Abstract
Here we report a unique situation in which an early and synchronized Epstein-Barr virus (EBV) reactivation was induced by a 6-day course of treatment with a humanized CD3-specific monoclonal antibody in patients with recent onset of type 1 diabetes. The virologic and immunologic analysis demonstrated that this reactivation was transient, self-limited, and isolated, associated with the rapid advent of an EBV-specific T-cell response. The anti-CD3 antibody administration induced short-lasting immunosuppression and minor yet clear-cut signs of T-cell activation that preceded viral reactivation. Early posttransplant monitoring of renal and islet allograft recipients showed that no comparable phenomenon was observed after the administration of full-dose immunosuppressive therapy. This EBV reactivation remains of no apparent clinical concern over the long term and should not preclude further development of therapeutic anti-CD3 antibodies. This phenomenon may also direct new research avenues to understand the still ill-defined nature of stimuli triggering EBV reactivation in vivo.
Introduction
Stimuli that trigger Epstein-Barr virus (EBV) reactivation, defined clinically as increased viral load in peripheral blood, are only partially identified, in contrast to our knowledge on the characteristics of EBV primary infection.1 The reactivation of EBV and its consequences have been studied in patients in whom it could be associated with the advent of EBV-related lymphomas. This reactivation essentially occurs in clinical situations associated with chronic immunosuppression secondary either to specific treatments, as in the case of organ or bone marrow transplantation,2-5 or to some chronic viral infections, such as HIV infection,6 a side effect that significantly improved with highly active antiretroviral therapy.7
Here we describe that transient EBV reactivation may occur in a totally different clinical setting, namely, autoimmune patients (ie, recently diagnosed with insulin-dependent diabetes) who received a short (1-week) treatment with a humanized CD3-specific monoclonal ChAglyCD3 antibody.8
The use of anti-CD3 antibodies in autoimmunity is a therapeutic strategy that was first established in the nonobese diabetic mouse model, which spontaneously develops a form of autoimmune insulin-dependent diabetes that closely resembles the human disease. We provided the first evidence demonstrating that when applied to overtly diabetic animals, a short-course low-dose CD3-specific antibody treatment induces durable disease remission as the result of restoration of self-tolerance to target β-cell autoantigens.9-11 Successful translation to the clinic was then achieved in patients presenting with recent-onset type 1 diabetes by the use of 2 different humanized Fc nonbinding CD3-specific antibodies.8,12 In the multicenter study we conducted, 80 patients with new-onset type 1 diabetes were randomly assigned to receive a 6-day course of ChAglyCD3 or placebo.8 ChAglyCD3 antibody treatment proved remarkably efficient in significantly preserving a functional β-cell mass and reducing exogenous insulin needs for at least 18 months after the single-week course.8
Results presented here show that transient EBV reactivation occurred in a majority of patients. In all of them, an EBV-specific immune response spontaneously developed. This observation is of general clinical relevance in the context of all innovative immunointervention strategies based on the use of monoclonal antibodies and fusion proteins targeting relevant lymphocyte receptors mediating cell signaling. These therapeutic tools may potentially induce EBV reactivation, which should not preclude further clinical development as long as the reactivation is controlled through preserved immunocompetence.
Methods
Patients
A phase 2 placebo-controlled study began in June 2000 and included 80 patients with recent-onset type 1 diabetes and treated either with the humanized aglycosylated CD3-specific antibody ChAglyCD3 (40 patients)13 or a placebo (40 patients).8 The first 9 patients (4 treated with ChAglyCD3 and 5 treated with placebo) received a first dose of 24 mg followed by 5 daily infusions of 8 mg. Because of flu-like side effects after the first infusions in the 4 patients who received active treatment, the first dose was reduced.8 Thus, the remaining 71 patients received 6 infusions of 8 mg/day. In March 2007, 65 patients (34 treated with ChAglyCD3 and 31 treated with placebo) were still participating in the study and reached the 4-year follow-up time point; some patients were followed up to 7 years. Blood samples were drawn before treatment (day 0) and after ChAGlyCD3 antibody therapy on days 2, 4, 7; weeks 2, 3, 4, 6; months 3, 6, 12, 18, 24, 36, and 48; and yearly thereafter. Day 2 and day 4 samples were available for cell phenotyping only. This work received approval from the institutional review boards of Vrije University in Brussels, Belgium, and the Institut für Diabetesforshung in Munich, Germany, and all patients gave informed consent in accordance with the Declaration of Helsinki.
Sixty-one additional patients who were either underwent transplantation for allogenic pancreatic islets (11 patients) or renal allograft (50 patients) were studied for EBV viral load in white blood cells during and after induction of immunosuppressive therapy (up to 6 months). In patients who underwent kidney transplantation, this induction included the use of rabbit antithymocyte globulins (ATG; 30 patients) or CD25-specific antibodies, namely basiliximab (14 patients, 20 mg per infusion on day 0 and day 4) or daclizumab (6 patients, 1 mg/kg per infusion on day 0 and day 14). Patients who underwent transplantation with islet allografts received ATG.14 Blood samples were drawn on days 0 (before initiation of immunosuppressive therapy), 4, and 7; weeks 2, 3, 4, and 6; and months 3 and 6. For in vitro studies, tonsils were obtained from children undergoing routine surgical tonsillectomy because of benign breathing-obstructive disorders.
Herpesvirus screening
A screening for common herpesviruses, that is, herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2), cytomegalovirus, EBV, varicella-zoster virus, and human herpesvirus 6 was performed on DNA from white blood cells by qualitative consensus polymerase chain reaction (PCR; Herpes consensus generic kit 67-090 and Hybridowell Herpes identification 67-050; Argene Biosoft).
EBV viral load
Measurement of EBV DNA load was performed on DNA extracted from white blood cells, plasma, or cultures of tonsillar cells through a quantitative (q) real-time PCR assay. This assay amplifies a highly conserved region in the thymidine kinase (BXLF1) gene by the use of a LightCycler instrument (Roche Diagnostics) as previously described.15 EBV DNA was quantified by the use of a serial 10-fold dilution of DNA extracted from Namalwa cells containing 2 integrated copies of EBV genome per cell. The results of quantitation were expressed as log copies per microgram of DNA. The sensitivity of this qPCR assay was of 10 copies per microgram of DNA. The assay has been developed in Grenoble in a laboratory that is a reference center for measurement of EBV viral load in France. It has been tested and validated by several EBV Quality Control for Molecular Diagnostic Proficiency Panels and by clinical studies.1,16 For the detection of free encapsidated viral particles, plasma DNA was treated before qPCR with DNase I as reported elsewhere.17
EBV serology
EBV-specific antibodies to the early antigen (EA; immunoglobulin G [IgG]), the viral capsid antigen (VCA; immunoglobulin M [IgM] and IgG), and the nuclear antigen 1 (EBNA-1) were measured by enzyme-linked immunosorbent assay (Platelia EBV; Bio-Rad Laboratories). Results were expressed as an OD index and considered positive if greater than 1. A 2-fold increase in antibody titer between 2 samples was considered significant.
Enumeration of lymphocyte subsets
Total lymphocytes, CD3+, CD4+, and CD8+ T-cell subsets were enumerated by flow cytometry by the use of the BD Multitest TruCount method (BD Biosciences). Immunophenotyping of T cells was performed with antibodies purchased from BD Biosciences (anti-CD3, -CD4, -CD8, -CD25, -CD69, -HLA-DR, -CD45RA, -CD45RO, and -CD27) and a FACSCalibur cytometer by use of the CellQuest software (BD Biosciences).
Isolation of mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (PAA Laboratories) centrifugation. Suspensions of tonsillar cells were prepared by first mincing surgical pieces of fresh tonsils in 1× phosphate-buffered saline (PBS)/0.5% bovine serum albumin (BSA), passing through a cell strainer, and subsequent Ficoll-Hypaque (PAA Laboratories) centrifugation.
Human leukocyte antigen/peptide multimer staining
Human leukocyte antigen (HLA) class I multimers (phycoerythrin-labeled Pro5 Pentamer) loaded with epitopes derived from EBV proteins expressed during the lytic cycle were purchased from ProImmune and used following the manufacturer's instructions. HLA-A*0201 multimers were loaded with epitopes GLCTLVAML (BMLF1-259-267) or YVLDHLIVV (BRLF1-109-117) and HLA-B*0801 with RAKFKQLL (BZLF1-190-197). In brief, 106 PBMCs were resuspended in PBS 0.1% sodium azide/0.1% BSA and incubated with 10 μL of Pro5 Pentamer for 30 minutes at room temperature. After 2 washes in PBS 0.1% sodium azide/0.1% BSA, cell samples were incubated with anti-CD8 antibody for 20 minutes at 4°C. After a further 2 washes, cells were fixed in PBS/1% fetal calf serum/2.5% formaldehyde for subsequent analysis by flow cytometry on FACSCalibur by use of the CellQuest software (BD Biosciences).
In vitro stimulation of EBV-specific T cells
PBMCs were cultured for 48 hours in the presence of GLC, YVL, or RAK epitopes specific for EBV lytic cycle. Interferon-γ (IFN-γ) was measured in culture supernatants with the use of a Quantikine enzyme-linked immunosorbent assay kit (R&D Systems Europe).
Activation of B lymphocytes
B lymphocytes were negatively selected from peripheral or tonsillar cell suspensions by the use of Dynabeads Untouched Human B Cells (Invitrogen). Autologous blood CD4+ T cells were purified by the use of CD4+ T-Cell Isolation Kit II (Miltenyi Biotec) according to manufacturer's instructions. Peripheral or tonsillar CD19+ B cells were labeled with 4μM carboxyfluorescein succinimidyl ester (CFSE) and cultured for 7 days with autologous irradiated (30 Gy) T cells with medium only, 1 μg/mL ChAGlyCD3 antibody, or 2.5 μ/mL CpG (InvivoGen Europe); 10 μg/mL F(ab′)2 goat anti–human IgM (Jackson ImmunoResearch Laboratories); and 50 UI/mL recombinant interleukin-2 (IL-2; kindly provided by Roussel Uclaf). On day 7, cells were harvested and stained for CD19, CD20, CD38, and immunoglobulin D (IgD). All antibodies used for staining were purchased from BD Biosciences.
Whole suspensions of tonsillar cells were cultured for 7 days in presence or absence of ChAGlyCD3 antibody (1 μg/mL). Cultures were harvested at different time points. Cells were pelleted and immediately frozen at −80°C for subsequent total DNA and total RNA extraction.
Quantitation of EBV protease transcripts
Total RNA from each sample was extracted with the High-Pure-RNA-Isolation-Kit (Roche Diagnostics), and residual DNA was removed by 20-minute incubations (n = 2) with 180 U of DNase I. Late EBV BVRF2 transcripts (coding for EBV protease) were quantified as described previously by use of the hydrolyze probe technology and an external RNA calibration curve with serial dilutions of an in vitro transcript.18 In brief, the reverse-transcription PCR mix (20 μL) included 100 ng of total RNA, 3.25mM Mn(OAC)2 (LightCycler-RNA-Master-Hybridization-Probes-Kit; Roche Diagnostics), 0.3μM each primer, and 0.3μM probe (Sigma-Aldrich). The standard RNA used for absolute quantitation was obtained by in vitro transcription (SP6/T7-Transcription-Kit; Roche Diagnostics) of 1 μg of pSPT18 plasmid containing a 100-bp reverse transcription PCR product from EBV protease mRNA expressed in B95-8 cell line. The results are expressed as EBV mRNA copies per microgram of total RNA.
Results
Transient EBV reactivation after ChAglyCD3 antibody treatment
The monitoring performed is detailed in Table 1.
Summary of the data collected in 40 ChAglyCD3-treated patients
| Patient . | VCA IgG ↗ . | VCA IgM ↗ . | EA IgG ↗ . | Serological EBV reactivation . | Increase of EBV viral load . | CD8 lymphocytosis . | Expansion of EBV-specific CD8+ T cells . |
|---|---|---|---|---|---|---|---|
| ID2 | nd | nd | nd | nd | nd | + | nd |
| ID10 | nd | nd | nd | nd | nd | + | nd |
| ID14 | nd | nd | nd | nd | nd | + | nd |
| ID18 | 0 | 0 | 0 | 0 | nd | 0 | nd |
| ID20 | + | 0 | 0 | + | nd | + | nd |
| ID24 | + | + | + | + | + | + | nd |
| ID27 | 0 | 0 | 0 | 0 | 0 | 0 | nd |
| ID33 | + | 0 | + | + | + | + | nd |
| ID38 | + | 0 | + | + | + | + | nd |
| ID41 | + | + | + | + | + | + | nd |
| ID44 | + | 0 | + | + | + | + | nd |
| ID51 | + | + | 0 | + | + | + | nd |
| ID56 | + | 0 | + | + | + | + | nd |
| ID76 | + | + | 0 | + | + | + | nd |
| ID80 | + | + | + | + | + | + | 0 |
| ID85 | + | 0 | + | + | + | + | 0 |
| ID90 | + | 0 | + | + | nd | + | + |
| ID97 | + | 0 | 0 | + | + | + | + |
| ID100 | + | 0 | 0 | + | + | + | + |
| ID110 | + | 0 | 0 | + | + | + | + |
| ID123 | + | 0 | + | + | nd | + | nd |
| ID124 | + | 0 | + | + | nd | + | nd |
| ID129 | + | 0 | 0 | + | nd | + | + |
| ID137 | + | + | + | + | nd | + | nd |
| ID149 | + | 0 | + | + | nd | + | + |
| ID153 | + | + | 0 | + | nd | + | + |
| ID157 | + | + | + | + | nd | + | + |
| ID159 | + | 0 | 0 | + | + | + | + |
| ID160 | + | 0 | 0 | + | nd | + | nd |
| ID164 | + | + | + | + | + | + | nd |
| ID169 | 0 | 0 | + | + | nd | + | 0 |
| ID176 | + | + | 0 | + | nd | + | + |
| ID179 | + | + | 0 | + | nd | + | nd |
| ID187 | + | 0 | 0 | + | + | + | + |
| ID190 | + | + | 0 | + | nd | + | + |
| ID196 | + | 0 | + | + | + | + | nd |
| ID197 | + | + | 0 | + | nd | + | + |
| ID200 | + | 0 | 0 | + | nd | + | + |
| ID209 | + | 0 | + | + | nd | + | + |
| ID210 | + | 0 | + | + | + | + | + |
| Patient . | VCA IgG ↗ . | VCA IgM ↗ . | EA IgG ↗ . | Serological EBV reactivation . | Increase of EBV viral load . | CD8 lymphocytosis . | Expansion of EBV-specific CD8+ T cells . |
|---|---|---|---|---|---|---|---|
| ID2 | nd | nd | nd | nd | nd | + | nd |
| ID10 | nd | nd | nd | nd | nd | + | nd |
| ID14 | nd | nd | nd | nd | nd | + | nd |
| ID18 | 0 | 0 | 0 | 0 | nd | 0 | nd |
| ID20 | + | 0 | 0 | + | nd | + | nd |
| ID24 | + | + | + | + | + | + | nd |
| ID27 | 0 | 0 | 0 | 0 | 0 | 0 | nd |
| ID33 | + | 0 | + | + | + | + | nd |
| ID38 | + | 0 | + | + | + | + | nd |
| ID41 | + | + | + | + | + | + | nd |
| ID44 | + | 0 | + | + | + | + | nd |
| ID51 | + | + | 0 | + | + | + | nd |
| ID56 | + | 0 | + | + | + | + | nd |
| ID76 | + | + | 0 | + | + | + | nd |
| ID80 | + | + | + | + | + | + | 0 |
| ID85 | + | 0 | + | + | + | + | 0 |
| ID90 | + | 0 | + | + | nd | + | + |
| ID97 | + | 0 | 0 | + | + | + | + |
| ID100 | + | 0 | 0 | + | + | + | + |
| ID110 | + | 0 | 0 | + | + | + | + |
| ID123 | + | 0 | + | + | nd | + | nd |
| ID124 | + | 0 | + | + | nd | + | nd |
| ID129 | + | 0 | 0 | + | nd | + | + |
| ID137 | + | + | + | + | nd | + | nd |
| ID149 | + | 0 | + | + | nd | + | + |
| ID153 | + | + | 0 | + | nd | + | + |
| ID157 | + | + | + | + | nd | + | + |
| ID159 | + | 0 | 0 | + | + | + | + |
| ID160 | + | 0 | 0 | + | nd | + | nd |
| ID164 | + | + | + | + | + | + | nd |
| ID169 | 0 | 0 | + | + | nd | + | 0 |
| ID176 | + | + | 0 | + | nd | + | + |
| ID179 | + | + | 0 | + | nd | + | nd |
| ID187 | + | 0 | 0 | + | + | + | + |
| ID190 | + | + | 0 | + | nd | + | + |
| ID196 | + | 0 | + | + | + | + | nd |
| ID197 | + | + | 0 | + | nd | + | + |
| ID200 | + | 0 | 0 | + | nd | + | + |
| ID209 | + | 0 | + | + | nd | + | + |
| ID210 | + | 0 | + | + | + | + | + |
EA indicates early antigen; EBV, Epstein-Barr virus; IgG, immunoglobulin G; IgM, immunoglobulin M; nd, not determined; VCA, viral capsid antigen; +, present; and 0, absent.
EBV load determination.
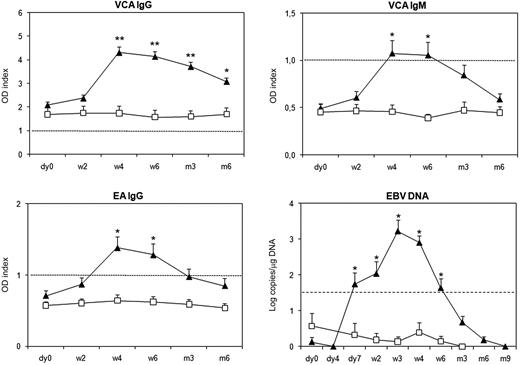
Qualitative PCR on white blood cells was used to assess for herpesviruses, including EBV, HSV-1, HSV-2, cytomegalovirus, varicella-zoster virus, and human Herpesvirus 6. Only EBV viral load was found transiently increased after ChAglyCD3 treatment (data not shown for the other viruses). These results were confirmed by the use of a real-time qPCR.15 Serial samples could be tested in 19 ChAGlyCD3- and 6 placebo-treated patients (Figure 1 and supplemental Figures 1-3, available on the Blood website; see the Supplemental Materials link at the top of the online article). A significant increase in EBV viral load was observed in 18 of 19 patients. EBV copy numbers peaked on day 21 (3.2 ± 0.3 log/μg; mean ± SEM) compared with placebo-treated patients (0.4 ± 0.1 log/μg; P < .001). Peak levels between 4 and 5 log/μg were observed in only 4 patients. Importantly, in all cases, EBV copies rapidly decreased, were back to normal by week 6 to 12, and remained so throughout long-term monitoring.
EBV viral load and EBV-specific humoral response in ChAglyCD3- and placebo-treated patients. Mean (± SEM) titers expressed as OD indexes of VCA IgG, VCA IgM, and EA IgG are shown for patients treated with ChAGlyCD3 (▴) or placebo (□). Black dotted lines represent the cut-off value for positive detection. Mean (± SEM) EBV copy numbers expressed as log/μg DNA are also shown for the 2 groups of patients. The threshold of 1.5 log EBV copies/μg DNA (mean value found in all patients at baseline ± 3 SD) is represented by a dotted line. *P < .05; **P < .001.
EBV viral load and EBV-specific humoral response in ChAglyCD3- and placebo-treated patients. Mean (± SEM) titers expressed as OD indexes of VCA IgG, VCA IgM, and EA IgG are shown for patients treated with ChAGlyCD3 (▴) or placebo (□). Black dotted lines represent the cut-off value for positive detection. Mean (± SEM) EBV copy numbers expressed as log/μg DNA are also shown for the 2 groups of patients. The threshold of 1.5 log EBV copies/μg DNA (mean value found in all patients at baseline ± 3 SD) is represented by a dotted line. *P < .05; **P < .001.
In 9 patients, plasma samples from day 0, week 2, and week 4 could be retrieved and used for the detection of DNase-resistant infectious viral particles. We did not detect any encapsidated virus at any time point except in 2 patients (2.5 log copies/mL on week 2 in 1 case and 2.8 log copies/mL on week 4 in the other).
Clinical symptoms.
Clinical symptoms are shown in Table 2. A single ChAglyCD3-treated patient experienced clinical signs typical of EBV reactivation, that is, cervical adenopathy and fever that lasted for 12 days and required corticotherapy. Fifteen other patients presented transient and moderate symptoms (lasting a few days) for which symptomatic treatment was instituted. These patients described sore throat (15 of 15), enlargement of 1 to 3 cervical lymph nodes (7 of 15), and hyperthermia (4 of 15). Table 2 also includes patients in whom symptoms were scored as mild because no palliative treatment was administered; this pattern was also observed in some placebo-treated patients. Long-term follow-up (48 to 84 months) showed that none of the ChAglyCD3-treated patients developed clinical or biologic evidence of EBV reactivation.
Incidence of clinical symptoms in ChAglyCD3-treated patients at the time of EBV reactivation as compared with placebo-treated patients in whom no EBV reactivation was observed
| Severity . | ChAglyCD3 . | Placebo . | ||||
|---|---|---|---|---|---|---|
| Mild (grade 1) . | Moderate (grade 2) . | Severe (grade 3) . | Mild (grade 1) . | Moderate (grade 2) . | Severe (grade 3) . | |
| Hyperthermia | 7 | 4 | 1 | 0 | 1 | 0 |
| Cervical lymph node enlargement | 2 | 7 | 1 | 3 | 0 | 0 |
| Sore throat | 13 | 15 | 1 | 1 | 2 | 0 |
| Severity . | ChAglyCD3 . | Placebo . | ||||
|---|---|---|---|---|---|---|
| Mild (grade 1) . | Moderate (grade 2) . | Severe (grade 3) . | Mild (grade 1) . | Moderate (grade 2) . | Severe (grade 3) . | |
| Hyperthermia | 7 | 4 | 1 | 0 | 1 | 0 |
| Cervical lymph node enlargement | 2 | 7 | 1 | 3 | 0 | 0 |
| Sore throat | 13 | 15 | 1 | 1 | 2 | 0 |
Symptoms were scored as mild, moderate, or severe according to the Common Terminology Criteria for adverse events, Version 3.0, Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, Department of Health and Human Services (March 31, 2003; http://ctep.cancer.gov), August 9, 2006. Grades refer to the severity of the adverse event and range from 1 to 5. None of the patients in this study experienced an adverse event of grade 4 (life threatening or disabling). Mild symptoms were spontaneously reversible (no treatment), and only symptomatic treatment was applied, when needed, in case of moderate symptoms.
EBV-specific antibody response in ChAGlyCD3 antibody-treated patients
EBV-specific antibodies were monitored in 37 of the 40 ChAglyCD3-treated patients. An increase in VCA IgG titers was observed in 34 patients, associated with increased titers of VCA IgMs and EA IgGs, or VCA IgMs only, or EA IgGs only in 6, 7, and 12 cases, respectively (Figure 1 and supplemental Figure 1; supplemental Table 1). Antibody levels generally peaked on day 28 after initiation of ChAglyCD3 therapy or later by day 42 in 6 patients. Two patients (ID18 and ID27) did not show any serologic evidence of EBV reactivation. In 9 placebo-treated patients monitored for EBV serology, antibody titers remained stable throughout the first 6 months of follow-up (Figure 1 and supplemental Figure 2).
EBV-specific CD8+ T-cell response in ChAglyCD3 antibody-treated patients
A CD8 lymphocytosis (833-12 303 cells/μL), maximal on day 21 to 28, was observed in 38 of the 40 antibody-treated patients (Figure 2). Only 2 patients (ID18 and ID27) did not show any significant change in CD8+ T-cell counts.
The EBV-specific CD8+ T-cell response in ChAglyCD3-treated patients. Each panel represents the CD8+ T-cell counts (curve, right y-axis) and the percentage of HLA/peptide multimer-positive cells among CD8+ T cells (histograms, left y-axis). Dotted lines indicate the absence of sampling for CD8+ T-cell counts at certain time points. nd indicates multimer analysis not performed.
The EBV-specific CD8+ T-cell response in ChAglyCD3-treated patients. Each panel represents the CD8+ T-cell counts (curve, right y-axis) and the percentage of HLA/peptide multimer-positive cells among CD8+ T cells (histograms, left y-axis). Dotted lines indicate the absence of sampling for CD8+ T-cell counts at certain time points. nd indicates multimer analysis not performed.
Nineteen HLA-A2– and/or HLA-B8–positive ChAGlyCD3-treated patients were assessed for EBV-specific CD8+ T cells detected with HLA/peptide multimers (Figure 2).19,20 Maximal proportions of multimer-positive CD8+ T cells also were found on day 21 to 28, correlating with the initiation of EBV viral load decrease (Figure 1 and supplemental Figure 3).
The frequency of HLA-B*0801-RAK–specific CD8+ T cells in 12 HLA-B8–positive patients ranged from 0.05% to 6.55% (median, 0.91%) at baseline and reached 8.33% to 41.6% of CD8+ T cells (median, 11.95%) by day 21 to 28. Then, HLA-B*0801-RAK–specific T cells progressively decreased (10-fold decrease on average between day 28 and month 6). In 1 patient (ID169), no increase in HLA-B*0801-RAK–specific T cells was observed at any time point during follow-up, although a slight increase in CD8+ T-cell counts and serologic evidence of EBV reactivation were found (Table 1).
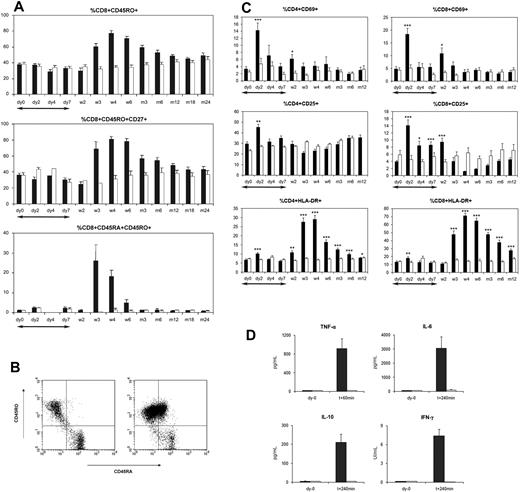
HLA-A*0201–restricted EBV-specific CD8+ T cells were studied in 12 ChAglyCD3-treated patients. Before therapy, HLA-A*0201-GLC–restricted T cells represented 0.2% to 4.8% of CD8+ subset (median, 0.85%) and HLA-A*0201-YVL–restricted T cells 0.3% to 1.4% of CD8+ subset (median, 0.6%). In 7 patients analyzed at the time of the CD8 lymphocytosis, significant expansions of EBV-specific T cells were observed, ranging from 1.6% to 3.8% (median, 2.5%) for HLA-A*0201-GLC–specific T cells and from 1.5% to 10% (median, 2.0%) for HLA-A*0201-YVL–specific T cells. CD8+ T-cell expression of both activation markers HLA-DR and CD45RO increased at the time of lymphocytosis (Figure 3). Most of the CD8+ T cells were HLA-DR+CD45RO+CD27+. A subset of CD8+ T cells transiently expressed high levels of both CD45RO and CD45RA at their surface (Figure 3B).
Phenotype of T cells in ChAglyCD3- and placebo-treated patients. (A) Median percentages of CD45RO+, CD45RO+CD27+, and CD45RA+CD45RO+ cells among CD8+ T cells in ChAglyCD3-treated (■) and placebo-treated (□) patients. (B) Flow cytometric analysis of CD45RA and CD45RO expression on gated CD8+ T cells in a representative ChAglyCD3-treated patient (ID157) on day 0 (left) and day 28 (right) after antibody therapy. (C) Mean percentages of CD4+ (left) and CD8+ (right) T cells expressing CD69, CD25, and HLA-DR in ChAGlyCD3- (■) and placebo-treated (□) patients up to month 12. *P < .05, **P < .001, ***P < .001 (comparison between ChAGlyCD3- and placebo-treated patients at each time point). (D) Mean (±SEM) serum concentrations of TNF-α, IL-6, IL-10, and IFN-γ at baseline (day 0) and after the first antibody infusion (T + 60 minutes for TNF-α, T + 240 minutes for IL-6, IL-10, and IFN-γ) in patients treated either with ChAGlyCD3 (■) or placebo (□). In panels A and C, the duration of ChAGlyCD3 therapy is represented by ↔.
Phenotype of T cells in ChAglyCD3- and placebo-treated patients. (A) Median percentages of CD45RO+, CD45RO+CD27+, and CD45RA+CD45RO+ cells among CD8+ T cells in ChAglyCD3-treated (■) and placebo-treated (□) patients. (B) Flow cytometric analysis of CD45RA and CD45RO expression on gated CD8+ T cells in a representative ChAglyCD3-treated patient (ID157) on day 0 (left) and day 28 (right) after antibody therapy. (C) Mean percentages of CD4+ (left) and CD8+ (right) T cells expressing CD69, CD25, and HLA-DR in ChAGlyCD3- (■) and placebo-treated (□) patients up to month 12. *P < .05, **P < .001, ***P < .001 (comparison between ChAGlyCD3- and placebo-treated patients at each time point). (D) Mean (±SEM) serum concentrations of TNF-α, IL-6, IL-10, and IFN-γ at baseline (day 0) and after the first antibody infusion (T + 60 minutes for TNF-α, T + 240 minutes for IL-6, IL-10, and IFN-γ) in patients treated either with ChAGlyCD3 (■) or placebo (□). In panels A and C, the duration of ChAGlyCD3 therapy is represented by ↔.
Proportions of HLA-B*0801-RAK–restricted CD8+ T cells expressing HLA-DR and CD45RO were 43% to 85% (median, 55%) and 47% to 100% (median, 85.7%), respectively, before therapy, and increased up to 92% to 99% and 88% to 99%, respectively, between days 21 and 28. Similar patterns of HLA-DR and CD45RO expression were observed for HLA-A*0201-GLC– and -YVL–restricted T cells.
The capacity of EBV-specific T cells to produce IFN-γ after stimulation with the RAK, GLC, and YVL EBV epitopes was examined. Before therapy, in vitro stimulation of PBMCs did not induce any IFN-γ production. In contrast, IFN-γ production (up to 2000 UI/mL after 48 hours of culture) was detected in PBMCs of antibody-treated patients at late time points, that is, months 3, 6, and 12, but not on day 28 at the peak of the EBV-specific CD8+ T-cell expansion. One possible interpretation for this may be the occurrence of apoptosis in highly activated specific T cells in the presence of the cognate antigen in vitro.
Biologic effects of ChAGlyCD3 antibody treatment
Transient cytokine release after the first injections of ChAglyCD3.
As previously reported,8 the first ChAGlyCD3 injections induced a rapid but transient cytokine release (Figure 3D and supplemental Figure 4). In all patients, cytokine levels were back to normal before the third antibody infusion. IL-6 levels were constantly found increased (> 250 pg/mL). By contrast, the increase of tumor necrosis factor-α (TNF-α), IFN-γ, and IL-10 concentrations varied among patients and was generally moderate or even absent.
Activation markers are expressed within the first few days of ChAglyCD3 treatment.
During the first days of ChAglyCD3 therapy, an increased expression of CD69 and CD25 on CD4+ and CD8+ T cells was observed (Figure 3C). The expression of HLA-DR, a late activation marker, did not significantly change during the first week of therapy.
ChAGlyCD3 therapy is associated with transient lymphopenia and CD3/TCR down-modulation.
As previously described, ChAglyCD3 treatment induced a transient decrease in peripheral CD2+ lymphocyte counts, most pronounced on day 2 and day 14 with partial recovery in between.8 During the first week, when circulating ChAGlyCD3 antibody was detected, the CD3/T-cell receptor (TCR) complex was down-modulated (T cells were CD2+CD3−CD4+ or CD2+CD3−CD8+).8 By day 14, the level of CD3/TCR expression on T cells was back to normal. In patients in whom it could be measured, a diminished, yet not totally abrogated, proliferative response to tetanus toxoid was found on day 7 compared with day 0 (median proliferation index, 259 on day 0 vs 25 on day 7).
EBV viral load in recipients of islet or renal allografts
Monitoring of EBV viral load was performed in 61 transplanted patients treated with monoclonal antibodies to CD25 (basiliximab or daclizumab, n = 20) or antithymocyte globulins (ATG, n = 41), in combination with conventional immunosuppressive regimen. A total of 432 samples from day 0 up to month 6 after transplantation were analyzed. No significant increase of EBV copy numbers compared with baseline was detected in any of the 3 subgroups of patients (supplemental Figure 5A). However, 4 patients (6.5%) showed a transient increase of EBV viral load above 3 log copies/μg early after transplantation (between day 8 and day 14). Interestingly, all of them had received ATG as part of their induction therapy. EBV copy numbers peaked between day 8 and day 21 then decreased down to pretreatment levels by month 3. In addition, all these 4 patients had detectable EBV load on day 0 (1.6-2.9 log copies/μg; supplemental Figure 5B).
In vitro effects of ChAglyCD3 on peripheral and tonsillar B cells
To address more directly the potential capacity of ChAglyCD3 to promote EBV reactivation, we established an in vitro model. In a first series of experiments we used B and T lymphocytes recovered from peripheral blood of EBV+ normal volunteers. In a second step, we moved to tonsil B cells surgically recovered from EBV+ subjects.
ChAGlyCD3-induced activation of peripheral B lymphocytes.
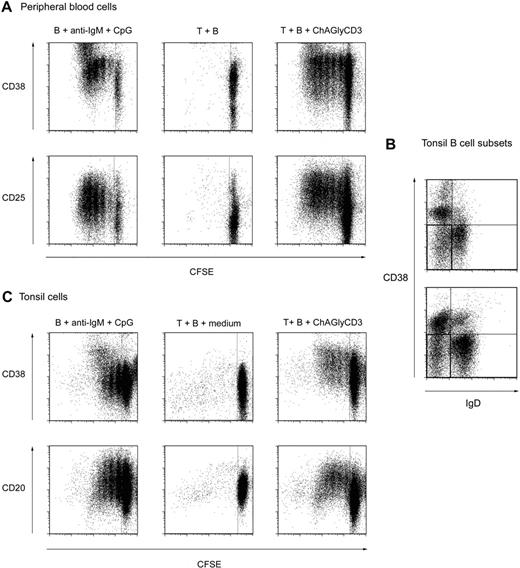
Cocultures of B lymphocytes and autologous T cells were performed in presence or absence of ChAglyCD3. The in vitro activating capacity of CD3-specific antibodies in general and ChAglyCD3 in particular is dependent on adequate CD3/TCR cross-linking achieved through immobilization of the antibody through its Fc portion (binding to Fc receptors on monocyte/macrophages or to plastic surface).11 As shown in Figure 4A, using peripheral blood mononuclear cells, we observed that ChAGlyCD3 antibody-treated T cells could provide bystander help to autologous B cells, inducing their activation and proliferation but without any detectable production of EBV copies (data not shown). One interpretation for this may be the very low frequency of latently infected memory B cells in the peripheral blood of healthy carriers (∼ 1 per 106 lymphocytes).21,22
Bystander T cells help provides activation signals for peripheral and tonsillar B cells. (A) Purified CD19+ peripheral blood B cells were CFSE-labeled and cultured for 7 days in the presence of CpG, anti–human IgM, and IL-2 (left) or irradiated autologous peripheral CD4+ T cells preactivated (right) or not (middle) with ChAGlyCD3 antibody. The CFSE dilution profiles of B cells (CD19+ gate) stained on day 7 for CD38 (top) or CD25 (bottom) are shown. (B) Tonsillar B-cell subsets in 2 representative tonsils as assessed by staining with CD19, CD20, CD38, and IgD antibodies. The quadrant corresponding to memory B cells is highlighted as the IgD-negative, CD38-negative/low subset (CD19+CD20+ gate). (C) Purified CFSE-labeled CD19+ tonsillar B cells were cultured for 7 days in the presence of CpG, anti–human IgM, and IL-2 (left) or irradiated autologous peripheral CD4+ T cells preactivated (right) or not (middle) with ChAGlyCD3 antibody. The CFSE dilution profiles of B cells (CD19+ gate) stained on day 7 for CD38 (top) and CD20 (bottom) are shown.
Bystander T cells help provides activation signals for peripheral and tonsillar B cells. (A) Purified CD19+ peripheral blood B cells were CFSE-labeled and cultured for 7 days in the presence of CpG, anti–human IgM, and IL-2 (left) or irradiated autologous peripheral CD4+ T cells preactivated (right) or not (middle) with ChAGlyCD3 antibody. The CFSE dilution profiles of B cells (CD19+ gate) stained on day 7 for CD38 (top) or CD25 (bottom) are shown. (B) Tonsillar B-cell subsets in 2 representative tonsils as assessed by staining with CD19, CD20, CD38, and IgD antibodies. The quadrant corresponding to memory B cells is highlighted as the IgD-negative, CD38-negative/low subset (CD19+CD20+ gate). (C) Purified CFSE-labeled CD19+ tonsillar B cells were cultured for 7 days in the presence of CpG, anti–human IgM, and IL-2 (left) or irradiated autologous peripheral CD4+ T cells preactivated (right) or not (middle) with ChAGlyCD3 antibody. The CFSE dilution profiles of B cells (CD19+ gate) stained on day 7 for CD38 (top) and CD20 (bottom) are shown.
ChAGlyCD3-induced activation of tonsil B lymphocytes.
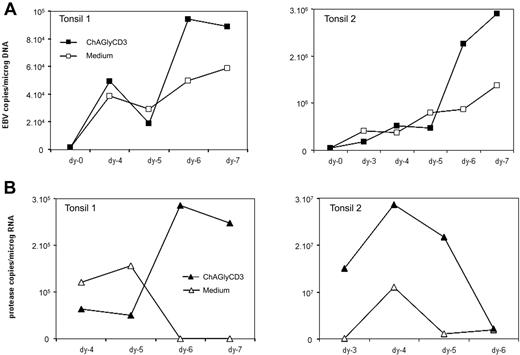
We then chose to study the in vitro effect of ChAGlyCD3 antibody-treated T cells on tonsil B cells isolated from EBV latently infected subjects. In fact, compared with peripheral blood, tonsils are particularly enriched in memory B cells (Figure 4B) that are the privileged reservoir of the virus.23 Using tonsil B cells, we confirmed the data obtained with peripheral B lymphocytes. Thus, as presented in Figure 4C, ChAGlyCD3 antibody-treated T cells provided bystander help to autologous tonsil B cells, promoting their activation, proliferation, and also their differentiation in plasmablasts with loss of CD20 expression and increase of CD38 expression. We then cultured whole suspensions of EBV-positive tonsillar cells containing both T and B cells (T/B ratio ranging from 0.25 to 1) for 7 days with or without ChAGlyCD3 antibody, and we quantified both intracellular EBV DNA and EBV protease mRNA transcripts, which are exclusively expressed at late stages of EBV lytic cycle (Figure 5), thus representing a reliable marker of complete EBV lytic cycle.24 We found that in the presence of ChAglyCD3, an enhancement of EBV copy viral load in the culture correlated with greater expression of protease mRNA.
Effect of ChAGlyCD3 antibody on tonsillar B cells in vitro. Whole suspensions of mononuclear cell prepared from tonsillectomies performed in EBV-positive subjects were cultured for 7 days in the presence of ChAGlyCD3 antibody or medium. (A) EBV viral load was quantified by qPCR. Results are expressed as numbers of EBV DNA copies per micrograms of cellular DNA. (B) EBV protease transcripts, induced during the lytic cycle of the virus, were quantified. Results are expressed as numbers of mRNA copies per micrograms of total RNA. Data from 2 representative tonsils are shown.
Effect of ChAGlyCD3 antibody on tonsillar B cells in vitro. Whole suspensions of mononuclear cell prepared from tonsillectomies performed in EBV-positive subjects were cultured for 7 days in the presence of ChAGlyCD3 antibody or medium. (A) EBV viral load was quantified by qPCR. Results are expressed as numbers of EBV DNA copies per micrograms of cellular DNA. (B) EBV protease transcripts, induced during the lytic cycle of the virus, were quantified. Results are expressed as numbers of mRNA copies per micrograms of total RNA. Data from 2 representative tonsils are shown.
Discussion
Our present data demonstrate that a transient EBV reactivation occurred in 35 of 37 type 1 insulin-dependent diabetic patients who were all healthy carriers of the virus at the time of inclusion and who received a short and single course treatment with a humanized CD3-specific monoclonal antibody, ChAglyCD3.8 In these patients, reactivation was detected only for EBV and not for other herpesviruses. A unique feature of this EBV reactivation was its synchronized kinetics. Increased EBV copy numbers were observed as early as day 7 after the first ChAglyCD3 injection, peaked generally on day 21, and were back to normal by month 3. In terms of clinical symptoms, a single patient experienced typical cervical adenopathy and fever that lasted for 12 days. In 15 other patients, careful interrogation and examination revealed transient symptoms that resolved within a few days and did only require symptomatic medication. Patients were followed for 4 to 7 years, and no other biologic or clinical signs of EBV reactivation or EBV-related disease have been observed.
During the reactivation, EBV replication was indirectly suggested by an increase in EA or VCA IgG titers (targeting respectively early and late antigens expressed during the lytic cycle), the appearance of VCA IgM, and the expansion of CD8 T cells recognizing early lytic antigens. Infectious encapsidated virus was found in the plasma of only 2 of 9 tested patients, demonstrating that, in some cases, viral replication might have taken place in the peripheral blood and suggesting that in most cases viral burden in leucocytes was accounted for by increased numbers of latently infected cells. Control of viral reactivation occurred spontaneously, as assessed by the rapid decrease of cellular EBV load in all patients. This finding correlated with the rapid advent of a specific CD8+ T-cell response. A CD8 lymphocytosis was observed during or shortly after the peak of reactivation and the expanded and activated (HLA-DR+) CD8+ T-cell pool included significant proportions of detectable EBV-specific T cells (up to 41.6%). Increased proportions of an unusual bright double-positive CD45RA+CD45RO+ CD8+ subset were observed. Such cells are present in normal human adenoids where they have been shown to be in S or G2/M phase of the cell cycle in vivo, indicating recent activation.25
The correlation between expansion of CD8+ T cells specific for lytic EBV antigens and decrease of EBV copies argues for the cytolytic activity of these CD8+ T cells, which is in keeping with the detected IFN-γ secretion in response to in vitro stimulation with the cognate peptides.20 However, there is evidence that CD8+ T-cell responses against lytic cycle antigens might not be very effective at target lysis because of viral mechanisms interfering with antigen presentation, such as TAP inhibition and surface HLA class I down-regulation.26 A role for natural killer cells in controlling EBV replication has been proposed on the basis of the correlation between sensitization to natural killer lysis of cell lines switching to lytic cycle and down-regulation of MHC class I molecules.27 CD8+ T-cell responses to latent antigens, which could not be analyzed in our study, also may participate to the control of reactivation by killing newly infected B cells expressing latent antigens and reducing the input in newly generated latently infected B cells into the blood compartment and subsequently EBV DNA cellular load.28
A phase 1/2 trial in recent type 1 diabetes also has been conducted with the use of another humanized CD3-specific antibody, that is, teplizumab (OKT3γ1 Ala-Ala), which included children and young adults (7-27 years).12,29 A CD8+ T-cell lymphocytosis also was observed in these patients that was ascribed to an effect of the antibody favoring expansion of CD8+ T cells expressing FoxP3 and endowed with regulatory properties.30 In none of these patients was any EBV-related symptoms reported, and EBV viral load or EBV-specific immune responses was not monitored.12,29 It must be noted that because of the younger age of the population in the US trial, only part of the patients were likely to be EBV carriers and hence potential candidates for reactivation. The presence of EBV-specific T cells within the expanded CD8+ T-cell pool is not incompatible with the presence of regulatory CD8+ T cells. Importantly, the cumulated dosage of teplizumab was lower than that used in our trial,8,12,29 which may explain the differences observed in terms of EBV reactivation. This difference may also explain that even if EBV reactivation occurred in a few patients from the US trial, it went clinically undetected. On the basis of this experience, further trials with these nonmitogenic CD3-specific antibodies should consider use of lower cumulated dosages to avoid such side effects.
One explanation for the EBV reactivation observed after CD3 antibody therapy is the transient immunosuppression induced by ChAGlyCD3. Although CD3 antibodies do not promote massive T-cell depletion, a lymphopenia is observed during treatment that is mostly the result of redistribution and marginalization of T cells, explaining the rapid reconstitution at the end of therapy.8,9,11 Remnant T cells undergo antigenic modulation of CD3/TCR, namely a redistribution of the complex at the T-cell membrane followed by internalization, which renders the cell “blind” to antigen recognition and functionally unresponsive.11,31,32 Antigenic modulation is fully reversible approximately 12 hours after the end of T-cell exposure to the antibody.11,31-33 This finding fits with both experimental and clinical data showing that when CD3 antibodies are administered alone, their immunosuppressive activity is rapidly reversible.10,11,34 In our patients, the ChAglyCD3 antibody had entirely disappeared from the circulation by 1 week after the last injection8 and lymphocytes counts as well as CD3 expression levels were back to normal by week 2 to 3.8 In keeping with the role of transient immunosuppression on its own in promoting viral reactivation, a short treatment with depleting CD45 antibodies was recently shown to induce 1 week later an increase in EBV viral load, which was subsequently controlled by the infusion of EBV-specific cytotoxic T lymphocytes.35
To further assess the contribution of immunosuppression on its own in the development of EBV reactivation, we monitored a series of 61 renal and islet allograft recipients during the early posttransplant period after induction plus full-dose triple therapy. These patients received either CD25 monoclonal antibodies or antilymphocyte serum, in association with FK506, corticosteroids, and mycophenolate. This combination represents a stronger and more durable immunosuppressive regimen compared with the 6-day course ChAGlyCD3 antibody treatment. In this setting, no EBV reactivation was detected in 57 of 61 patients. In only 4 of the kidney allograft recipients, a moderate and very transient EBV reactivation was observed on day 8 after transplant, which resolved within 1 week. These results indicate that intense immunosuppression is not systematically associated with early EBV reactivation and that, in the context of CD3 antibody therapy and on the basis of the data in the literature, additional mechanisms could be envisaged.
One particularity of ChAGlyCD3 antibody compared with other anti–T-cell antibodies lies in its immune-activating properties that, although significantly weaker than those of mitogenic CD3 antibodies such as OKT3,36,37 are still detectable. Typical activation markers, CD25 and CD69, were transiently observed on T lymphocytes within the first days of ChAglyCD3 treatment. In addition, measurable concentrations of TNF-α, IFN-γ, IL-10, and IL-6 were detected in all treated patients with variable patterns. Of note, the only transplanted patients in whom moderate increase of EBV viral load was detected had received ATG as induction therapy, a strong immunosuppressant that exhibits like ChAGlyCD3 immune cell activating properties (ie, in vivo cytokine release).38
The potential implication of T-cell activation in EBV reactivation is in keeping with our in vitro data showing that ChAglyCD3-activated T cells may provide bystander help to autologous tonsil B cells, promoting their activation, proliferation, and differentiation in plasmablasts. In these conditions, we could detect an increase in both EBV copies and EBV protease mRNA transcripts that are exclusively expressed at late stages of EBV lytic cycle and represent a marker for viral replication.24 However, further studies are still needed to unravel the underlying mechanisms of reactivation in these in vitro experiments. One may quote interactions between activated T cells and B cells via CD40L-CD40 costimulation.23,39 In this vein, EBV-specific CD4+ T cells were shown to provide non–HLA-restricted help for activation of resting B cells through the CD40–CD40L pathway, inducing expression of the early lytic transcript BZLF1 in latently infected B cells.40 In addition, T cell–derived cytokines such as IL-21 also may participate in the induction of the EBV lytic cycle.41
In the clinic, the role of lymphocyte activation in promoting EBV reactivation has been very well dissected in conditions such as HIV infection,42,43 primary cytomegalovirus infection,44 and systemic lupus erythematosus,45 a nonorgan-specific autoimmune disease. The case of HIV infection is particularly relevant to our discussion because it involves a major polyclonal B-46 and T-cell activation,47 also associated with increased cytokine levels.48 A greater EBV load is found in HIV-infected patients.42,43 One obvious explanation would be the HIV-related immunodeficiency. However, compelling evidence has been accumulated, notably from longitudinal studies in HIV-seroconverting subjects, demonstrating that the increase in EBV loads occurs early during HIV infection.49 Moreover, EBV load is similar in HIV+ patients progressing or not to full-blown AIDS and does not inversely relate to the decrease in CD4+ T-cell counts.49 Interestingly, changes in EBV viral load early after seroconversion were recently shown to correlate with several markers of CD4+ and CD8+ T-cell activation, suggesting that immune activation during HIV infection may be an essential factor leading to increase in EBV DNA load.50
To conclude, these data highlight that there may be many situations, in particular after the administration of “signal triggering” monoclonal antibodies or fusion proteins targeting key lymphocyte receptors, in which the induced early immune activation may participate, together with some degree of immunosuppression, in promoting transient EBV reactivation. However, this should not preclude further clinical development of these promising tools as long as the reactivation is controlled for the long term through preserved immunocompetence.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are indebted to M. J. Devaud, A. Leclerq, L. Vendrame, M. Bensaïd, and V. Sauvaget for skillful technical assistance and to Dr C. De Block for participation to the enrolment and follow-up of diabetic patients. We also thank Dr M. Baccard for assistance in the monitoring of EBV-specific antibodies; Dr Sylvie Larrat, Pr Patrice Morand, and Dr Raphaëlle Germi for assistance in quantitative PCR assays and helpful discussions; and Pr A. Fisher for critical review of the manuscript. The laboratory Inserm U580 and the Service de Transplantation Rénale Adulte of Necker Hospital are part of the Centaure network.
This work was supported by grants from Inserm, Fondation Day Solvay, and the Juvenile Diabetes Research Foundation.
Authorship
Contribution: B.K. acted as clinical coordinator and critically read the manuscript; S.C. and L. Chatenoud designed research, collected, analyzed, and interpreted data, and wrote the manuscript; S.F.-K., M.L.-V., and D.P. contributed analytical tools; A.Z. and C.M. acted as clinicians and critically read the manuscript; E.V., M.W., L. Crenier, E.T., and C.L. acted as clinicians; G.H., H.W., J.-F.B., and D.P. critically read the manuscript; and J.M.S. contributed analytical tools and critically read the manuscript.
Conflict-of-interest disclosure: H.W. and G.H. are cofounders of Tolerx Inc, the company developing the ChAGlyCD3 antibody (also known as TRX4), and recipients of royalties associated with the development of TRX4. H.W. is a director and chairman of the scientific advisory board of Tolerx Inc. The remaining authors declare no competing financial interests.
Correspondence: Pr Lucienne Chatenoud, Inserm U580, Hôpital Necker, 161 rue de Sèvres, 75015 Paris, France; e-mail: lucienne.chatenoud@inserm.fr.
References
Author notes
B.K. and S.C. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal