Abstract
Fetomaternal microchimerism suggests immunological tolerance between mother and fetus. Thus, we performed primary hematopoietic stem cell transplantation from a mismatched mother to thalassemic patient without an human leukocyte antigen–identical donor. Twenty-two patients with thalassemia major were conditioned with 60 mg/kg hydroxyurea and 3 mg/kg azathioprine from day −59 to −11; 30 mg/m2 fludarabine from day −17 to −11; 14 mg/kg busulfan starting on day −10; and 200 mg/kg cyclophosphamide, 10 mg/kg thiotepa, and 12.5 mg/kg antithymocyte globulin daily from day −5 to −2. Fourteen patients received CD34+-mobilized peripheral blood and bone marrow progenitor cells; 8 patients received marrow graft–selected peripheral blood stem cells CD34+ and bone marrow CD3/CD19-depleted cells. T-cell dose was adjusted to 2 × 105/kg by fresh marrow cell addback at the time of transplantation. Both groups received cyclosporine for graft-versus-host disease prophylaxis for 2 months after transplantation. Two patients died (cerebral Epstein-Barr virus lymphoma or cytomegalovirus pneumonia), 6 patients reject their grafts, and 14 showed full chimerism with functioning grafts at a median follow-up of 40 months. None of the 14 patients who showed full chimerism developed acute or chronic graft-versus-host disease. These results suggest that maternal haploidentical hematopoietic stem cell transplantation is feasible in patients with thalassemia who lack a matched related donor.
Introduction
The cure for thalassemia involves correcting the genetic defect in a hematopoietic stem cell that results in reduced or absent β-globin synthesis and an excess of α-globin dimers. Intracellular precipitation and accumulation of α-dimers results in ineffective erythropoiesis and hemolytic anemia. Replacing the abnormal thalassemic marrow with allogeneic normal or heterozygous stem cells carrying the functional gene restores appropriate β-globin chain synthesis. Eighty to ninety percent of patients receiving a transplant from a human leukocyte antigen (HLA)–identical sibling or parent become ex-thalassemic after transplantation.1,2
In the multiracial populations from the Mediterranean region, Middle East, and Arabian Gulf, the probability of having an HLA-identical related donor is 35% to 40%. Thus, the pool of potential donors must be expanded to cure most children with thalassemia or sickle cell anemia. The outcomes after HLA-matched unrelated donor transplantation for treating thalassemia are comparable with those after HLA-identical familial transplantation.3 The use of mismatched, unrelated cord blood hematopoietic stem cells is still experimental.
Haploidentical hemopoietic stem cell transplantation has been explored as an option for treating patients with leukemia who lack an HLA-identical sibling or parent donor. However, severe graft-versus-host disease (GVHD) and high graft failure/rejection rates have limited the application of this transplantation modality for patients with thalassemia. Advances that use high doses of T cell–depleted peripheral blood stem cells (PBSCs) and intensive pretransplantation conditioning regimens have helped to overcome these limitations.4 Grafts containing megadoses of enriched CD34+ progenitor cells can be achieved by combining bone marrow with granulocyte colony-stimulating factor–mobilized PBSCs. Thereafter, T cells can be removed by positive selection for CD34. Limiting the numbers of CD3+ cells in the graft might allow retention of rapid engraftment kinetics provided by the mobilized PBSCs while reducing the risk of extensive GVHD. In this pilot study, we used a similar approach involving megadose haploidentical positively selected CD34+ marrow and peripheral hematopoietic stem cell transplantation to treat patients with thalassemia who lack an HLA-identical familial or unrelated marrow donor. Positive selection of CD34+ stem cells results in an approximately 3- to 4-log reduction of CD3+ cells, which reduces the risk of GVHD but increases the risk of graft failure. Adding a defined dose of CD3+ marrow cells to the cellular suspension at the time of transplantation can help to reduce the graft rejection rate.
In contrast to positive selection of stem cells, marrow graft depleted of CD3+ and CD19+ cells contains significant amounts of monocytes, natural killer (NK) cells, dendritic cells, precursor T cells, and other cell types that may play important roles in engraftment while accelerating the posttransplantation immune reconstitution. Therefore, in a second prospective phase of this pilot study, we evaluated the use of haploidentical CD3+/CD19+-depleted marrow graft combined with CD34-selected mobilized PBSCs and CD3+ marrow cells that were added back at the time of infusion. Here, we report the outcomes of 22 children with thalassemia who received transplants from haploidentical donors (20 mothers and 2 brothers).
Methods
During 6 years, 2002 through 2008, 22 patients with thalassemia major received an HLA-haploidentical transplant. Signed informed consent was received before transplantation in accordance with the Declaration of Helsinki, and all procedures were performed according to our center's established protocols. The study protocol was approved by the institutional review board of the Mediterranean Institute of Hematology. The results for 7 patients included in this study have been reported previously and are updated here with longer follow-up.5,6
Risk assignment was performed according to published criteria.7,8 The system categorizes risk on the basis of hepatomegaly, the presence of portal fibrosis on pretransplantation liver biopsies, and the quality of previous chelation (regular: deferoxamine treatment was initiated within 18 months of the first transfusion and administered for 8 to 12 hours as a continuous daily subcutaneous infusion for at least 5 days per week; anything less was considered irregular chelation). The age in months when the patient first received regular chelation was recorded. A chelation index was used to describe the number of months that each patient did not receive regular chelation as a percentage of the number of months the patient should have received it. With this index, a completely satisfactory chelation history is represented by 0% and a completely unsatisfactory history is represented by 100%.5 Patient characteristics are presented in Table 1.
Patient characteristics at transplantation for 22 patients younger than 17 years
| Patient . | Age, y . | Sex . | Class . | Ferritin (ng/mL) . | GOT (U/L) . | GPT (U/L) . | FS . | No. Tx . | CI . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | F | 3 | 2168 | 33 | 27 | Mi | 44 | 100 | Alive and well |
| 2 | 7 | M | 3 | 2591 | 49 | 51 | Se | 23 | 100 | Alive and well |
| 3 | 6 | M | 2 | 6846 | 45 | 54 | Mi | 54 | 100 | Alive and well |
| 4 | 7 | M | 3 | 532 | 34 | 34 | Se | 58 | 100 | Alive and well |
| 5 | 4 | M | 2 | 2162 | 23 | 12 | No | 18 | 100 | Alive and well |
| 6 | 3 | M | 2 | 1390 | 35 | 23 | No | 12 | 49 | Rejection |
| 7 | 7 | F | 2 | 3048 | 24 | 48 | No | 19 | 100 | Alive and well |
| 8 | 6 | M | 3 | 3158 | 53 | 32 | Mi | 21 | 100 | Rejection |
| 9 | 8 | F | 3 | 2448 | 36 | 79 | Mi | 24 | 100 | Alive and well |
| 10 | 7 | F | 3 | 3231 | 45 | 41 | Mi | 48 | 100 | Rejection |
| 11 | 5 | M | 2 | 3492 | 31 | 31 | Se | 120 | 38 | Rejection |
| 12 | 13 | F | 3 | 4578 | 56 | 46 | Mi | 118 | 100 | Dead EBV NHL |
| 13 | 8 | F | 3 | 2120 | 42 | 64 | Mi | 58 | 100 | Alive and well |
| 14 | 11 | F | 3 | 2835 | 35 | 45 | Mo | 114 | 100 | Alive and well |
| 15 | 8 | F | 2 | 1967 | 43 | 32 | Mi | 82 | 100 | Alive and well |
| 16 | 6 | M | 3 | 1391 | 25 | 29 | Mo | 42 | 100 | Rejection |
| 17 | 11 | M | 3 | 3386 | 38 | 42 | Mo | 95 | 100 | Dead CMV sepsis |
| 18 | 12 | M | 3 | 19 801 | 16 | 28 | Mo | 50 | 100 | Alive and well |
| 19 | 3 | M | 1 | 578 | 35 | 23 | No | 12 | 49 | Rejection |
| 20 | 8 | F | 2 | 2980 | 36 | 27 | Mi | 51 | 80 | Alive and well |
| 21 | 6 | F | 1 | 1240 | 28 | 18 | Mi | 68 | 100 | Alive and well |
| 22 | 14 | M | 3 | 2568 | 31 | 23 | Mi | 125 | 100 | Alive and well |
| Patient . | Age, y . | Sex . | Class . | Ferritin (ng/mL) . | GOT (U/L) . | GPT (U/L) . | FS . | No. Tx . | CI . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | F | 3 | 2168 | 33 | 27 | Mi | 44 | 100 | Alive and well |
| 2 | 7 | M | 3 | 2591 | 49 | 51 | Se | 23 | 100 | Alive and well |
| 3 | 6 | M | 2 | 6846 | 45 | 54 | Mi | 54 | 100 | Alive and well |
| 4 | 7 | M | 3 | 532 | 34 | 34 | Se | 58 | 100 | Alive and well |
| 5 | 4 | M | 2 | 2162 | 23 | 12 | No | 18 | 100 | Alive and well |
| 6 | 3 | M | 2 | 1390 | 35 | 23 | No | 12 | 49 | Rejection |
| 7 | 7 | F | 2 | 3048 | 24 | 48 | No | 19 | 100 | Alive and well |
| 8 | 6 | M | 3 | 3158 | 53 | 32 | Mi | 21 | 100 | Rejection |
| 9 | 8 | F | 3 | 2448 | 36 | 79 | Mi | 24 | 100 | Alive and well |
| 10 | 7 | F | 3 | 3231 | 45 | 41 | Mi | 48 | 100 | Rejection |
| 11 | 5 | M | 2 | 3492 | 31 | 31 | Se | 120 | 38 | Rejection |
| 12 | 13 | F | 3 | 4578 | 56 | 46 | Mi | 118 | 100 | Dead EBV NHL |
| 13 | 8 | F | 3 | 2120 | 42 | 64 | Mi | 58 | 100 | Alive and well |
| 14 | 11 | F | 3 | 2835 | 35 | 45 | Mo | 114 | 100 | Alive and well |
| 15 | 8 | F | 2 | 1967 | 43 | 32 | Mi | 82 | 100 | Alive and well |
| 16 | 6 | M | 3 | 1391 | 25 | 29 | Mo | 42 | 100 | Rejection |
| 17 | 11 | M | 3 | 3386 | 38 | 42 | Mo | 95 | 100 | Dead CMV sepsis |
| 18 | 12 | M | 3 | 19 801 | 16 | 28 | Mo | 50 | 100 | Alive and well |
| 19 | 3 | M | 1 | 578 | 35 | 23 | No | 12 | 49 | Rejection |
| 20 | 8 | F | 2 | 2980 | 36 | 27 | Mi | 51 | 80 | Alive and well |
| 21 | 6 | F | 1 | 1240 | 28 | 18 | Mi | 68 | 100 | Alive and well |
| 22 | 14 | M | 3 | 2568 | 31 | 23 | Mi | 125 | 100 | Alive and well |
No. Tx indicates number of red cell transfusions before transplantation; CI, chelation index; GOT, glutamic-oxaloacetic transaminase; GPT, glutamic-pyruvic transaminase; and FS, portal fibrosis (Mi, mild; Mo, moderate; Se, severe; and Ci, cirrhotic).
Donors
Family members were assessed for HLA compatibility by serological methods or by high-resolution molecular analysis. All donors (20 mothers and 2 brothers) were identical for 1 haplotype and incompatible at 3 loci (HLA-A, -B, -DR) of the other, except for 2 who were mismatched at 2 loci (HLA-A, -B) on the unshared haplotype. One brother was mismatched for noninherited maternal antigens. The stem cell dose was achieved with a median of 3 leukaphereses (range, 1-5). Twenty-one donors had β-thalassemia minor, and one had sickle cell trait.
A total of 10 age- and sex-matched healthy donors were included as control subjects. The control subjects provided bone marrow aspirates, all of which were deemed normal. None of the control subjects had acute infections or were receiving medication at the time of the study.
Graft processing and transplantation procedures
All donors received recombinant human granulocyte colony-stimulating factor 15 μg/kg/d in 2 daily subcutaneous boluses to mobilize PBSCs. CD34+ cells from leukaphereses and bone marrow harvests were selected by the use of the CliniMACS one-step procedure (Miltenyi Biotec) for 14 donors. A 2-step selection (CD34+ selection leukapheresis followed by negative selection by the use of anti-CD3 and anti-CD19 monoclonal antibodies [mAbs]) of bone marrow cells was used for 8 donors. We attempted to suppress erythropoiesis by intensive hypertransfusion and chelation. Between day −59 and day −11 before the transplantation, 40 mg/kg deferoxamine was continuously infused through a central venous catheter each 24 hours. Red cells were transfused every 3 days to maintain the hemoglobin level between 140 and 150 g/L (14 and 15 g/dL). During this time interval hydroxyurea 60 mg/kg daily and azathioprine 3 mg/kg daily were administered to eradicate marrow, and growth factors, granulocyte colony-stimulating factor and erythropoietin, were given twice weekly to maintain stem cell proliferation in the face of hypertransfusion, thereby facilitating the effect of the hydroxyurea. Fludarabine was administered at a dosage of 30 mg/m2/d from day −17 through day −13. Starting on day −10, 14 doses of busulfan 1 mg/kg were administered orally 3 times daily during the course of 4 days (total dose 14 mg/kg during the course of 4 days) in the first 17 patients, and a corresponding dose of busulfan was given intravenously in the following 5 patients, followed by intravenous cyclophosphamide 50 mg/kg daily for each of the next 4 days (total dose, 200 mg/kg), and 10 mg/kg thiotepa and 12.5 mg/kg antithymocyte globulin.
All patients received cyclosporine for GVHD prophylaxis for the first 2 months after transplantation. Antifungal prophylaxis included liposomal amphotericin B (1 mg/kg daily) from day +8. Cytomegalovirus (CMV) prophylaxis consisted of 5 mg/kg acyclovir 3 times daily through day +60.
Tests for chimerism
Fluorescence in situ hybridization.
When the host and donor were sex mismatched, fluorescence in situ hybridization was performed on peripheral blood and bone marrow to detect marrow engraftment.9
DNA extraction.
High molecular weight DNA was extracted from peripheral blood or bone marrow by the use of a commercial DNA blood mini kit, in accordance with the manufacturer's instructions (QIAGEN).
Polymerase chain reaction.
To evaluate chimerism, 4 different minisatellite loci (33.6, SE33, APOB, and D1S80) were amplified by polymerase chain reaction (PCR). The PCR-amplified products were resolved on precast 10% nondenaturing polyacrylamide gel (NOVEX), and the gels were silver-stained. Mixed chimerism was estimated semiquantitatively by the comparison of recipient and donor band intensities with those of known standards.10
Graft content
Eight patients received T cell–depleted peripheral blood progenitor cells (CD34+ immunoselection) and CD3+- and CD19+-depleted bone marrow stem cells. Median infused cell doses per kilogram of recipient body weight were CD34+: 15.2 × 106 (range, 8.2-26 × 106); CD3+ T cells: 1.8 × 105 and 0.27 × 106/kg CD19.
Fourteen patients received CD34+-mobilized peripheral and bone marrow progenitor cells. Positive selection was performed by use of the CliniMACS procedure. The CD34+ grafts contained a median of 14.2 × 106/kg CD34+ cells (range, 5.4-39 × 106/kg), 2 × 105/kg CD3+ cells, and 0.19 × 106/kg CD19+. No side effects were associated with graft infusion.
Flow cytometric analysis of peripheral blood mononuclear cells.
For whole blood phenotype analysis, 500 μL of blood was lysed with 10 mL of Ortho-mune Lysing Reagent (Ortho Diagnostic Systems Inc) at room temperature, washed, and labeled with a cocktail of 4 mAbs for 30 minutes at 4°C. Anti–CD3-fluorescein isothiocyanate, anti–CD4-allophycocyanin, anti–CD8-peridinin chlorophyll protein, and anti–CD19-PE were purchased from BD Biosciences.
The NK phenotype of PBMCs was assessed by immunofluorescence and flow cytometry by the use of fluorescein isothiocyanate–conjugated anti-CD3 and PE-conjugated anti-CD56 mAbs (BD Biosciences). After staining, cells were washed once in phosphate-buffered saline containing 2% fetal bovine serum and analyzed on a FACSCalibur cytofluorometer (BD Biosciences) with the use of CellQuest software. Absolute lymphocyte counts were calculated by standard hemocytometry. To determine marker expression on CD4+ and CD8+ cells, total lymphocytes were first identified and gated by forward and side scatter, and then these cells were gated for CD4 or CD8 expression.
Statistical analysis
Estimates of overall survival and event-free survival were calculated by the Kaplan-Meier method.11 Rejection and nonrejection mortality were calculated as cumulative incidences.12 Rejection, recurrence of thalassemia, and death were recorded as events when estimating event-free survival. Rejection was defined as the development of complete marrow aplasia or recurrence of thalassemia (a return to the pretransplantation pattern of globin-chain synthesis).
Results
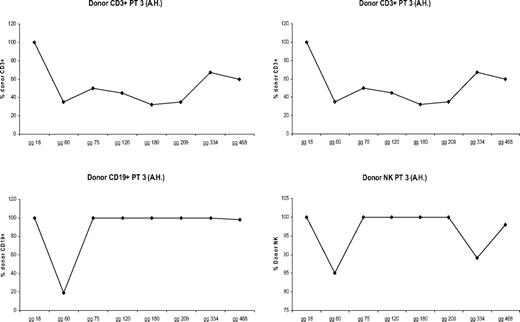
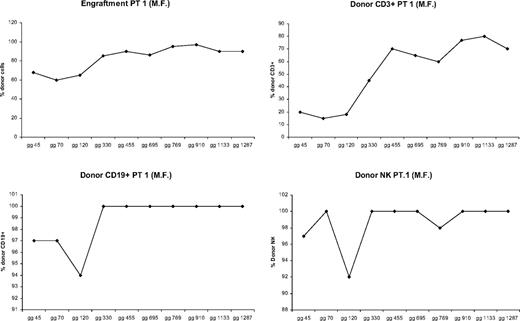
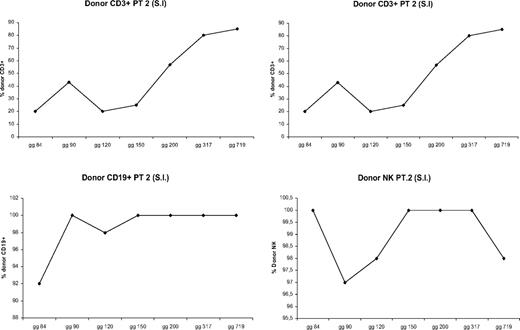
All patients showed donor chimerism by day 14 after HSCT. Granulocyte counts greater than 500 mL occurred after a median time of 13 days (range, 11-17 days). Six patients rejected their grafts; surviving with thalassemia, 3 patients showed early mixed chimerism (MC), which became persistent when observed, respectively, at 14, 38, and 42 months after the transplantation. In 14 cases the transplantation was successful with complete allogeneic reconstitution. In patients who showed allogeneic reconstitution, median time for granulocyte recovery was 13 days (range, 11-17 days), whereas median time for a self-sustained platelet recovery was 12 days (range, 9-17 days). There were 2 patients who died from transplantation-related causes: one of these patients died on day +114 of Epstein-Barr virus (EBV) cerebral lymphoma, and one died on day +92 from CMV pneumonia. In 6 cases, donor marrow was rejected with complete autologous reconstitution and return to pretransplantation clinical status. In 2 of these patients, rejection occurred after transient engraftment of donor cells. MC has already been described.13 MC was classified, according to the proportion of residual host cells present in the recipient, into MC level 1 (residual host cells < 10%), MC level 2 (residual host cells between 10% and 25%), and MC level 3 (residual host cells > 25%). Three patients experienced a status of MC early after bone marrow transplantation, which became persistent when observed, respectively, at 14, 38, and 42 months after the transplantation. To define the condition of MC better, we analyzed the proportion of donor engraftment in different lymphoid subsets at different times after bone marrow transplantation (BMT). Figures 1,Figure 2 through 3 report the MC condition in each of 3 patients.
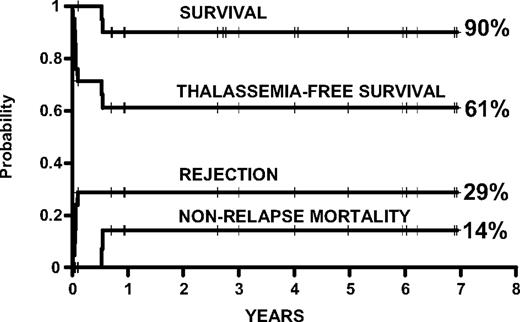
Kaplan-Meier probabilities of survival, thalassemia-free survival, and cumulative incidence of rejection and nonrelapse mortality in 22 thalassemic patients younger than 17 years of age.
Kaplan-Meier probabilities of survival, thalassemia-free survival, and cumulative incidence of rejection and nonrelapse mortality in 22 thalassemic patients younger than 17 years of age.
Proportion of donor engraftment in different lymphoid subsets at different times after BMT (patient 1).
Proportion of donor engraftment in different lymphoid subsets at different times after BMT (patient 1).
Proportion of donor engraftment in different lymphoid subsets at different times after BMT (patient 2).
Proportion of donor engraftment in different lymphoid subsets at different times after BMT (patient 2).
Fourteen patients developed functioning grafts at a median follow-up of 40 months. The 14 cured children are not transfusion dependent any longer, with hemoglobin levels ranging from 10.3 g/dL to 13.8 g/dL, and have an optimal quality of life.
Since the first transplantation performed by our group in Pesaro on February 15, 1989, more than 1000 thalassemic patients have successfully undergone transplantation. Their life is normal, that is, they are socially active, most have regained fertility, more and more are having children. A group working in Pescara has recently published their data.14 None of the children with full as well persistent MC developed GVHD (Figure 4).
Proportion of donor engraftment in different lymphoid subsets at different times after BMT (patient 3).
Proportion of donor engraftment in different lymphoid subsets at different times after BMT (patient 3).
On day +119, 1 patient developed varicella zoster meningoencephalitis (documented by PCR of cerebrospinal fluid) that responded to a combination of acyclovir and foscarnet. The 2 drugs were combined because the patient had been receiving acyclovir prophylaxis. On day +135, this patient also received a donor lymphocyte infusion (5 × 104 CD3+ donor cells/kg) to boost antivaricella zoster T cells. The donor, who had a history of varicella zoster, had been administered a dose of varicella zoster vaccine 1 week before peripheral lymphocyte collection. The patient recovered with no neurological sequelae or abnormalities on magnetic resonance imaging.
Fifteen patients demonstrated CMV infection without disease that resolved with preemptive gancyclovir treatment. Three patients had EBV reactivation with a high viral load that resolved after treatment with retuximab.
Immunological reconstitution
Delayed immune reconstitution after transplantation may be associated with a variety of functional and immunophenotypic abnormalities at bone marrow level as the result of augmented local production of inflammatory cytokines, increased T-cell activation, or intrinsic hematopoietic and stromal cell abnormalities. At day +20, 6 of 14 patients had significantly lower CD4+ T-cell counts than did the control subjects (1.9 ± 1.4% vs 47.5 ± 6%, respectively). This reduction was mainly in the CD45RA+CD62L+ (naive phenotype) subset (1.3 ± 2% in patients vs 52 ± 12% in control patients). A significant decrease in peripheral CD45RA+CD31+ Th cells (thymic-naive Th cells) was observed (0.5 ± 0.3% in patients vs 37 ± 10% in controls), whereas CD8+ T-cell numbers were similar in patients and control patients (24.2 ± 33.7% vs 20 ± 7%). NK cells were among the first lymphocytes to repopulate peripheral blood, and up to 70% of these cells were CD56bright, whereas CD56dimCD16+ NK cells were reduced.
On day +60, increases in the percentages of CD4+ T cells, naive CD4+ cells, and thymic-naive Th cells were observed (3 ± 1.2%, 2.9 ± 2.1%, and 2.7 ± 1%, respectively). CD8+ T cells were also increased (35 ± 27.5%). In addition, patients showed a significant increase in CD4+-cell activation markers (CD95, HLA-DR, and CCR5) that paralleled an increase in CD56dimCD16+ NK cells (potent cytotoxic effector cells), especially in the patients with full engraftment (47 ± 20% vs 28 ± 31% in MC).
The stromal layers cultured on chamber slides were positive for CD68, vimentin, and CD14 but negative for S100 and CD34, indicating cells of macrophage/monocyte lineage.21 In the patients with delayed immunohematological reconstitution, the majority (80%) of these cells appeared moderately large, rounded, and with abundant cytoplasm on light microscopy. In contrast, approximately 90% of stromal cells from control subjects were irregular or spindle shaped with branching cytoplasmic processes (fibroblast-like). Spontaneous stromal cell production of interleukin-7 (IL-7) was lower in patients than in control patients (0.3 ± 0.1 pg/mL vs 0.8 ± 0.1 pg/mL, respectively; P = .02).
Discussion
Hematopoietic stem cell transplantation offers the only chance of cure for patients with thalassemia. Haploidentical transplantation may extend this possibility to the 50% to 60% of the patients who lack a suitably matched familial donor or an HLA-identical unrelated donor.
The presence of fetal cells in maternal blood and of maternal cells in fetal blood (fetomaternal microchimerism) suggests that immunological tolerance may exist between mother and offspring.15,16 Van Rood et al demonstrated a lower rate of acute GVHD in sibling transplants mismatched for noninherited maternal antigens than in transplants mismatched for noninherited paternal antigens.17 We have reported the results of BMT in children with acute leukemia in relapse who are resistant to chemotherapy in which their haploidentical mother was used as the donor of nonmanipulated bone marrow.18
The combination of a megadose of purified CD34+ cells and a highly immunomyeloablative conditioning regimen is crucial for overcoming the barrier of residual antidonor cytotoxic T-lymphocyte precursors in T cell–depleted mismatched transplants.19 The immune regulatory role of CD34+ cells is supported by the observation that cells within the CD34+ population are endowed with veto activity20 ; early myeloid CD33+ cells may also have this potential.
The infusion of 2 × 105 cell/kg bone marrow mononuclear cells freshly obtained from the bone marrow of the donor requires cyclosporine prophylaxis for GVHD during the first 2 months after transplantation. However, the addition of bone-marrow mononuclear cells (including NK cells, mesenchymal stem cells, T cells) to a T cell–depleted allograft may help promote engraftment and control GVHD.
Haploidentical transplantation is associated with major posttransplantation immune deficiency resulting in significant morbidity and mortality from infection. Delayed immune reconstitution after transplantation may be associated with a variety of functional and immunophenotypic abnormalities at BM level because of augmented local production of inflammatory cytokines, increased T-cell activation, or intrinsic hematopoietic and stromal cell abnormalities. At 20 days after transplantation, a significant decrease in total lymphocyte counts and depletion of CD4+ T cells expressing predominantly the CD45RA+CD62L+ phenotype were observed. Also, in the CD4+CD45RA+CD31+ T-cell subset in vivo and in vitro, hematolymphopoiesis occurs in association with the complex network of cell types found in the stroma, including nonhematopoietic (fibroblasts, adipocytes, and endothelial cells) and hematopoietic cells (macrophages and T cells). Progenitor cell growth and differentiation depend on their interaction with stromal cells. The prevalence of macrophage-like cells in long-term bone marrow culture, rather than the typical “fibroblast-like” cells, suggests an altered composition of the bone-marrow stroma, possibly linked to an underlying inflammatory process within the bone marrow microenvironment.
A central function of stromal cells is IL-7 production. Recent evidence shows that IL-7 acts as a master regulator of T-cell homeostasis, expanding both the naive and memory T-cell populations. Compared with control patients, thalassemia patients exhibited altered stromal cytokine production at 20 days after transplantation, characterized by decreased IL-7 levels. We can hypothesize that the delayed immunoreconstitution of the T-cell compartment may be initially the result of altered generation of new T cells arising from hematopoietic progenitor cells with the interaction of impaired stromal cell function. NK CD56+bright cells develop more rapidly than other lymphocytes, but CD3−CD16+ NK cells (with cytotoxic potential) require more prolonged exposure to maturation factor (IL-2) in the bone marrow. Interestingly, we observed greater percentages of NK CD56+bright cells 20 days after transplantation in patients with full engraftment, suggesting a role for donor NK cells in improved engraftment and in prevention of rejection by an attack of the host lymphohematopoietic cells.
After 60 days after transplantation, a significant decrease in total lymphocyte counts and depletion of CD4+ T cells expressing predominantly the CD45RA+CD62L+ phenotype were observed. In addition, the CD4+CD45RA+CD31+ T-cell subset was significantly reduced in our cohort, suggesting a thymus involvement in these patients. Indeed, it is possible that the T-cell defect in thalassemia patients may occur at multiple levels, including egress from thymus.
NK CD56+bright cells develop more rapidly than other lymphocytes, but CD3−CD16+ NK cells (with cytotoxic potential) require more prolonged exposure to maturation factor (IL-2) in the bone marrow. The greater percentages of CD3−CD16+ in MC patients may have a possible role on control of host cell escape and in maintainer the chimerism condition.
NK cells (CD56+) developed more rapidly than other lymphocytes, but CD56dim CD16+ NK cells were increased at 60 days after transplantation, particularly in patients with full engraftment, suggesting a role for donor NK cells in bone marrow engraftment.21
The prevalence of macrophage-like cells in long-term bone-marrow culture as opposed to typical “fibroblast-like” cells suggests that the composition of the marrow stromal was altered, possibly the result of an underlying inflammatory process within the bone marrow microenvironment. Stromal cells produce IL-7, which acts as a growth and antiapoptotic factor for B- and T-cell precursors. This IL-7 production may be critical for the development of the new immune system from uncommitted progenitors infused with the graft. Stromal IL-7 production was decreased in transplant recipients, suggesting an important role for bone marrow accessory cells in immunohematological reconstitution after transplantation. We hypothesize that the recovery of the T-cell compartment resulted from deregulated production of new T cells from hematopoietic stem cells under the influence of the stromal microenvironment. The results of this study suggest that it may be possible to boost engraftment and immune recovery via the administration of specific cytokines (ie, IL-2 + IL-7) and/or mesenchymal stem cells. One patient died on day +114 of EBV cerebral lymphoma. The patient had low levels of CD8+ at the time of infection. No association between the number of CD19+ cells infused and occurrence of EBV reactivation was found. Despite the high incidence of CMV reactivation, only one patient died of CMV pneumonia. In conclusion, the transplantation protocol described herein appears to be well tolerated and effective for eradicating the hematopoietic system in patients with thalassemia.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We thank Gianluca Baldassarri for technical assistance.
Authorship
Contribution: P.S. and G.L. designed the study; A.I., J.G., P.P., K.P., M.M., M.D.S., A.R., A.M., C.A., G.D.A., C.G., B.E., G.I., F.Z., G.A., A.L., L.F., M.T., and M.A. performed research; and P.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Pietro Sodani, International Centre for Transplantation in Thalassemia and Sickle Cell Anemia, Mediterranean Institute of Hematology, Policlinico Tor Vergata, Viale Oxford, 81, Rome 00133, Italy; e-mail: p.sodani@fondazioneime.org.