Abstract
We retrospectively analyzed outcomes among 567 patients with hematologic malignancies who had hematopoietic cell transplantation from human leukocyte antigen-identical sibling donors between 2001 and 2007 for a correlation between statin use and risk of graft-versus-host disease (GVHD). Compared with allografts where neither the donor nor recipient was treated with a statin at the time of transplantation (n = 464), statin use by the donor and not the recipient (n = 75) was associated with a decreased risk of grade 3-4 acute GVHD (multivariate hazard ratio, 0.28; 95% confidence interval, 0.1-0.9). Statin use by both donor and recipient (n = 12) was suggestively associated with a decreased risk of grade 3 or 4 acute GVHD (multivariate hazard ratio, 0.00; 95% confidence interval, undefined), whereas statin use by the recipient and not the donor (n = 16) did not confer GVHD protection. Risks of chronic GVHD, recurrent malignancy, nonrelapse mortality, and overall mortality were not significantly affected by donor or recipient statin exposure. Statin-associated GVHD protection was restricted to recipients with cyclosporine-based postgrafting immunosuppression and was not observed among those given tacrolimus (P = .009). These results suggest that donor statin treatment may be a promising strategy to prevent severe acute GVHD without compromising immunologic control of the underlying malignancy.
Introduction
Therapy-refractory acute graft-versus-host disease (GVHD) contributes to morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT), and more effective prevention and treatment strategies are needed. In recent years, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, a widely used class of cholesterol-lowering drugs also termed “statins,” have been shown to alter immune function in ways that may contribute to their antiatherosclerotic effects. Statins also appear to reduce immune responses to autoantigens and alloantigens.1-3 Statins have been shown to affect immune responses through a variety of mechanisms, including induction of T-cell hyporesponsiveness by interference with intracellular signaling pathways, expansion of regulatory T cells (Treg), polarization toward an anti-inflammatory T cell phenotype (Th2), and down-regulation of antigen-presenting cell (APC) function.4-11
Zeiser et al observed reduced mortality related to acute GVHD when either donor or recipient mice were given atorvastatin for 10 days before major histocompatibility complex (MHC)-mismatched allogeneic HCT.6 Statin pretreatment of both donor and recipient enhanced the protective effect against acute GVHD-associated mortality in this study. Data regarding the potential role of statin treatment among recipients and/or donors and their potential impact on clinical GVHD outcomes are limited. In a recent retrospective analysis of 67 patients who received human leukocyte antigen (HLA)–matched or mismatched related or unrelated allografts for treatment of hematologic malignancies, Hamadani et al found a trend toward a decreased risk of grade 2-4 acute GVHD among the 10 recipients who were under treatment with a statin at the time of HCT, compared with those who were not.12 Definitive conclusions were limited by the small sample size and the heterogeneity of the patient population. In addition, effects of statin use by the donor were not evaluated in this study.
In the current retrospective analysis of 567 consecutive recipients of allografts from HLA-identical sibling donors, we analyzed the association of donor and recipient statin use with major transplantation outcomes. We found that donor statin treatment was significantly associated with protection against grade 3-4 acute GVHD. Moreover, the decreased risk of severe acute GVHD-associated with donor statin use was limited to patients given cyclosporine (CSP) prophylaxis and was not detected among those given tacrolimus (TAC).
Methods
Patients and donors
We retrospectively analyzed consecutive patients who had allogeneic HCT for hematologic malignancies from HLA-identical sibling donors at the Fred Hutchinson Cancer Research Center between January 2001 and December 2007. All recipients and donors had given written informed consent to treatment according to protocols approved by the Institutional Review Board at Fred Hutchinson Cancer Research Center, and all patients had given consent to the use of medical records for research related to transplantation outcomes in accordance with the Declaration of Helsinki. Only related donor/recipient pairs were included in the analysis to ensure access to medication histories of both recipients and their donors. To be included in the final analysis, recipients had to be at least 18 years old, and donors had to be at least 30 years old. The donor age threshold of 30 years was chosen to increase the likelihood of statin treatment in the donor group. During the period of study, 64 patients received transplantations from donors who were 18 to 29 years of age. Only one of the donors and none of these recipients was treated with a statin at the time of HCT. Medication data were obtained by retrospective review of medical records, and the quality of extracted data was independently confirmed by a pharmacist. Characteristics of recipients, donors, and treatment variables are shown in Table 1.
Recipient, donor, and transplantation characteristics according to statin treatment at the time of transplantation
| . | All transplantations . | Statin treatment at allograft . | |||
|---|---|---|---|---|---|
| R−/D− . | R+/D− . | R−/D+ . | R+/D+ . | ||
| N | 567 | 464 | 16 | 75 | 12 |
| Recipient (patient) characteristics | |||||
| Median age, y (range) | 51 (20-74) | 50 (20-74) | 59 (51-66) | 57 (35-72) | 60 (40-70) |
| Female sex, n (%) | 273 (48) | 232 (50) | 3 (19) | 37 (49) | 1 (8) |
| Disease risk,* n (%) | |||||
| Standard | 249 (44) | 207 (45) | 5 (31) | 33 (44) | 4 (33) |
| High | 318 (56) | 257 (55) | 11 (69) | 42 (56) | 8 (67) |
| Donor characteristics | |||||
| Median age, y (range) | 50 (30-83) | 49 (30-78) | 55 (39-62) | 57 (34-80) | 58 (42-83) |
| Sex, female (%) | 284 (50) | 239 (52) | 7 (44) | 33 (44) | 5 (42) |
| Donor/recipient pairs | |||||
| Female/male, n (%) | 144 (25) | 116 (25) | 6 (38) | 17 (23) | 5 (42) |
| Other combinations, n (%) | 423 (75) | 348 (75) | 10 (63) | 58 (77) | 7 (58) |
| Transplantation characteristics | |||||
| Conditioning, n (%) | |||||
| Myeloablative | 372 (66) | 306 (66) | 8 (50) | 52 (69) | 6 (50) |
| Bu/Cy | 193 (34) | 158 (34) | 6 (38) | 24 (32) | 3 (25) |
| Cy/TBI | 103 (18) | 90 (19) | 1 (6) | 11 (15) | 1 (8) |
| Other | 76 (13) | 58 (13) | 1 (6) | 17 (23) | 2 (17) |
| Nonmyeloablative | 195 (34) | 158 (34) | 8 (50) | 23 (31) | 6 (50) |
| Stem cell source, n (%) | |||||
| PBSCs | 553 (97) | 451 (97) | 16 (100) | 74 (99) | 12 (100) |
| BM | 14 (3) | 13 (3) | 0 | 1 (1) | 0 |
| GVHD prophylaxis, n (%) | |||||
| Cyclosporin | 417 (74) | 340 (73) | 13 (81) | 54 (72) | 10 (84) |
| Tacrolimus | 150 (26) | 124 (27) | 3 (19) | 21 (28) | 2 (16) |
| . | All transplantations . | Statin treatment at allograft . | |||
|---|---|---|---|---|---|
| R−/D− . | R+/D− . | R−/D+ . | R+/D+ . | ||
| N | 567 | 464 | 16 | 75 | 12 |
| Recipient (patient) characteristics | |||||
| Median age, y (range) | 51 (20-74) | 50 (20-74) | 59 (51-66) | 57 (35-72) | 60 (40-70) |
| Female sex, n (%) | 273 (48) | 232 (50) | 3 (19) | 37 (49) | 1 (8) |
| Disease risk,* n (%) | |||||
| Standard | 249 (44) | 207 (45) | 5 (31) | 33 (44) | 4 (33) |
| High | 318 (56) | 257 (55) | 11 (69) | 42 (56) | 8 (67) |
| Donor characteristics | |||||
| Median age, y (range) | 50 (30-83) | 49 (30-78) | 55 (39-62) | 57 (34-80) | 58 (42-83) |
| Sex, female (%) | 284 (50) | 239 (52) | 7 (44) | 33 (44) | 5 (42) |
| Donor/recipient pairs | |||||
| Female/male, n (%) | 144 (25) | 116 (25) | 6 (38) | 17 (23) | 5 (42) |
| Other combinations, n (%) | 423 (75) | 348 (75) | 10 (63) | 58 (77) | 7 (58) |
| Transplantation characteristics | |||||
| Conditioning, n (%) | |||||
| Myeloablative | 372 (66) | 306 (66) | 8 (50) | 52 (69) | 6 (50) |
| Bu/Cy | 193 (34) | 158 (34) | 6 (38) | 24 (32) | 3 (25) |
| Cy/TBI | 103 (18) | 90 (19) | 1 (6) | 11 (15) | 1 (8) |
| Other | 76 (13) | 58 (13) | 1 (6) | 17 (23) | 2 (17) |
| Nonmyeloablative | 195 (34) | 158 (34) | 8 (50) | 23 (31) | 6 (50) |
| Stem cell source, n (%) | |||||
| PBSCs | 553 (97) | 451 (97) | 16 (100) | 74 (99) | 12 (100) |
| BM | 14 (3) | 13 (3) | 0 | 1 (1) | 0 |
| GVHD prophylaxis, n (%) | |||||
| Cyclosporin | 417 (74) | 340 (73) | 13 (81) | 54 (72) | 10 (84) |
| Tacrolimus | 150 (26) | 124 (27) | 3 (19) | 21 (28) | 2 (16) |
Bu indicates busulfan; Cy, cyclophosphamide; TBI, total body irradiation; BM, bone marrow; and MTX, methotrexate.
Standard disease risk refers to chronic myeloid leukemia in chronic phase, myelodysplastic syndromes without excess blasts, and leukemia and lymphoma in remission. High disease risk refers to all other hematologic malignancies.
Preparative regimens and immunosuppression after transplantation
Myeloablative conditioning regimens (66%) included targeted oral busulfan (4 mg/kg per day for 4 consecutive days) and intravenous cyclophosphamide (60 mg/kg per day for 2 consecutive days, 34%); cyclophosphamide (60 mg/kg per day for 2 consecutive days) followed by fractionated total body irradiation (12 Gy; 18%); and other regimens (13%). Immunosuppression after myeloablative HCT consisted of a calcineurin inhibitor (CSP or TAC) in combination with methotrexate or mycophenolate mofetil (MMF).13 Nonmyeloablative conditioning regimens (34%) included low-dose total body irradiation (2-3 Gy) alone or in combination with fludarabine (30 mg/m2 body surface area/day, for 3 consecutive days). Patients prepared with a nonmyeloablative conditioning regimen were given a calcineurin inhibitor (CSP or TAC) in combination with MMF for immunosuppression after transplantation. In general, tapering schedules of immunosuppressive agents were modified at the discretion of the attending physicians for treatment of GVHD or persistent or recurrent malignancy.14,15 Whereas 97% of patients received granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells (PBSCs), 3% received bone marrow grafts (Table 1).
GVHD and donor chimerism
Grading of acute GVHD was performed according to established criteria.16,17 For the subgroup of recipients with nonmyeloablative allografts, the proportion of donor-derived T cells in the blood was assessed on days 28, 56, 180, and 365 days after HCT. Donor T-cell chimerism was measured by XY–fluorescence in situ hybridization analysis in sex-mismatched pairs and by DNA amplification of polymorphic microsatellite regions in sex-matched pairs as described previously.18
Analysis of graft composition
For recipients of G-CSF–mobilized PBSCs, graft composition was analyzed by flow cytometry for contents of CD34+ hematopoietic stem/progenitor cells, CD3+/CD4+ T helper cells, CD3+/CD8+ cytotoxic T cells, CD14+ monocytes, CD20+ B cells, CD3−/CD56+ NK cells, and type 1 and type 2 dendritic cells (DC1 and DC2), as previously described.19 Data were analyzed in a multiple-regression model that included donor statin treatment, age, and sex as covariates.
Statistical methods
Survival curves were estimated using the Kaplan-Meier method; cumulative incidence curves were estimated using methods previously described.20 Univariate and multivariate analysis of time-to-event endpoints was performed using Cox regression. Death was treated as a competing risk for analysis of relapse. Death and relapse were treated as competing risks for analysis of acute and chronic GVHD. Relapse was treated as a competing risk for the analysis of nonrelapse mortality (NRM). T-cell chimerism was compared between groups using 2-sample t tests. Statistical analysis of cell dose subsets was performed using standard linear regression. All P values were 2-sided.
Results
Prevalence of statin treatment among recipients and donors
Among the 567 transplantations included in this analysis, 28 recipients (5%) had used a statin daily for at least 3 consecutive months before HCT. The statin medications used by recipients included atorvastatin (n = 22), simvastatin (n = 5), and rosuvastatin (n = 1). Of these 28 recipients, 12 (43%) suspended statin treatment temporarily within 7 days before HCT, during the preparative regimen, and resumed treatment at a median of 16 days (range, 5-77 days) after HCT. Three recipients temporarily suspended statin treatment at 7, 11, and 59 days after HCT for clinical reasons (replacement of statin with gemfibrozil because of voriconazole treatment; suspected statin-induced myopathy and liver toxicity; increase in serum creatinine) and resumed treatment at 83, 77, and 105 days, respectively, after HCT. Eighty-seven donors (15%) had used a statin medication daily for at least 3 months immediately before stem cell donation. The statin medications used by donors included atorvastatin (n = 49), simvastatin (n = 23), rosuvastatin (n = 4), pravastatin (n = 6), lovastatin (n = 4), and fluvastatin (n = 1).
For the purpose of statistical comparisons of major transplantation outcomes according to statin exposure, donor/recipient pairs were divided into 4 groups: (1) neither the recipient nor donor used statin (R−/D−, n = 464, control group); (2) only the recipient used statin (R+/D−, n = 16); (3) only the donors used statin (R−/D+, n = 75); (4) both recipient and donor used statin (R+/D+, n = 12). Among both recipients and donors, statin use was associated with older age and male sex. Characteristics of recipients and donors and transplantation variables according to statin exposure are summarized in Table 1.
Association of statin use with acute and chronic GVHD
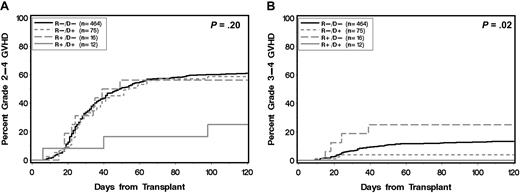
Compared with the R−/D− group, the R−/D+ group had a lower risk of grade 3-4 acute GVHD in univariate analysis (multivariate hazard ratio [HR], 0.28; 95% confidence interval [CI], 0.1-0.9; P = .03). This finding was confirmed in multivariate analysis after adjusting for recipient/donor sex mismatch (female donor/male recipient vs other), conditioning-intensity (myeloablative vs nonmyeloablative), recipient age (> 50 vs ≤ 50 years), donor age (> 50 vs ≤ 50 years), and standard versus high disease risk (HR, 0.28; 95% CI, 0.1-0.9; P = .03; see Table 2). Despite the reduction in risk of grade 3-4 acute GVHD, the R−/D+ group showed no statistically significant difference in risk of grade 2-4 acute GVHD compared with the R−/D− groups (Figure 1; Tables 2–3). The R+/D− group showed no statistically significant protection against grade 2-4 or grade 3-4 acute GVHD (Figure 1; Tables 2–3). The R+/D+ group showed trends toward protection against grade 2-4 acute GVHD (HR, 0.33; 95% CI, 0.1-1.0; P = .06) and grade 3-4 acute GVHD (HR, 0.00; 95% CI, undefined; P = .06) in multivariate analysis (Tables 2–3). Definitive conclusions for the R+/D− and R+/D+ groups, however, were limited by small sample size. Recipient or donor statin treatment was not associated with a decreased risk of extensive chronic GVHD (Tables 2–3).
Univariate and multivariate analyses for association of recipient and/or donor statin use with various outcomes compared with control group (R−/D−, n = 464)
| Endpoint . | Univariate analysis . | Multivariate analysis . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (R+/D−, n = 16) . | (R−/D+, n = 75) . | (R+/D+, n = 12) . | (R+/D−, n = 16) . | (R−/D+, n = 75) . | (R+/D+, n = 12) . | |||||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR* (95% CI) . | P* . | HR* (95% CI) . | P* . | HR* (95% CI) . | P* . | |
| Grade 2-4 GVHD | 0.90 (0.5-1.7) | .75 | 0.89 (0.6-1.2) | .47 | 0.33 (0.1-1.0) | .06 | 1.01 (0.5-2.0) | .98 | 0.89 (0.6-1.2) | .47 | 0.33 (0.1-1.0) | .06 |
| Grade 3-4 GVHD | 2.02 (0.7-5.5) | .17 | 0.28 (0.1-0.9) | .03 | 0.00 (undefined) | .07 | 1.78 (0.6-5.0) | .28 | 0.28 (0.1-0.9) | .03 | 0.00 (undefined) | .06 |
| Chronic GVHD | 1.01 (0.5-1.9) | .98 | 0.85 (0.6-1.2) | .31 | 0.61 (0.3-1.4) | .23 | 0.93 (0.5-1.8) | .83 | 0.82 (0.6-1.2) | .26 | 0.58 (0.3-1.3) | .19 |
| Relapse/disease progression | 0.85 (0.3-2.3) | .74 | 0.66 (0.4-1.2) | .15 | 0.66 (0.2-2.7) | .57 | 0.97 (0.4-2.7) | .96 | 0.63 (0.4-1.1) | .11 | 0.74 (0.2-3.0) | .67 |
| NRM | 0.77 (0.3-2.1) | .60 | 0.86 (0.5-1.4) | .53 | 0.91 (0.3-2.8) | .86 | 0.45 (0.2-1.2) | .12 | 0.72 (0.4-1.2) | .17 | 0.66 (0.2-2.0) | .49 |
| Overall mortality | 0.80 (0.4-1.7) | .57 | 0.86 (0.6-1.2) | .42 | 0.94 (0.4-2.3) | .88 | 0.58 (0.3-1.2) | .16 | 0.73 (0.5-1.1) | .10 | 0.77 (0.3-1.9) | .56 |
| Endpoint . | Univariate analysis . | Multivariate analysis . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (R+/D−, n = 16) . | (R−/D+, n = 75) . | (R+/D+, n = 12) . | (R+/D−, n = 16) . | (R−/D+, n = 75) . | (R+/D+, n = 12) . | |||||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR* (95% CI) . | P* . | HR* (95% CI) . | P* . | HR* (95% CI) . | P* . | |
| Grade 2-4 GVHD | 0.90 (0.5-1.7) | .75 | 0.89 (0.6-1.2) | .47 | 0.33 (0.1-1.0) | .06 | 1.01 (0.5-2.0) | .98 | 0.89 (0.6-1.2) | .47 | 0.33 (0.1-1.0) | .06 |
| Grade 3-4 GVHD | 2.02 (0.7-5.5) | .17 | 0.28 (0.1-0.9) | .03 | 0.00 (undefined) | .07 | 1.78 (0.6-5.0) | .28 | 0.28 (0.1-0.9) | .03 | 0.00 (undefined) | .06 |
| Chronic GVHD | 1.01 (0.5-1.9) | .98 | 0.85 (0.6-1.2) | .31 | 0.61 (0.3-1.4) | .23 | 0.93 (0.5-1.8) | .83 | 0.82 (0.6-1.2) | .26 | 0.58 (0.3-1.3) | .19 |
| Relapse/disease progression | 0.85 (0.3-2.3) | .74 | 0.66 (0.4-1.2) | .15 | 0.66 (0.2-2.7) | .57 | 0.97 (0.4-2.7) | .96 | 0.63 (0.4-1.1) | .11 | 0.74 (0.2-3.0) | .67 |
| NRM | 0.77 (0.3-2.1) | .60 | 0.86 (0.5-1.4) | .53 | 0.91 (0.3-2.8) | .86 | 0.45 (0.2-1.2) | .12 | 0.72 (0.4-1.2) | .17 | 0.66 (0.2-2.0) | .49 |
| Overall mortality | 0.80 (0.4-1.7) | .57 | 0.86 (0.6-1.2) | .42 | 0.94 (0.4-2.3) | .88 | 0.58 (0.3-1.2) | .16 | 0.73 (0.5-1.1) | .10 | 0.77 (0.3-1.9) | .56 |
Adjusted for female → male sex mismatch, conditioning intensity, donor age more than 50 years, patient age more than 50 years, TAC treatment after transplantation, and disease risk.
Cumulative incidence of acute GVHD according to statin treatment of donor and recipient. (A) Grade 2-4 and (B) grade 3-4 acute GVHD. (R) and (D) indicate patient and donor, respectively. (+) and (−) indicate absence or presence, respectively, of statin treatment. P values are derived from log-rank test.
Cumulative incidence of acute GVHD according to statin treatment of donor and recipient. (A) Grade 2-4 and (B) grade 3-4 acute GVHD. (R) and (D) indicate patient and donor, respectively. (+) and (−) indicate absence or presence, respectively, of statin treatment. P values are derived from log-rank test.
Multivariate analysis for association of recipient and/or donor statin use with various outcomes compared with control group (R−/D−, n = 340) among recipients with CSP-based GVHD prophylaxis
| Endpoint . | (R+/D−, n = 13) . | (R−/D+, n = 54) . | (R+/D+, n = 10) . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Grade 2-4 GVHD | 1.10 (0.5-2.3) | .79 | 0.79 (0.5-1.2) | .23 | 0.22 (0.1-0.9) | .03 |
| Grade 3-4 GVHD | 1.76 (0.6-5.0) | .29 | 0.00 (undefined) | < .001 | 0.00 (undefined) | .06 |
| Chronic GVHD | 0.79 (0.4-1.6) | .53 | 0.94 (0.6-1.4) | .76 | 0.59 (0.2-1.5) | .25 |
| Relapse/disease progression | 1.27 (0.4-3.6) | .66 | 0.72 (0.4-1.3) | .30 | 1.04 (0.3-4.3) | .95 |
| NRM | 0.36 (0.1-1.1) | .08 | 0.67 (0.4-1.2) | .15 | 0.86 (0.3-2.7) | .80 |
| Overall mortality | 0.53 (0.2-1.2) | .14 | 0.75 (0.5-1.1) | .18 | 1.00 (0.4-2.5) | .99 |
| Endpoint . | (R+/D−, n = 13) . | (R−/D+, n = 54) . | (R+/D+, n = 10) . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Grade 2-4 GVHD | 1.10 (0.5-2.3) | .79 | 0.79 (0.5-1.2) | .23 | 0.22 (0.1-0.9) | .03 |
| Grade 3-4 GVHD | 1.76 (0.6-5.0) | .29 | 0.00 (undefined) | < .001 | 0.00 (undefined) | .06 |
| Chronic GVHD | 0.79 (0.4-1.6) | .53 | 0.94 (0.6-1.4) | .76 | 0.59 (0.2-1.5) | .25 |
| Relapse/disease progression | 1.27 (0.4-3.6) | .66 | 0.72 (0.4-1.3) | .30 | 1.04 (0.3-4.3) | .95 |
| NRM | 0.36 (0.1-1.1) | .08 | 0.67 (0.4-1.2) | .15 | 0.86 (0.3-2.7) | .80 |
| Overall mortality | 0.53 (0.2-1.2) | .14 | 0.75 (0.5-1.1) | .18 | 1.00 (0.4-2.5) | .99 |
HR and P values were adjusted for female → male sex mismatch, conditioning intensity, donor age more than 50 years, patient age more than 50 years, and disease risk.
Cyclosporine prophylaxis in the recipient is required for statin-associated protection against severe acute GVHD
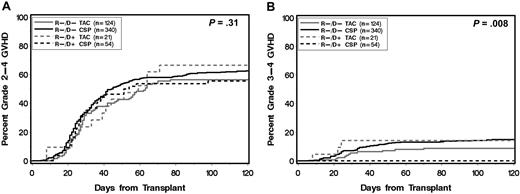
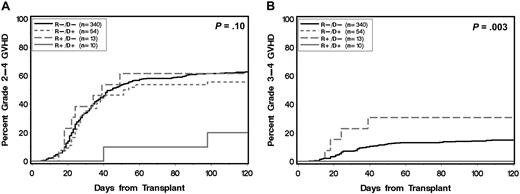
Among all patients, 417 (74%) received CSP-based postgrafting immunosuppression and 150 (26%) received TAC-based postgrafting immunosuppression. Further subgroup analysis showed that CSP-treated patients in the R−/D+ group were protected against grade 3-4 acute GVHD compared with CSP-treated patients in the R−/D− group (cumulative incidence at 120 days, 0% vs 16%, respectively; P = .003). This protection was not observed among TAC-treated patients (R−/D+ vs R−/D−, 15% vs 11%; P = .44; Figures 2–3). The decreased risk of grade 3-4 acute GVHD associated with donor statin treatment among recipients with CSP-based postgrafting immunosuppression was confirmed in multivariate analysis (Table 3).
Cumulative incidence of acute GVHD according to donor statin treatment and type of calcineurin inhibitor (CSP or TAC) for postgrafting immunosuppression. (R) and (D) indicate patient and donor, respectively. (+) and (−) indicate absence or presence, respectively, of statin treatment. P values are derived from log-rank test.
Cumulative incidence of acute GVHD according to donor statin treatment and type of calcineurin inhibitor (CSP or TAC) for postgrafting immunosuppression. (R) and (D) indicate patient and donor, respectively. (+) and (−) indicate absence or presence, respectively, of statin treatment. P values are derived from log-rank test.
Cumulative incidence of acute GVHD according to statin treatment of donor and recipient restricted to recipients with cyclosporine-based postgrafting immunosuppression. (A) Grade 2-4 and (B) grade 3-4 acute GVHD. (R) and (D) indicate patient and donor, respectively. (+) and (−) indicate absence or presence, respectively, of statin treatment. P values are derived from log-rank test.
Cumulative incidence of acute GVHD according to statin treatment of donor and recipient restricted to recipients with cyclosporine-based postgrafting immunosuppression. (A) Grade 2-4 and (B) grade 3-4 acute GVHD. (R) and (D) indicate patient and donor, respectively. (+) and (−) indicate absence or presence, respectively, of statin treatment. P values are derived from log-rank test.
Donor statin treatment confers preferential protection against severe GVHD of the gastrointestinal tract
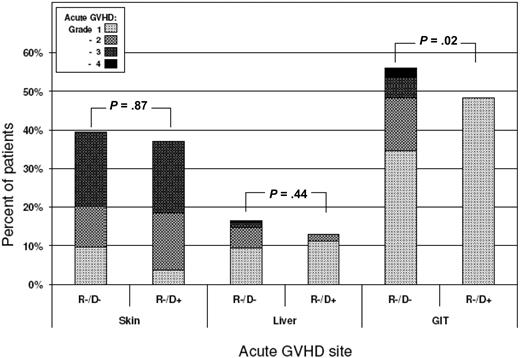
Further details of GVHD were analyzed in patients who received CSP-based immunosuppression to determine whether donor statin treatment conferred equivalent protection in all acute GVHD-target organs. GVHD stages in the skin, liver, and gut were evaluated in the 54 recipients who had grafts from statin-treated donors (R−/D+) and in the 340 controls where neither the donor nor recipient was treated with a statin at the time of transplantation (R−/D−). Donor statin treatment was associated with significant protection against acute GVHD in the gastrointestinal tract (P = .02) but not in the skin (Figure 4). The results also showed a weak trend suggesting reduced severity of GVHD in the liver.
Distribution of acute GVHD stages in the skin, liver, and gastrointestinal tract according to donor statin treatment. GVHD stages in target organs were evaluated in the 54 recipients with cyclosporine-based postgrafting immunosuppression who had grafts from statin-treated donors (R−/D+) and in the 340 controls where neither the donor nor recipient was treated with a statin at the time of transplantation (R−/D−). P values are derived from Wilcoxon rank-sum test.
Distribution of acute GVHD stages in the skin, liver, and gastrointestinal tract according to donor statin treatment. GVHD stages in target organs were evaluated in the 54 recipients with cyclosporine-based postgrafting immunosuppression who had grafts from statin-treated donors (R−/D+) and in the 340 controls where neither the donor nor recipient was treated with a statin at the time of transplantation (R−/D−). P values are derived from Wilcoxon rank-sum test.
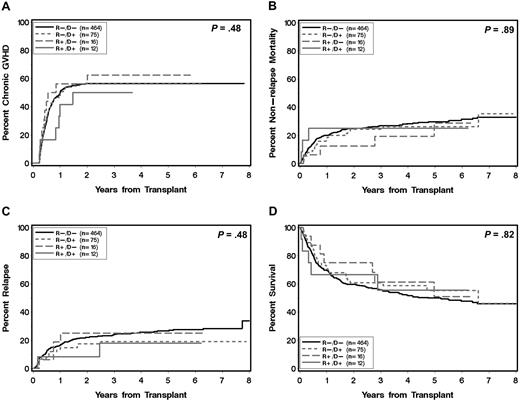
Association of statin treatment with other transplantation outcomes
Recipient or donor statin treatment at the time of HCT was not significantly associated with the risks of NRM, recurrent malignancy, and overall mortality (Figure 5; Tables 2–3). Among the 87 recipients of grafts from statin-treated donors, only one case of graft rejection (1%) occurred 5 months after HCT from an HLA-identical sibling donor in a recipient who had nonmyeloablative conditioning.
Major outcomes after allogeneic HCT according to statin treatment of donor and recipient. Cumulative incidence of chronic extensive GVHD (A), NRM (B), recurrent malignancy/progression (C), and Kaplan-Meier estimate for overall survival (D). (R) and (D) indicate patient and donor, respectively. (+) and (−) indicate absence or presence, respectively, of statin treatment. P values are derived from log-rank test.
Major outcomes after allogeneic HCT according to statin treatment of donor and recipient. Cumulative incidence of chronic extensive GVHD (A), NRM (B), recurrent malignancy/progression (C), and Kaplan-Meier estimate for overall survival (D). (R) and (D) indicate patient and donor, respectively. (+) and (−) indicate absence or presence, respectively, of statin treatment. P values are derived from log-rank test.
We evaluated the kinetics of donor T-cell chimerism among 142 recipients who had nonmyeloablative conditioning and received postgrafting CSP and MMF. The mean percentages of donor T-cell chimerism on days 28, 56, and 84 according to statin treatment groups were compared with controls (R−/D−, n = 111). Across the 3 time points evaluated, the R+/D+ group had significantly lower levels of T-cell chimerism (−18.8%, P = .02), but the reduction in donor T-cell chimerism did not reach statistical significance in the R−/D+ and R+/D− groups (−6.0%, P = .17; and −8.6%, P = .21, respectively).
Impact of donor statin treatment on graft composition
Comprehensive data on graft composition were available for 312 recipients of G-CSF–mobilized PBSCs. Donor statin treatment was significantly associated with a decreased median content of DC2 cells, whereas increased donor age was associated with an increased DC2 content (Table 4). Both donor age and female sex were associated with a decreased content of CD34+ cells, but donor statin treatment was not associated with CD34+ cell content. Donor statin treatment was not associated with the graft content of any other cell population evaluated.
Donor characteristics affecting cell content in PBSC grafts (n = 312)
| Donor characteristics . | CD34+ . | DC2 . | ||
|---|---|---|---|---|
| Estimate* (SE) . | P . | Estimate* (SE) . | P . | |
| Statin therapy | −0.78 (0.48) | .11 | −1.46 (0.46) | .002 |
| Age (per 10 y) | −.56 (0.18) | .002 | 0.66 (0.16) | < .001 |
| Female sex | −1.69 (0.34) | < .001 | 0.14 (0.32) | .65 |
| Donor characteristics . | CD34+ . | DC2 . | ||
|---|---|---|---|---|
| Estimate* (SE) . | P . | Estimate* (SE) . | P . | |
| Statin therapy | −0.78 (0.48) | .11 | −1.46 (0.46) | .002 |
| Age (per 10 y) | −.56 (0.18) | .002 | 0.66 (0.16) | < .001 |
| Female sex | −1.69 (0.34) | < .001 | 0.14 (0.32) | .65 |
Effect on cell dose estimated from multiple regression model; units are 106 cells per kilogram.
Discussion
Three principal findings can be derived from our study. First, whereas statin treatment of the donor alone or of both donor and recipient was associated with a profoundly decreased risk of severe (grade 3-4) acute GVHD, statin treatment of only the recipient did not have an appreciable protective effect. Second, the GVHD-protective effect associated with donor or donor/recipient statin use was limited to recipients with CSP-based postgrafting immunosuppression and was not seen among those given TAC. Third, statin-associated acute GVHD protection did not compromise immunologic control of the underlying malignancy.
Because of their potent cholesterol-lowering effects, statins are among the most widely prescribed drugs in the United States. In our study population, 15% of donors and 5% of recipients were using statin at the time of HCT. The higher prevalence of statin use among donors compared with recipients can best be explained by the probable tendency to avoid nonessential drugs in HCT recipients and the selection of donors who were at least 30 years of age.
The best characterized activity of statins involves inhibition of mevalonate and cholesterol biosynthesis, which is mediated by binding to HMG-CoA reductase. Beyond their well-documented cholesterol-lowering effects, statins have been shown to have complex immunomodulatory properties. Blocking mevalonate production, for example, inhibits not only cholesterol synthesis but also the synthesis of intermediates required for isoprenylation of guanosine triphosphate (GTP)–binding cell-signaling proteins, such as Ras, Rac, and Rho, thereby disrupting intracellular signaling pathways.3,8,11 Immune functions shown to be promoted by statins are Th2 polarization,6 Treg expansion,6 and trafficking to sites of inflammation.21 At the same time, statins inhibit APC function by preventing inflammatory cytokine-induced up-regulation of MHC class II molecules,7,9 and they block the binding of lymphocyte function-associated antigen-1 to its ligand, intercellular adhesion molecule-1. Blockade of lymphocyte function-associated antigen-1 to intercellular adhesion molecule-1 is independent of HMG-CoA reductase inhibition and may directly affect T-cell migration and costimulation.21
The potential of statin-mediated acute GVHD prevention was compellingly demonstrated by Zeiser et al,4,6 who showed that treatment of either the donor or the recipient with atorvastatin for 10 days before MHC-mismatched HCT protected recipient mice from acute GVHD lethality. They further showed that atorvastatin treatment of donor mice promoted Th2 polarization and inhibited an uncontrolled Th1 response while maintaining graft-versus-leukemia activity. In keeping with these preclinical findings, our retrospective study shows that donor statin treatment was associated with a substantially decreased risk of severe acute GVHD without significantly affecting chronic GVHD. In the subgroup of nonablative HCT recipients, donor statin use was also suggestively associated with decreased levels of donor T-cell chimerism between days 28 and 84 after HCT, which may argue for statin-mediated suppression of donor T-cell expansion or decreased elimination of recipient T cells by donor cells.
The role of recipient statin treatment in acute GVHD prevention cannot definitively be answered with our study because the proportions of allografts where both donor and recipient or the recipient alone were treated with statins were only 2% (n = 12) and 3% (n = 16), respectively. Statin use by CSP-treated recipients could have had an incremental effect by decreasing the risk of grade 2 acute GVHD, in addition to the reduction in risk of grade 3-4 GVHD associated with statin use by CSP-treated donors. Because most recipients resumed statin treatment soon after completion of conditioning, it remains unclear whether the incremental GVHD-protective effect associated with statin treatment of the recipient is mediated through modulation of host APC function or through continued exposure of donor T cells to statin in the recipient environment. In a retrospective analysis of 67 patients given related or unrelated allografts for acute leukemia, Hamandani et al reported a lower risk of grade 2-4 acute GVHD among recipients treated with a statin at the time of HCT.12 Even though their analysis did not account for donor statin exposure, their results suggest that recipient statin use may have an independent GVHD-protective effect that is additive to the protection associated with donor statin use.
