Abstract
This prospective study evaluated the risk of arterial thrombosis in 195 consecutive patients aged 18 to 65 years with newly diagnosed multiple myeloma (MM). All patients were treated with 3 cycles of VAD (vincristine, doxorubicin, and dexamethasone) or TAD (thalidomide-AD) or PAD (bortezomib-AD) in national trials, followed by high-dose melphalan and autologous stem cell transplantation. For a period of 522 patient-years, 11 of the 195 patients (5.6%) developed arterial thrombosis. The highest incidence was seen during induction chemotherapy courses. Median age at onset of arterial thrombosis was 59 years (range, 43-65 years). Hypertension and smoking were significantly associated with arterial thrombosis with a relative risk of 11.7 (2.23-61.2) and 15.2 (1.78-130), respectively. Factor VIII levels (FVIII:C) correlated significantly with age (P = .02) and higher International Scoring System (ISS) stage (P = .001). A higher FVIII:C was associated with arterial thrombosis (hazard ratio [HR] = 1.85; 95% confidence interval [CI] = 0.99-3.47) after adjustment for age, ISS score, and assigned treatment arm. MM patients have an increased risk for arterial thrombotic events during and after induction chemotherapy. Hypertension, smoking, and high factor VIII levels, possibly reflecting disease activity, contribute to the risk of arterial thrombosis.
Introduction
Patients with multiple myeloma (MM) are at high risk for venous thromboembolism.1 Thrombotic complications are most frequently seen in patients treated with thalidomide-based regimens. Several other risk factors for venous thromboembolism have been identified including abnormalities in coagulation factors, such as high factor VIII levels (FVIII:C), activated protein C resistance, and hypofibrinolysis.2-4 Only a few cases of arterial thrombosis have been reported in multiple myeloma patients and the true incidence of arterial thrombosis in MM is not known.5-9 Case reports of patients treated with thalidomide suggested that arterial thrombosis may be an adverse effect of this drug, which has been reported to increase the rate of venous thromboembolism. We performed a prospective analysis of arterial thrombosis in a cohort of newly diagnosed MM patients who were transplantation candidates and were included in national trials for induction chemotherapy followed by high-dose melphalan (HDM) with autologous stem cell transplantation. Data were assessed at entry to identify a possible association of cardiovascular and thrombophilic risk factors with the development of arterial thrombosis in MM patients
Methods
A total of 195 consecutive patients with untreated MM aged 18 to 65 years and admitted to the department of Hematology of the Erasmus MC Rotterdam, an academic tertiary referral hospital in The Netherlands were included. One hundred patients participated in the multicenter HOVON 50 trial, a prospective randomized phase 3 study comparing TAD (3 cycles of thalidomide, doxorubicin, and dexamethasone) with VAD (vincristine-AD), followed by HDM and maintenance therapy with interferon alpha (3 × 106 IU, 3 times week) or thalidomide 50 mg daily until relapse or progression,10 and 95 patients participated in the multicenter HOVON 65 trial, a prospective randomized phase 3 study, which compared 3 cycles of PAD (bortezomib, doxorubicin, and dexamethasone) with VAD, followed by HDM and autologous stem cell transplantation, followed by thalidomide 50 mg daily (VAD arm) or bortezomib 1.3 mg/m2 once every 2 weeks (PAD arm) for 2 years.11 Patients in the HOVON 50 study received thrombosis prophylaxis consisting of subcutaneous low-molecular-weight heparin (LMWH), nadroparin 2850 anti-Xa IU (or 5700 anti-Xa in case of body weight more than 90 kg) during TAD. No thrombosis prophylaxis was given during VAD. Patients were staged according to International Scoring System (ISS) criteria.12 The medical ethics committee of the Erasmus Medical Center approved the study, and written informed consent was obtained from all participants. The trials were performed according to the Declaration of Helsinki. The trials were registered as ISRCTN06413384 (HOVON 50) and ISRCTN64455289 (HOVON 65).
Data on cardiovascular risk factors were collected at diagnosis. Smoking was scored positive if the patient was smoking at diagnosis or had stopped smoking less than 10 years before inclusion. Hypertension, hypercholesterolemia, and diabetes mellitus were scored positive when stated in the medical information or when treated for this condition. Coronary and peripheral arterial disease had to be symptomatic and angiographically proven, whereas myocardial infarction had to be diagnosed according to clinical, enzymatic, and electrocardiographic criteria, with the exception of a documented silent myocardial infarction that was diagnosed based on electrocardiographic changes in patients with a normal electrocardiogram at study entrance. Ischemic stroke was defined as the onset of rapidly developing symptoms and signs of loss of cerebral function that lasted at least 24 hours and had no apparent nonvascular cause. Furthermore, it had to be confirmed by computed tomography or magnetic resonance imaging. If a cerebral event resolved completely within 24 hours without signs of cerebral lesions on scanning, it was classified by a neurologist as a transient ischemic attack. Blood samples were collected at time of diagnosis before start of treatment in 123 (63%) patients, and at a median of 3 months (range, 1-36 months) after start of treatment in55 (28%) of 195 patients. Venous blood was collected using a vacutainer system in citrate (0.105 M; Becton Dickinson) and centrifuged at 4°C at 2000g for 10 minutes. The collected plasma was additionally centrifuged for 10 minutes at 20 000g and stored in small aliquots at −70°C until use. Genomic DNA was isolated from the white cell fraction of citrated blood using a standard salting out procedure. FVIII:C was measured by a 1-stage clotting assay using Platelin (Organon; Teknika) and factor VIII–deficient plasma (Biopool). Von Willebrand factor antigen levels (VWF:Ag) were measured by an in-house sandwich enzyme-linked immunosorbent assay using rabbit anti–human VWF and horseradish peroxidase–conjugated anti–human VWF (DakoCytomation). All assays were calibrated with pooled normal plasma (factor assay control plasma; George King Bio-Medical). Fibrinogen was measured as described by Clauss.13 Antithrombin activity levels were determined using a chromogenic substrate. For the measurement of protein S, we used an assay (Staclot Protein S; Diagnostica Stago) that is known not be affected by FVIII when FVIII levels are less than 250%.14 The prothrombin 20210G>A gene variant and the factor V Leiden mutation were determined by polymerase chain reaction.
Continuous variables were summarized as median values and ranges; categoric data, as frequencies and percentages. Correlations between patient characteristics at enrollment were calculated with the Spearman rank correlation test. Univariate and multivariate Cox regression analysis was used to evaluate the impact of baseline characteristics on (time to) arterial thrombosis. All P values are 2-sided, and P values of .05 or less were considered statistically significant.
Results
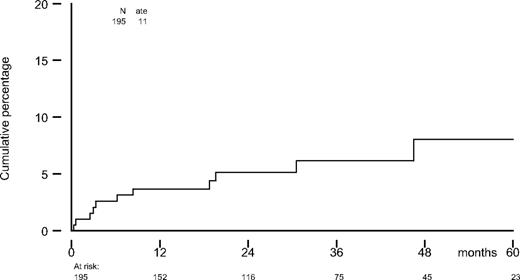
A total of 195 consecutive patients with MM was included. Median age was 56 years (range, 29-65 years), and 58% were males. According to ISS criteria, 47% had stage I; 29%, stage II; and 24%, stage III. For a period of 522 patient-years, 11 patients (5.6%) developed arterial thrombosis (Figure 1). Median age at onset of arterial thrombosis was 59 years (range, 43-65 years; Table 1). Of 11 events, 5 occurred during the induction courses (1 during TAD, 2 during PAD, and 2 during VAD). One event occurred between the induction courses and high-dose melphalan. All other events (n = 5) took place after HDM, of which 2 were during interferon alpha maintenance therapy (Table 2).
Cumulative incidence of arterial thrombosis in 195 multiple myeloma patients.
Characteristics of 195 multiple myeloma patients
| . | ATE, n = 11 . | No ATE, n = 184 . |
|---|---|---|
| Male sex, no. (%) | 9 (82) | 104 (57) |
| Median age at enrollment, y (range) | 58 (42-62) | 56 (29-65) |
| B2 microglobulin, mg/L | 2.4 (0.8-44.0) | 3.4 (1.4-11.8) |
| Monoclonal protein, IgD, IgA, and IgG, no. (%) | ||
| Kappa | 10 (91) | 127 (69) |
| Lambda | 1 (9) | 51 (28) |
| Unknown | 0 | 6 (3) |
| ISS stage, no. (%) | ||
| I | 5 (63) | 63 (46) |
| II | 2 (25) | 39 (29) |
| III | 1 (13) | 34 (25) |
| Treatment, no. (%) | ||
| VAD | 6 (55) | 97 (53) |
| TAD | 2 (27) | 42 (23) |
| PAD | 3 (27) | 45 (24) |
| Durie-Salmon, no. (%) | ||
| II | 2 (18) | 33 (18) |
| III | 9 (82) | 148 (81) |
| Long-term VKA or therapeutic LMWH,* no. (%) | 4 (36) | 24 (13) |
| Long-term aspirin,* no. (%) | 1 (9) | 7 (4) |
| Fibrinogen, g/L (range) | 3.50 (2.2-6.2) | 3.50 (1.60-7.70) |
| Factor VIII:C, IU/mL (range) | 2.93 (1.16-6.45) | 2.15 (0.74-6.48) |
| VWF Ag, U/mL (range) | 2.54 (0.88-7.26) | 2.09 (0.44-8.12) |
| Protein C activity, IU/mL (range) | 1.00 (0.79-1.54) | 0.97 (0.45-2.03) |
| Protein S activity, IU/mL (range) | 0.86 (0.44-1.37) | 0.76 (0.26-1.81) |
| Antithrombin, IU/mL (range) | 1.05 (0.73-1.50) | 0.99 (0.51-1.52) |
| PT 20210G>A, no. (%) | 0 (0) | 7 (5) |
| Factor V Leiden, no. (%) | 0 (0) | 5 (3) |
| Diabetes, no. (%) | 2 (18) | 12 (7) |
| Hypertension, no. (%) | 6 (55) | 40 (22) |
| Hyperlipidemia, no. (%) | 1 (9) | 12 (7) |
| Smoking history, no. (%) | 7 (64) | 42 (23) |
| . | ATE, n = 11 . | No ATE, n = 184 . |
|---|---|---|
| Male sex, no. (%) | 9 (82) | 104 (57) |
| Median age at enrollment, y (range) | 58 (42-62) | 56 (29-65) |
| B2 microglobulin, mg/L | 2.4 (0.8-44.0) | 3.4 (1.4-11.8) |
| Monoclonal protein, IgD, IgA, and IgG, no. (%) | ||
| Kappa | 10 (91) | 127 (69) |
| Lambda | 1 (9) | 51 (28) |
| Unknown | 0 | 6 (3) |
| ISS stage, no. (%) | ||
| I | 5 (63) | 63 (46) |
| II | 2 (25) | 39 (29) |
| III | 1 (13) | 34 (25) |
| Treatment, no. (%) | ||
| VAD | 6 (55) | 97 (53) |
| TAD | 2 (27) | 42 (23) |
| PAD | 3 (27) | 45 (24) |
| Durie-Salmon, no. (%) | ||
| II | 2 (18) | 33 (18) |
| III | 9 (82) | 148 (81) |
| Long-term VKA or therapeutic LMWH,* no. (%) | 4 (36) | 24 (13) |
| Long-term aspirin,* no. (%) | 1 (9) | 7 (4) |
| Fibrinogen, g/L (range) | 3.50 (2.2-6.2) | 3.50 (1.60-7.70) |
| Factor VIII:C, IU/mL (range) | 2.93 (1.16-6.45) | 2.15 (0.74-6.48) |
| VWF Ag, U/mL (range) | 2.54 (0.88-7.26) | 2.09 (0.44-8.12) |
| Protein C activity, IU/mL (range) | 1.00 (0.79-1.54) | 0.97 (0.45-2.03) |
| Protein S activity, IU/mL (range) | 0.86 (0.44-1.37) | 0.76 (0.26-1.81) |
| Antithrombin, IU/mL (range) | 1.05 (0.73-1.50) | 0.99 (0.51-1.52) |
| PT 20210G>A, no. (%) | 0 (0) | 7 (5) |
| Factor V Leiden, no. (%) | 0 (0) | 5 (3) |
| Diabetes, no. (%) | 2 (18) | 12 (7) |
| Hypertension, no. (%) | 6 (55) | 40 (22) |
| Hyperlipidemia, no. (%) | 1 (9) | 12 (7) |
| Smoking history, no. (%) | 7 (64) | 42 (23) |
ATE indicates arterial thrombosis; ISS, International Scoring System; IgD, immunoglobulin D; VAD, vincristine, doxorubicin, and dexamethasone; TAD, thalidomide, doxorubicin, and dexamethasone; PAD, bortezomib, doxorubicin, and dexamethasone; VKA, vitamin K antagonist; LMWH, low molecular weight heparin; VWF, von Willebrand Factor; and PT, prothrombin.
Long-term indicates at least 6 months of treatment.
Characteristics of arterial thrombosis patients
| Pt . | Type . | Age at onset, y . | Sex . | Onset in mo after inclusion . | Prior myeloma treatment . | Treatment at onset . | Risk factors . | FVIII:C level . | Anticoagulant therapy at onset . | VTE . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Stroke | 43 | Male | 2.5 | TAD | TAD courses | S, FO | 2.63 | Prophylactic LMWH | No |
| 2 | MI | 50 | Male | 19.7 | VAD-HDM | IFN alpha maintenance | S, HT, D | 1.16 | None | No |
| 3 | Silent MI | 43 | Male | 6.3 | TAD | After CAD | S | 1.49 | VKA | Yes* |
| 4 | Stroke | 58 | Male | 3.0 | PAD | PAD courses | HT | 6.45 | Clopidogrel | No |
| 5 | MI | 49 | Female | 0.3 | VAD | VAD | S | 5.71 | None | No |
| 6 | MI | 61 | Male | 0.6 | VAD | VAD | S, HT, HL | 1.61 | None | No |
| 7 | PAD | 63 | Male | 8.4 | VAD | Thal/dex | None | 3.7 | None | No |
| 8 | Stroke | 65 | Female | 46.5 | VAD-HDM | IFN alpha maintenance | S, HT, AF | 3.93 | VKA | No |
| 9 | Silent MI | 62 | Male | 3.3 | PAD | PAD courses | HT | 3.22 | Prophylactic LMWH | No |
| 10 | TIA | 59 | Male | 30.6 | VAD-HDM† | None | None | Missing | VKA | During FU |
| 11 | TIA | 63 | Male | 18.8 | PAD-HDM allogeneic Tx | None | S, HT, D, AF | 1.95 | VKA | No |
| Pt . | Type . | Age at onset, y . | Sex . | Onset in mo after inclusion . | Prior myeloma treatment . | Treatment at onset . | Risk factors . | FVIII:C level . | Anticoagulant therapy at onset . | VTE . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Stroke | 43 | Male | 2.5 | TAD | TAD courses | S, FO | 2.63 | Prophylactic LMWH | No |
| 2 | MI | 50 | Male | 19.7 | VAD-HDM | IFN alpha maintenance | S, HT, D | 1.16 | None | No |
| 3 | Silent MI | 43 | Male | 6.3 | TAD | After CAD | S | 1.49 | VKA | Yes* |
| 4 | Stroke | 58 | Male | 3.0 | PAD | PAD courses | HT | 6.45 | Clopidogrel | No |
| 5 | MI | 49 | Female | 0.3 | VAD | VAD | S | 5.71 | None | No |
| 6 | MI | 61 | Male | 0.6 | VAD | VAD | S, HT, HL | 1.61 | None | No |
| 7 | PAD | 63 | Male | 8.4 | VAD | Thal/dex | None | 3.7 | None | No |
| 8 | Stroke | 65 | Female | 46.5 | VAD-HDM | IFN alpha maintenance | S, HT, AF | 3.93 | VKA | No |
| 9 | Silent MI | 62 | Male | 3.3 | PAD | PAD courses | HT | 3.22 | Prophylactic LMWH | No |
| 10 | TIA | 59 | Male | 30.6 | VAD-HDM† | None | None | Missing | VKA | During FU |
| 11 | TIA | 63 | Male | 18.8 | PAD-HDM allogeneic Tx | None | S, HT, D, AF | 1.95 | VKA | No |
VTE indicates venous thromboembolism; S, history of smoking (present smoking or smoking until 10 years before inclusion); FO, foramen ovale; MI, myocardial infarction; IFN, interferon; HT, hypertension; D, diabetes; HL, hyperlipidemia; Thal, thalidomide; dex, dexamethasone; AF, atrial fibrillation; FU, follow-up; and Tx, transplantation.
Onset of silent MI in the same period as occurrence of VTE.
Rituximab twice and intrathecal chemotherapy and radiotherapy because of a combined non-Hodgkin lymphoma and multiple myeloma.
Arterial thrombosis occurred in patients treated according to all 3 treatment regimens: VAD (n = 6, 5.9%), TAD (n = 2, 4.5%), and PAD (n = 3, 6.4%).
Cardiovascular risk factors, such as hypertension and smoking, were strongly associated with the risk of arterial thrombosis: hazard ratios (HRs) of 3.7 (95% confidence interval [CI]: 1.13-12.2) and 6.25 (95% CI: 1.61-24.2), respectively. Chronic atrial fibrillation (AF) was an additional cardiovascular risk factor. At the time of inclusion, 2 patients had a history of AF; 1 of these patients developed an ischemic stroke. During follow-up, 5 patients developed chronic AF and 6 patients had temporary AF, 1 of whom developed a TIA.
Of the thrombophilic risk factors, only FVIII:C was associated with arterial thrombosis (HR = 1.92 for each IU/mL increase of FVIII:C; 95% CI = 1.17-3.14). FVIII:C correlated significantly with age (P = .02), higher ISS stage (P = .001), and Durie-Salmon stage (P = .001) but not with M-protein (P = .22). No differences in ISS stage and Durie-Salmon stage were observed between patients with and without arterial thrombosis. When adjusted for age, ISS stage, and treatment arm, hypertension (HR = 11.6; 95% CI: 2.23-61.2) and smoking (HR = 15.12; 95% CI 1.78-130) remained statistically significantly associated with arterial thrombosis, whereas HR for FVIII:C was 1.85 (95% CI: 0.99-3.47; Table 3). No association was found between fibrinogen, VWF:Ag, antithrombin levels, protein C and protein S activity, the presence of prothrombin 20210G>A, or factor V Leiden mutation and arterial thrombosis.
The influence of factor VIII:C, hypertension, and smoking on the risk of arterial thrombosis in 195 multiple myeloma patients
| Risk factor . | Univariate analysis, HR (95% CI) . | Multivariate analysis,* HR (95% CI) . |
|---|---|---|
| Factor VIII:C† | 1.92 (1.17-3.14) | 1.85 (0.99-3.47) |
| Hypertension | 3.70 (1.13-12.2) | 11.7 (2.23-61.2) |
| Smoking | 6.25 (1.61-24.2) | 15.2 (1.78-130) |
| Risk factor . | Univariate analysis, HR (95% CI) . | Multivariate analysis,* HR (95% CI) . |
|---|---|---|
| Factor VIII:C† | 1.92 (1.17-3.14) | 1.85 (0.99-3.47) |
| Hypertension | 3.70 (1.13-12.2) | 11.7 (2.23-61.2) |
| Smoking | 6.25 (1.61-24.2) | 15.2 (1.78-130) |
HR indicates hazard ratio; and CI, confidence interval.
Adjusted for age, ISS score, and assigned treatment arm.
For each IU/mL increase in FVIII level.
Discussion
Only a few cases of arterial thrombosis in MM patients have been reported. In this prospective analysis, we observed a high incidence of arterial thrombosis in MM patients. Considering an annual incidence of myocardial infarction in a comparable age group (55-59 years) of 0.40% in men and 0.06% in women15 and an annual incidence of cerebral ischemic events of 0.29% in men and 0.22% in women in the general Dutch population, aged 55 to 59 years,15 only 1 arterial thrombosis (myocardial infarction or cerebral ischemic event) each year would be expected in the entire study population. Of 11 events, 5 (45%) occurred during the induction courses (a 3-month period), suggesting a pathophysiologic role for induction chemotherapy in a disease that is characterized by activated prothrombotic factors.2
A few case reports have been published on arterial thrombosis in myeloma patients. Goz et al5 reported 2 patients who developed an arterial embolus shortly after start of thalidomide in addition to VAD therapy. Alkindi et al7 reported 4 cases of arterial thrombosis, 2 non-ST elevation myocardial infarctions, 1 stroke, and 1 distal arterial thrombosis in 32 myeloma patients treated with thalidomide alone or in combination with other chemotherapy. These events occurred 1 to 5 months after start of thalidomide. Altintas et al6 mentioned 2 cases of arterial thrombosis. The first patient had 5 VAD cycles followed by thalidomide. At 1 month after start of thalidomide, arterial thrombosis of the leg arteries occurred. The second patient developed arterial thrombosis shortly after the start of thalidomide and dexamethasone therapy. Scarpace et al8 described 1 TIA and 2 ischemic strokes in myeloma patients treated with thalidomide 4 to 5 years after diagnosis. In contrast to these observations, use of thalidomide was no risk factor for arterial thrombosis in our study group. However, the true incidence of arterial thrombosis could have been underestimated in the TAD group due to prophylactic use of LMWH. In our center, Raven et al reported a fatal case of thrombosis of the inferior mesenteric artery in a 49-year-old women with a smoking history after the first VAD induction course. Of great interest is the recent report of Kristinsson et al.16 In a large population study, the risk of arterial thrombosis was assessed in 18 627 MM patients and 70 991 controls. At 1, 5, and 10 years after MM diagnosis, the hazard ratio (95% CI) of developing arterial thrombosis was 1.9 (1.8-2.1), 1.5 (1.4-1.6), and 1.5 (1.4-1.5), respectively. These data support the theory that the incidence of arterial thrombosis is highest during the treatment phase.
van Marion et al17 studied the levels VWF, FVIII:C, fibrinogen, antithrombin, protein C, and protein S during VAD, TAD, and PAD induction courses and showed that levels of VWF:Ag, FVIII:C, and fibrinogen increased significantly during induction treatment. Of these factors, only fibrinogen levels were significantly different between the VAD and TAD group, with the highest levels in the TAD-treated patients. Minnema et al18 noticed extremely high VWF:Ag and FVIII:C levels in MM patients that were associated with disease activity but not with thalidomide treatment; plasma levels of protein C, protein S, antithrombin, and activated protein C were normal in patients on thalidomide. Only limited data are available on bortezomib and prothrombotic factors in patients treated with combination chemotherapy.17 Although there might be differences in prothrombotic profiles in VAD, TAD, and PAD, we did not observe an increased risk of arterial thrombosis during treatment with thalidomide or bortezomib. The 3 different induction regimens all contain doxorubicin and dexamethasone. Glucocorticoids may lead to changes in risk factors for coronary artery disease, including higher serum cholesterol levels, higher blood pressure, and weight gain.19 However, no data exist that high doses of glucocorticosteroids increase the risk of arterial thrombosis. On the other hand, doxorubicin, an anthracyclin, is a well-known cardiotoxic agent. Aleman et al20 demonstrated a significantly increased risk of late myocardial infarction during follow-up after treatment with anthracyclin-containing chemotherapy in Hodgkin lymphoma patients. Similar results were reported by Swerdlow et al.21 In this study, most myocardial infarctions occurred in patients receiving anthracyclins without additional radiotherapy during the first year of treatment.
As expected, arterial events in our study were strongly associated with classic risk factors for arterial thrombosis, including hypertension and smoking. An additional risk factor for ischemic stroke is AF. At the time of inclusion, 2 patients had a history of AF, which is in accordance with the prevalence (1.5%) in the general population for the same age group.22 During follow-up, 11 additional patients developed temporary or chronic AF, 1 of whom developed a TIA.
Of thrombophilic risk factors, only high factor VIII:C levels were associated with arterial thrombosis. This finding is in accordance with the results of a prospective family cohort study by Bank et al23 who reported a more than 4-fold increased risk for arterial thrombosis in previously healthy relatives with and without FVIII:C levels higher than 1.5 IU/mL. Our study population had relatively high FVIII:C levels, with a median of 2.17 IU/mL, as has been previously reported in multiple myeloma.18,24 FVIII:C levels were clearly associated with ISS stage as described before.2 This finding supports the hypothesis of Minnema et al,18 who postulated that high levels of factor VIII:C and von Willebrand factor antigen levels in MM patients are associated with disease activity, probably as a reflection of increased bone marrow angiogenesis. It also supports the theory that a higher tumor burden might lead to an increased risk of thrombosis as a result of an increased level of FVIII:C. However, we did not observe differences in Durie-Salmon and ISS stage in both symptomatic and asymptomatic patients, but this might be because of the small number of events. Interestingly, 4 patients developed an arterial thrombosis during vitamin K antagonist (VKA) treatment, which suggests that VKA is not sufficient as prophylactic treatment in MM patients at high risk for arterial thrombosis. The patients receiving a thalidomide-based regimen (n = 45) were prophylactically treated with low-dose LMWH during induction courses, to reduce venous thrombotic complications. Two patients developed arterial thrombosis despite LMWH prophylaxis. Platelet inhibitory treatment might be more effective and appropriate in reducing the incidence of arterial thrombosis than VKA or LMWH. Recently, guidelines were presented for antithrombotic prophylaxis in multiple myeloma patients treated with thalidomide- or lenalidomide-based regimens.25 These guidelines were developed because of the high incidence of venous thrombotic complications in newly diagnosed multiple myeloma patients. In these guidelines, it is advised to give aspirin for patients at low risk of thrombosis (≤ 1 risk factor), whereas patients at high risk are to be treated with LMWH in a prophylactic dose. This, however, is not based on randomized studies, and these studies are needed to make definite recommendations for the population of multiple myeloma patients. Considering our findings, these studies should have not only venous thrombosis as an end point, but also arterial thrombosis.
The strengths of our study are its prospective design in a well-defined cohort of multiple myeloma patients in whom we were able to identify clear risk factors for arterial thrombosis. Thrombophilic risk factors were measured at inclusion in most patients, avoiding possible bias from thrombotic events. It should be noted that FVIII:C levels in 2 symptomatic patients were not measured at baseline, but because these levels were measured at 2 and 4 months after the event, an increase of FVIII due to the event is unlikely.
A limitation of our study is the rather small number of events. Therefore, larger prospective studies are warranted with a longer follow-up time. Another limitation is the scoring of cardiovascular risk factors. Hyperlipidemia and hypertension were scored positively only when patients had known hyperlipidemia or hypertension or used medication to treat hyperlipidemia and or hypertension at the time of inclusion. This might underestimate the prevalence of these risk factors in the study population. TIA may occur due to causes other than a thromboembolus, which may lead to overestimation of the incidence of arterial thrombosis in our cohort, however in the 2 patients with TIA the clinical course is suggestive of a thromboembolic origin, because no other cause was found and 1 of them had known AF and radiologic evidence of a previous stroke.
In conclusion, our study indicates that multiple myeloma patients are at increased risk for arterial thrombotic events during and after induction chemotherapy. High factor VIII:C levels, possibly reflecting disease activity, contribute to the risk of arterial thrombosis especially in patients with known cardiovascular risk factors.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.J.L. collected and analyzed data and wrote the paper; P.S. and F.W.G.L. conceived and designed the study and revised the paper; B.v.d.H. performed statistical analysis and revised the paper; and M.P.M.d.M. contributed to laboratory assessment of thrombophilic data and revised the paper.
Conflict-of-interest disclosure: P.S. is a consultant for Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Frank W. G. Leebeek, MD, PhD, Department of Hematology (Rm L-435), Erasmus University Medical Centre, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: f.leebeek@erasmusmc.nl.