Abstract
It is difficult to imagine a greater challenge to the transplantation of haploidentical cells than thalassemia.
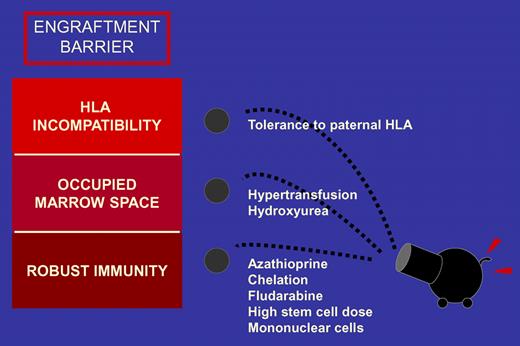
Thalassemia patients are not exposed to chemotherapy before transplantation. Therefore, they have robust immunity compared with most patients with hematologic malignancies. Chronic transfusions further heighten the immunologic barrier to engraftment by sensitizing the host to major and minor histocompatibility antigens. “Space” for engrafting cells is diminished as the compensatory erythropoiesis amplifies marrow cellularity. Furthermore, although total body irradiation provides the greatest ratio of immune suppression to toxicity, there is no therapeutic benefit to its use in conditioning for a nonmalignant disease. Sodani and colleagues have taken on many challenges in their effort to improve the lives of patients with thalassemia, and their report in this issue of Blood continues to improve care for these patients.1
Multiple lines of attack are needed to break down the barrier to engraftment of haploidentical cells. The initial assault comprises strategies that reduce immune competence and suppress hematopoiesis. The second wave of attack overwhelms the host with mega-dose stem cells and exploits maternal-fetal tolerance.
Multiple lines of attack are needed to break down the barrier to engraftment of haploidentical cells. The initial assault comprises strategies that reduce immune competence and suppress hematopoiesis. The second wave of attack overwhelms the host with mega-dose stem cells and exploits maternal-fetal tolerance.
The investigators combine a number of strategies to surmount the barriers to engraftment of haploidentical hematopoietic cells (see figure). A pretransplantation program of hypertransfusion was combined with azathioprine and hydroxyurea to suppress hematopoiesis and reduce marrow progenitors, effectively opening up marrow “space.” To augment the immunosuppressive properties of azathioprine, a course of fludarabine was tacked onto the conditioning regimen. The donor graft was engineered to enhance engraftment by manipulating marrow and peripheral blood stem cells to yield a high dose of hematopoietic stem cells and a targeted low dose of T cells.
Finally, selection of a maternal donor exploited the potential for feto-maternal microchimerism to induce immunologic tolerance. Taken altogether, these strategies allowed utilization of a relatively conventional regimen that did not contain total body irradiation. Although not perfect, the results are extremely promising—a reasonably small risk for mortality with a reasonably high rate of engraftment. Among patients who did not achieve full donor chimerism, disease symptoms were ameliorated in those with mixed donor-host chimerism and autologous hematopoiesis resumed in those who rejected their graft.
The fact that these results were obtained in a group of mostly class 2 to 3 thalassemia patients is even more remarkable.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal