Abstract
Human plasmacytoid dendritic cells (pDCs) are crucially involved in the modulation of adaptive T-cell responses in the course of neoplastic, viral, and autoimmune disorders. In several of these diseases elevated extracellular levels of the serine protease granzyme B (GrB) are observed. Here we demonstrate that human pDCs can be an abundant source of GrB and that such GrB+ pDCs potently suppress T-cell proliferation in a GrB-dependent, perforin-independent manner, a process reminiscent of regulatory T cells. Moreover, we show that GrB expression is strictly regulated on a transcriptional level involving Janus kinase 1 (JAK1), signal transducer and activator of transcription 3 (STAT3), and STAT5 and that interleukin-3 (IL-3), a cytokine secreted by activated T cells, plays a central role for GrB induction. Moreover, we find that the immunosuppressive cytokine IL-10 enhances, while Toll-like receptor agonists and CD40 ligand strongly inhibit, GrB secretion by pDCs. GrB-secreting pDCs may play a regulatory role for immune evasion of tumors, antiviral immune responses, and autoimmune processes. Our results provide novel information about the complex network of pDC–T-cell interactions and may contribute to an improvement of prophylactic and therapeutic vaccinations.
Introduction
Plasmacytoid dendritic cells (pDCs) represent a central link between innate and adaptive immunity and play a crucial role in viral, autoimmune, and neoplastic diseases.1-4 One of their most prominent features is the ability of pDCs to produce and secrete large amounts of type I interferons (IFNs), thereby initiating and orchestrating antiviral immune responses.1,5 pDCs can also function as antigen-presenting cells and stimulate effector T-cell responses.6 However, pDC effects on T-cell subsets can include both T-cell activation (immunogenic function)7 and induction of T-cell anergy (tolerogenic function).7,8 pDCs therefore play an important role in fine-tuning cellular immune responses depending on the microenvironment. Several studies suggest that both the activated T cell–derived cytokine interleukin-3 (IL-3)9 and the immunosuppressive cytokine IL-10 induce a rather tolerogenic pDC phenotype associated with suppression of T-cell responses.10-12 In contrast, activation of pDCs in the presence of ligands for the pDC-characteristic Toll-like receptors (TLRs) TLR7 and TLR91 and for CD4010 results in an immunogenic phenotype with such pDC triggering a proinflammatory immune response including T-cell activation and cytotoxicity.6,13,14
Several inflammatory diseases including viral and autoimmune diseases have been found to be associated with elevated levels of extracellular granzyme B (GrB). Granzymes including GrB have been found to be locally elevated in bronchoalveolar lavage fluid from patients with chronic allergic asthma and in synovial fluid from patients with rheumatoid arthritis.15 Infections with cytomegalovirus after renal transplantation, with the dengue fever virus or with HIV, have been associated with high serum levels of GrB.15 Granzymes such as GrB represent a major constituent of the granules of cytotoxic cells, including cytotoxic T lymphocyte (CTL) and natural killer (NK) cells. The classical function of GrB is induction of apoptosis in target cells recognized by CTLs.16,17 Evidence is growing that apart from its cytotoxic effects, granzymes may also have alternative functions. These include degradation of viral proteins important for assembly and replication,18 cytokine-like effects,19 receptor cleavage,20-22 immunosuppression,15,18,23,24 and matrix degradation and remodeling.25
It is not understood how GrB is secreted into the extracellular space in certain diseases. GrB may escape from the immunologic synapses as cytotoxic lymphocytes degranulate.15 An alternative explanation is the production of GrB from nonclassical sources. This possibility is supported by recent findings that immune cell subsets other than CTL or NK cells are able to produce and secrete active GrB under certain circumstances. These subsets include B cells,26,27 hematopoietic progenitor cells,28 basophils,29 mast cells,30 and IFN-α–activated monocyte-derived DCs.31 Interestingly, GrB expression has also been reported in pDCs,32-35 although its regulation and function in these cells is not clear.
In the present study, we demonstrate that activated human pDCs are an abundant source of GrB protein, and on a cell-by-cell basis, may produce GrB in amounts that exceed GrB produced by classical cytotoxic lymphocytes. In subjects with juvenile idiopathic arthritis we find not only significantly elevated levels of GrB in fresh synovial fluid specimens but also high GrB expression in the corresponding synovia-derived pDCs compared with healthy controls. Using pDCs from healthy subjects we investigate in detail how GrB production and secretion in pDCs is regulated by cytokines, TLR ligands, and costimulatory signals. Moreover, we show that pDC-derived GrB is directly delivered to T cells and suppresses T-cell proliferation in a cell contact–dependent and perforin-independent manner, a process reminiscent of regulatory T (Treg) cells.24,36 Our results provide novel insights into how pDCs may regulate antiviral, autoimmune, and antitumor immune responses.
Methods
Human subjects and cell culture
The present study was approved by the Ethics Committee at the University of Ulm. Peripheral blood from healthy volunteers was acquired after obtaining informed consent from each person in accordance with the Declaration of Helsinki. Alternatively, synovial fluid from pediatric patients with juvenile idiopathic arthritis was acquired during standard therapeutic procedures after obtaining informed consent from patients and parents. Mononuclear cells from peripheral blood (PBMC) or from synovial fluid were immediately isolated and red blood cells were removed according to standard procedures. For pDC isolation the BDCA4+ cell isolation kit II was used according to the manufacturer's instructions (Miltenyi Biotec), resulting in more than 95% cells with a lin-1−BDCA-2+CD123highMHC class IIhigh phenotype. For in vitro culture, cells were suspended in AIM-V medium (Gibco BRL) supplemented with 10 ng/mL of the human pDC growth factor IL-3 and incubated for 36 hours on 96-well flat-bottom plates (106 cells/mL, 200 μL/well) unless stated otherwise at 37°C and 5% CO2 atmosphere in the presence of various agents as indicated. Purity after 2 days was regularly more than 99% BDCA-2+ cells. For coculture experiments CD4+ T cells or pan-T cells from healthy persons were isolated using the CD4+ T cell isolation kit II or the pan-T cell isolation kit II, respectively (Miltenyi Biotec). T cells were resuspended in phosphate-buffered saline (PBS; 107 cells/mL) containing 5- (and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) at a final concentration of 1μM, incubated at 37°C for 10 minutes, and washed 3 times. T cells (105) were coincubated with allogeneic pDCs at a pDC:T-cell ratio of 1:250 for 6 days on a 96-well round-bottom plate in 200 μL of AIM-V medium as described.37
Reagents for functional assays
Human IL-3 (10 ng/mL) and IL-10 (50 ng/mL) were purchased from PeproTech. The CpG oligodeoxynucleotide (ODN) 2006 (2.5 μg/mL) with the specific sequence 5′-tcg tcg ttt tgt cgt ttt gtc gtt-3′ was purchased from Coley Pharmaceutical Group, and imiquimod (5 μg/mL) was purchased from InvivoGen. Phytohemagglutinin (PHA; 10 μg/mL), prostaglandin E2 (PGE2; 10−6M), cycloheximide (1 μg/mL), and Z-FA-FMK (50μM) were purchased from Sigma-Aldrich. Anti-CD3/CD28 antibody-coated beads (0.25 μL per 200 μL per well) were purchased from Dynal Biotech–Invitrogen. Brefeldin A (1 μg/mL) was purchased from Epicentre Technologies. Vasoactive intestinal peptide (VIP; 10−6M), pyridone 6 (P6; 1μM), Ly294002 (1μM), AKT inhibitor VIII (0.5μM), bafilomycin A (10nM), and lactacystin (0.1μM) were purchased from Calbiochem. CD40 ligand (1 μg/mL) and recombinant human active GrB were purchased from Axxora Deutschland. For GrB inhibition experiments, carrier- and preservative-free rabbit anti–human GrB polyclonal antibody (immunoglobulin G [IgG]) from USBiological was used at the concentrations indicated. ImmunoPure rabbit IgG from Pierce served as control IgG.
Flow cytometry
For fluorescence-activated cell sorter (FACS) analysis, cells were harvested at the indicated time points and stained as described previously.37 Fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, PE-Cy5–, peridinin chlorophyll protein–Cy5.5, PE-Cy7–, or allophycocyanin-labeled antibodies to lin-1, CD3, CD4, CD8, CD123, and MHC II were purchased from BD Biosciences; PE- or allophycocyanin-labeled antibodies to BDCA-2 (CD303) were purchased from Miltenyi Biotec, PE-labeled anti-perforin antibodies were purchased from eBioscience, and PE- or allophycocyanin-labeled anti-GrB antibodies (clone GB12) as well as the corresponding isotype controls were purchased from Invitrogen. For flow cytometric intracellular GrB or perforin detection, cells were incubated at 106/mL for 16 hours, brefeldin A (Epicentre Technologies) was added to a final concentration of 1 μg/mL, and cells were cultured for 4 hours. Intracellular staining was performed using fixation and permeabilization buffers from An Der Grub Bio Research. Briefly, cells were washed and resuspended in fixation buffer, incubated for 15 minutes at room temperature, and washed with PBS. Cells were then resuspended in permeabilization buffer, and FcR blocking reagent (Miltenyi Biotec) was added. After 10 minutes incubation, anti-GrB, anti-perforin, or isotype control monoclonal antibody was added. After another 15 minutes of incubation at room temperature, cells were washed with PBS. Flow cytometric analyses were performed on a FACScan, a FACSCalibur or an LSRII (BD Immunocytometry Systems) and data analyzed using FlowJo software (Version 8.7.1; TreeStar).
GrB ELISA
pDCc from healthy persons were magnetically purified to more than 95% purity as outlined above and incubated for 16 hours with either no treatment or in the presence of human IL-3 (10 ng/mL) with or without IL-10 (50 ng/mL). After incubation, supernatants were collected and GrB concentrations determined using a human GrB ELISA kit according to the manufacturer's instructions (Mabtech). Alternatively, GrB concentrations were detected in serum from healthy subjects or in synovial fluid from patients with juvenile idiopathic arthritis. Briefly, plates (MaxiSorp Nunc-Immuno Module; Thermo Fisher Scientific) were coated with the GrB coating antibody (clone GB10; 2 μg/mL) and incubated overnight at 4°C. Nonspecific reactions were blocked with blocking buffer (PBS, 0.5% Tween 20 and 1% bovine serum albumin) for 1 hour at 24°C. Supernatants, serum, synovial fluid (100 μL volume), or standards were placed on 96-well precoated enzyme-linked immunosorbent assay (ELISA) plates and incubated for 2 hours at 24°C. After washing, plates were incubated with equal volumes of the detection antibody (clone GB11-biotin, 1 μg/mL) for 1 hour at 24°C. Plates were then washed and incubated for 1 hour with streptavindin–horseradish peroxidase at 24°C. After addition of the substrate (R&D Systems) plates were analyzed in a Dynatech MR 7000 ELISA reader. The lower limit of GrB detection was 13 pg/mL.
GrB activity assay
For demonstration of enzymatic activity of secreted GrB in the supernatants of stimulated pDCs we used a highly specific GrB activity assay according to the manufacturer's specifications (SensiZyme; Sigma-Aldrich). Briefly, GrB from supernatants and GrB standards derived from active human recombinant GrB (Alexis) were captured on a 96-well plate precoated with anti-GrB antibody. After washing, substrate A (a proenzyme containing the GrB cleavage site) along with substrate B (a chromogenic substrate for the active substrate A) were added to the plate and the accumulating chromogenic product was detected in a Dynatech MR 7000 ELISA reader at 405 nm after incubation overnight at 37°C.
GrB ELISpot
Human GrB ELISpot kits were purchased from Cell Sciences, and polyvinylidene difluoride–bottom 96-well plates were from Millipore. The enzyme-linked immunospot (ELISpot) was performed following the manufacturer's instruction. Briefly, plates were prepared by adding the capture antibody and by blocking with 2% skim milk in PBS. Cells were plated in AIM-V medium at 105/100 μL/well (unless stated otherwise) in the presence of various agents as indicated and cultured for 16 hours. After culture, the detection antibody was added and plates were incubated for 1.5 hours. Streptavidin–alkaline phosphatase was distributed, and plates were incubated for 1 hour. Finally, 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium buffer was added and color was allowed to develop for 10 minutes at room temperature, followed by rinsing with distilled water. Plates were dried completely, and spots were read on an Immunospot series 1 analyzer using Immunospot 3 software, both from CTL Cellular Technology.
GrB and perforin RT-PCR
pDCs from healthy persons were magnetically isolated to a purity of more than 98% and incubated for 16 hours with either no treatment or in the presence of human IL-3 (10 ng/mL) and IL-10 (50 ng/mL). After incubation, the pDC were counted and mRNA from 6 × 105 to 9 × 105 cells per sample was prepared using a Dynabeads mRNA Direct kit (Invitrogen). Total RNA was reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (Invitrogen) in a 16-μL reaction volume. For the polymerase chain reaction (PCR), 5 μL of the generated cDNA was used per 25 μL of reaction with recombinant Taq DNA polymerase (0.02 U/μL; Invitrogen). Primers used for GrB were as follows: forward primer 5′-CCATCCATCCAAGCCTATAATCCTA-3′, reverse primer 5′-CCTGCACTGTCATCTTCACCT-3′ (biomers.net). Primers used for perforin were as follows: forward primer 5′-TGGAGTGCCGCTTCTACAGTT-3′, reverse primer 5′-GTGGGTGCCGTAGTTGGAGAT-3′. RPL-32 was used as a housekeeping gene to obtain comparable results. Primers used for RPL-32 were as follows: forward primer 5′-AGTTCCTGGTCCACAACGTC-3′, reverse primer 5′-GATGCCAGACAGTTTTT-3′. Five microliters of the reaction products were run on a 1.5% agarose gel along with a 100-bp ladder.
Western immunoblot
pDCs from healthy subjects were purified and incubated for the times indicated at 106 cells/500 μL/well with IL-3 (10 ng/mL) with or without IL-10 (50 ng/mL). Cell lysates were prepared and Western immunoblotting was performed according to standard procedures. After washing and blocking, primary antibodies were added, followed by a washing step and incubation with the secondary antibody conjugated to horseradish peroxidase for 1 hour. Finally, membranes were developed, dried, and exposed to a film. Primary antibodies against GrB, pJAK1, pSTAT1, pSTAT3, pSTAT5, and actin were purchased from Cell Signaling Technology, and the antibody against pJAK3 was purchased from Santa Cruz Biotechnology. Secondary antibodies were anti–goat IgG from Sigma-Aldrich Laborchemikalien, and anti–rabbit IgG and anti–mouse IgG were from GE Healthcare.
Fluorescence microscopy
Purified pDCs from healthy persons were cultured overnight on 96-well plates at 2 × 105 cells/200 μL per well in the presence of IL-3 (10 ng/mL) and IL-10 (50 ng/mL). Brefeldin A was added to a final concentration of 1 μg/mL, and cells were cultured for 4 hours. Subsequently, 1.2 × 106 cells were pooled and transferred into a FACS tube (BD Biosciences). Cells were then stained on their surface with FITC-labeled antibodies to BDCA-2 (Miltenyi Biotec) and intracellularly with PE-labeled antibodies to GrB or isotype control (both from Invitrogen) as described above for flow cytometry, but with prolonged incubation for 1 hour. After 2 washing steps, cells were transferred onto 8-well Lab-Tek II chamber slides (USA Scientific), settled for 20 minutes, and cover slips were attached with Vectashield mounting medium (Vector Laboratories) and examined using an Olympus AX70 fluorescence microscope.
Spinning disk confocal microscopy
Purified pDCs were cultured for 16 hours in the presence of IL-3 (10 ng/mL) and IL-10 (50 ng/mL). Brefeldin A was added to a final concentration of 1 μg/mL and cells were cultured for 4 hours. Then, pDCs were harvested, stained in FACS tubes with Cell Mask deep red membrane dye (Invitrogen) at 5 μg/mL for 15 minutes at 37°C and then incubated for 1 hour with 25 μL of GranToxiLux fluorogenic GrB substrate in 75 μL of AIM-V medium at room temperature (OncoImmunin). Alternatively, for total GrB staining, pDCs were fixed, permeabilized, blocked with FcR blocking reagent for 10 minutes (Miltenyi Biotec), and stained with anti-GrB-biotin (Abcam) for 15 minutes at room temperature. After washing, cells were resuspended in 100 μL of permeabilization buffer and stained for 15 minutes with streptavidin–Alexa Fluor 488 (Invitrogen). Finally, cells were resuspended in 200 μL of PBS, placed on 8-well Lab-Tek Permanox chamber slides (Nalge Nunc International) coated with fetal calf serum, and spun at 60g for 10 minutes. The sides of the chambers were removed, coverslips were attached with Vectashield mounting medium (Vector Laboratories), and the edges were sealed using Clarion permanent mounting medium (Biomeda). For pDC–T-cell–coculture experiments, isolated pDCs (> 95% purity) were cultured in the presence or absence of IL-3 and IL-10 for 60 hours. On day 3, 3 × 105 isolated CD4+ T cells (> 98% purity) in 60 μL of PBS were incubated for 2 hours on ibiTreat chamber slides (Integrated BioDiagnostics) for immobilization, stained with Cell Mask deep red membrane dye, and washed 3 times with PBS. pDCs (2 × 105) in 60 μL of PBS and 25 μL of GranToxiLux fluorogenic GrB substrate were added. Fluorescence images were acquired using the acquisition software Andor iQ 1.6 on a spinning disk confocal microscope assembled from individual components including a CSU10 scan head (Yokogawa), an inverted microscope (Axio Observer; Zeiss) with oil-immersion objective (UPlanSApo 60×/1.35; Olympus), environmental control (Pecon), an image-splitting unit (OptoSplit II; Cairn Research), and an EMCCD camera (DV-887; Andor). ImageJ software (National Institutes of Health) was used for subsequent image processing (2-dimensional parallel spectral deconvolution by generalized Tikhonov [reflexive] method and 3-dimensional reconstruction).
Statistics
Data are expressed as means plus or minus SEM. To determine statistical differences between the means of 2 data columns, the paired 2-tailed Student t test was used. P values were corrected using the Bonferroni method where applicable.
Results
IL-3–activated pDCs express GrB, an effect strongly enhanced by IL-10, but do not express perforin
Various inflammatory diseases including autoimmune arthritis have previously been associated with elevated levels of GrB15 as well as with increased frequencies of pDCs.3 We were able to confirm these findings in synovial fluid from subjects with idiopathic juvenile arthritis and could also show that GrB was highly expressed by pDCs from such synovial fluid (supplemental Figure 1A-B, available on the Blood website; see the Supplemental Materials link at the top of the online article). Because IL-3 is a primary survival factor for pDCs10 and has recently been reported to play an important role for collagen-induced arthritis in mice,38 we continued our experiments with isolated PBMCs from healthy donors, which we cultured in the presence of IL-3 and various additional cytokines. Production of GrB by the resulting pDCs was evaluated. Healthy pDC precursors did not express substantial levels of GrB. In contrast, IL-3–driven activation induced strong up-regulation of GrB protein expression (Figure 1A top panel). Similar responses were found when pDC precursors were purified before culturing in IL-3, suggesting that GrB induction was not a secondary effect resulting from stimulation of different cell populations (Figure 1A bottom panel). GrB production by pDCs was strongly enhanced by IL-10 (Figure 1A-B), revealing the combination of IL-3 and IL-10 as the most potent stimulus for GrB expression by pDCs. Immunofluorescence microscopy showed a scattered distribution pattern of GrB within the pDC cytoplasm (Figure 1C). Higher resolution imaging using spinning disk confocal microscopy revealed a perinuclear and granular localization of GrB (Figure 1D, supplemental Video 1). GrB protein expression by pDCs was inhibited by the translation inhibitor cycloheximide (Figure 1E), and GrB mRNA could be detected using reverse transcription (RT)–PCR (Figure 1F). This indicated that GrB was freshly produced during pDC activation and was not preformed. In contrast, pDC precursors and pDCs cultured with IL-3 or IL-3 plus IL-10 did not express perforin (supplemental Figure 2A-B).
pDCs activated in the presence of IL-3 with or without IL-10 express GrB mRNA and protein. PBMCs or purified pDC precursors (> 90%) were cultured for 16 hours in the presence of IL-3, either with or without IL-10. Freshly prepared unstimulated pDC precursors (0 hour) served as negative controls. (A) Zebra plots show GrB expression in pDCs from unfractionated PBMCs (top panel) or purified pDC preparations (bottom panel) as indicated by gating on the lin-1...CD123high populations. (B) Bar graphs indicate GrB expression as indicated by average median fluorescence intensities (MFI) from 6 independent experiments with purified pDCs. Error bars indicate SEM. (C) Fluorescence microscopy with ×60 magnification was performed with purified IL-3/IL-10–cultured pDCs stained with PE-labeled anti-GrB (orange) and FITC-labeled anti-BDCA-2 (green). (D) Spinning disk confocal microscopy was performed with purified pDCs cultured in the presence or absence of IL-3 and IL-10 and then stained with anti-GrB-biotin and streptavidin–Alexa Fluor 488 (green) as well as Cell Mask deep red membrane stain (red). The negative control shows staining with a biotinylated isotype control instead of anti-GrB-biotin. No green fluorescence was found when pDCs were cultured in the absence of IL-3 and IL-10 (not shown). (E) Line graphs and zebra plots show GrB expression by purified pDCs stimulated with IL-3 and IL-10 in the presence or absence of cycloheximide at 1 μg/mL (n = 3). (F) RT-PCR for GrB mRNA was performed using 6 × 105 and 9 × 105 purified pDCs isolated before (−) and after (+) incubation of pDCs for 16 hours with IL-3 and IL-10.
pDCs activated in the presence of IL-3 with or without IL-10 express GrB mRNA and protein. PBMCs or purified pDC precursors (> 90%) were cultured for 16 hours in the presence of IL-3, either with or without IL-10. Freshly prepared unstimulated pDC precursors (0 hour) served as negative controls. (A) Zebra plots show GrB expression in pDCs from unfractionated PBMCs (top panel) or purified pDC preparations (bottom panel) as indicated by gating on the lin-1...CD123high populations. (B) Bar graphs indicate GrB expression as indicated by average median fluorescence intensities (MFI) from 6 independent experiments with purified pDCs. Error bars indicate SEM. (C) Fluorescence microscopy with ×60 magnification was performed with purified IL-3/IL-10–cultured pDCs stained with PE-labeled anti-GrB (orange) and FITC-labeled anti-BDCA-2 (green). (D) Spinning disk confocal microscopy was performed with purified pDCs cultured in the presence or absence of IL-3 and IL-10 and then stained with anti-GrB-biotin and streptavidin–Alexa Fluor 488 (green) as well as Cell Mask deep red membrane stain (red). The negative control shows staining with a biotinylated isotype control instead of anti-GrB-biotin. No green fluorescence was found when pDCs were cultured in the absence of IL-3 and IL-10 (not shown). (E) Line graphs and zebra plots show GrB expression by purified pDCs stimulated with IL-3 and IL-10 in the presence or absence of cycloheximide at 1 μg/mL (n = 3). (F) RT-PCR for GrB mRNA was performed using 6 × 105 and 9 × 105 purified pDCs isolated before (−) and after (+) incubation of pDCs for 16 hours with IL-3 and IL-10.
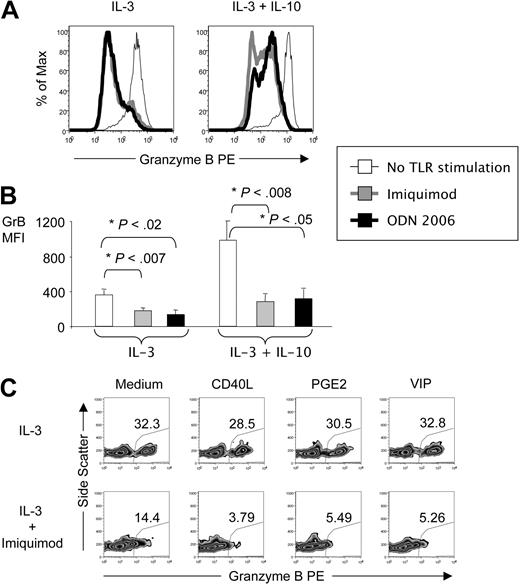
TLR agonists and CD40 ligand inhibit GrB expression in IL-3–activated pDCs
TLR agonists such as imiquimod and the CpG oligodeoxynucleotide ODN 2006 are recognized by pDCs via TLR7 and TLR9, respectively, and induce an immunogenic phenotype in pDCs.1 In the present study, both ODN 2006 and imiquimod inhibited production of GrB expression by pDCs activated with IL-3 alone and IL-3 plus IL-10 (Figure 2A-B). TLR agonist–mediated inhibition of GrB expression in pDCs was further enhanced by other known modulators of pDC phenotype, including CD40 ligand (CD40L), prostaglandin E2 (PGE2), and vasoactive intestinal peptide (VIP; Figure 2C).
pDC expression of GrB is negatively regulated by ligands for TLR7, TLR9, and CD40. pDCs were activated for 16 hours in the presence of IL-3 alone or IL-3 and IL-10 as well as the TLR9 ligand ODN 2006 or the TLR7 ligand imiquimod. Then they were stained with fluorescently labeled antibodies to pDC markers and GrB, and analyzed by FACS. (A) Histograms show GrB expression in pDCs from 1 representative donor. (B) Bar graphs illustrate average MFI for GrB expression from 3 independent experiments. (C) Zebra plots from pDCs activated in the presence of IL-3 and combinations of imiquimod with CD40L, PGE2, or VIP illustrate the percentages of GrB+ pDCs (representative for n = 3).
pDC expression of GrB is negatively regulated by ligands for TLR7, TLR9, and CD40. pDCs were activated for 16 hours in the presence of IL-3 alone or IL-3 and IL-10 as well as the TLR9 ligand ODN 2006 or the TLR7 ligand imiquimod. Then they were stained with fluorescently labeled antibodies to pDC markers and GrB, and analyzed by FACS. (A) Histograms show GrB expression in pDCs from 1 representative donor. (B) Bar graphs illustrate average MFI for GrB expression from 3 independent experiments. (C) Zebra plots from pDCs activated in the presence of IL-3 and combinations of imiquimod with CD40L, PGE2, or VIP illustrate the percentages of GrB+ pDCs (representative for n = 3).
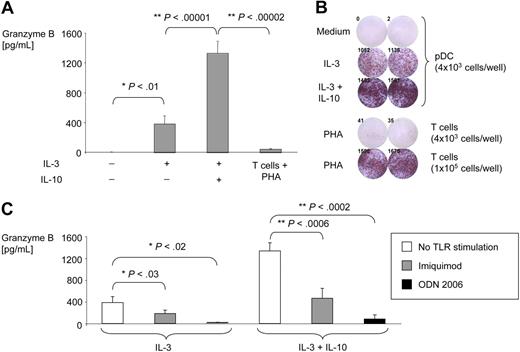
IL-3–activated pDCs secrete large amounts of active GrB
Classical GrB producers such as CTL or NK cells secrete GrB during the granule exocytosis phase after cells have recognized and docked to target cells.16,17 In contrast, pDC secretion of GrB occurred independently of other cells since highly purified pDCs were used for secretion experiments. pDCs activated with IL-3– and IL-10–secreted GrB levels up to 2 logs greater than that secreted by T cells stimulated with PHA (Figure 3A). The fraction of pDCs that produced GrB following IL-3 and IL-10 stimulation was much greater than the fraction of T cells that produced GrB in response to PHA as determined by ELISPOT (Figure 3B). Imiquimod and ODN 2006 inhibited secretion of GrB by mature pDCs (Figure 3C), demonstrating that these agents affect both production and secretion of GrB. Similar results were found with a highly sensitive enzyme-linked GrB activity ELISA demonstrating that pDC-derived GrB was enzymatically active (data not shown). Finally, enzymatic activity was further confirmed by visualization of cleaved GrB-specific substrate in the granules of highly purified pDCs using spinning disk confocal microscopy (supplemental Figure 3, supplemental Video 2).
IL-3–activated pDCs secrete large amounts of GrB. Purified pDCs were cultured for 16 hours in the absence or presence of IL-3 with or without IL-10. (A) GrB in supernatants was measured by ELISA. Bar graphs illustrate average GrB concentrations from 5 independent experiments (values normalized to 104 pDCs per well). (B) GrB secretion was determined by a GrB-specific ELISPOT using purified pDCs treated with IL-3 with or without IL-10 or using T cells treated with PHA (positive control). No spots were observed in samples with 4 × 103 pDCs per well in the presence of PHA (not shown). (C) GrB ELISA was performed from supernatants of purified pDCs treated with ODN 2006 or imiquimod. Bar graphs illustrate GrB concentrations normalized to 104 pDCs per well (n = 10).
IL-3–activated pDCs secrete large amounts of GrB. Purified pDCs were cultured for 16 hours in the absence or presence of IL-3 with or without IL-10. (A) GrB in supernatants was measured by ELISA. Bar graphs illustrate average GrB concentrations from 5 independent experiments (values normalized to 104 pDCs per well). (B) GrB secretion was determined by a GrB-specific ELISPOT using purified pDCs treated with IL-3 with or without IL-10 or using T cells treated with PHA (positive control). No spots were observed in samples with 4 × 103 pDCs per well in the presence of PHA (not shown). (C) GrB ELISA was performed from supernatants of purified pDCs treated with ODN 2006 or imiquimod. Bar graphs illustrate GrB concentrations normalized to 104 pDCs per well (n = 10).
pDC expression and secretion of GrB depend on JAK activation, endosomal acidification, and active intracellular transport
Induction of GrB in cytotoxic cells is known to depend on activation of the JAK/STAT pathway.39 The JAK inhibitor pyridone 6 (P6) efficiently suppressed GrB expression in pDCs. In contrast, no suppression was found with inhibitors of AKT (AKT inhibitor VIII) and PI3 kinase (Ly294002; Figure 4A-B). Bafilomycin A, which prevents protein acidification and degradation in endosomes, inhibited GrB expression by pDCs. Inhibition of cathepsin-dependent endosomal processing with the inhibitor Z-FA-FMK or of proteasomal processing with the inhibitor lactacystin had little effect. The secretion of GrB by pDCs was strongly dependent on active transport from the endoplasmic reticulum (ER) to the Golgi apparatus, as shown by complete abrogation of GrB secretion by brefeldin A (Figure 4C). P6 and cycloheximide also inhibited GrB secretion by pDCs, providing further evidence that GrB secretion is dependent on JAK phophorylation and protein translation (Figure 4C). Taken together, these data suggest that GrB expression and secretion by pDCs require JAK activation, protein translation, endosomal acidification, and active transport from the ER to the Golgi apparatus.
pDC expression and secretion of GrB depend on JAK activation, endosomal acidification, and active intracellular transport. pDCs were activated for 16 hours in the presence of IL-3 and IL-10 and various inhibitors. All inhibitors were nontoxic for pDC at the concentrations used. GrB expression in pDCs was analyzed by FACS. (A) Line graphs show GrB expression with and without various inhibitors as measured by intracellular staining for GrB from 4 individual experiments. (B) Zebra plots show GrB expression in pDCs from one representative experiment. (C) GrB secretion by purified pDCs was detected using a specific ELISPOT. Shown are results from 2 representative donors of 4.
pDC expression and secretion of GrB depend on JAK activation, endosomal acidification, and active intracellular transport. pDCs were activated for 16 hours in the presence of IL-3 and IL-10 and various inhibitors. All inhibitors were nontoxic for pDC at the concentrations used. GrB expression in pDCs was analyzed by FACS. (A) Line graphs show GrB expression with and without various inhibitors as measured by intracellular staining for GrB from 4 individual experiments. (B) Zebra plots show GrB expression in pDCs from one representative experiment. (C) GrB secretion by purified pDCs was detected using a specific ELISPOT. Shown are results from 2 representative donors of 4.
GrB expression by pDCs is associated with phosphorylation of JAK/STAT molecules
Further studies were performed to assess the involvement of other molecules in the JAK and STAT pathways, including Western immunoblots for the phosphorylated forms of JAK1, JAK2, JAK3, STAT1, STAT3, and STAT5. For phospho-JAK (pJAK) detection we used purified pDC precursors and pDCs stimulated with IL-3 with or without IL-10 for 10 minutes, whereas for pSTAT detection pDCs were stimulated for 30 minutes. GrB and β-actin blots from pDCs activated for 16 hours served as controls. IL-3 and IL-10 culture of pDCs resulted in strong up-regulation of GrB, pJAK1, pSTAT3, and pSTAT5, weak up-regulation of pJAK3 and pSTAT1, and no up-regulation of pJAK2 (Figure 5). These studies demonstrate induction of GrB in pDCs involves signaling pathways similar to those seen in NK cells or CTLs.39
Expression of GrB in pDCs is associated with phosphorylation of JAK/STAT members. pDC precursors were purified and evaluated either unstimulated or after stimulation with IL-3 with or without IL-10. Stimulation was performed for 16 hours for GrB detection, 10 minutes for pJAK detection, and 30 minutes for pSTAT detection. Protein was extracted and Western immunoblotting performed. Loading of equal protein amounts was assured by parallel blotting for β-actin. PBMCs stimulated with PHA served as positive controls (data not shown). One representative experiment of 3 is shown.
Expression of GrB in pDCs is associated with phosphorylation of JAK/STAT members. pDC precursors were purified and evaluated either unstimulated or after stimulation with IL-3 with or without IL-10. Stimulation was performed for 16 hours for GrB detection, 10 minutes for pJAK detection, and 30 minutes for pSTAT detection. Protein was extracted and Western immunoblotting performed. Loading of equal protein amounts was assured by parallel blotting for β-actin. PBMCs stimulated with PHA served as positive controls (data not shown). One representative experiment of 3 is shown.
pDC-derived active GrB is delivered to T cells and inhibits T-cell proliferation
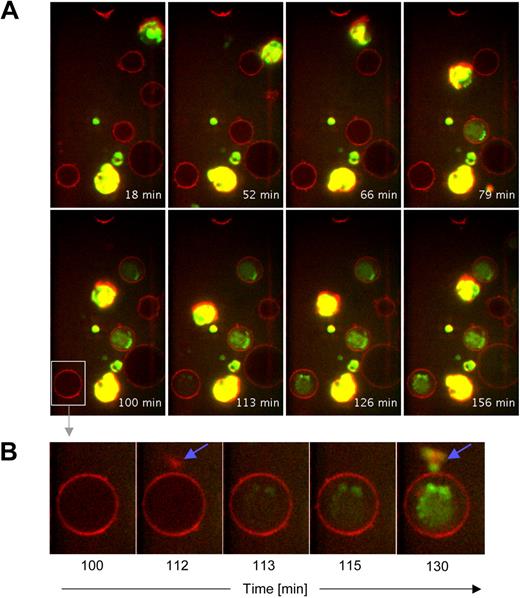
The intensity of GrB secretion by pDCs and its rigorous regulation strongly suggest a physiologic role in the modification of immune responses. Because the primary immune cells interacting with dendritic cells are T cells, we tested whether pDC-derived GrB can be delivered to T cells in its active form. To this end we preincubated pDCs in the presence or absence of IL-3 and IL-10 and then started coincubating them with CD4+ T cells in the presence of GrB-specific fluorogenic substrate. Using spinning disk confocal microscopy we demonstrated that 1 hour after the beginning of coincubation T cells started to internalize pDC-derived GrB into their cytoplasm. pDCs were highly mobile, and close interaction between GrB+ pDCs and T cells appeared to be required for GrB to be transferred to T cells (Figure 6, supplemental Video 3A-C). T cells coincubated with GrB− pDC (preincubated in the absence of IL-3 and IL-10) did not develop green fluorescence (supplemental Video 3D), confirming the notion that GrB was indeed pDC-derived and not T cell–derived.
pDCs matured in the presence of IL-3 and IL-10 deliver enzymatically active GrB to CD4+ T cells. pDCs from healthy donors were purified and cultured for 60 hours in the presence of IL-3 and IL-10. Then, pDC (> 95% purity) were harvested and added to chamber slides containing purified immobilized CD4+ T cells (> 98% purity) stained with Cell Mask deep red membrane dye. Finally, GranToxiLux fluorogenic GrB substrate was added to the chamber slides and cocultures were analyzed for 2.5 hours using spinning disk confocal microscopy. Images were deconvolved using ImageJ software. (A) GrB+ pDCs are displayed in green-yellow, cell membranes in red, and active GrB internalized by immobilized CD4+ T cells in green. No green or yellow fluorescence was found when pDCs were cultured in the absence of IL-3 and IL-10 (not shown). (B) Shown is a sequence of detailed images of 1 individual CD4+ T cell before and during contact to a GrB+ pDCs (blue arrows indicate visible contact). Note the rapid diffusion of active GrB within the T cell after first contact to GrB+ pDCs.
pDCs matured in the presence of IL-3 and IL-10 deliver enzymatically active GrB to CD4+ T cells. pDCs from healthy donors were purified and cultured for 60 hours in the presence of IL-3 and IL-10. Then, pDC (> 95% purity) were harvested and added to chamber slides containing purified immobilized CD4+ T cells (> 98% purity) stained with Cell Mask deep red membrane dye. Finally, GranToxiLux fluorogenic GrB substrate was added to the chamber slides and cocultures were analyzed for 2.5 hours using spinning disk confocal microscopy. Images were deconvolved using ImageJ software. (A) GrB+ pDCs are displayed in green-yellow, cell membranes in red, and active GrB internalized by immobilized CD4+ T cells in green. No green or yellow fluorescence was found when pDCs were cultured in the absence of IL-3 and IL-10 (not shown). (B) Shown is a sequence of detailed images of 1 individual CD4+ T cell before and during contact to a GrB+ pDCs (blue arrows indicate visible contact). Note the rapid diffusion of active GrB within the T cell after first contact to GrB+ pDCs.
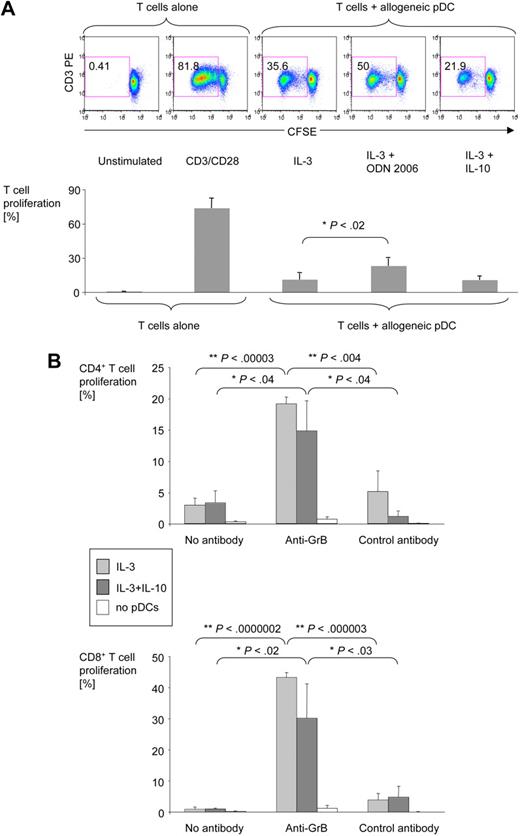
Further studies were done to determine whether pDC-derived GrB has an impact on the proliferative capacity of T cells. pDCs were activated in the presence of various stimuli for 36 hours, washed, and cocultured with allogeneic CFSE-labeled T cells for 6 days. T-cell proliferation was determined by CFSE dilution. pDCs activated in the presence of IL-3 and TLR7 or TLR9 ligands (GrBlow) induced a strong proliferative T-cell response. In contrast, activation of pDCs with IL-3 alone or IL-3 and IL-10 (GrB+) resulted in much lower T-cell proliferation (Figure 7A). To assess the role of GrB, a GrB-specific antibody was added to the cocultures. The anti-GrB antibody, but not a control antibody, enhanced the ability of IL-3/IL-10–activated GrB-secreting pDCs to induce proliferation of both CD4+ (Figure 7B top panels) and CD8+ (Figure 7B bottom panels) T cells. In the presence of anti-GrB antibody, T-cell proliferation in response to IL-3/IL-10–treated pDCs reached the same levels achieved with TLR ligand–activated pDCs.
pDC-derived GrB inhibits allogeneic T-cell proliferation. Purified pDCs were activated for 36 hours in the presence of IL-3, IL-10, and ODN 2006 as indicated. Activated pDCs were washed and coincubated for 6 days with CFSE-stained allogeneic T cells at a pDC:T cell ratio of 1:250. Cells were harvested, stained for CD3, CD4, and CD8, and analyzed by 4-color flow cytometry. (A) Dot plots from one representative experiment and bar graphs from 6 independent experiments illustrate the percentage of CD3+ T cells proliferating in response to allogeneic pDCs or anti-CD3/CD28 antibodies. Error bars indicate SEM. Similar responses were obtained with imiquimod instead of ODN 2006 (data not shown). (B) Bar graphs show average percentages of proliferating CD3+CD4+ and CD3+CD8+ T cells in the presence or absence of IL-3 with or without IL-10–cultured pDCs and anti-GrB or control antibodies (both at 50 μg/mL; n = 4). Error bars indicate SEM.
pDC-derived GrB inhibits allogeneic T-cell proliferation. Purified pDCs were activated for 36 hours in the presence of IL-3, IL-10, and ODN 2006 as indicated. Activated pDCs were washed and coincubated for 6 days with CFSE-stained allogeneic T cells at a pDC:T cell ratio of 1:250. Cells were harvested, stained for CD3, CD4, and CD8, and analyzed by 4-color flow cytometry. (A) Dot plots from one representative experiment and bar graphs from 6 independent experiments illustrate the percentage of CD3+ T cells proliferating in response to allogeneic pDCs or anti-CD3/CD28 antibodies. Error bars indicate SEM. Similar responses were obtained with imiquimod instead of ODN 2006 (data not shown). (B) Bar graphs show average percentages of proliferating CD3+CD4+ and CD3+CD8+ T cells in the presence or absence of IL-3 with or without IL-10–cultured pDCs and anti-GrB or control antibodies (both at 50 μg/mL; n = 4). Error bars indicate SEM.
Discussion
It is becoming increasingly evident that pDC biology is central to the regulation of a wide range of inflammatory immune responses. The studies presented above add yet another layer to the complexity of this network of interactions. Our studies demonstrate that pDCs can produce large amounts of enzymatically active GrB. GrB secretion by pDCs is not accompanied by perforin secretion but is highly regulated by immunomodulatory cytokines, costimulatory signals, and TLR agonists. In particular, IL-3, a cytokine secreted by eosinophils, mast cells, and activated T cells,9 and IL-10 strongly induce GrB secretion by pDCs. In contrast, CD40 ligand and TLR7 or TLR9 agonists such as imiquimod or CpG ODN are potent inhibitors of pDC-derived GrB. Importantly, pDC-derived GrB is not preformed or stored, but it is produced de novo upon appropriate stimulation. Its secretion requires activation of JAK1, STAT3, and STAT5 and involves protein translation, endosomal acidification, and an active transport from the ER to the Golgi apparatus.
pDCs are considered pivotal sensors of viral infections, critically involved in the initiation and orchestration of antiviral adaptive immune responses.1,2 Considerable attention has been paid to the mechanisms by which pDCs link innate and adaptive immunity, including production of type I IFN and presentation of antigens to T cells.1,6 After a viral infection, pDCs pick up antigen and migrate to the draining lymph nodes, where they present antigen to T cells. Therefore, highly specific activation of T cells occurs exclusively in the lymph nodes. In contrast, at the site of viral infection, where peripheral T cells are kept in a preactivated state by continuous self-antigen exposure,40-42 additional release and copresentation of autoantigens by viruses may result in the development of autoreactive T cells. Thus, expansion of peripheral T cells at the site of acute viral infection may not be desirable too early because of the potential for extensive autoreactivity. pDC-derived GrB may act as inhibitor of such early on-site T-cell expansion. The observation that extracellular GrB levels are particularly high in the acute phase of viral infections is consistent with this hypothesis.15 As soon as highly specific T cells have been selected in the lymph nodes, they express costimulatory molecules such as CD40L,43 which may then cooperate with released viral nucleic acids to abrogate further GrB release by pDCs, allowing for additional on-site T-cell expansion. The overall result may be the development of a strong antiviral immune response with a decreased chance of autoimmunity. This is in line with our finding that CD40L and TLR agonists are particularly effective at suppressing the production of GrB by pDCs.
In contrast to antiviral immune responses, antitumor responses are usually weaker due to the lack of danger signals such as TLR ligands.10 Moreover, the tumor environment can have an immunosuppressive impact by itself. It has become evident that pDCs play an important role for cancer immunity because they are found to infiltrate a series of tumors.4 The cytokines identified in the present study as being mainly responsible for the induction of GrB in pDCs, namely IL-3 and IL-10, can also be detected in the environment of neoplastic diseases.11,44,45 This suggests that GrB-secreting pDCs in such an environment could be involved in suppressing the expansion of tumor-specific T cells. Clearly, further studies are needed to investigate whether the regulatory effects of GrB-secreting pDCs on T cells may play a role for immune evasion of such neoplastic diseases.
Evidence is growing that pDCs regulate the intensity of a large variety of inflammatory immune responses. In particular, the role pDCs can play in autoimmune diseases3,46 may be due to both a pDC-based response to viruses pathogenetically involved in these diseases47,48 as well as a direct TLR-based recognition of autoantigens such as self nucleic acids by pDCs.1,49 For example, pDCs migrate into the joints of patients with rheumatoid arthritis, resulting in elevated frequencies of pDCs in the synovia and the synovial fluid of these patients.3,50 Similarly, various autoimmune diseases with cutaneous lesions, including lupus erythematosus, dermatomyositis, and psoriasis, have been demonstrated to exhibit enhanced frequencies of pDCs in affected areas.46 Importantly, all of these inflammatory diseases are characterized by both high pDC frequencies as well as elevated levels of extracellular GrB.15 The source of extracellular GrB has so far been elusive. Although the involvement of GrB release from cytotoxic cells has been discussed,15 this does not explain why extracellular GrB is not accompanied by the occurrence of extracellular perforin in such diseases. Further evaluation of the role of GrB in pDCs would benefit from evaluation of this finding in murine models; however, to date we have been unable to induce GrB production by murine pDCs, suggesting apparent species differences between mice and humans. Nevertheless, the data presented in this report demonstrate that human pDCs are a potential source of extracellular GrB in inflammatory conditions such as viral and autoimmune diseases in humans. This view is supported by our finding that pDCs in the synovial fluid of patients with juvenile idiopathic arthritis produce high levels of GrB.
Our studies suggest that GrB secretion by pDCs plays a central role in their ability to suppress T-cell proliferation. Evidence for the functional role of GrB is the finding that extracellular inhibition of GrB with specific antibodies completely restored the proliferative response of T cells to allogeneic pDCs. Our findings support the concept that IL-3 alone or in combination with IL-10 turns pDC precursors into tolerogenic pDCs, with GrB representing a key immunosuppressive effector molecule. Because we were able to directly visualize uptake of pDC-derived GrB by T cells, inhibition of T-cell proliferation may be due to either induction of T-cell apoptosis or to cleavage of molecules involved in cellular proliferation such as the ζ-chain of the T-cell receptor.20-22 The video microscopy images of pDC–T-cell cocultures and the fact that exogenous addition of active recombinant GrB did suppress T-cell proliferation only at higher concentrations (D.F., unpublished data, March 2009) suggest that close contact between pDCs and T cells is required for a suppressive effect of GrB-secreting pDCs.
On the other hand, our live imaging studies also showed that the involved pDCs were highly mobile and made several contacts before T cells started to develop GrB-dependent fluorescence. This suggests the cellular interactions between GrB-secreting pDCs and T cells are of low avidity and may be quite distinct from the tighter contact between a CTL and a target cell. Moreover, the uptake of pDC-derived GrB into T cells appears to occur independently of perforin, because the latter is not produced by pDCs. Alternative mechanisms of GrB uptake by T cells could include mannose-6-phosphate receptor–mediated or fluid-phase endocytosis.51-54 These saturable mechanisms may also explain why the antiproliferative effect of pDC-derived GrB appears to plateau with both IL-3–activated pDCs (that produce less GrB) and IL-3/IL-10–activated pDCs (that produce more GrB) having similar abilities to suppress T-cell proliferation. Although the exact role of perforin for Treg cell function is still under debate,24,36,55 GrB+ pDCs are similar to Treg cells in that both appear to be able to suppress effector T-cell proliferation in a GrB- and cell contact–dependent, but perforin-independent, manner.
In conclusion, the studies outlined in this report demonstrate that pDCs can secrete large amounts of GrB in response to IL-3 and IL-10. pDC-derived GrB suppresses T-cell proliferation in a perforin-independent manner, similar to what has been described for regulatory T cells. GrB-secreting pDCs may therefore contribute to the suppression of autoreactive T cells such as would be desirable at the site of acute viral infections. Additional studies are needed to assess what role pDC-derived GrB plays in modulating immune responses and how this finding might be used therapeutically. Incomplete activation and expansion of T cells represent a challenge not only in chronic viral infections and for antitumor immune responses, but also for several prophylactic and therapeutic vaccination approaches for viral and neoplastic diseases. Full activation and expansion of T cells may be inhibited by GrB-secreting pDCs, a process that would be beneficial in limiting autoimmunity, but not when induction of a robust antitumor or antiviral immune response is desired. Inhibition of pDC-derived GrB at the time of vaccination may therefore represent a novel strategy to induce more effective and comprehensive immune responses to vaccination.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank B. Böhm from the Department of Medicine II for permission to use the ELISpot reader, and A. Westhoff from the Department of Pediatrics for excellent assistance with fluorescence microscopy (University of Ulm, Ulm, Germany).
This work was supported by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation), from the Ministry of Science, Research, and the Arts Baden-Württemberg, and from the European Social Fund (ESF) within the Schlieben-Lange-Programm to D.F., as well as from the DFG, from the Deutsche José Carreras Leukämie-Stiftung eV, and from the Deutscher Akademischer Austauschdienst (DAAD; German Academic Exchange Service) to B.J. Further support came from the DFG through the Center for Functional Nanostructures (CFN) and SFB 497 to G.U.N. Finally, this work was supported by US Public Health Service Grant P50 CA97274 to G.W.
Authorship
Contribution: B.J., D.F., and G.J.W. generated the hypothesis; B.J. and D.F. designed the research; A.V., S.E.B., J.M., K.S., T.B., B.M., O.L., and K.T. performed experiments; B.J., D.F., A.V., S.E.B., K.S., T.B., B.M., O.L., and K.T. conducted the data analyses; G.U.N., T.S., G.J.W., and K.-M.D. provided key research tools; B.J., A.V., T.B., O.L., K.T., and D.F. prepared the figures; and B.J. and D.F. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dorit Fabricius, Department of Pediatrics, University of Ulm, Eythstrasse 24, 89075 Ulm, Germany; e-mail: dorit.fabricius@uni-ulm.de; or Bernd Jahrsdörfer, Institute of Pharmacology of Natural Products and Clinical Pharmacology, University of Ulm, Ulm, Germany; e-mail: bernd.jahrsdoerfer@uni-ulm.de.