Abstract
Novel strategies to control the binding of adhesion molecules belonging to the selectin family are required for the treatment of inflammatory diseases. We tested the possibility that synthetic monosaccharide analogs can compete with naturally occurring sugars to alter the O-glycan content on human leukocyte cell surface selectin-ligand, P-selectin glycoprotein ligand-1 (PSGL-1). Resulting reduction in the sialyl Lewis-X–bearing epitopes on this ligand may reduce cell adhesion. Consistent with this hypothesis, 50μM per-acetylated 4F-GalNAc added to the growth media of promyelocytic HL-60 cells reduced the expression of the cutaneous lymphocyte associated-antigen (HECA-452 epitope) by 82% within 2 cell doubling cycles. Cell binding to all 3 selectins (L-, E-, and P-selectin) was reduced in vitro. 4F-GalNAc was metabolically incorporated into PSGL-1, and this was accompanied by an approximately 20% reduction in PSGL-1 glycan content. A 70% to 85% reduction in HECA-452 binding epitope and N-acetyl lactosamine content in PSGL-1 was also noted on 4F-GalNAc addition. Intravenous 4F-GalNAc infusion reduced leukocyte migration to the peritoneum in a murine model of thioglycolate-induced peritonitis. Thus, the compound has pharmacologic activity. Overall, the data suggest that 4F-GalNAc may be applied as a metabolic inhibitor to reduce O-linked glycosylation, sialyl Lewis-X formation, and leukocyte adhesion via the selectins.
Introduction
The binding of adhesion molecules belonging to the selectin family to carbohydrate ligands facilitates the adhesion of blood leukocytes to activated endothelial cells, platelets, and other leukocytes in the human vasculature.1,2 Such molecular interactions play an important role in regulating leukocyte recruitment at sites of inflammation, cancer metastasis, and various cardiovascular disorders.3 Whereas numerous glycoproteins and glycolipids participate in selectin-mediated cell adhesion, interactions with carbohydrate epitopes expressed on the leukocyte glycoprotein P-selectin glycoprotein ligand-1 (PSGL-1, CD162) are particularly important because this ligand binds all 3 members of the selectin family (E-, P-, and L-selectin) with high affinity and under fluid flow conditions. Structural analysis of the glycans of PSGL-1 expressed on human promyelocytic leukemia HL-60 cells reveals that PSGL-1 is predominantly composed of core-2 based O-linked glycans.4,5 The prototypic selectin-binding carbohydrate structure sialyl Lewis-X (NeuAcα2,3Galβ1,4(Fucα1,3)GlcNAc-, sLeX; Figure 1A) is expressed on 2% to 14% of these O-glycans.
Glycan epitopes and monosaccharide analogs. (A) Putative structure of selectin-binding glycan located at the N-terminus of PSGL-1. This glycan has a terminal tetrasaccharide sLeX epitope and binding sites for DSA and AAL lectins. Neuraminidase cleaves sialic acid (NeuAc) residues to expose the terminal trisaccharide Lewis-X (LeX) epitope. Metabolic inhibition of these glycan structures may reduce leukocyte selectin-binding function. (B) Structure of per-acetylated compounds GalNAc, 4F-GalNAc, 4F-GlcNAc, and [14C]4F-GalNAc.
Glycan epitopes and monosaccharide analogs. (A) Putative structure of selectin-binding glycan located at the N-terminus of PSGL-1. This glycan has a terminal tetrasaccharide sLeX epitope and binding sites for DSA and AAL lectins. Neuraminidase cleaves sialic acid (NeuAc) residues to expose the terminal trisaccharide Lewis-X (LeX) epitope. Metabolic inhibition of these glycan structures may reduce leukocyte selectin-binding function. (B) Structure of per-acetylated compounds GalNAc, 4F-GalNAc, 4F-GlcNAc, and [14C]4F-GalNAc.
There is active interest in developing antagonists that control/block selectin-mediated cell adhesion using either competitive inhibitors or metabolic inhibitors. Competitive inhibitors attempt to block cell adhesion by regulating the ligand-binding epitope of either the selectin or its primary counter-receptor PSGL-1. Antagonists used for such inhibition include the tetrasaccharide sLeX and its glycomimetics,6 humanized antibodies directed against selectins,7-9 and soluble recombinant PSGL-1-Ig fusion protein.10 Only limited clinical success has been reported with these molecules, thus far.11 Although the use of competitive inhibitors is conceptually straightforward, in practice this is complicated by the overlapping functional redundancies among the members of the selectin family and their carbohydrate ligands, the multiple roles of selectins in both ligand binding and signaling, and the limited half-life in circulation of some classes of inhibitors.
The use of metabolic inhibitors is more recent and less well developed. These are mostly designed based on the growing knowledge of cellular glycosylation reactions and pathways. This approach uses small molecules that penetrate the cell to divert/block metabolic pathways that normally lead to the formation of selectin-binding carbohydrate epitopes. This strategy targets a group of related cellular reactions as opposed to a single pathway. Preliminary success has been noted using this approach. First, surrogate acceptors or decoys that act as unnatural substrates for glycosyltransferases have been introduced into cells. Glycosyltransferases act on such artificial substrates. This results in incomplete glycosylation of the natural glycoconjugates. Although an early approach showed that aryl-N-acetyl-α-galactosaminides (benzl, phenyl, p-nitrophenyl-α-GalNAc) added to cell culture media can alter glycans on mucinous glycoproteins, these reagents were applied at high concentrations (1-7.5mM).12 Later, it was demonstrated that per-acetylated forms of Galβ1,4GlcNAc-β-O-napthalenemethanol and GlcNAcβ1,3Gal-β-O-Galβ1,4GlcNAc-β-O-napthalenemethanol at 50μM can act as decoys/primers that block selectin-ligand formation.13-16 Second, glycosyltransferase inhibitors are also in development based on the structure of the sugar-nucleotide transition-state analogs17,18 and high throughput screens,19 although testing of these reagents has largely been performed in cell-free enzymatic assays. Third, per-acetylated, modified monosaccharides have been applied to cells as these may compete with the natural monosaccharides. Here, unnatural monosaccharides are incorporated into cellular glycoconjugates.20-24 Although analogs of galactose, GlcNAc, GalNAc, and mannose have been synthesized, only limited studies have been conducted in cellular assays.25-27 4F-GlcNAc is an example of this class of inhibitors. This molecule reduces selectin-mediated cutaneous lymphocyte-associated antigen (CLA+) T-cell adhesion in vitro,28 and in in vivo models of skin inflammation.29-31
Because O-linked glycans linked to PSGL-1 and other glycoproteins participate as important selectin ligands and because the attachment of GalNAc to serine/threonine residues on the peptide backbone is critical for the initiation of O-glycan assembly, we tested the hypothesis that modified monosaccharides based on GalNAc may be used to disrupt/alter the pattern of O-linked glycosylation. This can result in reduced selectin binding function. In this regard, unlike GlcNAc, which plays a major role in modifying both N- and O-linked glycans, heparan sulfates, and glycolipids, GalNAc is primarily important for the initiation of O-linked glycosylation and chondroitin sulfates.32 Here, we tested the effect of a synthetic analog of the natural GalNAc monosaccharide called per-acetylated 4F-GalNAc (2-acetamido-1,3,6-tri-O-acetyl-2,4-dideoxy-4-fluoro-D-galactopyranose; Figure 1B). This is an unnatural monosaccharide where fluorine replaced the hydoxyl group at the C-4 position of GalNAc. For simplicity, this per-acetylated molecule is referred to as “4F-GalNAc,” whereas its deacetylated analog 2-acetamido-2,4-dideoxy-4-fluoro-D-galactopyranose is called “nonacetylated 4F-GalNAc.” Results demonstrate that 50μM 4F-GalNAc added in cell culture media reduced selectin-mediated cell adhesion function in vitro; 4F-GalNAc also impaired leukocyte recruitment in vivo in a mouse inflammation model.
Methods
Supplemental data (available on the Blood website; see the Supplemental Materials link at the top of the online article) contain details on many procedures used in this work. Chemical synthesis of acetylated monosaccharides, fluorinated analogs, and radioactive [14C]4F-GalNAc (Figure 1B) is described here.
Cell culture
Human promyelocytic leukemia HL-60 cells were cultured in Iscove modified Dulbecco media (Invitrogen) with 20% fetal bovine serum (FBS) as recommended by ATCC. In many runs, per-acetylated forms of the natural monosaccharide GalNAc, monosaccharide analog (4F-GalNAc, 4F-GlcNAc), or radiolabeled [14C]4F-GalNAc at various doses (0-100μM) were added to cells at 0.2 × 106 cells/mL while they were in their exponential growth phase. The time when monosaccharide was added is designated t = 0 hours. In some cases, cells were treated with 0.05 U/mL Vibrio cholerae neuraminidase/sialidase (Sigma-Aldrich) for 1 hour at 37°C in 30mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer (pH 6.9) containing 10% fetal bovine serum. This enzyme cleaves α2,3/6/8-linked sialic acids. Residual neuraminidase was removed by washing before experimentation.
Flow cytometry
Flow cytometry measured cellular antigen expression (Lewis-X/LeX/CD15, sialyl Lewis-X/sLeX/CD15s/CSLEX-1, CLA/HECA-452, PSGL-1/CD162, Galβ1,4GlcNAc-/LacNAc/DSA [Datura stramonium] lectin binding and α1,3/4/6-linked fucose/AAL [Aleuria aurantia] lectin binding) and selectin (human P-/L-/E-selectin) fusion protein binding to leukocytes. Details are part of the supplemental data.
Cell adhesion under fluid flow
Parallel-plate flow chamber experiments were performed as described previously.33 Flow chamber substrate contained either a confluent monolayer of mouse fibroblast L cells that stably expressed human E-selectin and ICAM-1 (L-E/I)33 or recombinant P-selectin immunoglobulin G (IgG) fusion protein.34 HL-60 cells cultured for 38 hours either in the presence of 50μM 4F-GalNAc or vehicle control were perfused over the selectin-bearing substrate at 0.4 × 106/mL. The number of rolling cells, adherent cells, and cell rolling velocities were quantified as described in supplemental data.
Metabolic labeling studies performed with [14C]4F-GalNAc
HL-60 cells were cultured with per-acetylated [14C]4F-GalNAc (0.5 μCi/mL, 100μM) for 38 hours. The fate of the radioactive monosaccharide was analyzed by measuring (1) total radioactivity in various cell fractions, (2) monosaccharide status in cell culture supernatant, and (3) metabolic incorporation of [14C]4F-GalNAc into leukocyte PSGL-1. Detailed procedures are provided in supplemental data.
Cytometry-bead assay and Western blotting
The carbohydrate structure(s) on PSGL-1 were assessed by immunoprecipitating this protein from HL-60 lysates. Quantitative cytometry-bead assays and qualitative Western blot analysis were performed (supplemental data).35
Briefly, in each cytometry-bead experiment, PSGL-1 from 200 to 400 μg of cell lysate was immunoprecipitated onto “TB5 beads” (6-μm polystyrene beads with covalently immobilized anti–PSGL-1 monoclonal antibody [mAb] TB5). The binding of fluorescent reagents to these beads was measured. These includes fluorescein isothiocyanate (FITC)–conjugated HECA-452, FITC-DSA lectin, and unconjugated anti–PSGL-1 antibody (H-300, rabbit polyclonal antibody [pAb]) followed by FITC-conjugated F(ab′)2 mouse anti–rabbit IgG. Mean fluorescence intensity from control experiments performed in the absence of cell lysate was subtracted from all data presented in the figures.
For Western blots, PSGL-1 immunoprecipitated using 7 μg/mL anti-PSGL-1 (H-300) and protein A/G beads was separated using either 8% or 4% to 20% gradient Tris-sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel. In experiments with radiolabeled compounds, protein blotted onto nitrocellulose membrane was visualized using phosphorimaging. In other studies, the membrane was probed with anti–PSGL-1 mAb (KPL-1) or HECA-452 followed by appropriate horseradish peroxidase–conjugated secondary Abs, and chemiluminescence detection.
Mouse peritonitis model
Two types of experiments were performed (supplemental data). In some cases, mouse bone marrow cells (BMCs) recovered from donor C57BL/6 mice were cultured ex vivo for 2 days with acetylated monosaccharides or vehicle control. Antigen levels and selectin/IgG binding were measured using flow cytometry. BMCs were then reinjected into recipient mice, and peritonitis was induced by intraperitoneal injection of thioglycollate.36 In other cases, 100 mg/kg per day 4F-GalNAc or vehicle control was injected into mouse tail veins for 4 days before induction of peritonitis. Leukocyte counts in peritoneal lavage were measured in both experiments. All animal protocols were approved by the Institutional Animal Care and Use Committee of the Roswell Park Cancer Institute.
Statistics
Data are presented as mean plus or minus SEM for 3 or more independent experiments. In cases where results are presented in percentage form, normalization was performed with respect to vehicle control (< 0.25% dimethyl sulfoxide [DMSO]) runs conducted in parallel. Student t test (2-tailed) was performed for dual comparisons with respect to vehicle control. Analysis of variance was applied for comparison between multiple treatments. P values less than .05 were considered significant.
Results
We evaluated the ability of synthetic monosaccharide analogs, primarily 4F-GalNAc and also 4F-GlcNAc (Figure 1B), to alter carbohydrate structures on PSGL-1 and the selectin-binding function of leukocytes. All carbohydrates were acetylated to enhance their cell permeability. Acetyl groups are hydrolyzed by intracellular esterases.13
4F-GalNAc reduces leukocyte P-selectin binding and the expression of cell-surface CLA and sLeX epitopes
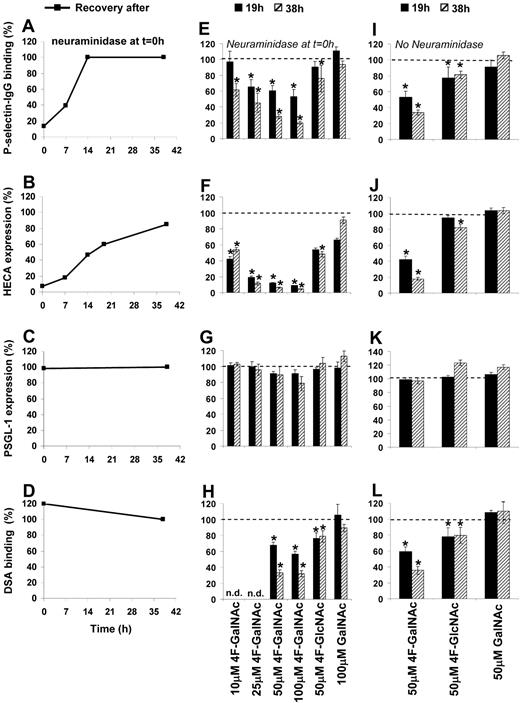
Two types of experiments were performed. Similar to a recent publication,28 in some cases, HL-60 cells were treated with neuraminidase to remove cell-surface sialic acid before addition of acetylated monosaccharide (Figure 2A-H). Neuraminidase reduced P-selectin binding to HL-60 by 85% (Figure 2A, data at t = 0 hours) and cell-surface CLA/HECA expression by 90% (Figure 2B). The anti-CLA mAb HECA-452 binds both CD15s/sLeX and other sLeX-like antigens37 (Figure 1A). No change in PSGL-1 levels was noted (Figure 2C), although LeX expression (data not shown) and DSA-lectin binding (Figure 2D) increased by 900% and 20%, respectively. Because DSA-lectin recognizes Galβ1,4GlcNAc structures (Figure 1A), the data suggest that removal of sialic acid exposes new terminal LacNAc structures. In other runs, monosaccharides were added without prior sialidase treatment (Figure 2I-L). Whereas the first set of experiments examine CLA and sLeX expression, and P-selectin binding function of newly synthesized PSGL-1 only, the latter also account for the recycling of PSGL-1 and the effects of cell division.
Effect of monosaccharides on epitope expression and P-selectin–binding function. The effect of fully acetylated natural monosaccharide (GalNAc) or monosaccharide analog (4F-GalNAc, 4F-GlcNAc) added to HL-60 cells during their exponential growth phase was assessed in terms of P-selectin–binding function (A,E,I), (2) CLA/HECA expression (B,F,J), PSGL-1 expression (C,G,K), and DSA-binding efficiency (D,H,L). Neuraminidase was used to remove cell surface sialic acids and selectin-binding function at t = 0 hours in panels A through H, but not panels I through L. P-selectin binding (A), CLA/HECA expression (B), and DSA binding (D) returned to baseline levels (denoted by 100%) after removal of neuraminidase. 4F-GalNAc was more effective than 4F-GlcNAc in reducing P-selectin binding (E), CLA/HECA expression (F), and DSA binding (H). Control monosaccharide GalNAc behaved similarly to vehicle (DMSO) control. Results similar to those shown in panels E-H were observed when HL-60 cells were treated with modified sugars in the absence of neuraminidase treatment at t = 0 hours (I-L). Data are percentages with respect to HL-60 cells treated with vehicle control and no neuraminidase. n.d. indicates not done. *P < .05 with respect to vehicle control.
Effect of monosaccharides on epitope expression and P-selectin–binding function. The effect of fully acetylated natural monosaccharide (GalNAc) or monosaccharide analog (4F-GalNAc, 4F-GlcNAc) added to HL-60 cells during their exponential growth phase was assessed in terms of P-selectin–binding function (A,E,I), (2) CLA/HECA expression (B,F,J), PSGL-1 expression (C,G,K), and DSA-binding efficiency (D,H,L). Neuraminidase was used to remove cell surface sialic acids and selectin-binding function at t = 0 hours in panels A through H, but not panels I through L. P-selectin binding (A), CLA/HECA expression (B), and DSA binding (D) returned to baseline levels (denoted by 100%) after removal of neuraminidase. 4F-GalNAc was more effective than 4F-GlcNAc in reducing P-selectin binding (E), CLA/HECA expression (F), and DSA binding (H). Control monosaccharide GalNAc behaved similarly to vehicle (DMSO) control. Results similar to those shown in panels E-H were observed when HL-60 cells were treated with modified sugars in the absence of neuraminidase treatment at t = 0 hours (I-L). Data are percentages with respect to HL-60 cells treated with vehicle control and no neuraminidase. n.d. indicates not done. *P < .05 with respect to vehicle control.
After neuraminidase removal at t = 0 hours, cells were allowed to grow in normal culture media containing either acetylated monosaccharides at indicated dose (Figure 2E-H) or vehicle control (Figure 2A-D). In vehicle control, P-selectin fusion protein binding returned to baseline levels by 14 hours (Figure 2A), whereas CLA/HECA took 36 hours (Figure 2B). Addition of 4F-GalNAc reduced P-selectin binding in a dose-dependent manner (Figure 2E). A total of 50μM 4F-GalNAc prevented the formation of any new CLA/HECA in HL-60 (Figure 2F) and reduced DSA-lectin (Figure 2H) and P-selectin binding by approximately 70% compared with vehicle/GalNAc control at 38 hours. 4F-GalNAc treatment of HL-60 also abrogated anti-sLeX mAb CSLEX-1 binding to cells. P-selectin and DSA-lectin binding to 4F-GalNAc–treated cells was higher at 19 hours (1 cell division) compared with 38 hours (2 cell divisions), suggesting that the effects of acetylated monosaccharides are more prominent after cell division. Whereas PSGL-1 expression was not affected by any treatment (Figure 2G), a 10% to 15% reduction in cell growth rate at 38 hours was noted compared with the vehicle/untreated control. In our experimental system, 4F-GalNAc was a superior inhibitor of selectin binding compared with 4F-GlcNAc (Figure 2E-H). Vehicle control runs with less than or equal to 0.25% DMSO behaved identically to cells grown in normal cell culture medium in all assays described in this study. The specificity of the P-selectin binding assay was confirmed in control studies (supplemental Figure 2).34,38
Observations similar to those shown in Figure 2E-H were made in runs where cells were not treated with neuraminidase before the addition of acetylated monosaccharide (Figure 2I-L). Here, too, 50μM 4F-GalNAc was more effective compared with 50μM 4F-GlcNAc at reducing P-selectin binding (66% vs 22% at 38 hours, Figure 2I), CLA/HECA (82% vs 20% at 38 hours, Figure 2J), and LacNAc (65% vs 22% at 38 hours, Figure 2L) epitope expression. No significant changes were observed in either GalNAc-supplemented cells or vehicle controls. Overall, 4F-GalNAc reduced both P-selectin binding to HL-60 and the expression of CLA/HECA and sLeX epitopes.
4F-GalNAc reduces L- and E-selectin fusion protein binding to HL-60
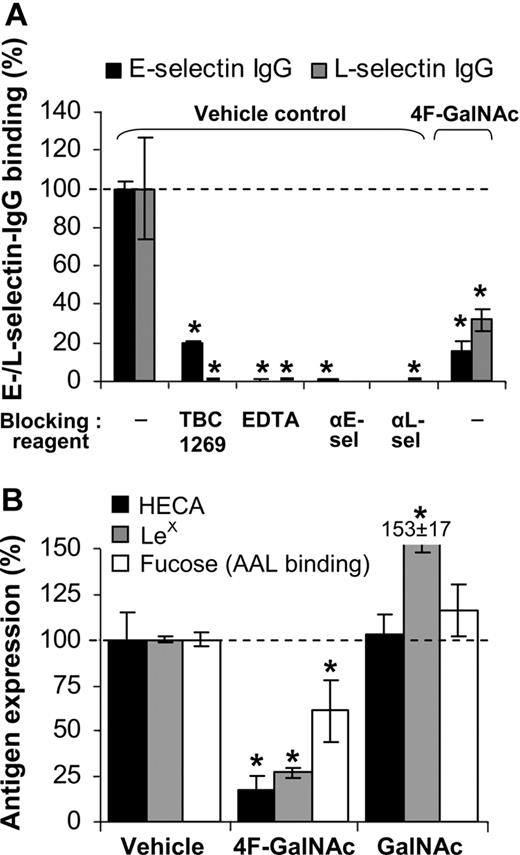
We tested whether E- and L-selectin IgG binding to monosaccharide-treated cells is also reduced (Figure 3A). Control runs verified the specificity of the measured interaction by demonstrating that selectin binding was blocked by 5mM ethylenediaminetetraacetic acid (EDTA), 2mM sLeX analog TBC1269, or blocking antibodies against individual selectins. Addition of 50μM 4F-GalNAc to growth medium resulted in 80% and 60% reduction in E- and L-selectin binding, respectively, to HL-60 cells at 38 hours, compared with vehicle control. Thus, 4F-GalNAc functions as a pan-selectin inhibitor.
E-/L-selectin binding. (A) E-/L-selectin–IgG fusion protein binding to HL-60 cells was measured at t = 38 hours after protocols identical to Figure 2I through L, in the absence of prior neuraminidase treatment. In vehicle control runs, selectin binding was blocked by 80% to 100% on addition of 3mM sLeX analog (TBC1269), 5mM ethylenediaminetetraacetic acid, or 30 μg/mL antiselectin Ab (either EP5C7 against E-selectin or DREG56 against L-selectin). Culturing cells in the presence of 50μM 4F-GalNAc reduced E-/L-selectin binding by more than 70%. (B) LeX, sLeX expression and AAL lectin (binds α1,3/4/6-linked fucose antigen) binding were measured at 38 hours. 4F-GalNAc reduced LeX expression and AAL lectin binding. Data are mean ± SEM (N ≥ 3 experiments). *P < .05 with respect to vehicle control with no blocking reagent.
E-/L-selectin binding. (A) E-/L-selectin–IgG fusion protein binding to HL-60 cells was measured at t = 38 hours after protocols identical to Figure 2I through L, in the absence of prior neuraminidase treatment. In vehicle control runs, selectin binding was blocked by 80% to 100% on addition of 3mM sLeX analog (TBC1269), 5mM ethylenediaminetetraacetic acid, or 30 μg/mL antiselectin Ab (either EP5C7 against E-selectin or DREG56 against L-selectin). Culturing cells in the presence of 50μM 4F-GalNAc reduced E-/L-selectin binding by more than 70%. (B) LeX, sLeX expression and AAL lectin (binds α1,3/4/6-linked fucose antigen) binding were measured at 38 hours. 4F-GalNAc reduced LeX expression and AAL lectin binding. Data are mean ± SEM (N ≥ 3 experiments). *P < .05 with respect to vehicle control with no blocking reagent.
Reduction in CLA and sLeX expression on 4F-GalNAc treatment could be the result of alteration in the extent of fucosylation in addition to the extent of sialylation. To test this possibility, cytometry studies were performed with antibodies and AAL, an α1,3/4/6-fucose binding lectin (Figure 1A). As seen, LeX epitope expression and AAL binding decreased by 73% and 40%, respectively, in 4F-GalNAc–supplemented cells compared with vehicle control (Figure 3B). AAL lectin-binding specificity for fucose was verified using 100mM L-fucose, which abrogated lectin-binding interaction. Thus, reduction in the extent of fucosylation may partly account for the reduced selectin binding.
Treatment with 4F-GalNAc increases leukocyte rolling velocity
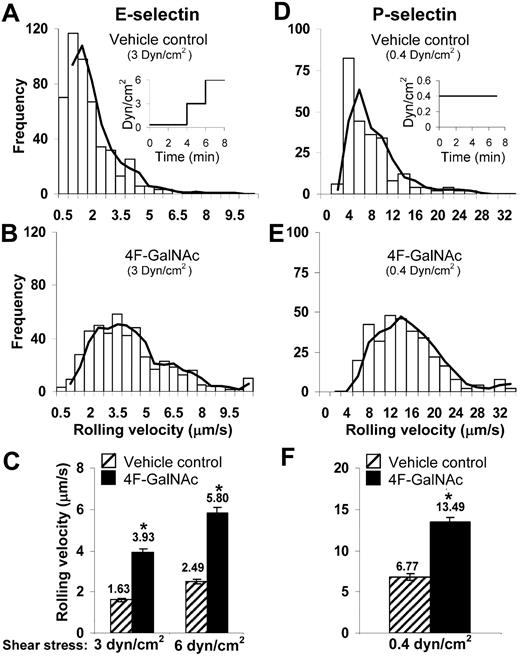
The ability of 4F-GalNAc to reduce selectin-mediated cell adhesion under fluid flow was measured using parallel-plate flow chambers (Figure 4). HL-60 cells were pretreated with neuraminidase before culture with either vehicle control or 4F-GalNAc for 38 hours. Thereafter, the binding of these cells to E-selectin–bearing cell monolayers (Figure 4A-C) or immobilized P-selectin fusion protein (Figure 4D-F) was measured.
Cell adhesion under flow. A total of 0.4 × 106/mL HL-60 cells cultured with 50μM 4F-GalNAc or vehicle control for 38 hours were perfused over either L-E/I (E-selectin) cell monolayers (A-C) or reconstituted P-selectin–bearing surfaces (D-F) in a parallel-plate flow chamber. Fluid shear was step increased from 0.35 dyn/cm2 to 3 and 6 dyn/cm2 in the case of E-selectin experiments as indicated (A inset). Wall shear stress was constant at 0.4 dyn/cm2 in P-selectin runs (D inset). Representative binned histograms of rolling velocities in vehicle control (A,D) and 4F-GalNAc–treated samples (B,E) are presented. (C,F) Rolling velocity data at indicated shear rates. 4F-GalNAc increased cell rolling velocity by 2.4-fold for E- and 2-fold for P-selectin–mediated rolling. *P < .05 with respect to vehicle control.
Cell adhesion under flow. A total of 0.4 × 106/mL HL-60 cells cultured with 50μM 4F-GalNAc or vehicle control for 38 hours were perfused over either L-E/I (E-selectin) cell monolayers (A-C) or reconstituted P-selectin–bearing surfaces (D-F) in a parallel-plate flow chamber. Fluid shear was step increased from 0.35 dyn/cm2 to 3 and 6 dyn/cm2 in the case of E-selectin experiments as indicated (A inset). Wall shear stress was constant at 0.4 dyn/cm2 in P-selectin runs (D inset). Representative binned histograms of rolling velocities in vehicle control (A,D) and 4F-GalNAc–treated samples (B,E) are presented. (C,F) Rolling velocity data at indicated shear rates. 4F-GalNAc increased cell rolling velocity by 2.4-fold for E- and 2-fold for P-selectin–mediated rolling. *P < .05 with respect to vehicle control.
Cell rolling velocity, number of rolling cells, and adherent cell density were quantified. The rolling cell density of HL-60 cultured with 4F-GalNAc was similar to vehicle control both in the case of E-selectin (∼ 110 cells/mm2) and P-selectin (∼ 145 cells/mm2) mediated cell adhesion. Cell rolling velocities were 2.4-fold higher for cells cultured with 4F-GalNAc compared with vehicle control for E-selectin–mediated rolling (Figure 4A-C). Similar results were observed for P-selectin–mediated rolling, where the rolling velocity for 4F-GalNAc–treated cells ranged from 4 to 20 μm/second compared with 2 to 10 μm/second for vehicle and GalNAc control. Although firmly attached cells were observed in vehicle control runs (77 ± 9 cells/mm2 at 3 dyn/cm2) in the E-selectin adhesion assay, adherent cell number was reduced (to 14 ± 8 cells/mm2) when HL-60 cells were cultured with 4F-GalNAc. The firmly attached cells were probably the result of the high density of E-selectin on the L-E/I monolayer because most of these cells could be detached on increasing the shear stress to 6 dyn/cm2. This interaction could also be blocked by anti–E-selectin mAb.
In control runs, cell rolling via both E- and P-selectin was absent on chelating calcium with 5mM EDTA. HL-60 also did not interact with substrates bearing rabbit anti–mouse F(ab′)2 antibody alone in the absence of P-selectin–IgG. Furthermore, HL-60 rolling/adhesive interactions to either P-selectin– or E-selectin–bearing substrates could be completely blocked on incubation of substrates with anti–P-selectin mAb G1 or anti–E-selectin mAb EP5C7. Experiments performed with control per-acetylated GalNAc resulted in cell rolling phenotype that was similar to vehicle control. Overall, 4F-GalNAc substantially increased E- and P-selectin–mediated cell rolling velocities. This may be the result of alterations in the carbohydrate structure of PSGL-1.
4F-GalNAc reduces the molecular weight of leukocyte PSGL-1 and the expression of CLA/HECA and LacNAc epitopes on this glycoprotein
We determined the effect of 4F-GalNAc on PSGL-1 glycosylation (Figure 5). With the goal of obtaining quantitative results and time-course data rapidly, polystyrene beads covalently linked with anti–PSGL-1 mAb TB5 (“TB5 beads”) and cytometry-based assays were developed (supplemental Figure 3). In these assays, PSGL-1 from cell lysate was first captured onto TB5 beads via immunoaffinity. After this, the beads were probed with (1) FITC-conjugated anti-CLA mAb HECA-452, (2) FITC-labeled DSA-lectin to detect LacNAc structures, or (3) anti–PSGL-1 antibody H-300 (rabbit IgG) along with FITC-labeled mouse anti–rabbit secondary Ab. Competitive binding assays, blocking studies, and isotype-control beads described in supplemental Figure 3 confirm that this assay specifically probes changes in glycan structures associated with PSGL-1.
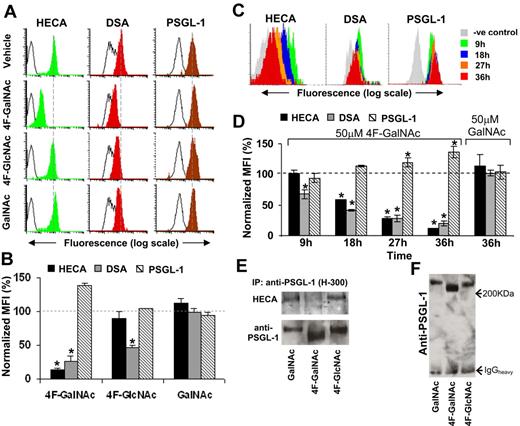
Glycan structures on PSGL-1. (A) HL-60 cells were cultured in the presence of 50μM acetylated monosaccharides (4F-GalNAc, 4F-GlcNAc, GalNAc) for 36 hours as outlined in Figure 2A through H. PSGL-1 was immunoprecipitated from 400 μg of cell lysate onto TB5 beads. Carbohydrate epitopes on this antigen were then probed using HECA-452 and DSA lectin. The number of immobilized PSGL-1 was quantified using anti-PSGL-1 polyclonal mAb H-300. Black-empty and color-filled histograms represent the fluorescence of beads resulting from Ab/lectin in the absence and presence of cell lysate, respectively. Dashed vertical line indicates peak fluorescence intensity in vehicle control. Representative histograms are shown. (B) Summary of results from panel A for 3 or more independent runs. Normalized geometric mean fluorescence intensity (%) = (MFI of sample − background MFI in the absence of cells)/(MFI of vehicle control − background MFI in the absence of cells). 4F-GalNAc reduces CLA/HECA expression on PSGL-1 and the amount of DSA-lectin recognition. *P < .05 with respect to GalNAc treatment. (C-D) Representative cytometry-bead histograms (C) and normalized MFI data (D) show temporal changes in PSGL-1 glycosylation on 50μM 4F-GalNAc or GalNAc treatment. Here, HL-60 cells were cultured in the absence of prior neuraminidase treatment (protocol in Figure 2I-L). CLA/HECA-452 expression, DSA-lectin binding, and PSGL-1 amount on beads were quantified after capture of PSGL-1 from same amount of lysate in all samples. *P < .05 with respect to t = 0 hours. (E) PSGL-1 from HL-60 cells cultured in 50μM 4F-GalNAc, 4F-GlcNAc, or GalNAc were immunoprecipitated using Ab H-300 and analyzed using Western blot analysis. Protein immunoprecipitated from 300 μg of lysate was loaded in each lane of 2 identical 8% polyacrylamide gel electrophoresis gels. One blot was probed with anti-PSGL-1 monoclonal antibody (KPL-1) and the other with HECA-452. CLA/HECA expression on PSGL-1 was reduced on 4F-GalNAc treatment. (F) 4F-GalNAc treatment reduced the molecular weight of PSGL-1 by approximately 10% to 15%.
Glycan structures on PSGL-1. (A) HL-60 cells were cultured in the presence of 50μM acetylated monosaccharides (4F-GalNAc, 4F-GlcNAc, GalNAc) for 36 hours as outlined in Figure 2A through H. PSGL-1 was immunoprecipitated from 400 μg of cell lysate onto TB5 beads. Carbohydrate epitopes on this antigen were then probed using HECA-452 and DSA lectin. The number of immobilized PSGL-1 was quantified using anti-PSGL-1 polyclonal mAb H-300. Black-empty and color-filled histograms represent the fluorescence of beads resulting from Ab/lectin in the absence and presence of cell lysate, respectively. Dashed vertical line indicates peak fluorescence intensity in vehicle control. Representative histograms are shown. (B) Summary of results from panel A for 3 or more independent runs. Normalized geometric mean fluorescence intensity (%) = (MFI of sample − background MFI in the absence of cells)/(MFI of vehicle control − background MFI in the absence of cells). 4F-GalNAc reduces CLA/HECA expression on PSGL-1 and the amount of DSA-lectin recognition. *P < .05 with respect to GalNAc treatment. (C-D) Representative cytometry-bead histograms (C) and normalized MFI data (D) show temporal changes in PSGL-1 glycosylation on 50μM 4F-GalNAc or GalNAc treatment. Here, HL-60 cells were cultured in the absence of prior neuraminidase treatment (protocol in Figure 2I-L). CLA/HECA-452 expression, DSA-lectin binding, and PSGL-1 amount on beads were quantified after capture of PSGL-1 from same amount of lysate in all samples. *P < .05 with respect to t = 0 hours. (E) PSGL-1 from HL-60 cells cultured in 50μM 4F-GalNAc, 4F-GlcNAc, or GalNAc were immunoprecipitated using Ab H-300 and analyzed using Western blot analysis. Protein immunoprecipitated from 300 μg of lysate was loaded in each lane of 2 identical 8% polyacrylamide gel electrophoresis gels. One blot was probed with anti-PSGL-1 monoclonal antibody (KPL-1) and the other with HECA-452. CLA/HECA expression on PSGL-1 was reduced on 4F-GalNAc treatment. (F) 4F-GalNAc treatment reduced the molecular weight of PSGL-1 by approximately 10% to 15%.
Our experiments monitored PSGL-1 in cells cultured in the presence of 50μM GalNAc, 4F-GalNAc, or 4F-GlcNAc (Figure 5A-B). After 36 hours, CLA/HECA expression and DSA lectin binding were reduced by 86% and 73%, respectively, on 50μM 4F-GalNAc treatment, compared with 10% and 53% for cells cultured with 4F-GlcNAc. Time-course studies demonstrated reduced CLA/HECA and LacNAc (detected by DSA-lectin) expression on PSGL-1 in 4F-GalNAc–treated cells beginning at 9 to 18 hours. No change was noted in GalNAc control (Figure 5C-D).
Immunoprecipitation of PSGL-1 followed by Western blot analysis verified results from cytometry-bead assays. CLA/HECA antigen associated with PSGL-1 was reduced by 4F-GalNAc, but not GalNAc or 4F-GlcNAc (Figure 5E). Gels run for extended duration show that the apparent molecular weight of PSGL-1 is reduced by 12.5% on 4F-GalNAc addition (Figure 5F). After accounting for the molecular weight of the peptide backbone, this translates to an approximately 20% reduction in the total glycan content of PSGL-1. Overall, the culture of cells with 4F-GalNAc results in a reduction in the LacNAc epitope, CLA/HECA expression, and molecular weight of PSGL-1.
4F-GalNAc is metabolically incorporated into PSGL-1
HL-60 cells were cultured with per-acetylated [14C]4F-GalNAc for 38 hours under standard conditions, and the fate of the radioactive compound was followed; 2% to 3% of the radioactivity was associated with the cellular glycoproteins, whereas a majority of the compound remained in the culture media (Table 1). With regard to this observation, because HL-60 cells (0.7 × 106 cells/mL, ∼ 15.6 μm diameter) constitute only 0.14% of the volume of the experimental well (1 mL), 3% of 4F-GalNAc associated with the cells represents a relatively high intracellular concentration of the modified monosaccharide (1.1mM).
Radioactivity associated with cells and cell components
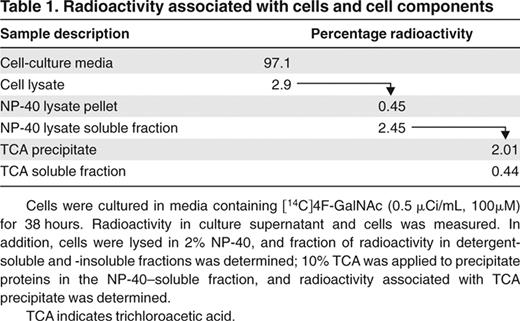
Cells were cultured in media containing [14C]4F-GalNAc (0.5 μCi/mL, 100μM) for 38 hours. Radioactivity in culture supernatant and cells was measured. In addition, cells were lysed in 2% NP-40, and fraction of radioactivity in detergent-soluble and -insoluble fractions was determined; 10% TCA was applied to precipitate proteins in the NP-40–soluble fraction, and radioactivity associated with TCA precipitate was determined.
TCA indicates trichloroacetic acid.
Because 97% of the total radioactivity was associated with the culture media (Table 1), additional studies followed the fate of [14C]4F-GalNAc in the growth media. Here, media containing [14C]4F-GalNAc was placed under standard growth conditions for 38 hours either in the presence or absence of HL-60 cells. Media recovered at 38 hours were separated using a Biogel P-2 column (Figure 6A-B). Negligible radioactivity was measured at the void volume (position 1), indicating that secreted proteins from HL-60 had negligible [14C]4F-GalNAc. Two major peaks were evident when HL-60 cells were present in culture (positions 2-3; Figure 6A), whereas only one peak appeared in the absence of cells (position 5; Figure 6B). Eluates corresponding to these peaks were separated using thin layer chromatography (TLC; Figure 6C), and their migration was compared with nonradiolabeled per-acetylated 4F-GalNAc and nonacetylated 4F-GalNAc (∼ 10 μg) under identical conditions. As seen, the first peak (positions 2 and 5) that is common between both runs contains primarily per-acetylated, 4F-GalNAc (Figure 6C lanes 2 and 5). Some product also appeared at intermediate distances between the per-acetylated and -deacetylated 4F-GalNAc, and this probably represents 4F-GalNAc that was partially hydrolyzed in culture media. In this regard, the original [14C]4F-GalNAc diluted and kept in DMSO under the same culture conditions appeared as a unique spot that migrated identically to fully acetylated 4F-GalNAc shown in lane 7 (Figure 6C). The second peak (position 3, lane 3), which is unique to experiments containing HL-60, migrated identically to nonacetylated 4F-GalNAc. This may represent 4F-GalNAc that was deacetylated inside cells before being secreted/diffusing out from the cells.
[14C]4F-GalNAc distribution in culture media and glycoproteins. Cell culture media containing [14C]4F-GalNAc (0.5 μCi/mL, 100μM) was placed under standard growth conditions either in the presence or absence of HL-60 cells for 38 hours. Growth media and cells were harvested separately. (A-B) Culture media harvested from cultures with (A) and without HL-60 (B) separated on Biogel P-2 column. (C) Samples at the void volume (samples 1 and 4), peak fractions in panel A (samples 2 and 3) and panel B (sample 5) were separated using TLC with chloroform/methanol (5:1) as mobile phase. Phosphorimaging detected radioactivity on TLC plates. Nonradiolabeled, nonacetylated 4F-GalNAc (lane 6) and per-acetylated 4F-GalNAc (lane 7) standards were run in parallel and developed using ethanol/sulfuric acid spray followed by charring. Addition of acetylated 4F-GalNAc to HL-60 cells leads to deacetylation as evidenced by prominent peak 3 in the chromatogram. (D) PSGL-1 was immunoprecipitated from HL-60 lysates cultured with [14C]4F-GalNAc using Ab H-300. This immunoprecipitate and PSGL-1–depleted cell lysate were resolved on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and blotted onto nitrocellulose membrane. Radioactivity was measured using scintillation counter in panels A and B; and using phosphorimaging in panels C and D. 4F-GalNAc is incorporated into leukocyte PSGL-1 and other glycoproteins.
[14C]4F-GalNAc distribution in culture media and glycoproteins. Cell culture media containing [14C]4F-GalNAc (0.5 μCi/mL, 100μM) was placed under standard growth conditions either in the presence or absence of HL-60 cells for 38 hours. Growth media and cells were harvested separately. (A-B) Culture media harvested from cultures with (A) and without HL-60 (B) separated on Biogel P-2 column. (C) Samples at the void volume (samples 1 and 4), peak fractions in panel A (samples 2 and 3) and panel B (sample 5) were separated using TLC with chloroform/methanol (5:1) as mobile phase. Phosphorimaging detected radioactivity on TLC plates. Nonradiolabeled, nonacetylated 4F-GalNAc (lane 6) and per-acetylated 4F-GalNAc (lane 7) standards were run in parallel and developed using ethanol/sulfuric acid spray followed by charring. Addition of acetylated 4F-GalNAc to HL-60 cells leads to deacetylation as evidenced by prominent peak 3 in the chromatogram. (D) PSGL-1 was immunoprecipitated from HL-60 lysates cultured with [14C]4F-GalNAc using Ab H-300. This immunoprecipitate and PSGL-1–depleted cell lysate were resolved on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and blotted onto nitrocellulose membrane. Radioactivity was measured using scintillation counter in panels A and B; and using phosphorimaging in panels C and D. 4F-GalNAc is incorporated into leukocyte PSGL-1 and other glycoproteins.
In another assay, radioactivity associated with PSGL-1 was determined by immunoprecipitating the protein using Ab H-300, separation on 4% to 20% gradient gel, and transfer to nitrocellulose membrane. Phosphorimaging of membrane was subsequently performed. Supernatant depleted of PSGL-1 was also analyzed in parallel. This study demonstrates the direct incorporation of 4F-GalNAc into leukocyte PSGL-1 and also other glycoproteins (Figure 6D). Radioactivity associated with leukocyte glycoproteins could be partially removed by α-N-acetylgalactosaminidase (supplemental Figure 4), and this suggests that the radioactivity measured is associated with O-glycans. Overall, 4F-GalNAc is metabolically active, and it can be incorporated into multiple cellular glycoproteins including PSGL-1.
4F-GalNAc reduced leukocyte recruitment to inflamed peritoneum
We determined whether 4F-GalNAc can reduce selectin-mediated leukocyte recruitment at sites of inflammation (Figure 7). BMCs from C57BL/6 mouse femur were cultured ex vivo with either vehicle control or per-acetylated monosaccharides. These BMCs consist predominantly of functional neutrophils.39-41 Consistent with these reports, we also observed, after 2 days of ex vivo culture in the presence of 20 ng/mL granulocyte colony-stimulating factor and 1 ng/mL IL-3, that a majority of LDS-751+ nucleated cells were positive for both granulocytic markers Gr-1 and 1A8/Ly-6-G (supplemental Figure 5). These cells, which appear in region R1 in the flow cytometry dot plot (Figure 7A), expressed mouse PSGL-1 and CD45, and they bound human P-selectin fusion protein (Figure 7B; supplemental Figure 5). Cells in region R2 represent 28% of the LDS-751+ cells, and these did not stain for granulocyte markers. Culture of BMCs with 25μM 4F-GalNAc, but not 25μM GalNAc or vehicle control, led to a 62% decrease in P-selectin–IgG binding to granulocytes (Figure 7B). Whereas CD45 expression was not altered under any of the growth conditions, a 22% and 14% reduction in PSGL-1 was noted on culture with per-acetylated 4F-GalNAc and GalNAc, respectively. In control runs, P-selectin–IgG binding to mouse cells could be abrogated by 10 μg/mL mouse antihuman P-selectin/CD62P blocking antibody G1, 3mM sLeX analog TBC1269, and 5mM EDTA.
Effect of 4F-GalNAc on selectin binding and leukocyte recruitment to peritoneum. BMCs were cultured in media containing granulocyte colony-stimulating factor and IL-3, and 25μM 4F-GalNAc, 25μM GalNAc, or vehicle control (0.125% DMSO). (A) After 2 days in culture, 2 cell populations (regions R1 and R2) stained positive for nuclear dye LDS-751 in the flow cytometer. Cells in R1 were neutrophils (Gr-1+1A8+). (B) PSGL-1 and CD45 antigen expression, and P-selectin IgG binding to Gr-1+ cells were measured. 4F-GalNAc, but not GalNAc or vehicle control, reduced P-selectin fusion protein binding to cells. Data are mean ± SEM with respect to vehicle control. *P < .05 with respect to all other treatments. (C) BMCs cultured for 2 days with 25μM 4F-GalNAc or vehicle control were labeled with either DiL or DiO dyes. Mixed-cell populations just before injection into C57BL/6 recipient mice contained approximately equal numbers of DiL-labeled vehicle control cells [(Vehicle)DiO] and DiO-labeled 4F-GalNAc–treated cells [(4F-GalNAc)DiL] or vice versa. (D) Peritoneal lavage sample from mice obtained 5 hours after intraperitoneal injection of thioglycollate shows substantial accumulation of neutrophils in the peritoneum. These cells were positive for LDS-751, Gr-1, and 1A8. DiO/DiL-labeled cells were not injected in this experiment. (E) Granulocytes from the peritoneal lavage of animals, which were tail-vein injected with mixed DiO + DiL BMCs just before induction of peritonitis. Number of DiL-labeled cells (vehicle control) exceeds DiO-labeled cells (4F-GalNAc–treated). (F) Ratio of 4F-GalNAc to vehicle control cells in infusion and peritoneum lavage sample. Data are mean ± SEM for 4 animals injected with DiL-labeled 4F-GalNAc–treated cells along with paired DiO-labeled vehicle controls. Four animals were injected with DiO/DiL labeling switched. 4F-GalNAc treatment reduced cell migration into peritoneum irrespective of whether these cells were labeled with DiO or DiL. *P < .05 with respect to infusion sample. (G) A total of 100 mg/kg/day 4F-GalNAc or vehicle control was injected into mice for 4 days before induction of peritonitis. Total leukocyte (Total), neutrophil (Neut), eosinophil (Eos), and macrophage (Mac) counts were determined in peritoneal lavage 5 hours after intraperitoneal injection of thioglycollate. Data are mean ± SEM for 4 animals for each treatment. *P < .05 with respect to vehicle control.
Effect of 4F-GalNAc on selectin binding and leukocyte recruitment to peritoneum. BMCs were cultured in media containing granulocyte colony-stimulating factor and IL-3, and 25μM 4F-GalNAc, 25μM GalNAc, or vehicle control (0.125% DMSO). (A) After 2 days in culture, 2 cell populations (regions R1 and R2) stained positive for nuclear dye LDS-751 in the flow cytometer. Cells in R1 were neutrophils (Gr-1+1A8+). (B) PSGL-1 and CD45 antigen expression, and P-selectin IgG binding to Gr-1+ cells were measured. 4F-GalNAc, but not GalNAc or vehicle control, reduced P-selectin fusion protein binding to cells. Data are mean ± SEM with respect to vehicle control. *P < .05 with respect to all other treatments. (C) BMCs cultured for 2 days with 25μM 4F-GalNAc or vehicle control were labeled with either DiL or DiO dyes. Mixed-cell populations just before injection into C57BL/6 recipient mice contained approximately equal numbers of DiL-labeled vehicle control cells [(Vehicle)DiO] and DiO-labeled 4F-GalNAc–treated cells [(4F-GalNAc)DiL] or vice versa. (D) Peritoneal lavage sample from mice obtained 5 hours after intraperitoneal injection of thioglycollate shows substantial accumulation of neutrophils in the peritoneum. These cells were positive for LDS-751, Gr-1, and 1A8. DiO/DiL-labeled cells were not injected in this experiment. (E) Granulocytes from the peritoneal lavage of animals, which were tail-vein injected with mixed DiO + DiL BMCs just before induction of peritonitis. Number of DiL-labeled cells (vehicle control) exceeds DiO-labeled cells (4F-GalNAc–treated). (F) Ratio of 4F-GalNAc to vehicle control cells in infusion and peritoneum lavage sample. Data are mean ± SEM for 4 animals injected with DiL-labeled 4F-GalNAc–treated cells along with paired DiO-labeled vehicle controls. Four animals were injected with DiO/DiL labeling switched. 4F-GalNAc treatment reduced cell migration into peritoneum irrespective of whether these cells were labeled with DiO or DiL. *P < .05 with respect to infusion sample. (G) A total of 100 mg/kg/day 4F-GalNAc or vehicle control was injected into mice for 4 days before induction of peritonitis. Total leukocyte (Total), neutrophil (Neut), eosinophil (Eos), and macrophage (Mac) counts were determined in peritoneal lavage 5 hours after intraperitoneal injection of thioglycollate. Data are mean ± SEM for 4 animals for each treatment. *P < .05 with respect to vehicle control.
We tested the hypothesis that 4F-GalNAc may reduce selectin-mediated leukocyte adhesion in mice. Thus, BMCs cultured ex vivo for 2 days in the presence of 25μM 4F-GalNAc or vehicle control were differentially labeled with lipophilic tracers, DiL (red fluorescence) or DiO (green fluorescence). These cells were mixed in 1:1 proportion and introduced into recipient C57BL/6 mice via tail-vein injection. Peritonitis was immediately induced by intraperitoneal injection of thioglycollate.36 In this model of inflammation, granulocytes constitute more than 70% of the cells in the peritoneal lavage 5 hours after induction of peritonitis.36 Flow cytometric analysis of granulocytes (Gr-1+) just after labeling (Figure 7C) and from the peritoneal lavage (Figure 7D-E) demonstrates that cells labeled with DiO (region R4, Figure 7C) and DiL (region R5) have distinct fluorescence compared with unlabeled native cells (region R3). The forward/side-scatter profile of the cultured cells and extravasated cells was similar. Figure 7E presents a representative experiment where cells cultured with 4F-GalNAc were labeled with DiO, whereas vehicle control cells were DiL-labeled. Here, the number of DiL-labeled cells exceeds the DiO-labeled population. Overall, a 48% reduction in the number of migrated leukocytes was observed with respect to vehicle control when 4F-GalNAc–treated cells were stained with DiL and vehicle controls were DiO-labeled (Figure 7F). Percentage reduction in cell migration was 60% when the labeling protocol was reversed, that is, 4F-GalNAc–treated cells were stained with DiO.
In other studies, 4F-GalNAc or vehicle control was tail-vein injected at 100 mg/kg per day into mice for 4 days (Figure 7G). There was no evidence of toxicity, outward signs of distress, or alteration in the alertness/movement of the animals treated with 4F-GalNAc. Pharmacologic activity of the monosaccharide analog was confirmed because there was a 40% to 50% reduction in total leukocyte, neutrophil, macrophage, and eosinophil count in the peritoneum in 4F-GalNAc–treated animals 5 hours after induction of peritonitis.
Discussion
Experiments were performed to test the possibility that 4F-GalNAc may alter O-linked glycosylation and reduce selectin-mediated cell adhesion. Consistent with this proposition, 4F-GalNAc addition to leukocytes reduced selectin fusion protein binding to HL-60 cells (Figures 2–3), increased HL-60 rolling velocity under fluid shear conditions (Figure 4), reduced the CLA/HECA epitope expression on the glycoprotein PSGL-1 (Figure 5), and inhibited leukocyte migration in a murine model of inflammation (Figure 7). In the last experiments, BMCs cultured ex vivo with 4F-GalNAc displayed reduced migration into the peritoneum during inflammation. Direct intravenous infusion of 4F-GalNAc also reduced leukocyte recruitment; thus, the compound is pharmacologically active.
Whereas rolling velocity doubled on 4F-GalNAc treatment compared with vehicle control in the flow-chamber assays and there was an approximately 75% to 80% reduction in the number of adherent cells, the total number of cells interacting with the selectin bearing substrate was not statistically different. In contrast, in the static assay, 4F-GalNAc reduced the binding of all 3 members of the selectin family (L-, E-, and P-selectin) to HL-60 cells by approximately 70%. One explanation for the more prominent reduction in fusion protein binding compared with cell adhesion under flow is that sLeX on other glycoproteins besides PSGL-1 may cooperate to stabilize soluble-selectin binding to leukocytes.42 Whereas blocking PSGL-1 alone is sufficient to abrogate molecular interactions under both static and shear-flow conditions, blocking sLeX/CLA formation with 4F-GalNAc is more effective under static conditions. Alternatively, the application of 4F-GalNAc may result in the appearance or exposure of other glycan structures that compensate for the loss of the sLeX/CLA epitope on PSGL-1. This change in the selectin-binding epitope may then lead to an increase in leukocyte rolling velocity. As shown by Jung et al,43 increasing cell rolling velocity reduces firm cell adhesion at sites of inflammation by reducing the time available for the leukocytes to integrate local chemoattractant signals. In our animal studies, also, we observed that 4F-GalNAc treatment reduced leukocyte migration into the inflamed peritoneum. Taken together, the data demonstrate that 4F-GalNAc can reduce selectin binding and leukocyte adhesion in disease models.
In addition to reducing cell adhesion, 4F-GalNAc dramatically reduced cell surface sLeX expression on HL-60 by approximately 82%, and it partially reduced the incorporation of fucose into cellular glycoconjugates. The reduction in sLeX expression was consistently observed using antibodies against CLA (HECA-452) and CD15s/sLeX (CSLEX-1). This inhibition was more prominent on addition of 50μM 4F-GalNAc compared with 50μM 4F-GlcNAc, or even 100 U/mL chymotrypsin.35 sLeX on HL-60 is primarily expressed on glycoproteins,44 although the exact protein scaffolds and glycan structures that bear this epitope remain unknown. Taken together, the data suggest that 4F-GalNAc may be a useful reagent in studies that examine the functional effects of sLeX/CLA located on glycoproteins. Given the absence of efficient tools to block O-glycosylation in cells, this reagent may also find utility in studies that examine the role of O-linked glycosylation in not only disease, but also immunity45 and development.46
Quantitative cytometry-bead assays coupled with qualitative Western blot analysis demonstrate that the glycans of PSGL-1 are modified on 4F-GalNAc treatment. In this regard, a 12.5% reduction in the apparent molecular weight of PSGL-1 was noted, along with a decrease in the expression of the CLA/HECA and Galβ1,4GlcNAc- (LacNAc) epitopes on this glycoprotein. These glycan changes probably occur in O-glycan linkages because PSGL-1 is a mucinous protein with 71 Ser/Thr sites for O-linked glycosylation and only 3-sites for N-glycosylation.2 GalNAc is also only seldom a part of N-glycans.32 A similar 14.5% to 16% reduction in the apparent molecular weight of PSGL-1 has been reported by others,47 who quantified PSGL-1 molecular weight in bone marrow neutrophils of Core 2-GlcNAcT-I–deficient mice compared with littermate controls. Core 2 O-glycans are absent in these transgenic mice, and selectin binding function is reduced. Thus, in both this published study and our work, the total glycan content of PSGL-1 is reduced by 20% to 25%. Alteration in PSGL-1 molecular weight is not noted in a study that examines the effect of 4F-GlcNAc on selectin function.28 Together, the data are consistent with the notion that 4F-GalNAc modifies critical O-linked glycans that are required for selectin recognition. Further, 4F-GalNAc and 4F-GlcNAc may reduce selectin-ligand interaction via different mechanisms.
Studies performed with radioactive, per-acetylated 4F-GalNAc demonstrate the direct incorporation of the modified monosaccharide into cellular glycoproteins, including PSGL-1. This suggests that 4F-GalNAc is metabolically active and it may undergo several metabolic transformations. First, acetylation increases molecular hydrophobicity, and this enhances cell permeability.13 Once in the cytoplasm, esterases hydrolyze O-acetyl groups.13 4F-GalNAc probably undergoes activation by uridine diphosphate (UDP) transfer to form UDP-4F-GalNAc. Such activation may allow facilitated transport of sugar nucleotide into the Golgi lumen via amino-sugar transporters.32 The efficiency of such transport is high resulting from the low KM (1-10μM) of these transporters and the relatively higher concentration (approximately millimolar) of modified sugar nucleotides in the cytoplasm. Unlike activated sugar-nucleotides, which may be transported into the Golgi via facilitated transport, passive diffusion into the Golgi may limit the efficacy of aryl-GalNAc molecules12 and decoys14,15 used by others. Finally, because the 4-OH moiety of GalNAc is important for the reversible conversion of GalNAc analogs to GlcNAc analogs via UDP-Gal/GalNAc-4-epimerase, it is possible that 4F-GalNAc cannot be transformed into other monosaccharides.32 Thus, the effect of 4F-GalNAc may be localized to a relatively fewer metabolic steps compared with 4F-GlcNAc. The later molecule may also interact with the N-acetyl mannosamine and sialic acid biosynthesis pathways.32
Besides the direct incorporation of 4F-GalNAc into the glycans of PSGL-1, other mechanisms may also contribute to the observed reduction in selectin-binding function. First, the attachment of 4F-GalNAc may reduce the extent of Core 2 glycan formation. In support of this, Brockhausen et al48,49 have shown that Core-2 forming enzyme β1,6GlcNAcT recognizes many of the −OH groups in the glycan core 1 (Galβ1,3GalNAc) structure. Hydroxyl groups at the C-4 and C-6 positions of both Gal and GalNAc along with the C-2 N-acetyl group are shown to be essential for full activity of this enzyme. Second, it may be possible that monosaccharide analogs may act as inhibitors of specific glycosyltransferases, like fucosyltransferases. In this regard, native 4F-GalNAc, its nucleotide form UDP-4F-GalNAc, or specific glycoconjugates formed because of 4F-GalNAc incorporation may display enzyme inhibition function. Third, besides the initial steps that regulate 4F-GalNAc incorporation into glycoconjugates, later steps, such as the time course of 4F-GalNAc accumulation in cells and cellular recycling/salvage pathways, may also be important at later times after the first cell division step. Whereas the aforementioned aspects are not specifically examined in this study, they could be the subject of future investigations.
Whereas 4F-GalNAc was well tolerated by cells in our study, we noted that the compound was also incorporated into other proteins besides PSGL-1. 4F-GalNAc, like 4F-GlcNAc, also altered the glycosaminoglycan content of cells (S.N., unpublished data, July 2009). In this regard, 4F-GalNAc reduces chondroitin sulfate biosynthesis. Others have shown that 4F-GlcNAc reduces both heparan sulfate and chondroitin sulfate biosynthesis.50 Thus, the pathways engaged are not specific to PSGL-1 alone, and the functional consequence of this remains to be studied. We also noted a 10% to 15% reduction in cell growth rate at 38 hours compared with the vehicle/untreated control. Such nonspecific effects are common to many other studies that use metabolic approaches to target cell adhesion. For example, Sharma et al22 demonstrate that both 4F-GalNAc and 4F-GlcNAc can reduce cell growth to a similar extent. A similar 10% to 15% reduction in cell growth is also reported in studies that use decoys Galβ1,4GlcNAc-NM and GlcNAcβ1,3Gal-NM to reduce selectin binding.14 Whereas reduction in cell growth is noted in these studies, metabolic labeling studies that use radioactive carbohydrate and amino acid precursors show that protein synthesis is only reduced at extracellular concentrations in the 0.3mM to 1mM range.22 Another study also notes that 100μM 4F-GalNAc and 4F-GlcNAc has no adverse effects on cellular protein biosynthesis.25
In conclusion, this study introduces 4F-GalNAc as a potential modifier of O-linked glycosylation. The in vitro and in vivo studies suggest that the compound can reduce the expression of the sLeX epitope on glycoproteins and diminish selectin-mediated cell adhesion.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grant HL63014, S.N.; grant CA35329, K.L.M.; and grant AI56082, J.T.Y.L.) and NYSTEM (NY State Stem Cell Science).
National Institutes of Health
Authorship
Contribution: D.D.M. designed research, performed experiments, and wrote the paper; A.B., E.V.C., J.X., R.D.L., and M.N. performed experiments; J.T.Y.L. designed research; K.L.M. provided key carbohydrate reagents and designed research; and S.N. designed research, performed experiments, coordinated project activities, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sriram Neelamegham, State University of New York, 906 Furnas Hall, Buffalo, NY 14260; e-mail: neel@eng.buffalo.edu.

![Figure 1. Glycan epitopes and monosaccharide analogs. (A) Putative structure of selectin-binding glycan located at the N-terminus of PSGL-1. This glycan has a terminal tetrasaccharide sLeX epitope and binding sites for DSA and AAL lectins. Neuraminidase cleaves sialic acid (NeuAc) residues to expose the terminal trisaccharide Lewis-X (LeX) epitope. Metabolic inhibition of these glycan structures may reduce leukocyte selectin-binding function. (B) Structure of per-acetylated compounds GalNAc, 4F-GalNAc, 4F-GlcNAc, and [14C]4F-GalNAc.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/6/10.1182_blood-2009-07-231480/4/m_zh89990948290001.jpeg?Expires=1769774783&Signature=1SEvjob2vDB-difpy6r1Wo0AADsFjg~cyRi5i706-n2-lN2iq3oWZPKvmy2eiTReB5DX5fYMiREkF~C2MrpGHjvnvgQ3zDdFTpK4NiSS0ZIKZlwEDryONiXaxeQY~mEat~n28Ui583savaqeDxNQ8~t84RTIFOck77SUJZK3yXBjrwMT5FjHOR3d0xvd9Jw~26e7ThRuYN8PUsm8s37H~g~xPKfiGZRJyy3yoGIQ~jSU5Hw4Aab1DSf7PxNkb4~nu7QYD6V9m9rzQuE5sNGm2FJR1S25-NnP~QLNB8hWqqYF80m0h2K6FdHUlXny4flev-lmjh3sUCe2Vn00PK8rBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. [14C]4F-GalNAc distribution in culture media and glycoproteins. Cell culture media containing [14C]4F-GalNAc (0.5 μCi/mL, 100μM) was placed under standard growth conditions either in the presence or absence of HL-60 cells for 38 hours. Growth media and cells were harvested separately. (A-B) Culture media harvested from cultures with (A) and without HL-60 (B) separated on Biogel P-2 column. (C) Samples at the void volume (samples 1 and 4), peak fractions in panel A (samples 2 and 3) and panel B (sample 5) were separated using TLC with chloroform/methanol (5:1) as mobile phase. Phosphorimaging detected radioactivity on TLC plates. Nonradiolabeled, nonacetylated 4F-GalNAc (lane 6) and per-acetylated 4F-GalNAc (lane 7) standards were run in parallel and developed using ethanol/sulfuric acid spray followed by charring. Addition of acetylated 4F-GalNAc to HL-60 cells leads to deacetylation as evidenced by prominent peak 3 in the chromatogram. (D) PSGL-1 was immunoprecipitated from HL-60 lysates cultured with [14C]4F-GalNAc using Ab H-300. This immunoprecipitate and PSGL-1–depleted cell lysate were resolved on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and blotted onto nitrocellulose membrane. Radioactivity was measured using scintillation counter in panels A and B; and using phosphorimaging in panels C and D. 4F-GalNAc is incorporated into leukocyte PSGL-1 and other glycoproteins.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/6/10.1182_blood-2009-07-231480/4/m_zh89990948290006.jpeg?Expires=1769774783&Signature=DpeNpXEUetmfoKzfSFiCQhJGsc90fo3I7S8lbCj9xdXVRPPY-i8jxRO-5kYwwc1ude~4PFtcZy53WKbNrnGlxm~rblfaUw30WFAyXB9yHRcAqlOtoSJ~5j6VCh6cHG03eOzZ-wtUMv1u7a~W~BMHrVODw3xRKDJFd1TDQo~CXUfFobf-Of0jOxtYuQ~vqoovp2zrFW4YA9V5gX6ZcbxiezG~W-duguwOWTFFqWyCQRRjMgwtOYDC2PZeTu6ZQXDHw9XzczFEkS-V3n0ywLgGwRWgJmhY2q5U2qDrp-5E6iYkpKgU~e09wdMEqyy-gJev0Qi~9q-Ovnyfv3u1H-ulww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Effect of 4F-GalNAc on selectin binding and leukocyte recruitment to peritoneum. BMCs were cultured in media containing granulocyte colony-stimulating factor and IL-3, and 25μM 4F-GalNAc, 25μM GalNAc, or vehicle control (0.125% DMSO). (A) After 2 days in culture, 2 cell populations (regions R1 and R2) stained positive for nuclear dye LDS-751 in the flow cytometer. Cells in R1 were neutrophils (Gr-1+1A8+). (B) PSGL-1 and CD45 antigen expression, and P-selectin IgG binding to Gr-1+ cells were measured. 4F-GalNAc, but not GalNAc or vehicle control, reduced P-selectin fusion protein binding to cells. Data are mean ± SEM with respect to vehicle control. *P < .05 with respect to all other treatments. (C) BMCs cultured for 2 days with 25μM 4F-GalNAc or vehicle control were labeled with either DiL or DiO dyes. Mixed-cell populations just before injection into C57BL/6 recipient mice contained approximately equal numbers of DiL-labeled vehicle control cells [(Vehicle)DiO] and DiO-labeled 4F-GalNAc–treated cells [(4F-GalNAc)DiL] or vice versa. (D) Peritoneal lavage sample from mice obtained 5 hours after intraperitoneal injection of thioglycollate shows substantial accumulation of neutrophils in the peritoneum. These cells were positive for LDS-751, Gr-1, and 1A8. DiO/DiL-labeled cells were not injected in this experiment. (E) Granulocytes from the peritoneal lavage of animals, which were tail-vein injected with mixed DiO + DiL BMCs just before induction of peritonitis. Number of DiL-labeled cells (vehicle control) exceeds DiO-labeled cells (4F-GalNAc–treated). (F) Ratio of 4F-GalNAc to vehicle control cells in infusion and peritoneum lavage sample. Data are mean ± SEM for 4 animals injected with DiL-labeled 4F-GalNAc–treated cells along with paired DiO-labeled vehicle controls. Four animals were injected with DiO/DiL labeling switched. 4F-GalNAc treatment reduced cell migration into peritoneum irrespective of whether these cells were labeled with DiO or DiL. *P < .05 with respect to infusion sample. (G) A total of 100 mg/kg/day 4F-GalNAc or vehicle control was injected into mice for 4 days before induction of peritonitis. Total leukocyte (Total), neutrophil (Neut), eosinophil (Eos), and macrophage (Mac) counts were determined in peritoneal lavage 5 hours after intraperitoneal injection of thioglycollate. Data are mean ± SEM for 4 animals for each treatment. *P < .05 with respect to vehicle control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/6/10.1182_blood-2009-07-231480/4/m_zh89990948290007.jpeg?Expires=1769774783&Signature=uCsLi7Sx65eLAy1Gw~LPzlL33hNeI37KVyhBwk1pWbNoIteOMAf4Yg~q209-pF3DLPCuYvU4FbiKRGg4Q~YCtbbVRIge5c3vVn33KfWCL04jAsmMxA2-Zn1LHnKa3JbW6FkmEaC5g4iMrX8IHadh9P~YGIQiVtIGjITYcVZK0HBvG9C39h~qVkmMl2-zLbhWPlCrerexyUJdkD4iZGj5QkMDyvStuGVpaRHMdnfBx6SibPQRqDm~cZN5af4syoAoCyKtwR0iFJoJ7pxjfY7wjSnVUR3FMVDq2c5vwuVbd6Fyl~p9xDM3Y9l1NtONQ8sSvrfpEeGtXk7GCCji9Qe33g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal