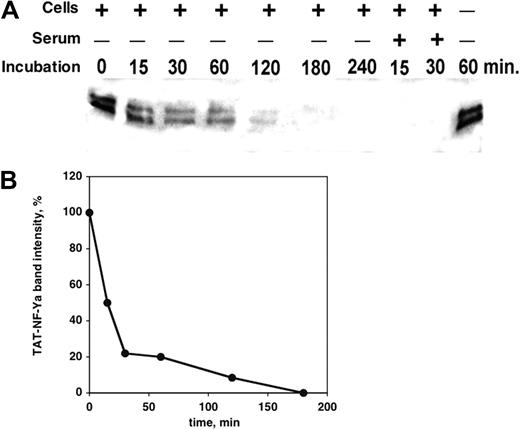
TAT-NF-Ya protein stability in cell culture
To determine its suitability for in vitro treatment of cultured hematopoietic cells, the stability of TAT-NF-Ya protein under tissue culture conditions was examined. K562 cells or normal human CD34+ peripheral blood cells were cultured with the fusion protein in Iscove modified Dulbecco medium, with or without 10% FBS for 15 minutes to 4 hours. Culture supernatants were harvested at different time points and analyzed by Western blot with anti-GST antibody to detect intact fusion protein. As little as 15 minutes of incubation in the presence of cells plus serum resulted in loss of detectable fusion protein, suggesting that FBS contains fusion protein-degrading enzymes (Figure 3A). The fusion protein was somewhat more stable in culture, including hematopoietic cells without serum, with approximately 50% of protein lost within 15 minutes and 75% lost within 30 minutes (Figure 3B). Protein incubated without cells at 37°C for at least 1 hour did not show any sign of degradation (Figure 3A, last right band), indicating that cell proteases also rapidly degrade the fusion protein.
Stability of TAT-NF-Ya in cell culture. (A) Western blot analysis with anti-GST antibody and (B) densitometric quantitation of immunoreactive NF-Ya after exposure to K562 or CD34+ peripheral blood cells, in the presence or absence of FBS. TAT-NF-Ya was stable in cell-free, serum-free medium, but the presence of serum in the culture led to complete disappearance of detectable NF-Ya within 15 minutes. In the culture medium without serum, inclusion of hematopoietic cells also led to TAT-NF-Ya degradation, but at a slower rate.
Stability of TAT-NF-Ya in cell culture. (A) Western blot analysis with anti-GST antibody and (B) densitometric quantitation of immunoreactive NF-Ya after exposure to K562 or CD34+ peripheral blood cells, in the presence or absence of FBS. TAT-NF-Ya was stable in cell-free, serum-free medium, but the presence of serum in the culture led to complete disappearance of detectable NF-Ya within 15 minutes. In the culture medium without serum, inclusion of hematopoietic cells also led to TAT-NF-Ya degradation, but at a slower rate.
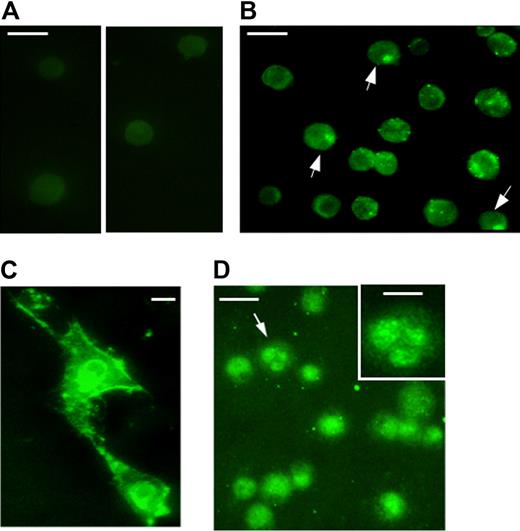
Cellular uptake of recombinant TAT-NF-Ya
We next asked whether the TAT-NF-Ya could be taken up by cells under conditions defined by this degradation analysis. As was found in the experiment with fusion protein stability, the optimal timing for TAT-NF-Ya delivery to the cytoplasm and nucleus in all tested types of cells (NIH/3T3, K562, and human CD34+) was every 15 minutes for a total 1.5 to 2 hours. Incubation of cells at the optimal concentration of fusion protein for 3 hours, or for 1.5 hours but at higher than optimal concentrations, showed significant cell death probably resulting from the protein toxicity. Transduction of cells with TAT-β-galactosidase (β-Gal) fusion protein at high concentration (2 μM for K562 cells or 120 nM for CD34+) for 4 hours did not affect cell viability, suggesting that NF-Ya, not TAT peptide, is toxic to the cells beyond optimal concentrations (not shown). NIH/3T3, K562, and human CD34+ cells were cultured in serum-free medium with TAT-NF-Ya, then washed, fixed, and stained with anti-GST antibody conjugated with AlexaFluor 488 for fluorescent microscopy as described in “TAT-NF-Ya protein delivery to the cells.” TAT-NF-Ya protein was visually detected throughout the cytoplasm and nucleus in each cell type. Control cells cultured without the fusion protein did not show fluorescent staining (Figure 4A).
Subcellular localization of transduced TAT-NF-Ya in different types of (A-B) Human peripheral blood cells, (C) NIH 3T3 cells, (D) K562 cells. Cells treated with TAT-NF-Ya were incubated with anti-GST AlexaFluor 488 conjugate. (A) Antibody-stained control cells that were not transduced; 2 areas of a single slide are shown. (B,D) Arrows indicate examples of nuclear staining. (D) Inset: Magnified image of the cell denoted by the arrow. (A-B,D) Bar represents 20 μm. (C,D inset) Bar represents 10 μm. Procedures for protein delivery and visualization are described in “TAT-NF-Ya protein delivery to the cells.”
Subcellular localization of transduced TAT-NF-Ya in different types of (A-B) Human peripheral blood cells, (C) NIH 3T3 cells, (D) K562 cells. Cells treated with TAT-NF-Ya were incubated with anti-GST AlexaFluor 488 conjugate. (A) Antibody-stained control cells that were not transduced; 2 areas of a single slide are shown. (B,D) Arrows indicate examples of nuclear staining. (D) Inset: Magnified image of the cell denoted by the arrow. (A-B,D) Bar represents 20 μm. (C,D inset) Bar represents 10 μm. Procedures for protein delivery and visualization are described in “TAT-NF-Ya protein delivery to the cells.”
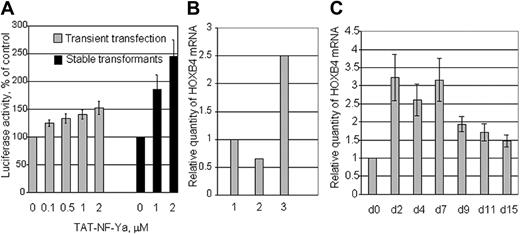
Transduced TAT-NF-Ya activates the HOXB4 promoter
To test whether GST-TAT-NF-Ya could activate the HOXB4 promoter in vivo, K562 cells were electroporated with a HOXB4 promoter-luciferase construct together with the plasmids that encode NF-Ya partners: NF-Yb, NF-Yc, USF1, USF2, and Renilla luciferase (control for transfection efficiency). After electroporation, the transfected K562 cells were cultured for 48 hours and then incubated with TAT-NF-Ya protein at different concentrations for 1.5 hours, and then analyzed for Firefly luciferase. Activity was normalized to Renilla luciferase and was also compared with luciferase activity of K562-HOXB4-luciferase stabletransformants.41 The results showed TAT-NF-Ya concentration-dependent stimulation of HOXB4-luciferase activity (Figure 5A). TAT-NF-Ya delivery to these cells enhanced HOXB4-luciferase activity 2 to 3 times more efficiently than in transiently transfected K562 cells at the same protein concentrations.
Activation of the HOXB4 promoter by TAT-NF-Ya. (A) K562 cells were electroporated with a HOXB4 promoter-luciferase construct together with the plasmids that encode NF-Ya partners: NF-Yb, NF-Yc, and USF1, USF2 (gray bars). K562 subline stably transformed with HOXB4-luciferase was also used (black bars). Transiently and stably transfected K562 cells were incubated with TAT-NF-Ya for 90 minutes and then analyzed for firefly luciferase activity. Activity was normalized to Renilla luciferase and then compared with the luciferase activity of control (transfected K562 cells without TAT-NF-Ya treatment). (B) Endogenous HOXB4 mRNA expression measured by real-time PCR in K562 unmanipulated cells (1), treated with TAT-β-Gal (2), or treated with TAT-NF-Ya (3). (C) Time course of HOXB4 mRNA induction after treatment with TAT-NF-Ya. Human PB CD34+ cells were treated with 60nM TAT-NF-Ya plus 250mM sucrose, then cultured with myeloid cytokines (“TAT-NF-Ya protein delivery to the cells”) from 2 to 15 days, and endogenous HOXB4 mRNA was measured by quantitative PCR. Compared with CD34+ cells cultured in cytokines without TAT-NF-Ya, HOXB4 mRNA increased 2.5- to 3-fold from day 2 to day 7 and remained elevated throughout the culture.
Activation of the HOXB4 promoter by TAT-NF-Ya. (A) K562 cells were electroporated with a HOXB4 promoter-luciferase construct together with the plasmids that encode NF-Ya partners: NF-Yb, NF-Yc, and USF1, USF2 (gray bars). K562 subline stably transformed with HOXB4-luciferase was also used (black bars). Transiently and stably transfected K562 cells were incubated with TAT-NF-Ya for 90 minutes and then analyzed for firefly luciferase activity. Activity was normalized to Renilla luciferase and then compared with the luciferase activity of control (transfected K562 cells without TAT-NF-Ya treatment). (B) Endogenous HOXB4 mRNA expression measured by real-time PCR in K562 unmanipulated cells (1), treated with TAT-β-Gal (2), or treated with TAT-NF-Ya (3). (C) Time course of HOXB4 mRNA induction after treatment with TAT-NF-Ya. Human PB CD34+ cells were treated with 60nM TAT-NF-Ya plus 250mM sucrose, then cultured with myeloid cytokines (“TAT-NF-Ya protein delivery to the cells”) from 2 to 15 days, and endogenous HOXB4 mRNA was measured by quantitative PCR. Compared with CD34+ cells cultured in cytokines without TAT-NF-Ya, HOXB4 mRNA increased 2.5- to 3-fold from day 2 to day 7 and remained elevated throughout the culture.
To confirm further that the internalized recombinant protein is functionally active, endogenous HOXB4 mRNA levels in K562 cells treated with 2 μM TAT-NF-Ya or with TAT-β-Gal were assayed by real-time PCR. Results showed a significant increase of HOXB4 mRNA level compare with unmanipulated cells (control) or cells treated with reporter protein (Figure 5B). Taken together, these data demonstrate that NF-Ya can be efficiently transduced into hematopoietic cells as a functionally active TAT-fusion protein.
Kinetics of HOXB4 up-regulation in CD34+ cells in response to TAT-NF-Ya
In preliminary experiments, we found that CD34+ cells from different sources (peripheral blood, cord blood, or bone marrow) are more sensitive to toxicity from TAT-NF-Ya than K562 cells: CD34+ cell viability was approximately 20% compared with approximately 80% cell viability for K562 cells at the same fusion protein concentration. To minimize cell toxicity, we took advantage of previously published studies46 showing that added lysosomotropic agents (eg, sucrose) facilitate nuclear uptake of added TAT-peptides at lower concentrations. Addition of 250 mM sucrose allowed greater uptake and activity of 60 to 80 nM TAT-NF-Ya, resulting in a 3-fold increase of HOXB4 mRNA at this lower dose while sustaining the viability of CD34+ cells. Under these conditions, HOXB4 expression in CD34+ cells was 3-fold higher over baseline for 7 days and remained increased for 14 days (Figure 5C).
TAT-NF-Ya stimulates expansion of hematopoietic progenitors in vitro
Human PB CD34+ cells were treated with TAT-NF-Ya (or not treated) for 1.5 hours and then cultured for 28 days in serum-free media supplemented with Flt-3/Flk-2L, SCF, thrombopoietin (100 ng/mL each), and IL-3 and IL-6 (20 ng/mL each). Cell growth was slow in the first week in both control and protein-treated cultures, then accelerated during the second week of culture with 4-fold higher cell numbers seen in TAT-NF-Ya-treated cells by day 15, and throughout the remainder of the cultures (Figure 6A). No decrease in cell death was observed in the GST-TAT-NF-Ya-treated cell cultures. Progenitor cell numbers were measured by methylcellulose assays performed at day 4, when there was yet no difference in cell growth between control and protein treatment, and at day 9, when the difference in overall cell growth was detected (Figure 6B). Cells plated on methylcellulose 4 days after protein delivery already showed an increase in total colony-forming cell number, primarily because of an increase in burst-forming unit-erythroid (BFU-E) and CFU-GEMM populations. By 9 days after protein delivery, the absolute number of total colony-forming cells was also higher for protein-treated cultures. The greatest effect on progenitor numbers was seen in the primitive CFU-GEMM compartment, with smaller increases seen in BFU-E and little change in CFU-GM. These results suggest that TAT-NF-Ya increases progenitors by stimulating the production and/or proliferation of the most primitive cells in the culture, and perhaps supports or stimulates HSC self-renewal. Because of the increase in erythroid and multilineage progenitor cells, the percentage of progenitor cells committed to granulocytes and/or macrophages declined in these cultures (Figure 6C).
Ex vivo expansion and colony-forming potential of peripheral blood CD34+ cells after treatment with TAT-NF-Ya. Human peripheral blood CD34+ cells were treated with TAT-NF-Ya (or not treated) for 1.5 hours and then cultured for 30 days in serum-free media supplemented with Flt-3/Flk-2L, SCF, thrombopoietin (100 ng/mL each), and IL-3 and IL-6 (20 ng/mL each). Total mononuclear cells (A) were counted; the number of colony-forming erythroid (ER indicates BFU-E), CFU-GM, and CFU-GEMM were measured by methylcellulose assays (B), and the relative contribution to the progenitor cell pool of each class of progenitors calculated (C).
Ex vivo expansion and colony-forming potential of peripheral blood CD34+ cells after treatment with TAT-NF-Ya. Human peripheral blood CD34+ cells were treated with TAT-NF-Ya (or not treated) for 1.5 hours and then cultured for 30 days in serum-free media supplemented with Flt-3/Flk-2L, SCF, thrombopoietin (100 ng/mL each), and IL-3 and IL-6 (20 ng/mL each). Total mononuclear cells (A) were counted; the number of colony-forming erythroid (ER indicates BFU-E), CFU-GM, and CFU-GEMM were measured by methylcellulose assays (B), and the relative contribution to the progenitor cell pool of each class of progenitors calculated (C).
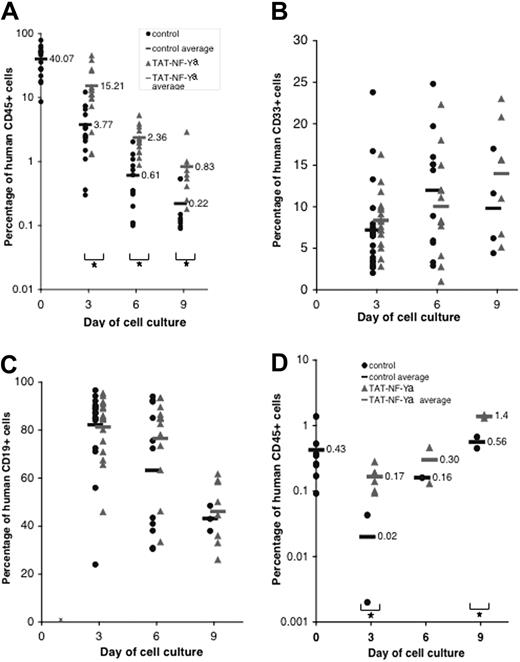
Effect of NF-Ya on repopulating ability of human hematopoietic progenitor cells
The ability of TAT-NF-Ya to stimulate the engraftment potential of ex vivo cultured CD34+ cells was evaluated by competitive repopulating activity in immunodeficient mice (Figure 7). Human bone marrow CD34+ cells were cultured in the presence of myeloid cytokines with or without TAT-NF-Ya peptide for 3, 6, or 9 days, and then 3 × 105 CD34+ cells from each culture were transplanted into sublethally irradiated NOD-Scid IL2Rγnull mice. Nine weeks later, the mice were killed, and bone marrow cells were analyzed for the presence of human (huCD45+), myeloid (huCD33+), and lymphoid (huCD19+) cells by flow cytometry. Cells treated with TAT-NF-Ya and cytokines provided significantly higher levels of human chimerism compared with the levels obtained with cells cultured with cytokines only. We observed a 4-fold increase in the percentage of huCD45+ cells recovered from the bone marrow of primary recipients (Figure 7A). TAT-NF-Ya had no effect on the differentiation pattern of Scid repopulating cells as the proportion of human lymphoid and myeloid cells was not statistically different between control and protein-treated culture (Figure 7B-C). Interestingly, retroviral expression of HOXB4 in mouse ES resulted in their differentiation into primitive hematopoietic cells capable of engrafting, but the recipients had hematopoiesis with enhanced myeloid and almost fully inhibited lymphoid development.47 This discrepancy may be explained by a very high level of expression of HOXB4 by retroviral vectors, and it may highlight the benefits of the protein transduction approach, which allows for better control over the protein of interest delivery. Alternatively, increasing NF-Y expression, by virtue of activating multiple normal regulatory pathways, may provide a more balanced, physiologic approach to hematopoietic cell activation than using a single HOX gene.
Engraftment of human bone marrow cells in NOD-Scid IL2Rγnull mice. (A-C) Primary transplantation. Human bone marrow CD34+ cells were cultured in the presence of myeloid cytokines with or without TAT-NF-Ya for 3, 6, or 9 days and then transplanted into sublethally irradiated NOD-Scid IL2Rγnull mice. (D) Secondary transplantation. Bone marrow recovered from primary recipient mice was transplanted in secondary recipients to evaluate the capacity of Scid repopulating cells. Nine weeks after primary or secondary transplantation, mice were killed and bone marrow cells were analyzed for the presence of human CD45+ (A,D), myeloid CD33+ (B), and lymphoid CD19+ (C) cells by flow cytometry. Results are pooled from 3 transplantation experiments. Increased recovery of huCD45+ cells was seen in the TAT-NF-Ya–treated groups in each experiment. The proportion of lymphoid and myeloid engrafted populations was not significantly different in control and protein-treated cells. *P < .05.
Engraftment of human bone marrow cells in NOD-Scid IL2Rγnull mice. (A-C) Primary transplantation. Human bone marrow CD34+ cells were cultured in the presence of myeloid cytokines with or without TAT-NF-Ya for 3, 6, or 9 days and then transplanted into sublethally irradiated NOD-Scid IL2Rγnull mice. (D) Secondary transplantation. Bone marrow recovered from primary recipient mice was transplanted in secondary recipients to evaluate the capacity of Scid repopulating cells. Nine weeks after primary or secondary transplantation, mice were killed and bone marrow cells were analyzed for the presence of human CD45+ (A,D), myeloid CD33+ (B), and lymphoid CD19+ (C) cells by flow cytometry. Results are pooled from 3 transplantation experiments. Increased recovery of huCD45+ cells was seen in the TAT-NF-Ya–treated groups in each experiment. The proportion of lymphoid and myeloid engrafted populations was not significantly different in control and protein-treated cells. *P < .05.
TAT-NF-Ya-induced increase in huCD45+ cells was observed after 3, 6, or 9 days in culture, suggesting that TAT-NF-Ya was able to maintain activity of Scid repopulating cells over time better than cytokines alone. Although the percentage of huCD45+ cells recovered from the mice fell over the time of culture, the inoculum for each mouse represented a progressively smaller fraction of the total number of cells harvested at each time point because of the expansion in cell numbers during culture. Indeed, quantitative analysis of huCD45+ cell engraftment, using 0.1% as the limit of reliable detection, suggests an increase in Scid repopulating cells of 5- to 10-fold, after incubation with TAT-NF-Ya. In the secondary transplantation experiment (Figure 7D), immunodeficient mice were injected with bone marrow cells isolated from mice after primary transplantation and after 9 weeks analyzed for the amount of engrafted huCD45+ cells. The results also showed higher levels of huCD45+ cells in secondary recipients of TAT-NF-Ya-treated cells compared with control untreated cells. These results suggest that TAT-NF-Ya better maintained the repopulating capacity of human progenitors compared with cytokines alone.
Discussion
The clinical potential for HSCs has led to many attempts to find techniques that will encourage stem and progenitor cell proliferation in vitro. Although some success with murine stem and progenitor cells has been achieved, success with human stem cells has proven more elusive. In this study, we sought to take advantage of 3 previous lines of research: (1) several studies have demonstrated that overexpression of HOX4 transcription factors, and especially HOXB4, in mouse HSCs can trigger real HSC expansion, as demonstrated by competitive repopulating assays in congenic mice1,48,49 ; (2) each of the HOX4 genes, as well as several additional genes important to HSC proliferation, are downstream transcriptional targets of NF-Ya; and (3) TAT-peptide technology offers the potential to directly deliver functional transcription factors to the nucleus of live cells. TAT-peptide technology, if it can be effectively developed, would have the dual advantages of providing a temporary effect without permanent alteration of the stem cell genome. Of course, nonintegrating vectors exploiting episomal gene expression could provide an alternative approach to avoiding insertional mutagenesis, but regulated gene expression would be more problematic than protein administration.
In this study, we provide data that TAT-NF-Ya delivered in combination with standard concentrations of myeloid cytokines in serum-free culture condition is able to increase the proliferation of human hematopoietic cells in vitro. These conditions support increased production of hematopoietic precursors and progenitors, and also increase the potency of the cells in human-to-immunodeficient mouse transplantation assays. We propose that TAT-peptide and perhaps other CPP technology hold significant potential for human HSC application. Of note, not only does the method avoid concerns about retrovirally mediated insertional leukemogenesis, but also the short half-life of the TAT-peptide in the cell culture allows for careful cell dosing.
Several features of this TAT-peptide approach are of interest. First, we found that GST-fusion protein can be isolated at significantly higher yield than His-tagged proteins, and purified proteins were stable when stored at −80°C for several months. GST-TAT-NF-Ya bound appropriately to HOXB4 promoter constructs in vitro in collaboration with NF-Yb, NF-Yc, and USF1/2. Once added to cultured CD34+ cells for 90 minutes, purified TAT-NF-Ya up-regulated endogenous HOXB4 expression for more than 7 days. This allowed for minimal exposure to any potential toxicities from the serum-free peptide incubation. The degree of induction observed (2- to 4-fold) is within the same range as seen in hematopoietic cells in which NF-Ya is overexpressed after retroviral expression.12 Importantly, we found that TAT-NF-Ya was effective at maintaining progenitor activity and possibly inducing their proliferation for all sources tested, including bone marrow, peripheral blood, and umbilical cord blood. This is significant because previous efforts to maintain and increase human HSC/progenitor activity in vitro have been far more successful with umbilical cord blood than with bone marrow, and even less successful with peripheral blood.
Finally, we appreciate that the present study probably does not represent the most efficient protocol that might be achieved. Clearly, the efficiency of TAT-NF-Ya delivery is probably limited by its degradation both outside the cell and within, after TAT-mediated uptake. The intracellular concentration of NF-Ya is regulated by the ubiquitin/proteasome degradation mechanism. For example, substitution of 4 lysines in the C-terminus of NF-Ya, which are potential ubiquitylation sites for arginines, has been found to markedly increase protein stability.50 Future studies will hopefully be able to exploit and modify the contributions made in the current protocol to increase the efficiency and efficacy of this approach.
In conclusion, we find that the TAT-NF-Ya protein can be successfully purified and delivered to human hematopoietic cells, as detected by the activation of the HOXB4 gene. This treatment directly stimulates the proliferation of primitive hematopoietic progenitor cells over several days in culture. We think that this approach offers the possibility for application to a variety of experimental protocols, many of which can be evaluated in vitro and/or in vivo in immunodeficient mouse models to test and improve their efficacy before any clinical application.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Steven Dowdy (University of California, San Diego) for kindly providing pTAT-HA and pTAT-β-Gal vectors, Jiang Zhu for NF-Y plasmids and HOXB4 probe for EMSA, and Anthony Secreto, Cathy Keefer, and Cezary Swider from the Stem Cell and Xenograft Core at the University of Pennsylvania for the excellent technical assistance.
This work was supported by National Institutes of Health (grant RO1-CA090833). M.P.C. was supported by Sanofi Aventis Corporation and Agios Pharmaceuticals.
National Institutes of Health
Authorship
Contribution: A.D.D. conceived and designed the study, collected, assembled, analyzed, and interpreted the data, and wrote the manuscript; G.D.-D. designed the study and analyzed and interpreted the data; A.A. collected the data; M.P.C. interpreted the data; and S.G.E. conceived and designed the study, interpreted the data, wrote the manuscript, and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen G. Emerson, 101 Founders Hall, Haverford College, Haverford, PA 19041; e-mail: semerson@haverford.edu.