Abstract
The European Organisation for Research and Treatment of Cancer 58951 trial for children with acute lymphoblastic leukemia (ALL) or non-Hodgkin lymphoma (NHL) addressed 3 randomized questions, including the evaluation of dexamethasone (DEX) versus prednisolone (PRED) in induction and, for average-risk patients, the evaluation of vincristine and corticosteroid pulses during continuation therapy. The corticosteroid used in the pulses was that assigned at induction. Overall, 411 patients were randomly assigned: 202 initially randomly assigned to PRED (60 mg/m2/d), 201 to DEX (6 mg/m2/d), and 8 nonrandomly assigned to PRED. At a median follow-up of 6.3 years, there were 19 versus 34 events for pulses versus no pulses; 6-year disease-free survival (DFS) rate was 90.6% (standard error [SE], 2.1%) and 82.8% (SE, 2.8%), respectively (hazard ratio [HR] = 0.54; 95% confidence interval, 0.31-0.94; P = .027). The effect of pulses was similar in the PRED (HR = 0.56) and DEX groups (HR = 0.59) but more pronounced in girls (HR = 0.24) than in boys (HR = 0.71). Grade 3 to 4 hepatic toxicity was 30% versus 40% in pulses versus no pulses group and grade 2 to 3 osteonecrosis was 4.4% versus 2%. For average-risk patients treated according to Berlin-Frankfurt-Muenster–based protocols, pulses should become a standard component of therapy. This trial was registered at www.clinicaltrials.gov as #NCT00003728.
Introduction
Continuation treatment in childhood acute lymphoblastic leukemia (ALL) relies on a combination of daily thiopurine and weekly methotrexate. Some evidence supports a relation between higher doses of these drugs during continuation therapy and a better outcome.1,2 Clinical studies performed in the 1970s and 1980s showed that treatment intensification during maintenance improved the outcome of nonintensive protocols.3,4
Among the combinations added to the thiopurine-methotrexate basic continuation therapy, reinductions of vincristine (VCR) and prednisone (PRED) have often been used and have improved treatment outcome for some patients.5 A large retrospective analysis by the Childhood ALL Collaborative Group6 suggested that for patients at standard or intermediate risk of relapse receiving a treatment regimen of moderate intensity, the addition of VCR + PRED pulses to continuation therapy did improve the disease-free survival (DFS) from 59.5% to 68.9%.
The Dutch Childhood Leukemia Study Group ALL VI nonrandomized study for non–high-risk patients, based on the Pinkel St Jude Total Therapy approach, used dexamethasone (DEX) instead of PRED during induction and frequent VCR + DEX reinduction pulses throughout continuation therapy (14 days every 7 weeks).7 The high cure rate achieved in this study—an event-free survival at 8 years of 81% (standard error [SE], 3%)—and the low incidence of central nervous system (CNS) relapse were partially attributed to the use of DEX in induction and in pulses.
Pulses of VCR and PRED had been evaluated in one randomized Berlin-Frankfurt-Muenster (BFM) trial, but the results were inconclusive because the insufficient patient accrual precluded the achievement of adequate statistical power.8 In the late 1990s, the European Organisation for Research and Treatment of Cancer–Children's Leukemia Group (EORTC-CLG) started a randomized trial in patients with ALL and lymphoblastic non-Hodgkin lymphoma (NHL) addressing 3 questions: (1) the value of PRED (60 mg/m2/d) versus DEX (6 mg/m2/d) in induction for all patients; (2) the value of prolonged courses of L-asparaginase throughout consolidation and late intensification in non–very-high-risk patients; (3) the value of VCR + corticosteroid pulses added to continuation therapy in patients of average risk (AR). To prevent an interaction between the first and the third randomization, the corticosteroid of the pulses was that assigned at first randomization. Moreover, the patients in the DEX arm were to be included in a large intergroup trial (I-BFM-SG ALL IR 95)9 addressing the same question, ie, whether VCR + DEX pulses could improve the DFS of intermediate risk (IR) patients. At the end of 2002, however, a preliminary analysis of the intergroup trial results suggested that the pulses would fail to provide any benefit. Therefore, the EORTC regulatory authorities decided to stop the CLG trial for futility. However, although the total accrual of patients has accordingly been markedly reduced compared with that initially expected, the final analysis of the CLG trial now shows data robust enough to belie this prediction of futility.
Methods
Patients
Patients younger than 18 years of age with previously untreated ALL or NHL were eligible for the trial. The diagnosis of ALL or of NHL was based on morphologic, cytochemical, and immunophenotypical criteria. ALL of French-American-British L3 morphology and diffuse large cell B-cell lymphoma, Burkitt lymphoma, and high-grade B-cell lymphoma Burkitt-like were excluded, as well as patients previously treated with corticosteroids for more than 7 days.
Immunophenotype was determined with standard techniques, and positivity for a marker required its presence in more than 20% of the lymphoblasts if on the surface or in more than 10% if in the cytoplasm. Chromosome analysis used standard techniques. Bone marrow (BM) smears, immunophenotypes, and cytogenetics were reviewed centrally.
Patients were assigned to different risk groups: very low risk (VLR), AR, and very high risk (VHR). VLR was defined as white blood cell (WBC) counts below 10 × 109/L, hyperdiploid karyotype (> 50 chromosomes in each leukemic cell), and DNA index greater than 1.16, and no CNS or gonadal involvement. VHR criteria consisted of blast counts in peripheral blood of 1 × 109/L or greater at completion of the prephase, t(9;22), t(4;11), or another mixed-lineage leukemia rearrangement, near-haploidy (< 34 chromosomes), acute undifferentiated leukemia, failure to achieve complete remission, or minimal residual disease greater than 10−2 (> 1000 blasts/100 000 mononuclear cells) at completion of induction.10,11 AR patients were all children without VLR or VHR characteristics, and they were subdivided in AR1 and AR2 patient groups.
The AR1 group consisted of all patients with B-cell lineage ALL with WBC counts below 100 × 109/L or with initial surreptitious or hemorrhagic cerebrospinal fluid (CSF) becoming negative at day 4 of the prephase. Patients with B-cell lineage ALL with WBC counts above 100 × 109/L or patients with T-cell lineage ALL were classified as AR2. All patients with gonadal involvement, overt or nonequivocal CNS involvement at diagnosis or any CNS involvement at day 4 were also included in the AR2 group.
Informed consent from the parents or the legal guardian was provided before entry in the study according to the Declaration of Helsinki. The protocol was approved by the EORTC Protocol Review Committee and by the local institutional ethical committees in each participating center.
Definitions
CNS disease was graded according to the classification proposed by Pui and Howard.12 CNS-1 was defined as no detectable blast cells in the CSF. CNS-2 accounted for fewer than 5 leucocytes/μL with detectable blast cells in the centrifuged preparation of CSF, and CNS-3 was for 5 or more leucocytes/μL with identifiable blast cells or the presence of ALL or NHL-related cranial nerve palsy.
Complete remission (CR) was defined as disappearance of all symptoms and physical and radiologic signs related to the leukemia or to the NHL in combination with fewer than 5% leukemic cells in the BM with hematologic recovery and absence of blasts in the CSF.
Remission failure was defined as failure to reach CR at the completion of consolidation. Relapse was defined as the reappearance of more than 20% leukemic cells in the BM or of any leukemic cell at another site.
Toxicity was graded according to World Health Organization criteria.13
Treatment
In this trial, the value of DEX versus PRED during induction and maintenance was evaluated, as well as the value of an increased number of administrations of L-asparaginase during consolidation (protocol IB) and during late intensification (protocol II) regarding outcome. Patients with AR characteristics were eligible for the third randomization: VCR + corticosteroid pulses or no pulses during continuation treatment.
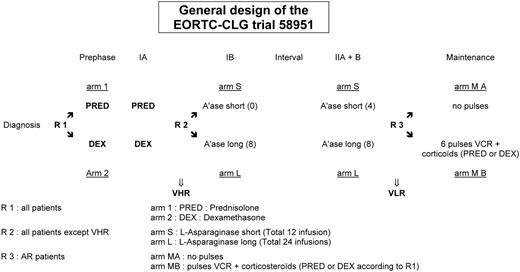
The protocol was based on a BFM-like protocol, without cranial or local irradiation. The general scheme of the protocol is shown in Figure 1. The strategy for AR patients is summarized in Table 1 and is based on induction-consolidation, CNS-directed therapy with high-dose methotrexate (MTX) and late intensification, followed by a continuation therapy of 74 weeks' duration.
EORTC-CLG treatment protocol for AR (AR1 and AR2) patients
| Drug . | Dose . | Days of administration . |
|---|---|---|
| Induction: protocol IA | ||
| Methotrexate (IT)* | 1 | |
| According to randomization | ||
| PRED (PO) | 60 mg/m2 | 1-7 (prephase) |
| DEX (PO) | 6 mg/m2 | 1-7 (prephase) |
| According to randomization | ||
| PRED (PO) | 60 mg/m2 | 8-28, tapered over 9 d |
| DEX (PO) | 6 mg/m2 | 8-28, tapered over 9 d |
| AR1 | ||
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Daunorubicin (IV) | 30 mg/m2 | 8, 15, 22, 29 |
| Triple chemotherapy (IT)* | 8, 22 | |
| Escherichia coli asparaginase | 10 000 IU/m2 | 12, 15, 18, 22, 25, 29, 32, 35 |
| AR2 | ||
| Cyclophosphamide | 1000 mg/m2 | 9 |
| Methotrexate (24 h)† | 5 g/m2 | 8 |
| Triple chemotherapy (IT)* | 9, 22 | |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Daunorubicin (IV) | 40 mg/m2 | 15, 22, 29 |
| E coli asparaginase | 10 000 IU/m2 | 12, 15, 18, 22, 25, 29,3 2, 35 |
| Consolidation: protocol IB | ||
| Cyclophosphamide (IV) | 1000 mg/m2 | 36, 63 |
| Cytarabine (IV) | 75 mg/m2 | 38-41, 45-48, 52-55, 59-62 |
| Triple chemotherapy (IT)* | 38, 52 | |
| 6-Mercaptopurine (PO) | 60 mg/m2 | 36-63 |
| According to randomization | ||
| No asparaginase | ||
| E coli asparaginase | 5000 IU/m2 | 38, 41, 45, 48, 52, 55, 59, 62 |
| Interval therapy | ||
| 6-Mercaptopurine (PO) | 25 mg/m2 | 1-56 |
| Methotrexate (24 h)† | 5000 mg/m2 | 8, 22, 36, 50 |
| Triple chemotherapy (IT)* | 9, 23, 37, 51 | |
| Late intensification: protocol II | ||
| DEX (PO) | 6 mg/m2 | 1-21, taper over 9 d |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Doxorubicin (IV) | 30 mg/m2 | 8, 15, 22, 29 |
| Triple chemotherapy (IT)* | 38 | |
| Cyclophosphamide (IV) | 1000 mg/m2 | 36 |
| Cytarabine (IV) | 75 mg/m2 | 38-41, 45-48 |
| 6-Thioguanine (PO) | 60 mg/m2 | 36-49 |
| According to randomization | ||
| E coli asparaginase | 10 000 IU/m2 | 8, 11, 15, 18 |
| E coli asparaginase | 10 000 IU/m2 | 8, 11, 15, 18 |
| 5000 IU/m2 | 22, 25, 29, 32 | |
| Maintenance (74 wk) | ||
| AR1 | ||
| 6-Mercaptopurine (PO) | 50 mg/m2 | Daily |
| Methotrexate (PO) | 20 mg/m2 | Weekly |
| Triple chemotherapy (IT)* | Every 70 d, starting D22, 6 times | |
| According to randomization | ||
| No pulses | ||
| PRED (PO)‡ | 60 mg/m2 | Every 70 d, for 7 d, starting D57-63, 6 times |
| DEX (PO)‡ | 6 mg/m2 | Every 70 d, for 7 d, starting D57-63, 6 times |
| Vincristine | 1.5 mg/m2 (max 2 mg) | Every 70 d, on D57 and D63, 6 times |
| AR2 | ||
| 6-Mercaptopurine (PO) | 50 mg/m2 | Daily |
| Methotrexate (PO) | 20 mg/m2 | Weekly |
| Methotrexate (24 h)† | 5000 mg/m2 | Every 70 d, starting D22, 6 times |
| Triple chemotherapy (IT)* | Every 70 d, starting D23, 6 times | |
| E coli asparaginase | 25 000 IU/m2 | Every 70 d, starting D23, 6 times |
| According to randomization | ||
| No pulses | ||
| PRED (PO)‡ | 60 mg/m2 | Every 70 d, for 7 d starting D57-63, 6 times |
| DEX (PO)‡ | 6 mg/m2 | Every 70 d, for 7 d, starting D57-63, 6 times |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | Every 70 d, on D57 and D63, 6 times |
| Drug . | Dose . | Days of administration . |
|---|---|---|
| Induction: protocol IA | ||
| Methotrexate (IT)* | 1 | |
| According to randomization | ||
| PRED (PO) | 60 mg/m2 | 1-7 (prephase) |
| DEX (PO) | 6 mg/m2 | 1-7 (prephase) |
| According to randomization | ||
| PRED (PO) | 60 mg/m2 | 8-28, tapered over 9 d |
| DEX (PO) | 6 mg/m2 | 8-28, tapered over 9 d |
| AR1 | ||
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Daunorubicin (IV) | 30 mg/m2 | 8, 15, 22, 29 |
| Triple chemotherapy (IT)* | 8, 22 | |
| Escherichia coli asparaginase | 10 000 IU/m2 | 12, 15, 18, 22, 25, 29, 32, 35 |
| AR2 | ||
| Cyclophosphamide | 1000 mg/m2 | 9 |
| Methotrexate (24 h)† | 5 g/m2 | 8 |
| Triple chemotherapy (IT)* | 9, 22 | |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Daunorubicin (IV) | 40 mg/m2 | 15, 22, 29 |
| E coli asparaginase | 10 000 IU/m2 | 12, 15, 18, 22, 25, 29,3 2, 35 |
| Consolidation: protocol IB | ||
| Cyclophosphamide (IV) | 1000 mg/m2 | 36, 63 |
| Cytarabine (IV) | 75 mg/m2 | 38-41, 45-48, 52-55, 59-62 |
| Triple chemotherapy (IT)* | 38, 52 | |
| 6-Mercaptopurine (PO) | 60 mg/m2 | 36-63 |
| According to randomization | ||
| No asparaginase | ||
| E coli asparaginase | 5000 IU/m2 | 38, 41, 45, 48, 52, 55, 59, 62 |
| Interval therapy | ||
| 6-Mercaptopurine (PO) | 25 mg/m2 | 1-56 |
| Methotrexate (24 h)† | 5000 mg/m2 | 8, 22, 36, 50 |
| Triple chemotherapy (IT)* | 9, 23, 37, 51 | |
| Late intensification: protocol II | ||
| DEX (PO) | 6 mg/m2 | 1-21, taper over 9 d |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Doxorubicin (IV) | 30 mg/m2 | 8, 15, 22, 29 |
| Triple chemotherapy (IT)* | 38 | |
| Cyclophosphamide (IV) | 1000 mg/m2 | 36 |
| Cytarabine (IV) | 75 mg/m2 | 38-41, 45-48 |
| 6-Thioguanine (PO) | 60 mg/m2 | 36-49 |
| According to randomization | ||
| E coli asparaginase | 10 000 IU/m2 | 8, 11, 15, 18 |
| E coli asparaginase | 10 000 IU/m2 | 8, 11, 15, 18 |
| 5000 IU/m2 | 22, 25, 29, 32 | |
| Maintenance (74 wk) | ||
| AR1 | ||
| 6-Mercaptopurine (PO) | 50 mg/m2 | Daily |
| Methotrexate (PO) | 20 mg/m2 | Weekly |
| Triple chemotherapy (IT)* | Every 70 d, starting D22, 6 times | |
| According to randomization | ||
| No pulses | ||
| PRED (PO)‡ | 60 mg/m2 | Every 70 d, for 7 d, starting D57-63, 6 times |
| DEX (PO)‡ | 6 mg/m2 | Every 70 d, for 7 d, starting D57-63, 6 times |
| Vincristine | 1.5 mg/m2 (max 2 mg) | Every 70 d, on D57 and D63, 6 times |
| AR2 | ||
| 6-Mercaptopurine (PO) | 50 mg/m2 | Daily |
| Methotrexate (PO) | 20 mg/m2 | Weekly |
| Methotrexate (24 h)† | 5000 mg/m2 | Every 70 d, starting D22, 6 times |
| Triple chemotherapy (IT)* | Every 70 d, starting D23, 6 times | |
| E coli asparaginase | 25 000 IU/m2 | Every 70 d, starting D23, 6 times |
| According to randomization | ||
| No pulses | ||
| PRED (PO)‡ | 60 mg/m2 | Every 70 d, for 7 d starting D57-63, 6 times |
| DEX (PO)‡ | 6 mg/m2 | Every 70 d, for 7 d, starting D57-63, 6 times |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | Every 70 d, on D57 and D63, 6 times |
IT indicates intrathecally; PO, orally; PRED, prednisolone; DEX, dexamethasone; IV, intravenously; and D/d, day.
Methotrexate: younger than 1 year, 6 mg; 1 year, 8 mg; 2 years, 10 mg; 3 years and older, 12 mg. Cytarabine: younger than 1 year, 15 mg; 1 year, 20 mg; 2 years 25 mg; 3 years and older, 30 mg. Hydrocortisone: younger than 1 year, 7.5 mg; 1 year, 10 mg; 2 years, 12.5 mg; 3 years and older, 15 mg.
Leucovorin rescue 12 mg/m2 every 6 hours starts at hour 36.
Same corticosteroid as initial randomization (protocol IA).
The AR patients in CR at the end of the late intensification were randomly assigned to receive or not to receive, in addition to daily 6-mercaptopurine (6-MP) and weekly MTX for all, 6 “pulses” at intervals of 10 weeks during the first 60 weeks of continuation therapy. The pulses consisted of 7 days of corticosteroids, either PRED 60 mg/m2/d or DEX 6 mg/m2/d depending on the first randomization, and VCR 1.5 mg/m2 on day 1 and day 7.
After 60 weeks, the same continuation chemotherapy, without pulses, was pursued during 14 more weeks. Because the addition of pulses and their toxicity could possibly jeopardize the continuous administration of 6-MP and MTX, to ensure that patients in both arms would nevertheless eventually receive the same cumulative dose of these drugs, the duration of continuous therapy was prolonged by the number of days during which the treatment had to be interrupted.
Statistical analysis
Patients were prospectively registered in the study at the time of diagnosis. At the time of beginning of continuation, patients eligible for the pulse question were randomly assigned at the EORTC Data Center, Brussels; randomization was stratified according to the treatment arm allocated at the first and second step, whether the prephase was randomized or not, risk group (AR1 or AR2), patient's sex, and center, using a minimization technique.
The primary end point was DFS for patients randomly assigned for the pulses or no pulses question. DFS was computed from the date of randomization until date of relapse or date of death or date last known to be alive (censored observation).
Overall survival (OS), secondary end point, was computed from date of randomization until date of death or date last known to be alive (censored observation). The additional secondary end point was evaluation of toxicity.
The Kaplan-Meier technique was used to compute the actuarial curve, and the SEs of the estimates were obtained by the Greenwood formula. The differences between curves were tested for statistical significance with the use of the 2-tailed log-rank test. To summarize the overall difference, the hazard ratio (HR) of having an event per time in one group versus the one in another group, along with its 95% or 99% confidence interval (CI), was estimated with the Cox proportional hazards model.14 All analyses were performed according to the intent-to-treat principle. The SAS 9.1 software (SAS Institute Inc) has been used for the statistical analyses.
Results
From June 1999 until November 2002, 411 consecutive AR patients (384 ALL and 27 NHL) included in the EORTC 58951 trial were randomly assigned either to receive or not to receive pulses of VCR and corticosteroids during maintenance. The proportion of these AR patients was approximately 74.4% of the whole population; 12.6% and 13% were VLR and VHR patients, respectively.
Of the 205 patients in the no pulses group, 101 (49.3%) had been initially randomly assigned for PRED and 100 (48.8%) for DEX. In the pulses group, 101 of 206 children (49.0%) had been previously randomly assigned for PRED and 101 of 206 children for DEX and received the same steroid as the one they were originally assigned to. Eight patients registered in the trial but not randomly assigned for the first question had been assigned to PRED in induction.
The distribution of patient and disease characteristics was quite well balanced in the 2 treatment groups (Table 2). A slight imbalance regarding the initial WBC count was noticed.
Patient and disease characteristics and previous randomized arm by treatment group
| . | No pulses, N = 205, n (%) . | Pulses, N = 206, n (%) . |
|---|---|---|
| EORTC risk group given at randomization | ||
| AR1 | 159 (77.6) | 160 (77.7) |
| AR2 | 46 (22.4) | 46 (22.3) |
| Sex | ||
| Female | 98 (47.8) | 100 (48.5) |
| Male | 107 (52.2) | 106 (51.5) |
| First randomization | ||
| PRED | 101 (49.3) | 101 (49.0) |
| DEX | 100 (48.8) | 101 (49.0) |
| Not randomized | 4 (2.0) | 4 (1.9) |
| Second randomization | ||
| Short asparaginase | 99 (48.3%) | 99 (48.1) |
| Long asparaginase | 94 (45.9%) | 94 (45.6) |
| Not randomized | 12 (5.9) | 13 (6.3) |
| Age, y | ||
| Younger than 1 | 0 (0.0) | 2 (1.0) |
| 1 to younger than 2 | 15 (7.3) | 14 (6.8) |
| 2 to younger than 6 | 98 (47.8) | 81 (39.3) |
| 6 to younger than 10 | 42 (20.5) | 51 (24.8) |
| 10 or older | 50 (24.4) | 58 (28.2) |
| WBC count, ×109/L | ||
| Fewer than 25 | 141 (68.8) | 164 (79.6) |
| 25 to fewer than 100 | 51 (24.9) | 28 (13.6) |
| 100 or more than 100 | 13 (6.8) | 14 (6.8) |
| NCI risk group | ||
| Standard risk | 134 (65.4) | 128 (62.1) |
| High risk | 71 (34.6) | 78 (37.9) |
| Disease | ||
| ALL | 195 (95.1) | 189 (91.7) |
| NHL | 10 (4.9) | 17 (8.3) |
| Immunophenotype | ||
| B-lineage | 175 (85.4) | 172 (83.5) |
| T-lineage | 30 (14.6) | 34 (16.5) |
| CSF status12 | ||
| CNS-1 | 180 (87.8) | 184 (89.3) |
| CNS-2 + TLP | 22 (10.7) | 21 (10.2) |
| CNS-3 | 3 (1.5) | 1 (0.5) |
| . | No pulses, N = 205, n (%) . | Pulses, N = 206, n (%) . |
|---|---|---|
| EORTC risk group given at randomization | ||
| AR1 | 159 (77.6) | 160 (77.7) |
| AR2 | 46 (22.4) | 46 (22.3) |
| Sex | ||
| Female | 98 (47.8) | 100 (48.5) |
| Male | 107 (52.2) | 106 (51.5) |
| First randomization | ||
| PRED | 101 (49.3) | 101 (49.0) |
| DEX | 100 (48.8) | 101 (49.0) |
| Not randomized | 4 (2.0) | 4 (1.9) |
| Second randomization | ||
| Short asparaginase | 99 (48.3%) | 99 (48.1) |
| Long asparaginase | 94 (45.9%) | 94 (45.6) |
| Not randomized | 12 (5.9) | 13 (6.3) |
| Age, y | ||
| Younger than 1 | 0 (0.0) | 2 (1.0) |
| 1 to younger than 2 | 15 (7.3) | 14 (6.8) |
| 2 to younger than 6 | 98 (47.8) | 81 (39.3) |
| 6 to younger than 10 | 42 (20.5) | 51 (24.8) |
| 10 or older | 50 (24.4) | 58 (28.2) |
| WBC count, ×109/L | ||
| Fewer than 25 | 141 (68.8) | 164 (79.6) |
| 25 to fewer than 100 | 51 (24.9) | 28 (13.6) |
| 100 or more than 100 | 13 (6.8) | 14 (6.8) |
| NCI risk group | ||
| Standard risk | 134 (65.4) | 128 (62.1) |
| High risk | 71 (34.6) | 78 (37.9) |
| Disease | ||
| ALL | 195 (95.1) | 189 (91.7) |
| NHL | 10 (4.9) | 17 (8.3) |
| Immunophenotype | ||
| B-lineage | 175 (85.4) | 172 (83.5) |
| T-lineage | 30 (14.6) | 34 (16.5) |
| CSF status12 | ||
| CNS-1 | 180 (87.8) | 184 (89.3) |
| CNS-2 + TLP | 22 (10.7) | 21 (10.2) |
| CNS-3 | 3 (1.5) | 1 (0.5) |
AR indicates average risk; PRED, prednisolone; DEX, dexamethasone; WBC, white blood cell; NCI, National Cancer Institute; CSF, cerebrospinal fluid; CNS, central nervous system; and TLP, traumatic lumbar puncture.
Treatment applicability
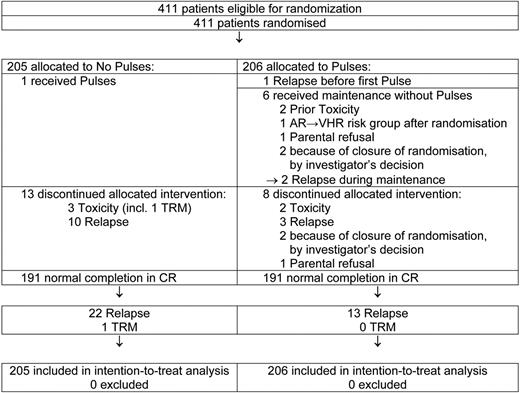
Among 205 patients allocated to the no pulses group, 1 received 6 pulses by mistake (Figure 2). A total of 191 patients completed the continuation therapy period; 10 discontinued maintenance because of an early relapse and 3 stopped treatment because of excessive toxicity (infection, hepatotoxicity, death due to varicella).
Among 206 patients allocated to the pulses group, 7 did not receive pulses. One child had an early relapse, 5 weeks after the randomization, and the first pulse should start on day 57. Two children did not start pulses because of previous toxicity; for one case the risk group changed from AR to VHR after randomization, and for another patient the parents refused the administration of pulses. Finally, 2 children were randomly assigned right before the amendment (closure of the third randomization) arrived, and their physician decided not to give pulses during maintenance.
A total of 191 patients completed the continuation therapy period with pulses and 8 discontinued the therapy period: 3 had an early relapse, 2 stopped pulses because of excessive toxicity (infection), 1 refused further pulses, and in 2 cases the randomization period ended after the fourth pulse and the physicians did not give the fifth and sixth pulses.
The mean daily administered dose of 6-MP (50 mg/m2/d) and MTX (20 mg/m2/wk) was not influenced by the administration of pulses. Their total cumulative doses were not substantially affected and were close to the expected doses (data not shown).
Efficacy
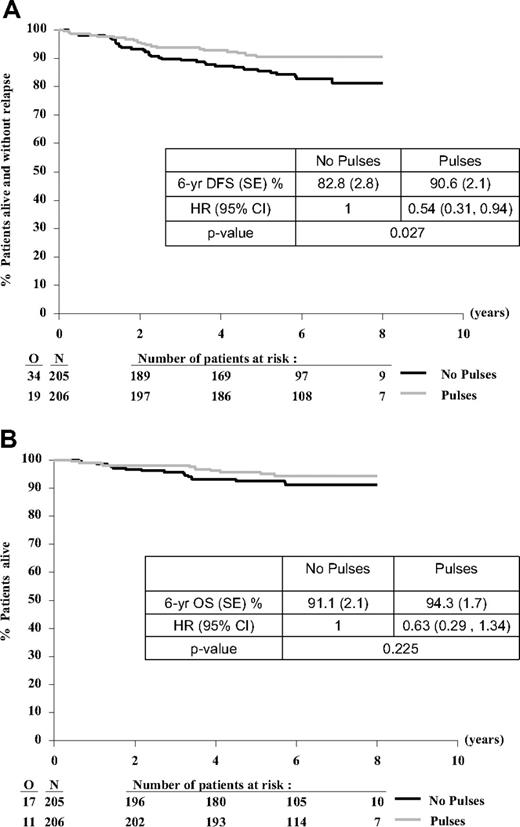
The DFS and OS curves, with a median follow-up of 6.3 years, are shown in Figure 3A and B. The 6-year DFS rate from randomization was 90.6% (SE, 2.1%) in the pulses group and 82.8% (SE, 2.8%) in the no pulses group (HR = 0.54; 95% CI, 0.31-0.94; 2-sided log-rank P = .027). There were 19 versus 34 DFS events for the pulses versus no pulses comparison: BM relapses (10 vs 16), CNS relapses (1 vs 4), another isolated relapse (2 vs 3), combined BM + CNS relapses (2 vs 5), combined BM + other relapses (4 vs 4), death in CR (0 vs 2) (Table 3). One child died of a varicella infection in first CR during continuation phase and another child had a secondary myelodysplasia and died of treatment-related mortality (TRM) after stem cell transplantation. The 6-year OS rate was 94.3% (SE, 1.7%) in the pulses group and 91.1% (SE, 2.1%) in the no pulses group (HR = 0.63; 95% CI, 0.29-1.34; 2-sided log-rank P = .225).
Treatment comparison for DFS and OS from time of randomization. (A) DFS and (B) OS. O indicates observed number of events; and N, number of patients randomly assigned.
Treatment comparison for DFS and OS from time of randomization. (A) DFS and (B) OS. O indicates observed number of events; and N, number of patients randomly assigned.
Type of first event and survival status by treatment group
| . | No pulses, N = 205, n (%) . | Pulses, N = 206, n (%) . |
|---|---|---|
| DFS status | ||
| CCR | 171 (83.4) | 187 (90.8) |
| Relapse | 32 (15.6) | 19 (9.2) |
| BM | 16 (7.8) | 10 (4.9) |
| CNS | 4 (2.0) | 1 (0.5) |
| Other isolated | 3 (1.5) | 2 (1.0) |
| BM + CNS | 5 (2.4) | 2 (1.0) |
| BM + other | 4 (2.0) | 4 (1.9) |
| Death without relapse | 2 (1.0) | 0 |
| Survival status | ||
| Alive | 188 (91.7) | 195 (94.7) |
| Dead | 17 (8.3) | 11 (5.3) |
| . | No pulses, N = 205, n (%) . | Pulses, N = 206, n (%) . |
|---|---|---|
| DFS status | ||
| CCR | 171 (83.4) | 187 (90.8) |
| Relapse | 32 (15.6) | 19 (9.2) |
| BM | 16 (7.8) | 10 (4.9) |
| CNS | 4 (2.0) | 1 (0.5) |
| Other isolated | 3 (1.5) | 2 (1.0) |
| BM + CNS | 5 (2.4) | 2 (1.0) |
| BM + other | 4 (2.0) | 4 (1.9) |
| Death without relapse | 2 (1.0) | 0 |
| Survival status | ||
| Alive | 188 (91.7) | 195 (94.7) |
| Dead | 17 (8.3) | 11 (5.3) |
DFS indicates disease-free survival; CCR, continuous complete remission; BM, bone marrow; and CNS, central nervous system.
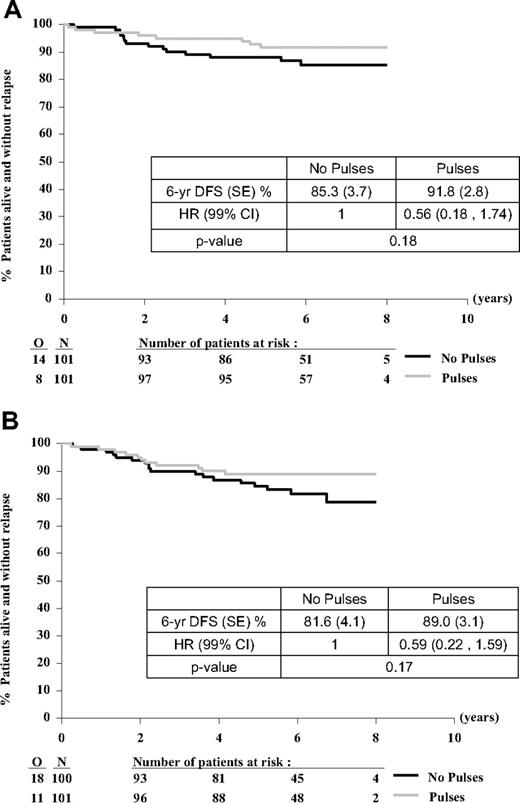
The effect of pulses on DFS was similar in the PRED group (HR = 0.56; 99% CI, 0.18-1.74; P = .18) and the DEX group (HR = 0.59; 99% CI, 0.22-1.59; P = .17) (Figure 4A-B; Table 4).
Treatment comparison for DFS from time of randomization according to type of corticosteroid (allocated at first randomization). PRED (A) or DEX (B). O indicates observed number of events (relapse or death without relapse); and N, number of patients randomly assigned.
Treatment comparison for DFS from time of randomization according to type of corticosteroid (allocated at first randomization). PRED (A) or DEX (B). O indicates observed number of events (relapse or death without relapse); and N, number of patients randomly assigned.
Type of first event and survival status by treatment group according to type of corticosteroid (allocated at first randomization)
| . | PRED . | DEX . | ||
|---|---|---|---|---|
| No pulses (N = 101) . | Pulses (N = 101) . | No pulses (N = 100) . | Pulses (N = 101) . | |
| DFS status | ||||
| CCR | 87 | 93 | 82 | 90 |
| Relapse | 14 | 8 | 16 | 11 |
| BM | 5 | 3 | 10 | 7 |
| CNS | 3 | 1 | 1 | 0 |
| Other isolated | 1 | 1 | 2 | 1 |
| BM + CNS | 3 | 1 | 1 | 1 |
| BM + other | 2 | 2 | 2 | 2 |
| Death without relapse | 0 | 0 | 2 | 0 |
| Survival status | ||||
| Alive | 94 | 97 | 91 | 94 |
| Dead | 7 | 4 | 9 | 7 |
| . | PRED . | DEX . | ||
|---|---|---|---|---|
| No pulses (N = 101) . | Pulses (N = 101) . | No pulses (N = 100) . | Pulses (N = 101) . | |
| DFS status | ||||
| CCR | 87 | 93 | 82 | 90 |
| Relapse | 14 | 8 | 16 | 11 |
| BM | 5 | 3 | 10 | 7 |
| CNS | 3 | 1 | 1 | 0 |
| Other isolated | 1 | 1 | 2 | 1 |
| BM + CNS | 3 | 1 | 1 | 1 |
| BM + other | 2 | 2 | 2 | 2 |
| Death without relapse | 0 | 0 | 2 | 0 |
| Survival status | ||||
| Alive | 94 | 97 | 91 | 94 |
| Dead | 7 | 4 | 9 | 7 |
PRED indicates prednisone; DEX, dexamethasone; DFS, disease-free survival; CCR, continuous complete remission; BM, bone marrow; and CNS, central nervous system.
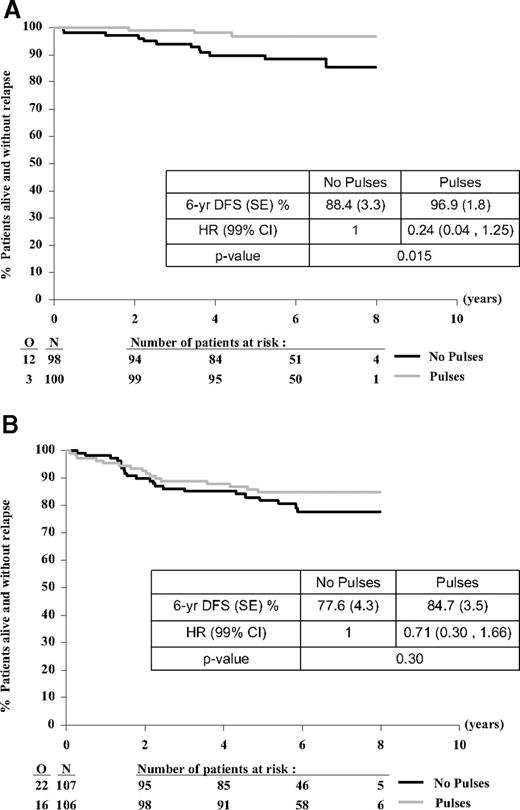
Overall, girls have a better outcome than boys with a 6-year DFS rate of 92.6% compared with 81.2% (HR = 0.40; 95% CI, 0.22-0.73; P = .002) and an OS of 95.7% compared with 90.0% (HR = 0.43; 95% CI, 0.19-0.97; P = .035). The pulses effect seemed to be more pronounced in girls (HR = 0.24; 99% CI, 0.04-1.25; P = .015) than in boys (HR = 0.71; 99% CI, 0.30-1.66; P = .30) (Figure 5A-B). Remarkably, there seems to be a sex effect for the compartment in which the pulses effect was noticed. In girls the pulses effect was entirely because of a reduction of BM relapses, whereas the incidence of (isolated) CNS relapse was low in both arms. In contrast, in boys, the pulses reduced the incidence of isolated CNS and combined CNS relapses, whereas the BM relapses remained similar in the 2 arms (Table 5).
Treatment comparison for DFS from time of randomization according to patient's sex. Girl (A) or boy (B). O indicates observed number of events (relapse or death without relapse); and N, number of patients randomly assigned.
Treatment comparison for DFS from time of randomization according to patient's sex. Girl (A) or boy (B). O indicates observed number of events (relapse or death without relapse); and N, number of patients randomly assigned.
Type of first event and survival status by treatment group according to patient's sex
| . | Girls . | Boys . | ||
|---|---|---|---|---|
| No pulses (N = 98) . | Pulses (N = 100) . | No pulses (N = 107) . | Pulses (N = 106) . | |
| DFS status | ||||
| CCR | 86 | 97 | 85 | 90 |
| Relapse | 11 | 3 | 21 | 16 |
| BM | 7 | 1 | 9 | 9 |
| CNS | 1 | 0 | 4 | 1 |
| Other isolated | 0 | 0 | 2 | 2 |
| BM + CNS | 1 | 2 | 4 | 0 |
| BM + other | 2 | 0 | 2 | 4 |
| Death without relapse | 1 | 0 | 1 | 0 |
| Survival status | ||||
| Alive | 92 | 98 | 96 | 97 |
| Dead | 6 | 2 | 11 | 9 |
| . | Girls . | Boys . | ||
|---|---|---|---|---|
| No pulses (N = 98) . | Pulses (N = 100) . | No pulses (N = 107) . | Pulses (N = 106) . | |
| DFS status | ||||
| CCR | 86 | 97 | 85 | 90 |
| Relapse | 11 | 3 | 21 | 16 |
| BM | 7 | 1 | 9 | 9 |
| CNS | 1 | 0 | 4 | 1 |
| Other isolated | 0 | 0 | 2 | 2 |
| BM + CNS | 1 | 2 | 4 | 0 |
| BM + other | 2 | 0 | 2 | 4 |
| Death without relapse | 1 | 0 | 1 | 0 |
| Survival status | ||||
| Alive | 92 | 98 | 96 | 97 |
| Dead | 6 | 2 | 11 | 9 |
DFS indicates disease-free survival; CCR, continuous complete remission; BM, bone marrow; and CNS, central nervous system.
Two hundred forty-seven patients corresponded to IR criteria used in the intergroup trial9 (I-BFM-SG ALL IR 95), ie, WBC count of 20 × 109/L or greater and age younger than 1 year or 6 years or older. One hundred twenty-eight and 119 of these patients were randomly assigned for the VCR + PRED pulses and for the VCR + DEX pulses, respectively. In IR patients there was an advantage of pulses on DFS, both in the VCR + PRED group (HR = 0.28;99% CI, 0.06-1.22) as in the VCR + DEX group (HR = 0.51; 99% CI, 0.16-1.69). (Additional data provided on the Web site; curves and 6-year DFS rates: PRED arm, 93.7% [SE, 3.0%] vs 78.0% [SE, 5.5%]; DEX arm: 87.4% [SE, 4.4%] vs 77.0% [SE, 5.8%]; supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Toxicity
Data on toxicity, according to National Cancer Institute common toxicity criteria, were available for all patients: 206 patients in the pulses group and 205 in the no pulses group. Grade 3 to 4 hepatic toxicity rate was lower in the pulses group (30% vs 40%). However, a higher grade 2 to 3 osteonecrosis rate was found in the pulses group (n = 9; 4.4%) than in the no pulses group (n = 4, 2%) as well as a higher rate of pancreas enzyme elevation (n = 10, 4.9% vs n = 6, 2.9%) and of grade 3 to 4 infections (n = 29, 14.1%, and n = 20, 9.8%). Symptomatic acute pancreatitis requiring medical intervention was not seen.
Discussion
Although a subset of the patients described has been included previously in the I-BFM intergroup trial9 that examined the value of VCR + DEX pulses in continuation therapy, the EORTC-CLG presents its findings on VCR + corticosteroid pulses in patients with AR characteristics after a longer follow-up.
Previous studies have shown that additional therapy during maintenance improved the outcome of patients treated by nonintensive protocols.3,5,6 In the late 1990s, at a time when AR groups of patients treated by BFM-based protocols had an event-free survival of approximately 75% (ie, 15%-20% higher than in the early 1980s), the question raised whether the benefit provided by the addition of pulses in continuation therapy would hold on in patients treated with current, more intensive, regimens. The AR group of patients experiencing the greatest cumulative incidence of relapses in absolute numbers was believed to be the most appropriate patient group for the addition of pulses to the basic continuation treatment.
Because of the lower incidence of CNS relapse in patients treated with DEX15 and the favorable result of the moderately intensive Dutch Childhood Leukemia Study Group Total Therapy-based ALL VI trial, applying frequent VCR + DEX pulses in non–high-risk ALL patients,7 the EORTC-CLG as well as several groups substituted DEX for PRED in induction and maintenance.9,16-18 Because the expected number of AR patients randomly assigned for the pulses question was expected to be too low for detecting with a sufficient statistical power a relatively small difference between the 2 treatment arms, this randomization was closed in 2002. The EORTC-CLG group participated in the prospective intergroup study of the I-BFM and 81 patients with IR characteristics randomly assigned to receive DEX pulses were included in this intergroup trial (I-BFM-SG ALL IR 95). Conter et al9 reported an overall 4-year DFS rate of 80.6% (SE, 0.8%) in IR patients, and no additional benefit by VCR + DEX pulses on DFS was seen.
In the EORTC-CLG 58951 trial, the overall 6-year DFS rate for AR patients was 82.8% (SE, 2.8%). Despite this result, administration of VCR + corticosteroid pulses led to an improvement of DFS, ie 90.6% (SE, 2.1%), which is in contrast with the results of the I-BFM intergroup trial. The effect on DFS was similar for VCR + PRED pulses and for VCR + DEX pulses. The slight imbalance of the initial WBC count between the 2 treatment arms had no real effect because treatment comparison adjustments for possible confounding factors (sex, WBC count, risk group) yielded results that were similar to the overall result (unadjusted comparison; data not shown).
Our data suggest that, even after major improvement of treatment results obtained through intensification of early therapy courses, a modification of continuation therapy may further improve the long-term outcome of the patients. The difference in terms of OS was less important and was not statistically significant. This may be because of the effect of second-line treatment after relapse or to a lack of statistical power in our series.
The difference in patient follow-up time might partially explain the discrepancies between our results and those of the I-BFM intergroup trial. The median follow-up of the EORTC patients in the intergroup trial was only 3.3 years, whereas the current DFS result was found after a median follow-up of 6 years. Moreover, in the intergroup trial, some heterogeneity in outcome was noticed between participating treatment groups, particularly higher TRM was seen in the pulses arm of some participating groups.
Subgroup analyses suggested that girls did better than boys in the control arm and that the beneficial effect of pulses was greater in girls than in boys. Moreover, our data indicate a preferential benefit of pulses on occurrence of BM relapses in girls and on isolated plus combined CNS relapses in boys. These results have to be confirmed in larger patient groups.
The dose intensity of 6-MP is proven to be an important pharmacologic factor influencing treatment outcome.19 The addition of pulses did not jeopardize the continuous administration of 6-MP and MTX in our study. Moreover, to ensure that patients in both arms would receive the same cumulative dose of these drugs, the duration of continuous therapy was prolonged by the number of days during which the treatment had to be interrupted. In the EORTC-CLG 58951 trial, the duration of maintenance was strictly adjusted after an interruption of chemotherapy, which was not always the case in the different study groups participating in the I-BFM meta-analysis.
Both VCR and steroids can add to the toxic effects of continuation therapy.20-22 There was no influence of the administration of pulses on behavior, and only a slight enhancement in occurrence of grade 3 to 4 infections, osteonecrosis, and elevated pancreas enzymes were noted. A recent report on the DCOG-ALL 9 trial,20 using intensive VCR + DEX pulses, alerts to a higher frequency of infectious deaths. In our study, only 2 patients died of infection/TRM, and these children were randomly assigned not to receive pulses.
In conclusion, VCR + steroid pulses during maintenance therapy are of benefit after initial intensive chemotherapy in patients with average risk characteristics and improve DFS after long follow-up, without increasing toxic effects. The EORTC-CLG suggests reintroducing pulses in BFM-based protocols for AR patients.
Presented in abstract form at the 50th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 7, 2008.23
The online version of this article contains a data supplement.
Acknowledgments
We thank the EORTC-CLG study group for its participation in the study and the EORTC data managers (Gabriel Solbu, Murielle Dardenne, Christine Waterkeyn, and others) involved in the 58951 trial.
This work was supported by the National Cancer Institute (grants 5U10 CA11488-29 through 5U10 CA11488-39), by TéléVie (grant no. 7.4561.01), and by donations from the Vlaamse Liga Tegen Kanker, the Belgian Federation Against Cancer, the Kinderkankerfonds from Belgium, and the EORTC Charitable Trust.
The content of this article is solely the responsibility of the authors and does not necessarily reflect the official views of the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: B.D.M. checked the data and wrote the manuscript; S.S. was the study statistician and analyzed the data and designed the figures and tables; Y. Bertrand and Y. Benoit are previous and present chairmen of the EORTC-CLG; L.B. was the coordinating study physician at the EORTC headquarters; Y. Bertrand, Y. Benoit, and L.B. planned and coordinated the study; J.O. designed and coordinated the 58951 trial and supervised and checked data, analyses, and reports, and carefully reviewed this paper; B.D.M., Y. Bertrand., F. Mazingue, A.R., A.U., K.Y., A.F., G.M., P.L., M.M., N.S., L.N., P.B., D.P., F. Millot, P.P., Y. Benoit, and J.O. included patients in the trial and provided clinical data. All authors participated in study supervision, participated in data interpretation, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Children's Leukemia Group of the European Organisation for Research and Treatment of Cancer appears in the Appendix below.
Correspondence: Barbara De Moerloose, Ghent University Hospital, Department of Pediatric Hematology-Oncology, De Pintelaan 185, B-9000 Ghent, Belgium; e-mail: barbara.demoerloose@ugent.be.
Appendix
Participating institutions and investigators of EORTC-CLG include the following: EORTC Data Center, Brussels: L. Baila, S. Suciu; CHR La Citadelle, Liège: M. F. Dresse, C. Hoyoux; CHR, Saint-Jacques Hospital, Besançon: V. Laithier, E. Plouvier, P. Rohrlich; CHR Hôtel-Dieu, Nantes: F. Méchinaud; CHR La Tronche, Grenoble: D. Plantaz; CHR, Lille: M. Fournier, F. Mazingue, B. Nelken; CHRU, Caen: P. Boutard, O. Minckes; CHU, Angers: X. Rialland; CHU de Purpan, Toulouse: N. Dastugue, G. Plat, A. Robert; CHU Hôpital Bernard, Poitiers: F. Millot; CHC-Espérance, Montegnée: N. Francotte, P. Philippet; CHU de Nice, Hôpital de l'Archet, Nice: A. Deville, M. Poirée, N. Sirvent, C. Soler; Hôpital Universitaire Hautepierre, Strasbourg: P. Lutz; Hôpital Américain, Reims: C. Béhar, M. Munzer; Hôpital Robert Debré, Paris: D. Adjaoud, H. Cavé, J. H. Dalle, B. Lescoeur, K. Yakouben; Hôpital Reine Fabiola, Brussels: C. Devalck, A. Ferster; Hôpital A. De Villeneuve, Montpellier: G. Margueritte; Hôpital Debrousse, Lyon: Y. Bertrand, S. Girard, K. Kebaili, N. Philippe; Hospital Escolar San Joao, Porto: L. Norton; IPO Gentil, Porto: S. Borges; UZ Brussel, Brussels: J. Otten, A. Van Damme, J. Van der Werff; UZ Gent, Ghent: Y. Benoit, B. De Moerloose, C. Dhooge, G. Laureys; UZ Gasthuisberg, Leuven: M. Renard, A. Uyttebroeck; ZNA Middelheim, Antwerp: S. Bravo, P. Maes.