Whereas 74% and 26% of recipients received postgrafting CSP and TAC, respectively, statin-associated protection against acute GVHD was restricted to CSP-treated recipients (significance of effect modification, CSP vs TAC, P = .009). The CSP/TAC distribution in the postgrafting immunosuppressive regimens used in our study was similar to that in the study by Hamadani et al (70% vs 30%).12 Reasons for the unexpected effect association between statins and CSP remain unclear. Both CSP and TAC suppress T-cell activation by inhibiting calcineurin and consequently preventing the nuclear import of nuclear factor of activated T cells, a transcription factor necessary for T-cell activation, but they have distinct proximal cellular targets.22 Whereas TAC binds to FK-binding protein, for example, CSP interacts with several cyclophilins, including cyclophilin D, which regulates the calcium-dependent mitochondrial membrane permeability transition (MPT), known to play a pivotal role in the regulation of apoptosis.23 Because statins have also been shown to affect the calcium-dependent MPT,24 one could speculate that CSP and statins synergize by inducing T-cell hyporesponsiveness or apoptosis at the level of the MPT. The validity of this hypothesis will have to be tested in carefully designed in vitro studies.
The reasons for statin-mediated preferential protection against severe gut GVHD with much less effect on liver GVHD and no detectable effect of skin GVHD are unclear. The findings could be interpreted to suggest that (1) GVHD pathophysiology in the gastrointestinal tract differs from that in other target organs and (2) statin conditioning of the donor preferentially interferes with a GVHD-mediating mechanism that is prone to causing end-organ damage in the gastrointestinal tract.
Although the retrospective nature of our analysis precluded mechanistic studies of statin-mediated protection against severe acute GVHD, we were able to assess the impact of statin treatment on graft composition. By limiting the analysis to recipients of G-CSF–mobilized PBSCs, which composed 97% of the study population, and after controlling for donor age and gender, we found that donor statin use was associated with lower numbers of DC2 cells in the graft. Even though these data suggest that donor statin use has the potential of modifying graft composition, we found no obvious association between the numbers of DC2 cells in the graft and the risk of GVHD. Hence, it appears doubtful that any statin-associated reduction in DC2 dose is mechanistically linked to the GVHD-preventive effect. Data on Treg content or Th2-polarization in grafts were not available.
Statin-associated protection against acute GVHD was not associated with increased rates of recurrent malignancy. The absence of any evident impairment of graft-versus-leukemia activity might be explained by absence of any statin-associated effect on chronic GVHD. The finding that statin use did not significantly lower rates of NRM and overall mortality, even though grade 3-4 acute GVHD was virtually eliminated, may best be explained by the fact that mortality related to acute GVHD typically accounts for only a small proportion of overall NRM after allografting.
In conclusion, within the limitations of a retrospective study, our data provide strong evidence that donor or donor/recipient statin use confers protection against severe acute GVHD without compromising immunologic control of the underlying malignancy. Given the well-established toxicity profile of statins, our retrospective study might motivate the design of confirmatory prospective trials. Several questions, however, should be addressed in laboratory and preclinical studies before designing prospective clinical trials. (1) Do all statins have the same immunomodulatory properties? Even though atorvastatin was the most frequently used statin in our study, a meaningful comparison of differential statin efficacy was not possible because of sample size limitations. (2) What is the optimal statin dose and treatment duration? (3) Is statin-associated protection against acute GVHD limited to HCT with PBSC grafts, or does it also apply to HCT with marrow grafts? (4) What mechanisms account for the association between CSP prophylaxis and statin-mediated protection against GVHD? Pursuit of the latter question might also help elucidate novel molecular mechanisms involved in the pathogenesis of severe acute GVHD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Gary Schoch for assistance with data retrieval; Dr Mary Flowers for chronic GVHD evaluation; Helen Crawford, Bonnie Larson, and Sue Carbonneau for assistance with manuscript preparation; and all the patients who participated in these protocols and the nurses and staff who cared for them.
This work was supported by the Dana Foundation (P01-CA18029, CA78902, CA15704, HL36444, and TE 4540).
National Institutes of Health
Authorship
Contribution: M.R. contributed to study design, collected the data, analyzed and interpreted the data, and drafted the manuscript; B.E.S. performed the statistical analysis; S.H. performed the graft composition analysis; R.F.S. contributed to study design and edited the manuscript; A.P. confirmed quality of extracted medication data; P.J.M. assisted in data interpretation and edited the manuscript; B.M.S., D.G.M., and H.J.D. wrote the transplantation protocols; F.R.A. assisted in data interpretation; and M.M. designed the study, analyzed and interpreted the data, and wrote and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Mielcarek, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, PO Box 19024, Seattle, WA 98109; e-mail: mmielcar@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal