Abstract
Forty patients were enrolled in this phase 2 study combining radioimmunotherapy (RIT) using yttrium-90-ibritumomab-tiuxetan (15 MBq [0.4 mCi]/kg) with reduced-intensity conditioning (RIC) using fludarabine (90 mg/m2) and 2 Gy total body irradiation followed by allogeneic hematopoietic cell transplantation (HCT) from related (n = 13) or unrelated (n = 27) donors for the treatment of advanced non-Hodgkin lymphoma. Diagnoses were follicular lymphoma (n = 17), chronic lymphocytic leukemia (n = 13), mantle cell lymphoma (n = 8), marginal zone lymphoma (n = 1), and lymphoplasmacytic lymphoma (n = 1). Median age was 55 years (range, 34-68 years). All patients were high risk with refractory disease or relapse after preceding autologous HCT. No additional toxicities attributable to RIT were observed. Engraftment was rapid and sustained. Incidences of acute graft-versus-host disease 2-4 and chronic graft-versus-host disease were 43% and 53%, respectively. Kaplan-Meier–estimated nonrelapse mortality was 45% at 2 years. Twenty-two of 40 patients (55%) are alive, resulting in a Kaplan-Meier–estimated 2-year survival of 51% for all, 67% for follicular lymphoma, 49% for chronic lymphocytic leukemia, and 37% for mantle cell lymphoma patients. The combined use of RIT with RIC is feasible with acceptable toxicity, even in elderly and heavily pretreated patients. This study is registered at www.clinicaltrials.gov as #NCT00302757.
Introduction
Patients with low-grade lymphomas are currently incurable by standard chemotherapy regimens.1 There are several reports of long-term disease-free survival after allogeneic hematopoietic cell transplantation (HCT) in patients with refractory low-grade non-Hodgkin lymphoma (NHL).2 Allogeneic HCT offers a number of potential advantages over standard chemotherapy regimens, including autologous HCT, namely, a potent graft-versus-lymphoma (GVL) effect, a stem cell source free of neoplastic cells, and a marrow graft without exposure to chemotherapeutic mutagens. These advantages have to compete with the higher treatment-related mortality (TRM) of up to 40% and the complications of graft-versus-host disease (GVHD).3-5 Because the peak incidence of low-grade NHL is in the sixth decade of life,6 most patients, even with a human leukocyte antigen (HLA)–identical donor, are not offered a conventional allogeneic HCT because of age or comorbidities. Reduced- intensity conditioning (RIC) regimens have been developed to overcome these limitations and to allow allogeneic HCT in elderly patients with contraindications for conventional myeloablative HCT.7 Because of the low antilymphoma efficacy of RIC, the burden of antilymphoma activity is shifted to the allogeneic graft. The Seattle group developed a nonmyeloablative conditioning regimen using fludarabine and 2 Gy total body irradiation (Flu/TBI) before HCT, followed by post-transplantation immunosuppression with cyclosporine (CSP) and mycophenolate mofetil (MMF). This approach seems to be the least dose intense of the RIC regimens used for allogeneic HCT in patients with NHL so far. Early reports on Flu/TBI in patients with advanced follicular lymphoma (FL), chronic lymphocytic leukemia (CLL), and mantle cell lymphoma (MCL) showed promising results, especially in patients with low tumor burden.8-10 A new strategy to increase the limited antilymphoma activity of RIC without increasing the toxicity might be the use of radioimmunotherapy (RIT) in the conditioning regimen. The radiolabeled anti-CD20 antibody (yttrium-90 [90Y] ibritumomab tiuxetan; Zevalin; Bayer Health Care) has been shown to have potent activity in patients with NHL.11-14 In this phase 2 study, we prospectively evaluated the use of targeted radiotherapy with 90Y-ibritumomab tiuxetan in combination with nonmyeloablative allogeneic HCT.
Methods
Study protocol and centers
The patients reported have been enrolled in a multicenter phase 2 trial between 2006 and 2008 (EudraCT 2005-002206-37; www.clinicaltrials.gov, #NCT00302757). Participating centers were the Medical Centers of the Universities of Tuebingen, Leipzig, Ulm, Dresden, Hannover, Berlin, Wuerzburg, Goettingen, and Essen, Germany. The study protocol was approved by all participating institutional review boards and the German federal agency Paul Ehrlich Institute responsible for approval of trials in HCT. All patients signed informed consent.
Eligibility criteria
Inclusion critera were diagnoses of CLL, MCL, FL grade 1 or 2, or marginal zone lymphoma (MZL). Patients with advanced CD20+ disease relapsing after more than 2 preceding chemotherapy regimens, including rituximab or with relapse after autologous HCT, could be enrolled. Further inclusion criteria were age more than 18 years but less than 70 years, Karnofsky score more than 60%, availability of an HLA-compatible (9-10 of 10) match-related donor (MRD) or unrelated donor (URD), and disease stage at inclusion of complete remission (CR), partial remission (PR), or stable disease (SD).
Exclusion criteria were rapidly progressive disease, less than 3 months after preceding HCT, central nervous system involvement with disease, uncontrolled fungal infections, liver function abnormalities with bilirubin more than 2 mg/dL, transaminases more than 2 times the upper limit of normal, chronic active viral hepatitis, ejection fraction less than 40%, patients with more than grade 2 hypertension by Common Toxicity Criteria, creatinine clearance less than 50 mL/min, respiratory failure necessitating supplemental oxygen or diffusing capacity for carbon monoxide less than 30%, allergy against murine antibodies, HIV infection, pregnancy or breastfeeding, no adequate contraceptive method, pleural effusion, or ascites.
Patients
Forty patients with advanced NHL specified as FL (n = 17), CLL (n = 13), MCL (n = 8), MZL (n = 1), and lymphoplasmacytic lymphoma (n = 1) were enrolled in the study (Table 1). Median age was 55 years (range, 34-68 years). All patients were high risk with relapsed or refractory disease (n = 28) and/or relapse after preceding autologous HCT (n = 22). The patients had a median of 4 (range, 2-7) preceding lines of chemotherapy. A total of 36 patients had previously received rituximab with a median of 6 cycles (range, 1-15 cycles). Median European Group for Blood and Marrow Transplantation (EBMT)–HCT risk score15 was 6 (range, 4-7). Disease stage at time of transplantation was CR = 7, PR = 27, and SD = 6. Seven patients (4 CLL, 2 FL2, and 1 MCL) had bulky disease with lymph nodes of more than 5 cm diameter at time of HCT. Lactate dehydrogenase was elevated (≥ 250 U/L) in 15 patients.
Patient characteristics
| Characteristic . | No. of patients (n = 40) . |
|---|---|
| Sex | |
| Male | 30 |
| Female | 10 |
| Age, y | |
| Median | 55 |
| Range | 34-68 |
| Prior treatment | |
| No. of lines of CTX | |
| Median | 4 |
| Range | 2-8 |
| Prior autologous HCT | 22 |
| Donor | |
| Related | 13 |
| Unrelated | 27 |
| Diagnosis/histology | |
| Follicular NHL grade 1 or 2 | 17 |
| CLL | 13 |
| MCL | 8 |
| MZL | 1 |
| Lymphoplasmacytic lymphoma | 1 |
| LDH at inclusion | |
| Less than 250 U/mL | 25 |
| 250 U/mL or more | 15 |
| EBMT-HCT score15 | |
| 4 | 2 |
| 5 | 8 |
| 6 | 27 |
| 7 | 3 |
| Disease status at HCT | |
| CR | 7 |
| PR | 27 |
| SD | 6 |
| Less than 5 cm largest LN | 33 |
| 5 cm or more largest LN | 7 |
| Characteristic . | No. of patients (n = 40) . |
|---|---|
| Sex | |
| Male | 30 |
| Female | 10 |
| Age, y | |
| Median | 55 |
| Range | 34-68 |
| Prior treatment | |
| No. of lines of CTX | |
| Median | 4 |
| Range | 2-8 |
| Prior autologous HCT | 22 |
| Donor | |
| Related | 13 |
| Unrelated | 27 |
| Diagnosis/histology | |
| Follicular NHL grade 1 or 2 | 17 |
| CLL | 13 |
| MCL | 8 |
| MZL | 1 |
| Lymphoplasmacytic lymphoma | 1 |
| LDH at inclusion | |
| Less than 250 U/mL | 25 |
| 250 U/mL or more | 15 |
| EBMT-HCT score15 | |
| 4 | 2 |
| 5 | 8 |
| 6 | 27 |
| 7 | 3 |
| Disease status at HCT | |
| CR | 7 |
| PR | 27 |
| SD | 6 |
| Less than 5 cm largest LN | 33 |
| 5 cm or more largest LN | 7 |
CTX indicates chemotherapy; LDH, lactate dehydrogenase; and LN, lymph node.
HLA typing, matching, stem cell mobilization, and collection
Patients and their donors were tested for HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 by high-resolution molecular typing methods. A single allele/antigen disparity was allowed. Granulocyte colony-stimulating factor mobilized peripheral blood stem cell (PBSC) grafts from HLA-compatible (9-10 of 10) MRD or URD were used.
Dosimetry, conditioning regimen, and transplantation
The first 5 patients enrolled received dosimetry using 111In-ibritumomab tiuxetan on day −21 to meet radiation safety requirements of this approach according to German federal regulations. For dosimetry, sequential whole-body scans in anterior and posterior projections were performed using a γ camera immediately after 111In-ibritumomab tiuxetan administration and repeated 3 hours postinjection, on day 1, day 3 or 4, and on day 6.
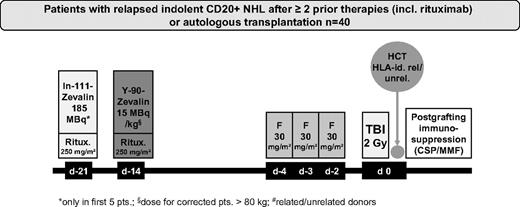
Before RIT, the patients received 2 infusions of 250 mg/m2 rituximab. In patients receiving dosimetry, the first rituximab infusion was given in combination with 185 MBq (5 mCi) 111In-ibritumomab tiuxetan for dosimetry on day −21. The second infusion of rituximab, administered 1 week later (day −14), was followed immediately by 15 MBq/kg (0.4 mCi/kg) of 90Y-ibritumomab tiuxetan (maximum, 1184 MBq or 32 mCi). After the first 5 patients with dosimetry, the remaining patients received only rituximab (250 mg/m2), administered on days −21 and −14 followed by 90Y-ibritumomab tiuxetan on day −14. On days −4, −3, and −2, the patients received 30 mg/m2 per day fludarabine followed on day 0 by 200 cGy TBI at 6 to 7 cGy/min from a linear accelerator and subsequent transfusion of allogeneic PBSC (Figure 1). Postgrafting immunosuppression included CSP and MMF as described previously.16,17 Starting on day −3, CSP was given at a dose of 5.0 mg/kg orally twice a day. All patients received MMF 15 mg/kg every 8 hours if transplanted from URD and every 12 hours if transplanted from MRD. MMF was stopped for recipients of MRD on day 30. For recipients of URD, MMF was switched to every 12 hours dosing on day 30 and tapered by day 100. CSP was tapered from day 56 to day 100 for recipients of MRD; in URD, CSP was continued to day 100 followed by tapering to day 180. Supportive care, including antimicrobial prophylaxis and prophylaxis against cytomegaly virus, was administered according to the standard of care of the participating centers.
Monitoring of patients for engraftment, chimerism, immune reconstitution, disease, toxicity, and donor lymphocyte infusions
Hematopoietic donor cell chimerism was monitored every 4 weeks in all patients using microsatellite markers as described.18 Engraftment was assessed by peripheral blood cell counts. Engraftment was defined as the first day on which the absolute neutrophil count (ANC) was consistently more than 500 ANC/μL. Platelet engraftment was defined as the first day with consistently more than 20 000 platelets/μL.
For disease staging and to assess disease response, all patients received computed tomography scans or positron emission tomography-computed tomography scans within 4 weeks of the start of conditioning and on day 30, day 100, and after 1 year or if clinically indicated. Response was assessed using criteria of the international working group.19 GVHD was assessed and graded as described.20 Toxicities were scored according to the National Cancer Institute Common Toxicity Criteria (Version 3.0). Patients were eligible to receive additional donor lymphocyte infusions in case of relapse or progression of underlying disease.
Statistical analysis
The primary objectives of this study were feasibility and treatment-related toxicity of the combined use of RIT and RIC for allogeneic HCT. Disease response, survival, and GVHD were evaluated as secondary objectives. For statistical analysis, SAS (Version 9.1.3; SAS Institute) biostatistical software was used. Actuarial curves were estimated according to the Kaplan-Meier method; the log-rank test was used for the comparison of Kaplan-Meier estimates between different groups of patients. Overall survival (OS) was measured as the number of days from transplantation to death from any cause. Patients who were still alive at follow-up were censored at the last follow-up date. Time to nonrelapse mortality (NRM) included only deceased patients who died without preceding relapse. Event-free survival (EFS) was calculated as the number of days from HCT until relapse/progression/death. Patients without evidence for relapse/progression were censored at last follow-up date or date of death. Risk factors for survival, relapse/progression, and NRM were evaluated using univariate comparisons and Cox regression. Cox regression was only performed if more than 10 events for the factor evaluated occurred. The following factors were included in the regression models: sex, age (< 60 years vs ≥ 60 years), prior autologous HCT, achievement of CR before HCT (yes vs no), grade of acute GVHD (aGVHD; < 3 vs ≥ 3), chronic GVHD (cGVHD), diagnosis (FL vs MCL/CLL/MZL or lymphoplasmacytic lymphoma), chemotherapy (number of prior lines of chemotherapy [< 3 vs ≥ 3 lines]), duration of disease (< 2 years vs ≥ 2 years), MRD versus URD, and bulky disease (> 5 cm vs ≤ 5 cm). The final model includes only factors that had P values less than .1. All P values are 2-sided.
Results
Transplantation details
All patients received HCT with granulocyte colony-stimulating factor-mobilized PBSC grafts. The median CD34+ cell dose was 6 × 106 CD34+ cells/kg (range, 1.7-18 × 106 CD34+ cells/kg). HLA-compatible (9-10/10) grafts were either from MRD (n = 13) or URD (n = 27). Among the 27 patients transplanted from URD, in 2 patients one antigen mismatches in HLA-C, in 6 patients allelic mismatches in either HLA-A (n = 1), HLA-B (n = 1), HLA-C (n = 2), or HLA-DR (n = 2) were present.
Engraftment
The median time to more than 500 ANC/μL was 13 days (range, 0-69 days), and to more than 20 000 platelets/μL 9 days (range, 0-69 days). Median ANC nadir was 100 cells/μL (range, 0-1300 cells/μL), and median nadir of platelets was 15 000 cells/μL (range, 1000-75 000 cells/μL). In 13 patients, platelets never dropped less than 20 000/μL and in 1 patient ANC never less than 500/μL. All patients engrafted with full donor chimerism by day 28 after HCT. Twenty-four of 40 patients (60%) required platelet support (median, 1; range 0-107). Twenty-nine of 40 (73%) patients received red blood cell transfusions (median, 2; range, 0-25). We observed no cases of graft rejection.
Dosimetry
Ninety percent of the energy of the β-emitter 90Y is absorbed within a radius of 5.3 mm,21 which enables administration of 90Y-ibritumomab tiuxetan in an outpatient setting. Because the amount of radioactivity given to patients was approved for clinical use without dosimetry, imaging with 111In-ibritumomab tiuxetan was only performed in the first 5 patients to fulfill German federal radiation safety regulation. Because of technical reasons (software failure), radiation dose was only evaluable in 4 patients. The mean organ dose (± SEM) determined was 2.73 Gy (0.36 Gy) to liver, 3.3 Gy (0.96 Gy) to kidney, 1.78 Gy (1.25 Gy) to red marrow, 4.07 Gy (0.50 Gy) to spleen, and 0.7 Gy (0.15 Gy) to lung (Table 2).
Dosimetry
| Patient no. . | Liver . | Kidney . | Marrow . | Spleen . | Lung . |
|---|---|---|---|---|---|
| 1 | 2.98 | 3.63 | 0.36 | 5.08 | 0.52 |
| 2 | 1.97 | 4.27 | 0.36 | 3.8 | 0.53 |
| 3 | 2.36 | 0.5 | 0.88 | 2.78 | 1.15 |
| 4 | 3.6 | 4.8 | 5.5 | 4.6 | 0.6 |
| Mean ± SEM | 2.73 ± 0.36 | 3.3 ± 0.96 | 1.78 ± 1.25 | 4.07 ± 0.50 | 0.7 ± 0.15 |
| Patient no. . | Liver . | Kidney . | Marrow . | Spleen . | Lung . |
|---|---|---|---|---|---|
| 1 | 2.98 | 3.63 | 0.36 | 5.08 | 0.52 |
| 2 | 1.97 | 4.27 | 0.36 | 3.8 | 0.53 |
| 3 | 2.36 | 0.5 | 0.88 | 2.78 | 1.15 |
| 4 | 3.6 | 4.8 | 5.5 | 4.6 | 0.6 |
| Mean ± SEM | 2.73 ± 0.36 | 3.3 ± 0.96 | 1.78 ± 1.25 | 4.07 ± 0.50 | 0.7 ± 0.15 |
Dose (Gy) values are given.
Organ toxicity
Combination of RIT with RIC seemed not to increase the toxicity comparison with the previous experience in patients conditioned with fludarabine/2 Gy TBI alone.17 In particular, we observed no cases of veno-occlusive disease, no increased rate of mucositis or nephrotoxicity. Hematologic toxicity was moderate with 13 patients with a platelet nadir more than or equal to 20 000/μL and 1 patient with an ANC nadir more than or equal to 500/μL.
GVHD
The observed incidence of grade 2-4 aGVHD was 43% with grade 2 in 10 patients (25%), grade 3 in 5 patients (13%), and grade 4 in 2 patients (5%). cGVHD occurred in 21 patients (53%), which was extensive in 12 patients (30%).
NRM
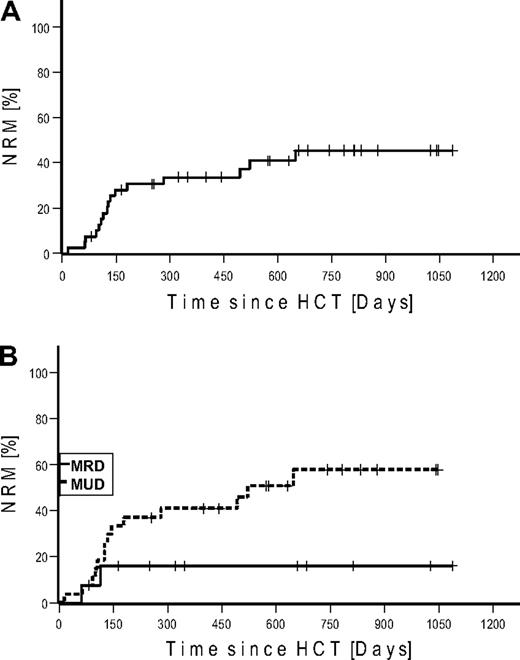
NRM up to day 100 was 10% (n = 4) and 40% (n = 16) for the whole observation period. Kaplan-Meier estimated NRM was 34% at 1 and 45% at 2 years, respectively (Figure 2). NRM for patients transplanted from MRD was 16% versus 58% from URD (P = .073). Causes of deaths were infections (n = 8), GVHD (n = 7), and cerebral bleeding (n = 1; Table 3). The lethal infections were the result of sepsis (n = 6), aspergillosis (n = 1), and viral encephalitis (n = 1).
Nonrelapse mortality. (A) Cumulative. (B) Stratified for match-related donor and unrelated donor.
Nonrelapse mortality. (A) Cumulative. (B) Stratified for match-related donor and unrelated donor.
Causes and timing of NRM
| Category . | Cause/grade/site . | Days from HCT to death . |
|---|---|---|
| Infections (n = 8) | Sepsis, MOF | 16 |
| Sepsis, MOF | 93 | |
| Sepsis, MOF | 101 | |
| Invasive fungal infection | 114 | |
| Sepsis, MOF | 127 | |
| Sepsis | 126 | |
| Sepsis, MOF | 282 | |
| Sepsis, MOF | 521 | |
| GVHD (n = 7) | Grade IV, skin, pneumonia | 62 |
| Grade IV, gut | 64 | |
| Grade III, gut, skin, sepsis | 107 | |
| Grade IV, gut, skin, liver | 134 | |
| Grade III, gut, BK + HSV encephalitis | 147 | |
| Extensive cGVHD gut, skin, sepsis | 180 | |
| Extensive cGVHD, ascites, cirrhosis | 493 | |
| Other (n = 1) | Cerebral bleeding | 648 |
| Category . | Cause/grade/site . | Days from HCT to death . |
|---|---|---|
| Infections (n = 8) | Sepsis, MOF | 16 |
| Sepsis, MOF | 93 | |
| Sepsis, MOF | 101 | |
| Invasive fungal infection | 114 | |
| Sepsis, MOF | 127 | |
| Sepsis | 126 | |
| Sepsis, MOF | 282 | |
| Sepsis, MOF | 521 | |
| GVHD (n = 7) | Grade IV, skin, pneumonia | 62 |
| Grade IV, gut | 64 | |
| Grade III, gut, skin, sepsis | 107 | |
| Grade IV, gut, skin, liver | 134 | |
| Grade III, gut, BK + HSV encephalitis | 147 | |
| Extensive cGVHD gut, skin, sepsis | 180 | |
| Extensive cGVHD, ascites, cirrhosis | 493 | |
| Other (n = 1) | Cerebral bleeding | 648 |
MOF indicates multiorgan failure; and HSV, herpes simplex virus.
Disease response, EFS, and OS
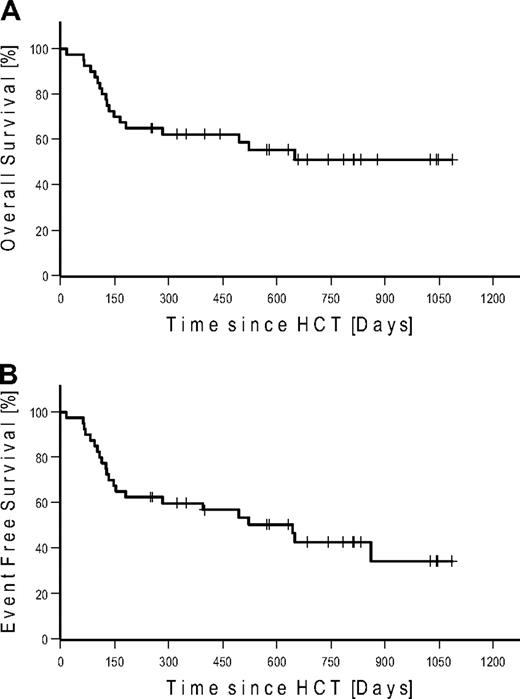
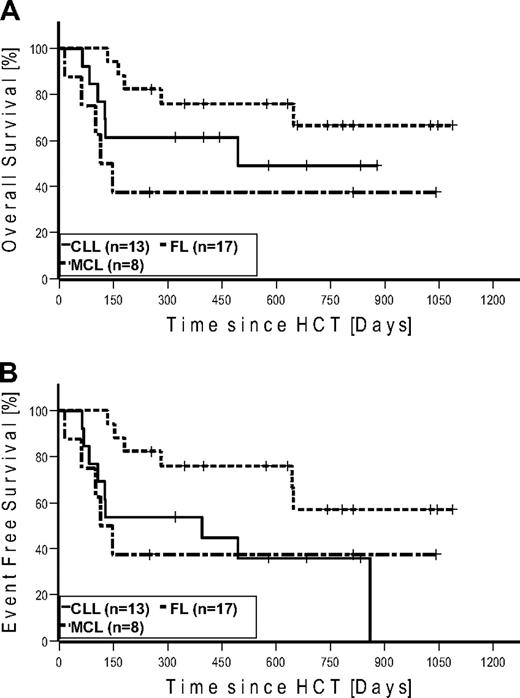
Seven patients (4 FL, 2 CLL, and 1 MCL) were in CR at the time of HCT. None of these patients relapsed after HCT. Four of these patients (2 CLL and 2 FL) died of NRM. Of the 27 patients in PR at the time of HCT, 15 converted to CR, 11 remained in PR, and 1 patient progressed until day 100. At last follow-up in August 2009, 17 of 27 patients in PR converted to CR, 6 remained in PR, and 4 progressed. Of the 6 patients with SD at the time of HCT, 2 achieved CR and 3 PR on day 100. At last follow-up, 1 additional patient achieved PR as best response. Among the 7 patients with bulky disease, 4 patients achieved PR and 3 patients CR. Table 4 gives details on outcome by disease histology. Twenty-two patients were alive: with 12 patients in CR, 4 patients in PR, 2 patients with SD, and 4 patients with relapse. A total of 6 patients relapsed/progressed, resulting in a Kaplan-Meier estimate of relapse of 36%. Median follow-up of patients alive was 672 days (range, 251-1086 days). The Kaplan-Meier estimated OS at 2 years was 51% (Figure 3A). The Kaplan-Meier estimated EFS at 2 years after HCT was 43% (Figure 3B). Kaplan-Meier estimated OS and EFS for the different diagnosis at 2 years were 67% and 57% for FL, 49% and 36% for CLL, and 37% and 37% for MCL, respectively (Figure 4). Kaplan-Meier estimated OS after HCT from MRD was 69% versus 42% from URD (P = .303).
HCT outcome by disease histology
| Disease . | n . | Response . | Outcome: alive . | Causes of death . | |||||
|---|---|---|---|---|---|---|---|---|---|
| CR . | PR . | SD . | PD . | Relapse . | Toxicity/infection/other . | GVHD . | |||
| FL | 17 | 13 | 1 | 2 | 1 | 12 | 1 | 3 | 1 |
| CLL | 13 | 5 | 4 | 0 | 4 | 7 | 1 | 2 | 3 |
| MCL | 8 | 6 | 2 | 0 | 0 | 3 | 0 | 3 | 2 |
| MZL | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| LL | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Disease . | n . | Response . | Outcome: alive . | Causes of death . | |||||
|---|---|---|---|---|---|---|---|---|---|
| CR . | PR . | SD . | PD . | Relapse . | Toxicity/infection/other . | GVHD . | |||
| FL | 17 | 13 | 1 | 2 | 1 | 12 | 1 | 3 | 1 |
| CLL | 13 | 5 | 4 | 0 | 4 | 7 | 1 | 2 | 3 |
| MCL | 8 | 6 | 2 | 0 | 0 | 3 | 0 | 3 | 2 |
| MZL | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| LL | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
PD indicates progressive disease; and LL, lymphoplasmacytic lymphoma.
Kaplan-Meier estimates in all patients. (A) Overall survival. (B) Event-free survival.
Kaplan-Meier estimates in all patients. (A) Overall survival. (B) Event-free survival.
Kaplan-Meier estimates in patients with follicular lymphoma (n = 17), chronic lymphocytic leukemia (n = 13), and mantle cell lymphoma (n = 8). (A) Overall survival. (B) Event-free survival.
Kaplan-Meier estimates in patients with follicular lymphoma (n = 17), chronic lymphocytic leukemia (n = 13), and mantle cell lymphoma (n = 8). (A) Overall survival. (B) Event-free survival.
Donor lymphocyte infusions
One patient with CLL received 3 doses of donor lymphocyte infusion after allogeneic HCT for progressive disease and is still alive.
Risk factors for survival and NRM
In a univariate analysis of risk factors for OS, EFS, and NRM, several possible factors could be identified (Table 5). Decreased OS was associated with non-FL histology (P = .038), aGVHD more than or equal to 3 (.001), and no cGVHD (P = .004). EFS was decreased in patients without FL (P = .013), aGVHD more than or equal to 3 (.004) or in patients without cGVHD (P = .001) as was NRM (non-FL histology (P = .029), aGVHD more than or equal to 3 (< .001), no cGVHD (P = .016).
Risk factors for OS, EFS, and NRM
| Variable (risk) . | Number under risk . | Univariate log-rank test: P . | Cox regression . | ||
|---|---|---|---|---|---|
| Hazard ratio . | 95% CI . | P . | |||
| OS | |||||
| Male sex | 30 | .242 | — | — | — |
| Age 60 y or more | 10 | .371 | — | — | — |
| No CR before HCT | 33 | .536 | — | — | — |
| No FL | 17 | .038 | 4.0 | 1.3-11.7 | .013 |
| Less than 3 cycles of chemotherapy | 8 | .195 | — | — | — |
| Duration of disease 2 y or more | 31 | .566 | — | — | — |
| Autologous HCT | 22 | .762 | — | — | — |
| Limited/extensive cGVHD | 19 | .004 | 0.3 | 0.1-0.8 | .022 |
| Acute GVHD 3 or more | 7 | .001 | 3.0 | 0.9-9.8 | .062 |
| Unrelated donor | 27 | .303 | — | — | — |
| Bulky disease | 7 | .844 | — | — | — |
| EFS | |||||
| Male sex | 30 | .237 | — | — | — |
| Age 60 y or more | 10 | .364 | — | — | — |
| No CR before HCT | 33 | .931 | — | — | — |
| No FL | 17 | .013 | 4.5 | 1.4-14.8 | .013 |
| Less than 3 cycles of chemotherapy | 8 | .150 | — | — | — |
| Duration of disease 2 y or more | 31 | .332 | — | — | — |
| Autologous HCT | 22 | .816 | — | — | — |
| Limited/extensive cGVHD | 19 | .001 | 0.3 | 0.1-1.2 | .096 |
| Acute GVHD 3 or more | 7 | .004 | 4.3 | 1.2-15.5 | .026 |
| Unrelated donor | 27 | .237 | — | — | — |
| Bulky disease | 7 | .476 | — | — | — |
| NRM | |||||
| Male sex | 30 | .359 | — | — | — |
| Age 60 y or more | 10 | .517 | — | — | — |
| No CR before HCT | 33 | .392 | — | — | — |
| No FL | 17 | .029 | 4.2 | 1.3-13.8 | .019 |
| Less than 3 cycles of chemotherapy | 8 | .110 | — | — | — |
| Duration of disease 2 y or more | 31 | .742 | — | — | — |
| Autologous HCT | 22 | .887 | — | — | — |
| Limited/extensive cGVHD | 19 | .016 | 0.4 | 0.1-1.4 | .141 |
| Acute GVHD 3 or more | 7 | < .001 | 3.9 | 1.1-14.2 | .041 |
| Unrelated donor | 27 | .073 | 1.9 | 0.4-9.0 | .429 |
| Bulky disease | 7 | .545 | — | — | — |
| Variable (risk) . | Number under risk . | Univariate log-rank test: P . | Cox regression . | ||
|---|---|---|---|---|---|
| Hazard ratio . | 95% CI . | P . | |||
| OS | |||||
| Male sex | 30 | .242 | — | — | — |
| Age 60 y or more | 10 | .371 | — | — | — |
| No CR before HCT | 33 | .536 | — | — | — |
| No FL | 17 | .038 | 4.0 | 1.3-11.7 | .013 |
| Less than 3 cycles of chemotherapy | 8 | .195 | — | — | — |
| Duration of disease 2 y or more | 31 | .566 | — | — | — |
| Autologous HCT | 22 | .762 | — | — | — |
| Limited/extensive cGVHD | 19 | .004 | 0.3 | 0.1-0.8 | .022 |
| Acute GVHD 3 or more | 7 | .001 | 3.0 | 0.9-9.8 | .062 |
| Unrelated donor | 27 | .303 | — | — | — |
| Bulky disease | 7 | .844 | — | — | — |
| EFS | |||||
| Male sex | 30 | .237 | — | — | — |
| Age 60 y or more | 10 | .364 | — | — | — |
| No CR before HCT | 33 | .931 | — | — | — |
| No FL | 17 | .013 | 4.5 | 1.4-14.8 | .013 |
| Less than 3 cycles of chemotherapy | 8 | .150 | — | — | — |
| Duration of disease 2 y or more | 31 | .332 | — | — | — |
| Autologous HCT | 22 | .816 | — | — | — |
| Limited/extensive cGVHD | 19 | .001 | 0.3 | 0.1-1.2 | .096 |
| Acute GVHD 3 or more | 7 | .004 | 4.3 | 1.2-15.5 | .026 |
| Unrelated donor | 27 | .237 | — | — | — |
| Bulky disease | 7 | .476 | — | — | — |
| NRM | |||||
| Male sex | 30 | .359 | — | — | — |
| Age 60 y or more | 10 | .517 | — | — | — |
| No CR before HCT | 33 | .392 | — | — | — |
| No FL | 17 | .029 | 4.2 | 1.3-13.8 | .019 |
| Less than 3 cycles of chemotherapy | 8 | .110 | — | — | — |
| Duration of disease 2 y or more | 31 | .742 | — | — | — |
| Autologous HCT | 22 | .887 | — | — | — |
| Limited/extensive cGVHD | 19 | .016 | 0.4 | 0.1-1.4 | .141 |
| Acute GVHD 3 or more | 7 | < .001 | 3.9 | 1.1-14.2 | .041 |
| Unrelated donor | 27 | .073 | 1.9 | 0.4-9.0 | .429 |
| Bulky disease | 7 | .545 | — | — | — |
— indicates not applicable.
In multivariate Cox regression analysis, non-FL histology resulted in an increased hazard ratio (HR) for OS (HR = 4.0, P = .013), EFS (HR = 4.5, P = .013), and NRM (HR = 4.2, P = .019; Table 5). aGVHD was associated with an increased HR for OS (HR = 3.0, P = .062), EFS (HR = 4.3, P = .026), and NRM (HR = 3.9, P = .041). cGVHD decreased the HR for OS (HR = 0.3, P = .022) and EFS (HR = 0.3, P = .096).
Discussion
Radiolabeled antibodies are potentially ideal agents for combination with RIC in the context of allogeneic HCT for lymphoma patients. RIT targets continuous low-dose rate radiation to the disease site while sparing normal surrounding tissue. Dose-limiting toxicity of 90Y-ibritumomab tiuxetan is hematologic toxicity,22 which is reversed by the stem cell rescue with an allogeneic graft. The vast majority of studies using RIT in the context of HCT have been performed in autologous HCT, proving that RIT does not hamper engraftment if an adequate time interval between RIT administration and stem cell transfer is assured. A number of studies combined standard dose 90Y-ibritumomab tiuxetan or 131I-tositumobmab with high-dose conditioning using BEAM followed by autologous HCT with moderate toxicity and promising disease response.23-25 Dose escalation studies of RIT with 90Y-ibritumomab tiuxetan as a single agent were also performed, showing an acceptable safety profile up to a dose of 45 MBq/kg.26,27
In allogeneic HCT, so far only 1 small phase 1 or 2 study and 1 case report evaluated the combined approach of RIT with RIC.28,29 Shimoni et al reported 12 patients with low- and high-grade NHL RIT using 90Y-ibritumomab tiuxetan followed by RIC with fludarabine/busulphan/melphalan.28 Fietz et al reported 2 additional patients combining 90Y-ibritumomab tiuxetan with RIC using fludarabine/cyclophosphamide.29 Moderate toxicity of this approach was observed, with main toxicities related to RIC and allogeneic HCT but not attributable specifically to RIT.
The study reported here is the largest prospective trial of the combined use of RIT and RIC for allogeneic HCT so far. The primary objective of this study was to assess feasibility, safety, and toxicity of the combined use of fludarabine/2 Gy TBI RIC with RIT using 90Y-ibritumomab tiuxetan. This study demonstrates the feasibility and safety of the combined use of standard-dose RIT with RIC before allogeneic HCT in patients with advanced NHL. Dosimetry performed in 4 patients confirmed radiation doses to be in the range previously reported.22 The standard dose of 15 MBq/kg used in this trial was approved for use without additional dosimetry. No additional toxicities attributable to RIT compared with toxicities reported previously with this regimen without RIT17 were observed. The engraftment rate was high with no rejections and robust hematologic reconstitution in all patients. The high number of patients without severe thrombopenia after RIT-RIC illustrates the nonmyeloablative nature and specific targeting of this approach.
Despite the moderate acute toxicity of the regimen, NRM was relatively high in our study with a Kaplan-Meier estimate of 45% at 2 years. This high NRM was not entirely unexpected given an average EBMT risk score of 6, which is associated with a NRM of approximately 40% for RIC-HCT.15 The high EBMT score reflects the age of the patients, the advanced stage of their disease and the predominant use of unrelated donors. NRM in the group of patients transplanted from MRD was substantially lower. Furthermore, in a similar cohort of patients with the same RIC but without additional RIT, Rezvani et al reported an estimated 3-year NRM of 42%.16 Half of the NRM in our study is attributable to a high incidence of GVHD. This may be related to an early taper of immunosuppression to induce a GVL effect. In addition, two-thirds of our patients received grafts from URD, 8 from a mismatched donor. GVHD 3-4 was mainly gastrointestinal in our study. Shimoni et al also observed a high incidence of severe gastrointestinal GVHD in their study of RIT combined with RIC.28 Whether this is influenced by the use of RIT in the conditioning remains unclear. However, our rates of acute grade 2-4 and extensive chronic GVHD are comparable with the incidences (acute grade 2-4 = 62% and extensive chronic GVHD = 47%) reported with Flu/TBI alone.16 NRM might be improved by prolonged administration of immunosuppression in future studies.
This trial was designed to evaluate feasibility and toxicity and therefore disease response, and survival should be interpreted very cautiously. Our results for OS and EFS at 2 years for patients with FL and CLL seem comparable with data published so far using Flu/TBI without additional RIT.16,30 Given the heterogeneity of our patient population and the high incidence of GVHD, it is almost impossible to assess the contribution of RIT as opposed to the GVL effect to disease control. Multiple trials have shown that single-agent 90Y-ibritumomab tiuxetan can induce impressive disease responses with overall response rates of 73% to 83% and CR rates of 15% to 51%, leading to long-term remission in patients with refractory indolent and high-grade NHL.11-13,31-33 In patients with FL, a recent phase 3 study showed high PR-to-CR conversion rates, even in patients with advanced FL.14 In our study, the rapid achievement of CR in patients with PR before transplantation (day 100 response) and the fact that, in contrast to the Seattle study, bulky disease was not associated with a poor outcome16,33 could be regarded as indirect evidence of efficacy.
In patients with MCL, our results seem less favorable than those reported by Maris et al.10 However, such comparisons are difficult to interpret given the low number of patients with MCL in our study and the potent influence of patient selection. One potentially confounding factor is the extensive use of rituximab in our patient cohort, which might have impacted targeting by 90Y-ibritumomab tiuxetan.34
In view of the not entirely satisfactory disease response data in MCL, how could this regimen be improved? The first approach could be to use this approach earlier in the course of disease. Alternatively, dose escalation of RIT to 22 or 30 MBq/kg might enable better lymphoma control and less relapses. The feasibility and safety of such dose escalation have been shown in the setting of autologous HCT.26,27 We have recently completed a phase 1/2 study evaluating such an approach for high-grade NHL in the allogeneic setting and dose escalated RIT prooved to be safe (W.A.B., D.B., et al, unpublished data, December 2009).
Instead of adding RIT to a nonmyeloablative conditioning, dose escalation of RIC is an alternative strategy. Morris et al used a regimen of fludarabine, melphalan, and alemtuzumab for allogeneic HCT in patients with NHL.35 TRM at day 100 was 14% and progression-free survival was 65% for low-grade and 34% for high-grade NHL. The M. D. Anderson group has reported on the use of fludarabine/cyclophosphamide/rituximab as conditioning for HCT mainly from MRDs in patients with FL who failed prior autologous HCT.36 They observed a TRM of 15% and an excellent progression-free survival of 83% at 5 years in this selected patient group having chemosensitive disease and a good performance status. However, variations in patient characteristics, patient and donor selection, and conditioning regimens make direct comparisons between different approaches of RIC for indolent NHL difficult.
In conclusion, our study demonstrated the safety, moderate toxicity, and feasibility of a combination of RIT with 90Y-ibritumomab tiuxetan and an RIC for allogeneic HCT. Disease response and survival data seem promising in a heavily pretreated patient cohort. This study sets the stage for future randomized studies to evaluate the additional impact provided by RIT in the conditioning regimen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the nurses and staff of the transplantation units taking part in the study; the departments of nuclear medicine participating in the study, in particular R. Bares (Tuebingen), S. N. Reske (Ulm), J. Kotzerke (Dresden), and O. Sabri (Leipzig); and Dagmar Frisch and Anja Junker for data management and monitoring of the study.
This work was supported by the German José Carreras Foundation and Bayer Health Care GmbH (an unrestricted research grant).
Authorship
Contribution: W.A.B. and D.B. conceived and designed the clinical trial, provided the patients, collected and assembled the data, analyzed and interpreted data, wrote and edited the manuscript, and gave final approval of the manuscript; T.L., S.v.H., B.F., M. Stadler, L.U., M. Stelljes, G.W., R.T., V.V., C.F., W.V., and L.K. provided patients, collected and assembled data, and gave final approval of the manuscript; C.M. and H.D. analyzed and interpreted data, wrote and edited the manuscript, and gave final approval of the manuscript; and M.B. and S.K. provided patients, collected and assembled data, edited the manuscript, and gave final approval of the manuscript.
Conflict-of-interest disclosure: W.A.B. received honoraria and research funding from Bayer Health Care GmbH. M.B. received honoraria from Hoffmann La Roche, Novartis, and Celgene and research funding Hoffmann La Roche and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Wolfgang A. Bethge, Medical Center University of Tuebingen, Department of Hematology & Oncology, Otfried-Mueller Strasse 10, 72076 Tuebingen, Germany; e-mail: wolfgang.bethge@med.uni-tuebingen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal