Abstract
The HSP90 client chaperone interaction stabilizes several important enzymes and antiapoptotic proteins, and pharmacologic inhibition of HSP90 results in rapid client protein degradation. Therefore, HSP90 inhibition is an attractive therapeutic approach when this protein is active, a phenotype commonly observed in transformed but not normal cells. However, preclinical studies with HSP90 inhibitors such as 17-AAG demonstrated depletion of only a subset of client proteins and very modest tumor cytotoxicity in chronic lymphocytic leukemia (CLL) cells. Herein, we describe another HSP90 inhibitor, 17-DMAG, which is cytotoxic to CLL but not normal lymphocytes. Treatment with 17-DMAG leads to depletion of the HSP90 client protein IKK, resulting in diminished NF-κB p50/p65 DNA binding, decreased NF-κB target gene transcription, and caspase-dependent apoptosis. Furthermore, treatment with 17-DMAG significantly decreased the white blood cell count and prolonged the survival in a TCL1-SCID transplant mouse model. The ability of 17-DMAG to function as an NF-κB inhibitor is of great interest clinically, as few currently available CLL drugs target this transcription factor. Therefore, the effect of 17-DMAG on NF-κB signaling pathways represents a novel therapy warranting further clinical pursuit in this and other B-cell lymphoproliferative disorders.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the United States. CLL is a disease of mature B cells expressing the T-cell antigen CD5 that are resistant to apoptosis and accumulate over time.1 Therapy available for the treatment of CLL includes chemotherapeutic agents, such as chlorambucil, cyclophosphamide, fludarabine, and bendamustine; and immunotherapy, including rituximab and alemtuzumab.2 Although rituximab-based chemoimmunotherapy3-7 has improved the outcome for patients with CLL, no therapies for CLL are curative, with the exception of allogeneic hematopoietic stem cell transplantation.8 The complex genetic diversity of the disease makes it difficult to determine which therapies will be most beneficial to patients; furthermore, many patients are either resistant to treatment or respond initially but eventually develop refractory disease. These difficulties have prompted an ongoing interest in identifying new, more effective drug targets in CLL.
One class of drugs being explored in leukemia and other cancers are those targeting the heat shock proteins. Heat shock protein 90 (HSP90) is a molecular chaperone protein that interacts with client proteins,9 thereby preventing their degradation. To serve as a chaperone protein, HSP90 has to be in an active conformation, which is commonly seen in transformed but not normal cells.10 In the absence of HSP90 binding, rapid degradation of client proteins occurs via the proteasome. Therefore, this increased HSP90 activity provides a rationale for pursuing therapeutic agents that target this specific enzyme. Proteins stabilized by interaction with HSP90 have been implicated in leukemia transformation, tumor cell survival, and disease progression, such as fusion kinases like BCR-ABL in chronic myelogenous leukemia.11 Furthermore, it has been demonstrated that the HSP90 inhibitor geldanamycin is cytotoxic to CLL cells independently of p53 function, indicating the value of this class of drugs to a broad class of patients with limited therapeutic options.12
The HSP90 inhibitor geldanamycin has shown preclinical efficacy in the treatment of CLL; geldanamycin destabilizes AKT, targets it for degradation, and confers sensitivity to chlorambucil and fludarabine.13 A derivative of geldanamycin, 17-allylamino 17-demethoxygeldanamycin (17-AAG, tanespimycin), has previously been reported by our laboratory as well as others to demonstrate effective cytotoxicity in vitro against CLL cells.14,15 However, the activity of both geldanamycin and 17-AAG is limited to specific client proteins, and the poor solubility and difficulty of delivery of these compounds have prompted the development of more clinically applicable agents. 17-Dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG, alvespimycin) has been reported to exhibit better solubility and less toxicity to normal cells; in addition, the drug is now available in an oral form, which facilitates administration and probably increases patient compliance in treatment.16 These advantages have prompted further efforts to determine whether 17-DMAG effectively depletes HSP90 client proteins critical to CLL survival, examine whether this drug offers an advantage over other HSP90 inhibitors, and better characterize the molecular mechanisms by which 17-DMAG mediates death in these tumor cells. Such studies are needed to support the clinical development of 17-DMAG as a potential therapeutic agent in CLL.
An HSP90 client that is important in CLL but has not yet been explored with pharmacologic antagonists is the I-κ-B kinase (IKK) complex, the activating component of the nuclear factor-κB (NF-κB) family of transcription factors. NF-κB is constitutively active in many types of cancer and is considered a major factor in disease severity and progression.17 NF-κB activity is increased in CLL,18 and this has recently been reported to correlate with in vitro survival in CLL.19 NF-κB has been shown to positively regulate a variety of important antiapoptotic proteins and oncogenes, such as BCL2, XIAP, c-FLIP, and MCL1.20,21 Given the importance of these genes in initiating or enhancing CLL cell survival, targeting NF-κB via depletion of IKK represents an attractive target for CLL treatment. Geldanamycin has been shown to interfere with both the activity and stability of IKK,22 although we have previously found that 17-AAG had little activity against this family of proteins.15 In the present study, we demonstrate that 17-DMAG, in contrast to 17-AAG, effectively depletes both subunits of IKK in CLL cells, inhibits NF-κB DNA binding, and down-regulates expression of target genes that prevent apoptosis. Furthermore, we show that, by targeting the NF-κB family, 17-DMAG selectively mediates cytotoxicity against CLL cells in vitro and in vivo, but not normal T cells or NK cells important for immune surveillance. Our findings provide strong justification for the clinical development of 17-DMAG in the treatment of CLL.
Methods
Patients, cell separation, culture conditions, and reagents
For in vitro studies, written, informed consent was obtained to procure cells from patients with previously diagnosed CLL as defined by the modified National Cancer Institute criteria.23 Mononuclear cells from CLL patients and normal volunteers were isolated and placed in culture as previously described by our group.24,25 17-DMAG and 17-AAG were obtained from the Division of Cancer Treatment and Diagnosis, National Cancer Institute (Bethesda, MD). CD3-coated plates were purchased from BD Biosciences, and z-VAD-fmk was purchased from MP Biomedicals.
Animal studies
SCID mice were housed in a clean environment and supplied with sterile food and water ad libitum. CD19-selected transformed B cells (1 × 106) from the spleen of a TCL1 transgenic mouse with active leukemia were engrafted by tail vein into an SCID mouse. Ten or 11 mice in each treatment group were treated with dimethyl sulfoxide (vehicle control) or 10 mg/kg of 17-DMAG administered by intraperitoneal injection 5 times per week beginning 2 weeks after injection of leukemia cells. Blood was collected at various time points throughout treatment for RNA isolation and assessment of gene expression. Mice were killed on development of hind-limb paralysis or other disease criteria causing discomfort. All experiments were carried out under protocols approved by the Ohio State University Institutional Animal Care and Use Committee. Overall survival was determined as described in “Statistical analysis.”
Viability assays
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were performed to determine cytotoxicity. A total of 1 × 106 CD19-selected B cells from CLL patients were incubated for 24 or 48 hours in 17-DMAG, 17-AAG, or vehicle. MTT reagent (Sigma-Aldrich) was then added, and plates were incubated for an additional 24 hours before spectrophotometric measurement. Apoptosis was determined by staining with annexin V-fluorescein isothiocyanate and propidium iodide (PI). After exposure to drugs, cells were washed with phosphate-buffered saline and stained in 1 time binding buffer (BD Biosciences). Cell death was assessed by flow cytometry using a Beckman-Coulter model EPICS XL cytometer. Data were analyzed with the System II software package (Beckman-Coulter). A total of 10 000 cells were counted for each sample. Mitochondrial membrane potential changes were assessed by staining with the lipophilic cationic dye JC-1 (Invitrogen) and analysis by flow cytometry.
Immunoblot analysis
Nuclear and cytoplasmic lysates were prepared with NE-PER Nuclear and Cytoplasmic Extraction kit (Pierce Protein Research Products). Antibodies used for immunoblots included AKT, p-IκB, and poly-(ADP-ribose) polymerase (PARP; Cell Signaling); p65, p50, RELB, MCL1, BCL2, IκBα, and actin (Santa Cruz Biotechnology); and IKKα and IKKβ (Imgenex). Protein (50 μg/lane) was separated on polyacrylamide gels and transferred onto nitrocellulose. After antibody incubations, proteins were detected with chemiluminescent substrate (Pierce Chemical) and quantified using a ChemiDoc system with Quantity One software (Bio-Rad Laboratories).
Electrophoretic mobility shift assay
32P-labeled probe containing an NF-κB consensus binding site (5′ AGT TGA GGG GAC TTT CCC AGG C 3′; Santa Cruz Biotechnology) was labeled using the Nick Translation System (Invitrogen). A total of 5 μg of nuclear protein was incubated for 30 minutes at room temperature in 1 time binding buffer (5 times: 50mM Tris-HCl, pH 7.5, 5mM ethylenediaminetetraacetic acid, 20% Ficoll, 5mM dithiothreitol, 375mM KCl) plus 250 ng of poly (dI-dC; Sigma-Aldrich). Complexes were separated on 5.5% polyacrylamide gels in Tris borate ethylenediaminetetraacetic acid buffer (89mM Tris-base, 89mM boric acid, 2mM ethylenediaminetetraacetic acid), dried, and autoradiographed. For shift experiments, antibodies to the p65 or p50 subunit (Santa Cruz Biotechnnology) of NF-κB were incubated with nuclear extract for 10 minutes before the addition of 32P-labeled probe.
Real-time PCR
RNA was extracted by phenol chloroform isolation using TRIzol reagent (Invitrogen) and purified using QIAGEN RNeasy columns (QIAGEN). cDNA was prepared with SuperScript First-Strand Synthesis System for reverse-transcriptase polymerase chain reaction (PCR; Invitrogen). Real-time PCR was performed using commercially available primers (Applied Biosystems) or custom-designed primers.26 Primer sequences are given in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Detection was performed using an ABI Prism 7700 sequence detection system (Applied Biosystems). Average relative expression (treatment compared with media) was normalized to the internal control genes TATA-box binding protein (TBP) or 18S.
Statistical analysis
All reported statistical evaluation was done by the Center for Biostatistics at Ohio State University. Because many of the measurements used samples from the same patients, linear-mixed effects models were used for analysis to take into consideration the dependency of these observations. To stabilize the variance, the raw threshold cycle value of real-time PCR data was normalized to internal control, and the standardized data were analyzed using linear mixed-effects models. Holm procedure27 was used to correct for multiple comparisons when it is needed. Type I error is strongly controlled at α = 0.05 for single comparisons and with adjustment for multiple comparisons. Survival in the TCL1-SCID model was compared using a log-rank test, and the result was displayed using the Kaplan-Meier plots.
Results
17-DMAG promotes selective apoptosis in CLL cells
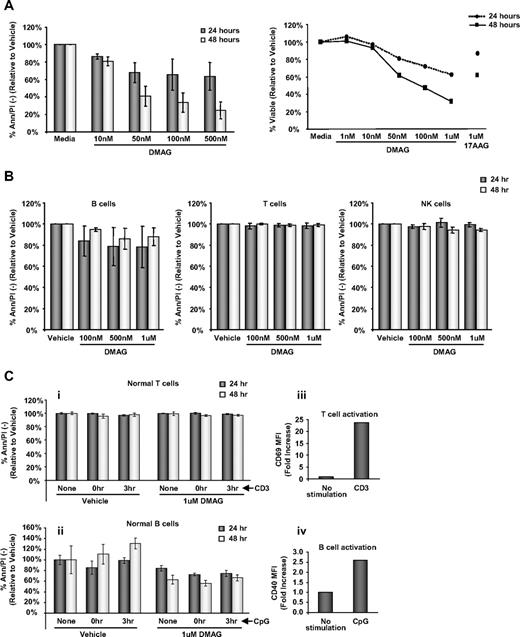
Our laboratory and others have previously shown that 17-AAG is modestly cytotoxic toward CLL cells.14,15 In Figure 1A, we demonstrate that, compared with the vehicle control, 17-DMAG exerts dose-dependent apoptosis (P < .001 averaged across 24- and 48-hour time points) at concentrations of 50nM to 500nM, which represent pharmacologically attainable doses. Similar to many other agents, 17-DMAG also demonstrates time-dependent apoptosis (P < .001, averaged across all doses) in CLL cells with extended exposure from 24 to 48 hours. In addition, 17-DMAG is much more potent after 24 and 48 hours of treatment than 17-AAG (Figure 1A right).
Apoptosis is significantly increased by 17-DMAG. (A) Left: Viability of CLL patient cells (n = 6) treated with vehicle control or increasing doses of 17-DMAG for 24 and 48 hours determined by annexinV/PI (Ann/PI) staining. Ann/PI double-negative cells are considered live cells. Right: Viability of CLL patient cells (n = 7) treated with vehicle control or increasing doses of 17-DMAG or 1μM 17-AAG for 24 and 48 hours determined by MTT assay. (B) Viability of normal B cells (n = 12), T cells (n = 8), and NK cells (n = 8) treated with vehicle control or increasing doses of 17-DMAG for 24 and 48 hours determined by Ann/PI. (C) Normal T cells (i) and B cells (ii) (n = 3) either unstimulated, or stimulated with CD3 (T cells) or CpG (B cells) concurrently or 3 hours before treatment with vehicle control or 1μM 17-DMAG for 24 and 48 hours. Viability is determined by Ann/PI staining, and Ann/PI double-negative cells are considered live cells. The T-cell and B-cell activation is demonstrated by increased CD69 (iii) and CD40 (iv) mean fluorescence intensity (MFI) surface staining, respectively.
Apoptosis is significantly increased by 17-DMAG. (A) Left: Viability of CLL patient cells (n = 6) treated with vehicle control or increasing doses of 17-DMAG for 24 and 48 hours determined by annexinV/PI (Ann/PI) staining. Ann/PI double-negative cells are considered live cells. Right: Viability of CLL patient cells (n = 7) treated with vehicle control or increasing doses of 17-DMAG or 1μM 17-AAG for 24 and 48 hours determined by MTT assay. (B) Viability of normal B cells (n = 12), T cells (n = 8), and NK cells (n = 8) treated with vehicle control or increasing doses of 17-DMAG for 24 and 48 hours determined by Ann/PI. (C) Normal T cells (i) and B cells (ii) (n = 3) either unstimulated, or stimulated with CD3 (T cells) or CpG (B cells) concurrently or 3 hours before treatment with vehicle control or 1μM 17-DMAG for 24 and 48 hours. Viability is determined by Ann/PI staining, and Ann/PI double-negative cells are considered live cells. The T-cell and B-cell activation is demonstrated by increased CD69 (iii) and CD40 (iv) mean fluorescence intensity (MFI) surface staining, respectively.
One disadvantage of several currently used therapies for CLL, including fludarabine and alemtuzumab, is the adverse effect on the normal lymphocyte population. To ascertain the influence of 17-DMAG treatment on normal immune cells, we treated B, T, and NK cells from healthy volunteers with increasing concentrations of this agent (Figure 1B). Similar to findings previously reported with 17-AAG,14,15 17-DMAG does not demonstrate significant toxicity to normal T cells and NK cells (P = .26 and P = .86, respectively) after 24 or 48 hours of treatment compared with the vehicle control. 17-DMAG is minimally toxic to normal B cells; however, most CLL patients have a relatively small normal B-cell population. In addition, we found that stimulation of B cells with CpG oligodeoxynucleotides or T cells with CD3 to mimic a more in vivo activated state does not enhance the susceptibility to 17-DMAG treatment (B cells, P = .32; T cells P = .63; Figure 1C). Together, these data suggest that 17-DMAG may be less immunosuppressive than other cytotoxic agents currently used for the treatment of CLL.
17-DMAG mediates apoptosis via caspase activation
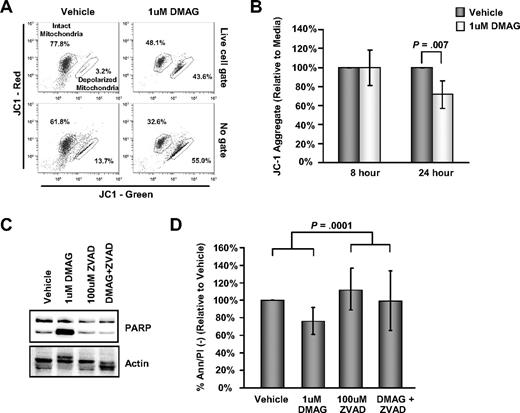
We next determined whether 17-DMAG–mediated cell death was dependent on the intrinsic pathway of apoptosis and caspase activation. Figure 2A-B demonstrates that 17-DMAG leads to a disruption of JC-1 aggregation, an indication of increased mitochondrial membrane depolarization and apoptosis. Furthermore, 17-DMAG treatment leads to increased processing of PARP in CLL cells, which is prevented by treatment with the universal caspase inhibitor z-VAD-fmk, indicating that 17-DMAG induces caspase activation (Figure 2C). The cytotoxic effect of 17-DMAG on CLL cells is also rescued by caspase inhibition, as there is significantly greater viability in cells treated with 17-DMAG in the presence of z-VAD versus 17-DMAG alone, which is not explained by the slight increase in cell viability resulting from the ZVAD effect (interaction contrast, P < .001; Figure 2D). These findings indicate that the dominant mechanism of 17-DMAG induced death is caspase-dependent apoptosis.
17-DMAG–mediated cytotoxicity is caspase dependent. (A) Representative JC-1 flow diagram of CLL patient cells treated with vehicle or 1μM 17-DMAG for 24 hours. The percentage of cells containing intact mitochondria and depolarized mitochondria are indicated. The data are shown gated on live cells by forward and side scatter properties (top row) or ungated (bottom row). (B) Analysis of mitochondrial membrane potential in vehicle control or 1μM 17-DMAG–treated CLL at 8 and 24 hours by JC-1 staining (n = 6). Values shown in this experiment were calculated as a decrease in the JC-1 aggregate relative to the vehicle control. (C) Western blot analysis for PARP in whole cell lysate prepared from CLL patient cells treated with vehicle control or 1μM 17-DMAG for 24 hours in the presence or absence of 100μM caspase inhibitor z-VAD-fmk. Blots are probed with actin as a loading control. Results shown are representative of at least 6 patient samples. (D) Viability of CLL patient cells (n = 12) treated with vehicle control or 1μM 17-DMAG for 24 hours in the presence or absence of 100μM caspase inhibitor z-VAD-fmk determined by Ann/PI staining.
17-DMAG–mediated cytotoxicity is caspase dependent. (A) Representative JC-1 flow diagram of CLL patient cells treated with vehicle or 1μM 17-DMAG for 24 hours. The percentage of cells containing intact mitochondria and depolarized mitochondria are indicated. The data are shown gated on live cells by forward and side scatter properties (top row) or ungated (bottom row). (B) Analysis of mitochondrial membrane potential in vehicle control or 1μM 17-DMAG–treated CLL at 8 and 24 hours by JC-1 staining (n = 6). Values shown in this experiment were calculated as a decrease in the JC-1 aggregate relative to the vehicle control. (C) Western blot analysis for PARP in whole cell lysate prepared from CLL patient cells treated with vehicle control or 1μM 17-DMAG for 24 hours in the presence or absence of 100μM caspase inhibitor z-VAD-fmk. Blots are probed with actin as a loading control. Results shown are representative of at least 6 patient samples. (D) Viability of CLL patient cells (n = 12) treated with vehicle control or 1μM 17-DMAG for 24 hours in the presence or absence of 100μM caspase inhibitor z-VAD-fmk determined by Ann/PI staining.
17-DMAG leads to degradation of IKK-α and β
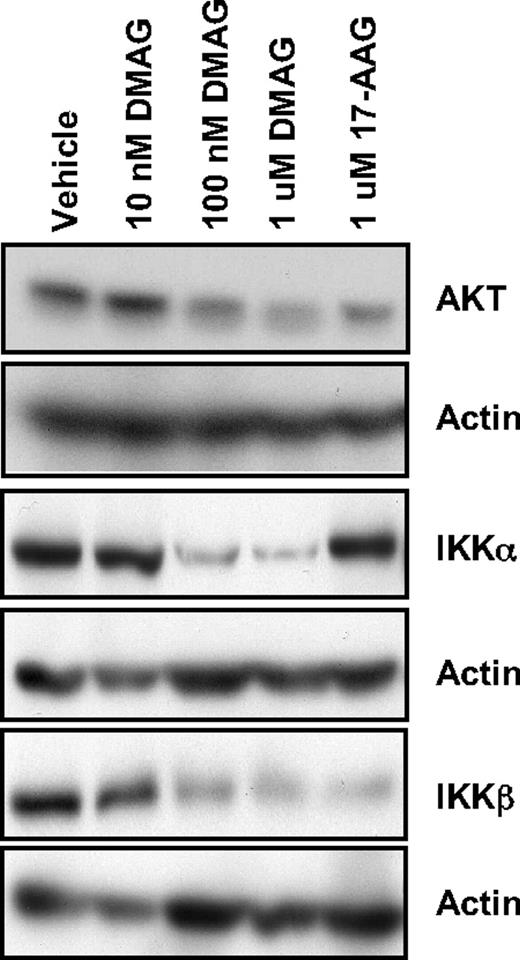
HSP90 has been shown to chaperone multiple client proteins relevant to human CLL.14 Of these client proteins, the NF-κB pathway is of great interest in CLL, given its constitutive activation and contribution to cell survival and resistance to therapy in this disease.19,28 To date, pharmacologic attempts to target this important transcription factor clinically have not been successful because of redundancy in its regulation and the ability to bypass specific inhibitors of the activating regulatory kinases IKKα and IKKβ. Activation of NF-κB is prevented by binding of inhibitor of NF-κB (IκB) proteins to active NF-κB complexes, thereby confining them to the cytoplasm. Both IKKα and IKKβ represent kinases that phosphorylate IκB proteins and lead to their degradation by the proteasome. In Figure 3, we demonstrate that 17-DMAG leads to a strong decrease in both IKKα and IKKβ, whereas 17-AAG results in depletion of only IKKβ. The differential effects of 17-DMAG and 17-AAG are specific to IKKα, as another HSP90 client protein AKT is depleted by both of these inhibitors. In addition, a higher dose of 17-AAG is required for the effect on IKKα and AKT (1μM 17-AAG produces a similar response to 100nM 17-DMAG), consistent with a more potent influence of 17-DMAG on HSP90 client proteins. Thus, our data demonstrate that 17-DMAG offers the advantage of deregulation of both the IKKβ-mediated classic pathway as well as the IKKα-mediated alternative pathway of NF-κB activation. The ability of 17-DMAG to target this family makes it an attractive therapeutic option in CLL; therefore, we subsequently focused our investigation on the downstream effects of inhibition of this pathway.
17-DMAG down-regulates NF-κB signaling through IKKα and IKKβ. Western blot analysis for AKT, IKKα, and IKKβ in whole cell lysate prepared from CLL patient cells treated with vehicle control or increasing doses of 17-DMAG for 24 hours. Blots are probed with actin as a loading control. Results shown are representative of 8 patient samples.
17-DMAG down-regulates NF-κB signaling through IKKα and IKKβ. Western blot analysis for AKT, IKKα, and IKKβ in whole cell lysate prepared from CLL patient cells treated with vehicle control or increasing doses of 17-DMAG for 24 hours. Blots are probed with actin as a loading control. Results shown are representative of 8 patient samples.
17-DMAG depletion of IKK results in inhibition of NF-κB activation and signaling
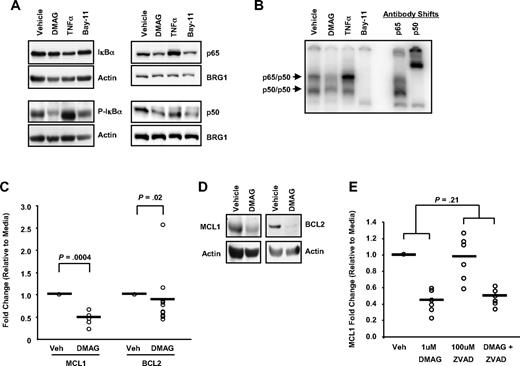
Given that both IKKα and IKKβ are depleted by 17-DMAG in CLL cells as described in Figure 3, we sought to confirm that this leads to downstream inhibition of NF-κB DNA binding and transcriptional activation of antiapoptotic target genes. In Figure 4A, we demonstrate that 17-DMAG treatment results in reduced phosphorylation of the IKK target IκBα, resulting in maintained or stabilized levels of total IκBα protein. Tumor necrosis factor-α (TNF-α), a known activator of NF-κB, has the opposite effect. The results with 17-DMAG on IKK inhibition are also similar to a known IKK inhibitor, Bay-11. Therefore, by preventing IκBα phosphorylation and subsequent degradation, 17-DMAG may function to turn off this signaling pathway. We also found that treatment with 17-DMAG prevents nuclear localization of p65 and p50, the major components of the active NF-κB complex (Figure 4A); and as a result, DNA binding to an NF-κB consensus binding site is also reduced (Figure 4B).
17-DMAG regulates NF-κB activity and target gene transcription. (A) Western blot analysis for IκBα and phosphorylated IκBα (P-IκBα) in cytoplasmic cell lysate and p65 and p50 in nuclear cell lysates prepared from CLL patient cells treated with vehicle control or 1μM 17-DMAG for 24 hours, 20 ng/mL TNF-α for 30 minutes, or 10μM Bay-11 for 1 hour. Blots are probed with actin or BRG1 as a loading control. Results shown are representative of at least 6 patient samples. (B) Electrophoretic mobility shift assay analysis with nuclear extract prepared from cells treated with vehicle control or 1μM 17-DMAG for 24 hours, 20 ng/mL TNF-α for 30 minutes, or 10μM Bay-11 for 1 hour using a radiolabeled oligonucleotide containing a consensus NF-κB-binding site. Antibody shifts are performed with nuclear extract prepared from vehicle control sample incubated with antibodies specific to the p65 or p50 subunits of NF-κB. The p65/p50 and p50/p50 complexes are indicated. Results shown are representative of 6 CLL samples. (C) Real-time PCR for MCL1 and BCL2 (n = 5 and n = 11, respectively) after 24 hours 1μM 17-DMAG treatment. Data are normalized to 18S transcript and represented as fold change in expression of 17-DMAG treated relative to the vehicle control. ○ represents individual patient samples. Bar represents the average of all patient samples. (D) Western blot analysis for MCL1 and BCL2 in cytoplasmic cell lysate prepared from CLL patient cells grown treated with vehicle control or 1μM 17-DMAG for 24 hours. Blots are probed with actin as a loading control. Results shown are representative of at least 6 patient samples. (E) Real-time PCR for MCL1 (n = 6) in CLL patient cells treated with vehicle control or 1μM 17-DMAG for 24 hours in the presence or absence of 100μM caspase inhibitor z-VAD-fmk. Data are normalized to TBP or 18S transcript and represented as fold change in expression of 17-DMAG treated relative to the vehicle control. ○ represents individual patient samples. Bar represents the average of all patient samples.
17-DMAG regulates NF-κB activity and target gene transcription. (A) Western blot analysis for IκBα and phosphorylated IκBα (P-IκBα) in cytoplasmic cell lysate and p65 and p50 in nuclear cell lysates prepared from CLL patient cells treated with vehicle control or 1μM 17-DMAG for 24 hours, 20 ng/mL TNF-α for 30 minutes, or 10μM Bay-11 for 1 hour. Blots are probed with actin or BRG1 as a loading control. Results shown are representative of at least 6 patient samples. (B) Electrophoretic mobility shift assay analysis with nuclear extract prepared from cells treated with vehicle control or 1μM 17-DMAG for 24 hours, 20 ng/mL TNF-α for 30 minutes, or 10μM Bay-11 for 1 hour using a radiolabeled oligonucleotide containing a consensus NF-κB-binding site. Antibody shifts are performed with nuclear extract prepared from vehicle control sample incubated with antibodies specific to the p65 or p50 subunits of NF-κB. The p65/p50 and p50/p50 complexes are indicated. Results shown are representative of 6 CLL samples. (C) Real-time PCR for MCL1 and BCL2 (n = 5 and n = 11, respectively) after 24 hours 1μM 17-DMAG treatment. Data are normalized to 18S transcript and represented as fold change in expression of 17-DMAG treated relative to the vehicle control. ○ represents individual patient samples. Bar represents the average of all patient samples. (D) Western blot analysis for MCL1 and BCL2 in cytoplasmic cell lysate prepared from CLL patient cells grown treated with vehicle control or 1μM 17-DMAG for 24 hours. Blots are probed with actin as a loading control. Results shown are representative of at least 6 patient samples. (E) Real-time PCR for MCL1 (n = 6) in CLL patient cells treated with vehicle control or 1μM 17-DMAG for 24 hours in the presence or absence of 100μM caspase inhibitor z-VAD-fmk. Data are normalized to TBP or 18S transcript and represented as fold change in expression of 17-DMAG treated relative to the vehicle control. ○ represents individual patient samples. Bar represents the average of all patient samples.
We looked at the effect on 2 NF-κB targets that are involved in caspase-dependent apoptosis, BCL2 and MCL1, and found that both are down-regulated by treatment with 17-DMAG at both the transcript level (BCL2, P = .02; MCL1, P < .001; Figure 4C) and at the protein level (Figure 4D). Furthermore, we found that, although the presence of the caspase inhibitor ZVAD prevents 17-DMAG–induced cell death (as shown in Figure 2D), it does not significantly impair the 17-DMAG–mediated decrease in MCL1 transcript (DMAG alone vs DMAG + ZVAD; P = .21; Figure 4E), indicating that the decrease in transcript is probably mediated through IKKα and IKKβ client protein regulation, rather than a nonspecific consequence of cell death.
17-DMAG prevents CD40L- and CpG-induced NF-κB activity
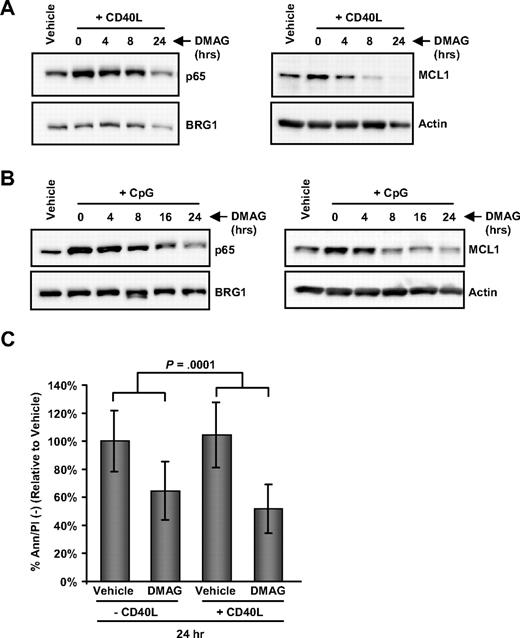
The tumor microenvironment has been demonstrated to play a significant role in the survival of CLL cells,29 as well as resistance to many therapies. This protective effect provided by the microenvironment involves activation of the cells through CD40 or CpG, both of which are potent activators of NF-κB. We therefore investigated the effect of 17-DMAG on NF-κB induced by stimulation from the microenvironment. We found that pretreatment of CLL patient cells with 17-DMAG blocks the ability of CD40L to induce p65 nuclear localization as well as increase MCL1 protein levels (Figure 5A). A similar result is evident with 17-DMAG treatment followed by CpG-containing oligodeoxynucleotide stimulation (Figure 5B). Furthermore, we found that coculture of CLL cells with CD40L is not able to rescue 17-DMAG–induced cell death; and indeed, the addition of CD40L significantly enhanced the cytotoxic effect of 17-DMAG (Figure 5C; P < .001).
17-DMAG prevents CD40L- and CpG-induced NF-κB activity in CLL and prevents CD40L-mediated viability. (A) Western blot analysis for MCL1 in cytoplasmic cell lysate and p65 in nuclear cell lysate prepared from CLL patient cells treated with vehicle control, or 1μM 17-DMAG for 0, 4, 8, or 24 hours, followed by stimulation with 500 ng/mL CD40L for 1 hour. Blots shown are representative of 6 patient samples, and actin and BRG1 were probed as loading controls. (B) Western blot analysis for MCL1 in cytoplasmic cell lysate and p65 in nuclear cell lysate prepared from CLL patient cells treated with vehicle control, or 1μM 17-DMAG for 0, 4, 8, 16, or 24 hours, followed by stimulation with 3.2μM CpG oligodeoxynucleotides for 3 hours. Blots shown are representative of 6 patient samples, and blots are stripped and probed with actin and BRG1 as loading controls. (C) Viability by Ann/PI at 24 hours in CLL patient cells treated with vehicle control or 17-DMAG with and without 1 μg/mL CD40L.
17-DMAG prevents CD40L- and CpG-induced NF-κB activity in CLL and prevents CD40L-mediated viability. (A) Western blot analysis for MCL1 in cytoplasmic cell lysate and p65 in nuclear cell lysate prepared from CLL patient cells treated with vehicle control, or 1μM 17-DMAG for 0, 4, 8, or 24 hours, followed by stimulation with 500 ng/mL CD40L for 1 hour. Blots shown are representative of 6 patient samples, and actin and BRG1 were probed as loading controls. (B) Western blot analysis for MCL1 in cytoplasmic cell lysate and p65 in nuclear cell lysate prepared from CLL patient cells treated with vehicle control, or 1μM 17-DMAG for 0, 4, 8, 16, or 24 hours, followed by stimulation with 3.2μM CpG oligodeoxynucleotides for 3 hours. Blots shown are representative of 6 patient samples, and blots are stripped and probed with actin and BRG1 as loading controls. (C) Viability by Ann/PI at 24 hours in CLL patient cells treated with vehicle control or 17-DMAG with and without 1 μg/mL CD40L.
17-DMAG prolongs survival in a mouse model of CLL
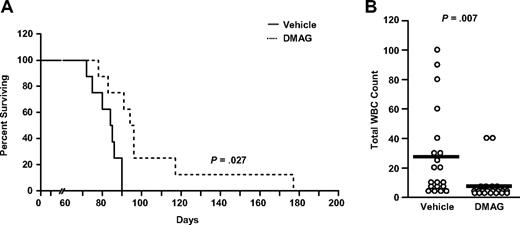
Finally, we determined the in vivo significance of 17-DMAG regulation of NF-κB using a TCL1-SCID mouse transplantation model derived from the transgenic mouse model of CLL that we have described previously as a therapeutic tool for this disease.30,31 TCL1 leukemia cells were engrafted into SCID mice, which were started on treatment with vehicle (dimethyl sulfoxide) or 17-DMAG (10 mg/kg) 16 days after engraftment. We found that 17-DMAG treatment significantly prolongs the survival of these mice (Figure 6A; median survival, 75 days vs 66 days, P = .027, n = 10/group). Furthermore, we found that treatment with 17-DMAG significantly reduces the number of white blood cells in these animals (Figure 6B; P = .007). Collectively, these data suggest that 17-DMAG represents a novel therapeutic agent that targets NF-κB in the TCL1-SCID model and provides support for clinical development in CLL.
17-DMAG prolongs survival in a mouse model of CLL. (A) Survival curve for TCL1-SCID mice treated with 10 mg/kg 17-DMAG treatment or vehicle control (n = 10/group). (B) White blood cell count (WBC) in 17-DMAG and vehicle control treated TCL1-SCID mice (n = 20/group). Count is determined at day 55 after initiation of treatment by hematoxylin and eosin-stained peripheral blood smear.
17-DMAG prolongs survival in a mouse model of CLL. (A) Survival curve for TCL1-SCID mice treated with 10 mg/kg 17-DMAG treatment or vehicle control (n = 10/group). (B) White blood cell count (WBC) in 17-DMAG and vehicle control treated TCL1-SCID mice (n = 20/group). Count is determined at day 55 after initiation of treatment by hematoxylin and eosin-stained peripheral blood smear.
Discussion
Herein, we demonstrate that the well-characterized HSP90 inhibitor 17-DMAG represents the first therapeutic agent to target both NF-κB activating kinases IKKα and IKKβ in primary CLL cells. Our data demonstrate that effective degradation of IKKα and IKKβ results in enhanced cytoplasmic retention of IκBα, with diminished DNA binding of nuclear p50 and p65 and associated down-regulation of NF-κB target genes (MCL1 and BCL2) that prevent apoptosis in CLL cells. Although Bay-11 seems to have an even more pronounced effect than 17-DMAG, the 10μM dose is relatively high for leukemia cells. However, it was used because we wanted to ensure complete inhibition as this was a control for reduced NF-κB DNA binding. The dose of 17-DMAG used is a physiologic attainable dose and therefore is not as probable to cause the same degree of inhibition as Bay-11 by electrophoretic mobility shift assay. Nevertheless, we show that inhibition of 17-DMAG induced NF-κB activity does functionally lead to inhibition of downstream NF-κB signaling in CLL cells.
We did not observe any effect on NF-κB activity after 17-DMAG treatment in normal peripheral blood mononuclear cells (data not shown), which are characterized by low or absent nuclear NF-κB.18 Therefore, the tumor-specific mechanism of 17-DMAG killing may be attributable to the agent's lack of effect in cells with low levels of NF-κB activity. Accordingly, we demonstrate that 17-DMAG promotes caspase-dependent apoptosis in a dose- and time-dependent fashion in CLL cells, with only minimal toxicity toward normal NK cells and T cells at all concentrations and durations of treatment examined. Furthermore, normal B cells do not become more susceptible to 17-DMAG even when transiently stimulated with CpG, which is able to induce NF-κB activity, suggesting the constitutively activated NF-κB in CLL cells is important for the sensitivity to 17-DMAG. One of the major toxicities of commonly used therapies in CLL, including fludarabine and alemtuzumab, is depletion of cellular and/or innate immune effector cells, which predisposes patients to opportunistic infections. The lack of T-cell or NK-cell apoptosis makes 17-DMAG a potentially attractive agent in CLL, as this drug does not exacerbate the immune suppression associated with this disease.
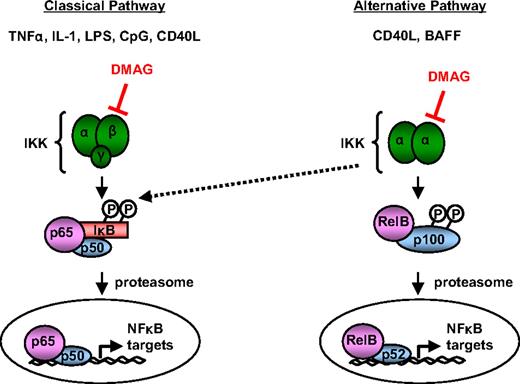
Many available agents that target NF-κB exhibit specificity for IKKβ and are unable to inhibit IKKα.32 In addition to activating a distinct NF-κB complex and set of target genes through the alternative pathway, the activation of IKKα has been demonstrated to compensate for classic signaling on inhibition of IKKβ33 (illustrated in Figure 7). Thus, regulation of both of these proteins is important therapeutically. Both IKKα and IKKβ can be induced by CD40L, an important in vivo survival signal for CLL cells.34,35 We found that 17-DMAG is able to prevent activation by CD40L and CpG signaling, as demonstrated by decreased nuclear localization of p65 and induction of MCL1 (Figure 5), suggesting that 17-DMAG may be able to prevent survival signals produced by the surrounding tumor microenvironment. Indeed, we found that CD40L is not able to rescue the 17-DMAG–mediated cytotoxicity, and even enhances the effect of 17-DMAG. However, it has been shown that CD40L can induce mitogen-activated protein kinase-mediated apoptosis36 ; therefore, preventing CD40L-induced NF-κB activation with 17-DMAG may allow a mitogen-activated protein kinase or similar pathway to activate cell death in CLL cells. Further study of the mechanism of synergy of CD40L and 17-DMAG is underway at this time. We also studied the effect of 17-DMAG treatment in CLL cells in culture with or without a bone marrow-derived stromal cell line (HS5). We found that the HS5 stromal cells offer some degree of protection against 17-DMAG treatment (data not shown). However, given that this system does not accurately reflect in vivo treatment, we extended these in vitro studies with 17-DMAG to an in vivo system to address the effect of the surrounding microenvironment. We used an adapted model of our TCL1 transgenic model in which tumor cells derived from an elderly TCL1 mouse with active leukemia are engrafted into an SCID mouse. Over time, development of progressive CD19/CD5+ blood lymphocytosis, splenomegaly, and ultimately death occurs. 17-DMAG treatment significantly extends the survival in this model compared with vehicle-treated control mice, despite the protective in vivo microenvironment. In addition, after 17-DMAG treatment, we detected a decrease in the total number of leukemic cells compared with vehicle-treated mice. Together, these studies provide evidence that the TCL1-SCID transgenic model can be used to test new therapies and validate the in vivo influence, as well as provide support for future clinical development of 17-DMAG as an NF-κB inhibitor in CLL.
The proposed effect of 17-DMAG on NF-κB activity. Diagram of NF-κB signaling through the alternative and classic pathways. Classic signaling occurs after stimulation of IKKα/IKKβ heterodimers with inducers, such as TNF-α, interleukin-1, lipopolysaccharide, and CpG oligodeoxynucleotide, to activate p65/p50 complexes. Alternative signaling occurs after stimulation of IKKα homodimers with inducers, such as CD40L and BAFF, to activate RelB/p52 complexes. The proposed level of 17-DMAG intervention with NF-κB signaling is through the down-regulation of IKKα and IKKβ protein levels.
The proposed effect of 17-DMAG on NF-κB activity. Diagram of NF-κB signaling through the alternative and classic pathways. Classic signaling occurs after stimulation of IKKα/IKKβ heterodimers with inducers, such as TNF-α, interleukin-1, lipopolysaccharide, and CpG oligodeoxynucleotide, to activate p65/p50 complexes. Alternative signaling occurs after stimulation of IKKα homodimers with inducers, such as CD40L and BAFF, to activate RelB/p52 complexes. The proposed level of 17-DMAG intervention with NF-κB signaling is through the down-regulation of IKKα and IKKβ protein levels.
Whereas the initial success of targeted therapy in chronic myelogenous leukemia with imatinib mesylate (Gleevec) prompted much excitement for the potential role of selective agents for cancer therapy, it quickly became recognized that agents directed at single pathways are much less probable to be successful in other forms of cancer, where multiple signaling pathways are disrupted. This has prompted investigators to turn their attention to new cancer therapeutic agents, which broadly inhibit and target multiple pathways that are deregulated in several tumor types. In this regard, 17-DMAG and other HSP90-inhibiting agents represent the ideal prototypes of multiple targeting agents. However, one concern about 17-DMAG as a potential therapeutic agent for CLL is that, by depleting multiple client protein targets, this therapy may have significant toxicity. It has been shown that differences in the level of activated HSP90 in tumor compared with normal cells results in increased client protein binding in the tumor so that these cells that become dependent on HSP90.10 Similarly, the amount of constitutively active NF-κB is increased in CLL, and recent studies have described a correlation among the amount of nuclear p65, cell survival, and response to therapy.19,37 These characteristics may explain why CLL cells are more susceptible than normal B cells to HSP90 and NF-κB inhibition. Furthermore, BCL2 and MCL1 are both present at increased levels in CLL. Overexpression of antiapoptotic proteins, which overcome normal compensatory death signals, may predispose cancer cells to undergo death when these survival signals are inhibited. Thus, overexpression of BCL2 and MCL1 may produce “oncogene addiction”38 in CLL cells, and a decrease in these proteins could explain the enhanced sensitivity of CLL cells to 17-DMAG compared with normal lymphocytes. Therefore, therapies, such as 17-DMAG, which target both of these survival proteins, are of great clinical interest.
To date, the clinical experience with 17-DMAG and other less potent HSP90 inhibitors in CLL has been limited. No clinical study of an HSP90 inhibitor has been reported in CLL. However, 17-DMAG has been explored in other hematologic malignancies using several intravenous schedules with demonstration of a half-life of approximately 24 plus or minus 15 hours. In one such phase 1 study in acute myelogenous leukemia, clinical responses were observed.39 In these studies, dose-limiting toxicity consisted of acute myocardial infarction and elevation of troponin at the highest dose level (32 mg/m2). These toxicities fortunately were not observed at doses less than the maximum concentration of 291 ng/mL,39 which corresponds approximately to a 471nM in vitro dose, where CLL cytotoxicity was clearly evident in our study. This suggests that we probably will be able to attain the concentrations observed in our in vitro studies that promote significant apoptosis in human CLL cells.
More recently, an oral formulation of 17-DMAG has demonstrated similar pharmacologic properties as the intravenous compound and, more importantly, can provide continuous HSP90 inhibition. Preliminary results of a phase 1 study of this oral formulation in solid tumors were recently reported with acceptable toxicity and pharmacologic properties.40 At the recommended phase 2 dose of the oral compound, 2-fold HSP90 inhibition was observed, similar to the biologic activity obtained in prior studies of the intravenous formulation of 17-DMAG.41 Thus, the oral formulation of 17-DMAG appears to be as biologically active as the parental intravenous compound while offering patients a more convenient route of administration, which will hopefully improve treatment compliance. Further exploration of the oral form of 17-DMAG in hematologic malignancies has not been pursued to this point but is warranted.
Based on the data herein, we think that 17-DMAG is a potent inhibitor of both classic (IKKβ) and alternative (IKKα) activators of NF-κB, and subsequently effective in regulating NF-κB target genes involved in cell survival. Given its role as an NF-κB inhibitor, we have initiated a clinical study in CLL through the Cancer Therapy Evaluation Program of the National Cancer Institute that will soon begin clinical evaluation of this agent.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute (P01 CA95426; CLL Research Consortium P01 CA81534-02; Leukemia SPORE P50-CA140158, T32 Grant T-32-5CA009338), The Leukemia & Lymphoma Society, and the D. Warren Brown Foundation. A.J.J. is a Paul Calabresi Scholar.
National Institutes of Health
Authorship
Contribution: E.H. designed and performed research, analyzed data, and wrote the paper; A.J.W., C.A.R., and D.A.W. designed and performed research; J.J., T.S.L., K.J.M., and J.C.B. designed the research concept and provided samples; W.H.T. and V.M.G. performed research; X.Z. and D.J. analyzed and interpreted data; C.M.C. provided materials; and A.J.J. designed the research concept, analyzed and interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amy J. Johnson, Ohio State University, 410 West 12th Ave, CCC Bldg, Room 455C, Columbus, OH 43210; e-mail: amy.johnson@osumc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal