Abstract
T-cell development in the thymus depends on continuous supply of T-cell progenitors from bone marrow (BM). Several extrathymic candidate progenitors have been described that range from multipotent cells to lymphoid cell committed progenitors and even largely T-lineage committed precursors. However, the nature of precursors seeding the thymus under physiologic conditions has remained largely elusive and it is not known whether there is only one physiologic T-cell precursor population or many. Here, we used a competitive in vivo assay based on depletion rather than enrichment of classes of BM-derived precursor populations, thereby only minimally altering physiologic precursor ratios to assess the contribution of various extrathymic precursors to T-lineage differentiation. We found that under these conditions multiple precursors, belonging to both multipotent progenitor (MPP) and common lymphoid progenitor (CLP) subsets have robust T-lineage potential. However, differentiation kinetics of different precursors varied considerably, which might ensure continuous thymic output despite gated importation of extrathymic precursors. In conclusion, our data suggest that the thymus functions to impose T-cell fate on any precursor capable of filling the limited number of progenitor niches.
Introduction
T-cell development in the thymus depends on continuous supply of T-cell progenitors from bone marrow (BM) via the circulation. In the thymus T-cell precursors pass through a series of defined developmental stages, with the most immature thymocytes residing in the double-negative (DN) subset, characterized by the absence of the surface markers CD4 and CD8. Thymocyte differentiation then proceeds through the CD4+CD8+ double-positive (DP) stage, after which thymocytes become either CD4 or CD8 single positive (SP) and leave the thymus to enter the mature T-cell pool. The most immature T-cell progenitors in the thymus are lineage negative (lin−), CD44+, CD25−, Sca-1high, CD117 (c-kit)high (LSK), and CD127−/lo (IL-7Rα) early T-lineage progenitors (ETPs),1 which constitute a subfraction of the CD44+CD25− DN1 population. These cells were shown to have high T but only limited B and some myeloid potential.2,3 ETPs could be further subdivided according to their expression levels of CD135 (Fms-like tyrosine kinase receptor 3 [Flt3])4 and loss of CD135 expression correlated with loss of B-cell potential.
Like all hematopoietic lineages T cells are ultimately derived from hematopoietic stem cells (HSCs) residing in BM. HSCs can generate CD135+ multipotent progenitors (MPPs), which are likewise of the LSK phenotype,5 as well as more committed precursors such as RAG-1–positive early lymphoid progenitors (ELPs)6 and L-selectin–positive progenitors (LSPs),7 both of which constitute subsets of MPPs. Common lymphoid progenitors (CLPs),8 which are lin−Sca-1+CD117+/loCD127+CD135+, and lin−Sca-1+CD117−CD127+CD135+B220+ CLP-29 differ from MPP subsets in their lack of myeloid potential,10 and lin−Sca-1+CD117+/loCD127+CD90+ circulating T-lineage committed progenitors (CTPs) lack even B-lineage potential.11
Despite extensive characterization of these progenitor populations, the nature of precursors seeding the thymus under physiologic conditions has remained largely elusive. Furthermore, it is not known whether there is only one physiologic T-cell precursor population or many.12 Based on phenotypic similarity to ETPs, multipotent LSK cells or a subfraction thereof in blood has been suggested to constitute physiologic thymic immigrants in adult mice.1,13,14 Furthermore, it has recently been reported that ETPs, on a clonal level, could generate both T and myeloid cells and might contribute considerably to the pool of thymic macrophages, suggesting a closer relationship of ETPs to multipotent progenitors than to lymphoid-restricted populations, such as CLPs.2,3 However, ETPs differ from circulating LSK cells with respect to their dependence on Notch signals: Abrogation or reduction of Notch signals led to a massive reduction in ETP numbers, whereas BM and blood LSK cells were not affected, suggesting that Notch signals are required for the generation of ETPs, but not LSK cells.4,15
CLPs or CLP-2 cells may also constitute populations of thymic progenitors.9,16-18 CLP-2 cells, originating from CLPs, efficiently enter the thymus upon intravenous transfer and give rise to a single wave of T cells, indicating limited self-renewal capacity.9,19 Both CLPs and CLP-2 cells progress developmentally along the T lineage in vitro with kinetics similar to ETPs, whereas MPPs display delayed developmental progression compared with ETPs.20 Although CLP-2 cells, in contrast to CLPs, have not yet been detected in the circulation under steady-state conditions, it has been shown that early thymic immigrants after BM transfer are CD117− and enriched for B220+ cells.21 It has been suggested that ETPs constitute the central pool of intrathymic progenitors from which the majority of cells of later developmental stages and, ultimately, mature T cells originate,22,23 thus arguing against CLPs and CLP-2 cells as physiologic T-cell precursors based on phenotypical differences compared with ETPs. However, we have demonstrated earlier that extrathymic precursors display a marked phenotypic plasticity upon exposure to Notch signals,20 and others showed that, upon intrathymic or intravenous transfer, CLPs readily assume an ETP phenotype.17
CTPs isolated from peripheral blood of adult mice are largely T-lineage committed and lack robust B and myeloid potential. These cells were shown to settle the thymus and give rise to T cells with rapid kinetics.11 However, a bone marrow–resident precursor of CTPs has not been characterized yet.
Although many candidate T-cell precursors have been characterized extensively, the contribution of each of these precursor populations to T-lineage differentiation in the presence of physiologic ratios of other candidate precursor populations has not been analyzed so far. Most in vivo experiments to analyze T-lineage potential and thymus homing have been based on adoptive transfer of highly enriched precursor cells resulting in a competitive advantage of such cells over endogenous precursors usually present at low numbers in peripheral blood. Thus, the physiologic role of candidate precursors might be overestimated in certain cases.
In this study, we developed an assay based on depletion rather than enrichment of classes of BM-derived precursor populations. Lin− BM cells were depleted of candidate precursor populations and mixed with complete lin− BM cells from congenic mice, thus generating mixtures of precursors that retain physiologic precursor ratios with the exception of a 50% reduction in frequency of the population of interest. These mixtures were then analyzed for their T-lineage potential in vivo. We found that under these conditions multiple precursors from BM and peripheral blood, belonging to both MPP and CLP subsets, have robust T-lineage potential. However, differentiation kinetics of different precursors varied considerably, which might ensure continuous thymic output despite gated importation of extrathymic precursors. In conclusion, our data suggest that the thymus functions to impose T-cell fate on any precursor capable of filling the limited number of progenitor niches.
Methods
Mice
Il7ra-deficient mice (B6.129S7-Il7rtm1Imx/J) and B6.SJL-Ptprca Pepcb/BoyJ mice (C57BL/6 mice carrying the CD45.1 allele, termed B6 CD45.1 throughout this paper) were purchased from The Jackson Laboratory. C57BL/6J mice (CD45.2), (C57BL/6J × B6 CD45.1)F1 mice (CD45.1/CD45.2 heterozygous), and Il7ra-deficient mice expressing CD45.1 were bred at the animal facility of Hannover Medical School. Animals were maintained under specific pathogen–free conditions. All animal experiments were conducted in accordance with local and institutional guidelines and were approved by the ethical committee of Hannover Medical School.
Antibodies and flow cytometry
Monoclonal antibodies specific for CD4 (RM4-5, GK1.5), CD8 (53-6.7), CD25 (PC61), CD44 (IM7), Gr-1 (RB6-8C5), erythroid cell marker (Ter-119), CD19 (1D3), CD11b (M1/70), pan-NK (DX5), CD45.1 (A20), CD45.2, (104) B220 (RA3-6B2), CD117 (2B8, ACK2), Sca-1 (E13-161.7), CD90.2 (53-2.1), CD135 (A2F10), CD127 (A7R34), CCR7 (4B12), and CCR9 (7E7-1-1) were used purified or as biotin, Pacific Blue, eFluor450, fluorescein isothiocyanate, Alexa488, phycoerythrin (PE), peridinin chlorophyll protein–Cy5.5 (PerCP-Cy5.5), PE-Cy7, allophycocyanin (APC), APC-Cy7, or APC–Alexa 750 conjugates. Antibodies were purified from hybridoma supernatants using standard procedures or were purchased from eBioscience, BD Biosciences, or Biolegend. PE–Texas Red– or PE-Cy7–conjugated streptavidin (BD Biosciences) was used to reveal staining with biotinylated monoclonal antibody. Qdot565-coupled anti–rat immunoglobulin (Ig) was purchased from Invitrogen. Recombinant P-selectin–Ig fusion protein was purchased from R&D Systems. Flow cytometric analysis was performed on a BD LSRII. Data were analyzed with FlowJo software (TreeStar). For analysis, dead cells and debris were excluded by appropriate gating of forward and sideward scatter. Lineage-negative cells were isolated from total BM by staining cell suspensions with a lineage-specific antibody cocktail (anti-CD4, anti-CD8, anti-CD19, anti-CD11b, anti–Gr-1, Ter-119, and DX5) followed by incubation with anti–rat IgG–conjugated magnetic beads (Dynal; Invitrogen) and magnetic bead depletion of mature lineages. Enriched cell suspensions were surface stained with Qdot565-coupled anti–rat Ig. Cells were sorted using a FACSAria (BD). For depletion of precursor populations 95% or more of the respective target population was removed by sorting. For isolation, MPPs and CLPs were resorted; sorted cells were of 99% or higher purity, as determined by postsort analysis. Isolation of blood cells was performed as previously described.11
Competitive adoptive transfers
Lin− BM cells and lin− BM cells depleted of populations expressing CD27, CD135, both CD27 and CD135, high levels of CD117, CD127, CD90, or cells expressing any of the latter 3 markers, were prepared as described in the previous section. Lin− BM competitor cells (CD45.2; 1.5 × 106) were mixed with lin− BM test cells (CD45.1) depleted of certain populations at numbers corresponding to 1.5 × 106 lin− BM cells adjusted according to the frequency of depleted cells, thus maintaining largely 1:1 ratios of progenitor cells. In some experiments, test or competitor cells were CD45.1/CD45.2 heterozygous to exclude potential recipient-derived effects or possible effects due to different CD45 alleles. No significant recipient-derived effects or variances due to different CD45 alleles were observed. Mixtures of competitor and test cells were transferred intravenously into Il7ra-deficient hosts, which allow continuous thymus seeding, while maintaining an intact thymic architecture. Thymi were analyzed flow cytometrically 2 and 4 weeks after transfer. For competitive adoptive transfers of lin− BM cells and lin− blood cells depleted of populations expressing high levels of CD117 or CD127, lin− BM competitor cells (CD45.1) were mixed with lin− blood test cells (CD45.2) depleted of certain populations from 20 donors per recipient. Mixtures of competitor and test cells were transferred intravenously into sublethally irradiated (4 Gy) Il7ra-deficient hosts (CD45.1).
Intrathymic transfers
MPPs or CLPs (6 × 103) isolated from B6 CD45.2 mice were injected into thymi of nonirradiated B6 CD45.1 mice. Thymi were analyzed for donor-derived cells 7, 14, 21, and 28 days after transfer.
OP9-DL1 cocultures
OP9-DL1 coculture assays were essentially performed as described.24 Five days after adoptive transfer, total thymocytes from Il7ra-deficient mice were plated onto subconfluent OP9-DL1 monolayers at 5 × 104 cells/well in a 24-well plate. Cocultures were performed in the presence of 1 ng/mL IL-7 and 5 ng/mL Flt3 ligand (Flt3L). At day 4 of differentiation, the culture medium was exchanged and at day 7 thymocytes were seeded onto fresh OP9-DL1 monolayers.
Statistical analysis
All analysis was performed using GraphPad Prism software. Data are represented as mean plus or minus SEM. Analysis of significance between 2 groups of mice was performed using unpaired t tests.
Results
A depletion approach to analyze thymocytopoiesis under competitive conditions with near-normal precursor ratios
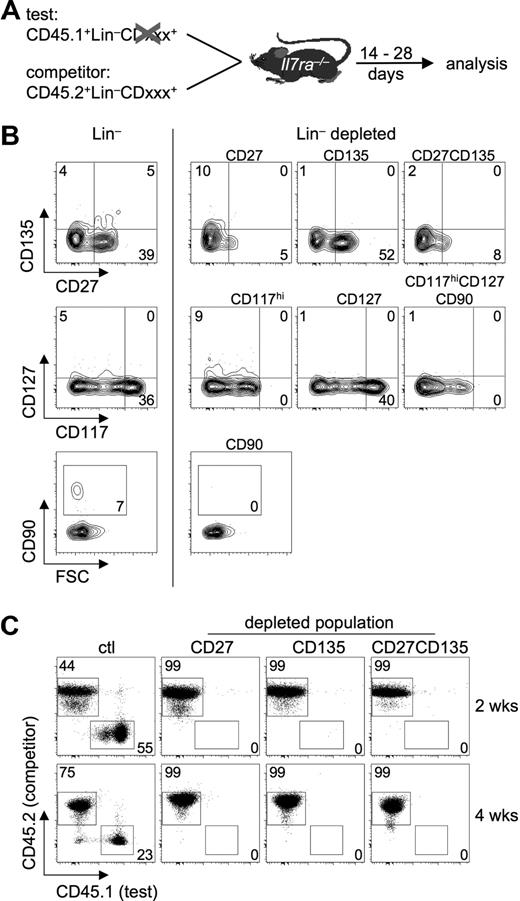
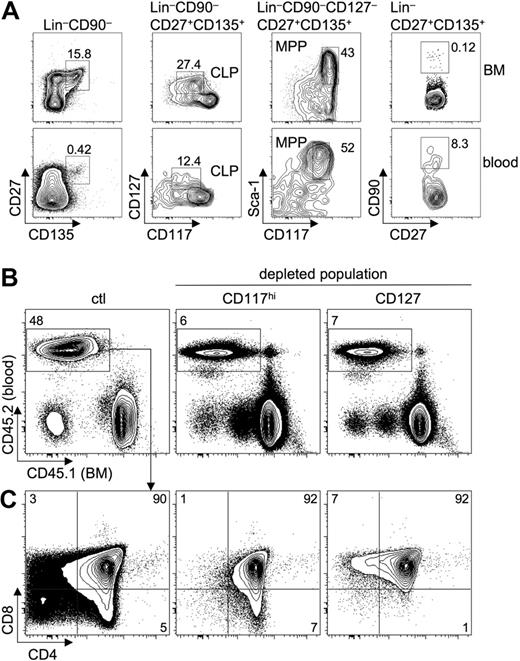
Adoptive transfer of highly enriched putative T-cell precursors massively skews the ratio of circulating candidate precursors beyond those normally observed in peripheral blood. Thus, analyzed this way the contribution of certain precursors to the T lineage is likely to be overestimated. To circumvent this problem, we devised an experimental strategy that only minimally alters precursor ratios. Lin− BM cells were depleted of cells expressing characteristic markers of various candidate precursor populations by fluorescence-activated cell sorting (FACS) and mixed with lin− BM cells from congenic mice containing all candidate precursors. Thus, in this mixture the ratios of precursor populations remain largely intact, except for a 50% reduction of the total amount of the population of interest (Figure 1A). Markers used for FACS-based depletion were CD27, CD135, CD117, CD127, and CD90, thus encompassing virtually all candidate T-cell precursors currently discussed. Figure 1B shows postsort analyses of cells used in competitive adoptive transfer experiments. CD135-expressing cells comprise both MPP and CLP populations. Depletion of cells expressing high levels of CD117 (defined as levels comparable with those of the LSK population) results in the absence of precursors retaining myeloid potential such as HSCs, MPPs, lymphoid-primed MPPs, ELPs, and LSPs, whereas depletion of cells expressing CD127 leads to deficiency in CLP-1 and CLP-2 populations. Finally, cells expressing CD90 are likely to represent T lineage–restricted cells, possibly precursors of CTPs.
A depletion approach to analyze thymocytopoiesis under competitive conditions with near-normal precursor ratios. (A) Outline of the experimental approach. For details see “Results.” (B) Postsort analysis of lin− BM cells depleted of populations expressing various surface markers by FACS sorting (right panels). (Left panels) Sorted lin− BM cells. Numbers in gates and quadrants indicate the percentage of cells. (C) T-cell precursors are confined to CD27- and CD135-expressing cells under competitive conditions. Lin− BM cells from B6 CD45.1 mice (test) were depleted of CD27+ cells, CD135+ cells, or both, mixed with lin− BM cells from B6 CD45.2 mice (competitor), and transferred intravenously into Il7ra-deficient hosts. Ctl indicates mixture of nondepleted B6 CD45.1 lin− BM cells with B6 CD45.2 lin− BM cells. Donor-derived thymocytopoiesis was analyzed flow cytometrically 2 and 4 weeks after transfer. Representative FACS plots of test versus competitor ratios 2 weeks (top panels) and 4 weeks (bottom panels) after transfer. Numbers in gates indicate the percentage of cells. Combined analysis of 2 independent experiments with 2 mice per group.
A depletion approach to analyze thymocytopoiesis under competitive conditions with near-normal precursor ratios. (A) Outline of the experimental approach. For details see “Results.” (B) Postsort analysis of lin− BM cells depleted of populations expressing various surface markers by FACS sorting (right panels). (Left panels) Sorted lin− BM cells. Numbers in gates and quadrants indicate the percentage of cells. (C) T-cell precursors are confined to CD27- and CD135-expressing cells under competitive conditions. Lin− BM cells from B6 CD45.1 mice (test) were depleted of CD27+ cells, CD135+ cells, or both, mixed with lin− BM cells from B6 CD45.2 mice (competitor), and transferred intravenously into Il7ra-deficient hosts. Ctl indicates mixture of nondepleted B6 CD45.1 lin− BM cells with B6 CD45.2 lin− BM cells. Donor-derived thymocytopoiesis was analyzed flow cytometrically 2 and 4 weeks after transfer. Representative FACS plots of test versus competitor ratios 2 weeks (top panels) and 4 weeks (bottom panels) after transfer. Numbers in gates indicate the percentage of cells. Combined analysis of 2 independent experiments with 2 mice per group.
In a first set of experiments, we sought to identify markers characteristic for all T-lineage precursor populations. CD135 might constitute such a marker, as it is expressed on both MPP and CLP populations and signaling via CD135 is critical for expression of CCR9.17,18 Lin− BM cells were depleted of CD135+ cells (test population, CD45.1), mixed with lin− BM from congenic mice (competitor population, CD45.2), and adoptively transferred into Il7ra-deficient recipients. Il7ra knockout mice were used as recipients, because they represent a model of continuous thymic receptivity for T-cell precursors.25 Two and 4 weeks after transfer, thymocytes were analyzed for the relative contribution of test and competitor populations to thymocytopoiesis (Figure 1C). After both 2 and 4 weeks, no contribution of BM cells depleted of CD135+ cells was detected, whereas both congenic BM fractions contributed equally without prior depletion of CD135+ cells (Figure 1C left panels), indicating that indeed all T-lineage precursors are confined to the CD135+ population of lin− BM cells. Recently, Serwold et al proposed CD27 as a marker characterizing all T-cell precursor populations in BM and blood,18 as transfer of CD27− cells failed to generate donor-derived T-cell development. Thus, we subjected lin− BM cells depleted of CD27+ cells to a competitive in vivo differentiation assay as described for Figure 1A. Lin−CD27− BM cells were essentially unable to contribute to T-cell differentiation. Similarly, lin− BM cells depleted of CD135+ as well as CD27+ cells were unable to contribute to thymocytopoiesis (Figure 1C). These results indicate that FACS-based depletion of precursors is sufficiently sensitive to completely remove distinct populations from lin− BM and further substantiates the hypothesis that all physiologic T-lineage precursors reside within a CD27+CD135+ fraction of BM cells.
Depletion of CD117hi, CD127+, or CD90+ precursors does not abrogate T-cell differentiation
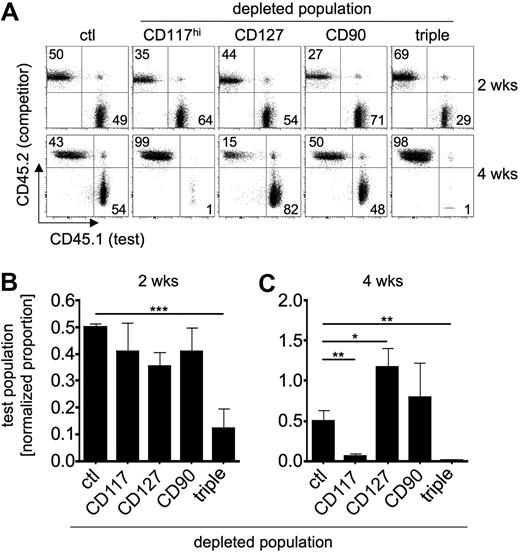
CD135+ cells within lin− BM contain multiple candidate T-lineage precursor populations such as CD117hi MPPs, LSPs, or ELPs or CD127+ CLP-1 and CLP-2 cells. Using a Ptcra reporter mouse, we identified a small population of reporter-positive CD117+CD90+ cells within BM that could constitute a direct precursor of CTPs from blood (A.K. and H.v.B., unpublished observations, March 2007). Thus, we applied the competitive depletion approach described in the previous section to assess the relative contribution of these different precursor populations to T-cell differentiation. Lin− BM cells from B6 CD45.1 mice were depleted of cells expressing high levels of CD117, or those positive for CD127 or CD90. These cells were then mixed with complete lin− BM cells from B6 CD45.2 mice and adoptively transferred into Il7ra-deficient mice. Analysis of the contribution of test and competitor populations to thymocytopoiesis was performed after 2 and 4 weeks. Two weeks after transfer, all test populations had contributed to T-cell differentiation to virtually identical extents and somewhat less, although not statistically significant, compared with nondepleted control populations (Figure 2A top panel and B). Of note, depletion of all 3 CD117hi, CD127+, and CD90+ populations did not result in complete abrogation of T-lineage differentiation from the remaining cells, suggesting that additional precursor populations may exist. Analysis of donor-derived thymocytopoiesis 4 weeks after transfer revealed some striking differences. Whereas depletion of CD117hi cells from lin− BM resulted in a marked competitive disadvantage compared with total lin− BM, depletion of CD127+ cells as well as CD90+ resulted in greater contribution of CD127- or CD90-depleted lin− BM to T-lineage differentiation (Figure 2A bottom panel and C). Depletion of all 3 candidate populations resulted in the virtual absence of T-lineage cells 4 weeks after transfer, suggesting that the competitive advantage observed in populations lacking CD127+ or CD90+ cells was due mainly to a relative enrichment of CD117hi precursors. Taken together, these data indicate that several candidate T-cell precursors can contribute to T-lineage differentiation under competitive conditions with near-normal precursor ratios. Nevertheless, these precursors display different abilities to give rise to various numbers of thymocytes over time.
Depletion of CD117hi, CD127+, or CD90+ precursors does not abrogate T-cell differentiation. Lin− BM cells from B6 CD45.1 mice (test) were depleted of CD117hi cells, CD127+ cells, CD90+ cells, or all of them combined (triple), mixed with lin− BM cells from B6 CD45.2 mice (competitor), and transferred intravenously into Il7ra-deficient hosts. Ctl indicates mixture of nondepleted B6 CD45.1 lin− BM cells with B6 CD45.2 lin− BM cells. Donor-derived thymocytopoiesis was analyzed by flow cytometry 2 and 4 weeks after transfer. (A) Representative FACS plots of test versus competitor ratios 2 weeks (top panels) and 4 weeks (bottom panels) after transfer. Numbers in quadrants indicate the percentage of cells. (B) Thymic reconstitution 2 weeks after transfer. Combined analysis of 5 independent experiments with 2 mice per group. Proportions of test populations were normalized to ctl. Data are shown as mean ± SEM; ***P < .001 (C) Thymic reconstitution 4 weeks after transfer. Combined analysis of 5 independent experiments with 2 mice per group. Proportions of test populations were normalized to ctl. Data are shown as mean ± SEM; *P < .02; **P < .01.
Depletion of CD117hi, CD127+, or CD90+ precursors does not abrogate T-cell differentiation. Lin− BM cells from B6 CD45.1 mice (test) were depleted of CD117hi cells, CD127+ cells, CD90+ cells, or all of them combined (triple), mixed with lin− BM cells from B6 CD45.2 mice (competitor), and transferred intravenously into Il7ra-deficient hosts. Ctl indicates mixture of nondepleted B6 CD45.1 lin− BM cells with B6 CD45.2 lin− BM cells. Donor-derived thymocytopoiesis was analyzed by flow cytometry 2 and 4 weeks after transfer. (A) Representative FACS plots of test versus competitor ratios 2 weeks (top panels) and 4 weeks (bottom panels) after transfer. Numbers in quadrants indicate the percentage of cells. (B) Thymic reconstitution 2 weeks after transfer. Combined analysis of 5 independent experiments with 2 mice per group. Proportions of test populations were normalized to ctl. Data are shown as mean ± SEM; ***P < .001 (C) Thymic reconstitution 4 weeks after transfer. Combined analysis of 5 independent experiments with 2 mice per group. Proportions of test populations were normalized to ctl. Data are shown as mean ± SEM; *P < .02; **P < .01.
Depletion of various precursors results in thymocytopoiesis progressing with different kinetics
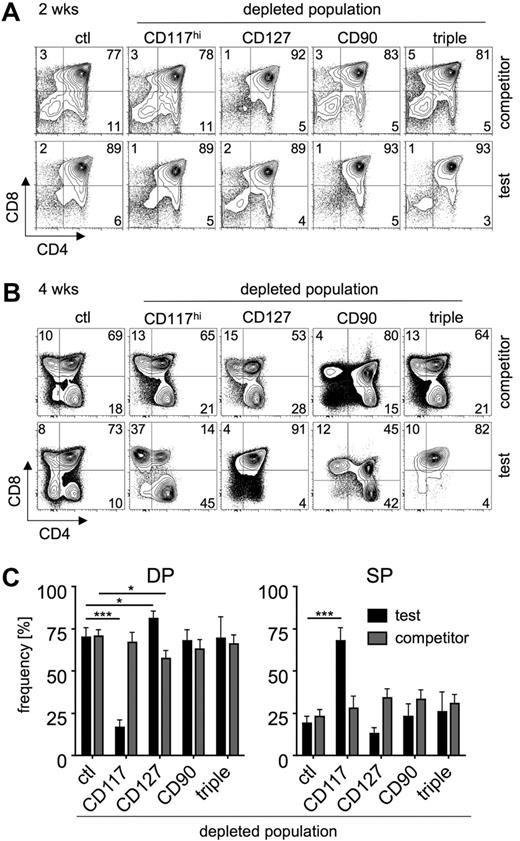
Multiple reasons could account for the competitive advantage over extended periods of time of populations devoid of CD127+ or CD90+ and thus enriched in CD117hi precursors. Intrathymic T-cell differentiation involves several distinct developmental stages including both selection steps as well as stages of proliferative bursts. CD117hi cells within BM comprise more immature stages of precursor cells with broader lineage potential compared with mostly lymphoid-restricted CD127+ or CD90+ cells. Therefore, we hypothesized that after 4 weeks thymocytes derived from populations depleted of CD117hi cells might represent different developmental stages than thymocytes derived from CD127- or CD90-depleted populations. To address this hypothesis, we analyzed the developmental stages of the various test populations compared with nondepleted lin− BM competitor cells flow cytometrically 2 and 4 weeks after transfer (Figure 3). Two weeks after transfer, no major differences could be observed in comparison with nondepleted competitor populations, irrespective of the various depleted populations (Figure 3A). However, 4 weeks after transfer thymocytes derived from donor cells depleted of CD117hi populations were largely SP and displayed significantly reduced numbers of DP thymocytes compared with competitor cells or nondepleted controls, indicating more advanced developmental progression (Figure 3B-C). In contrast, thymocytes derived from CD127-depleted donor cells were still mostly DP and the DP compartment was increased compared with competitor cells and nondepleted controls. Taken together, these data indicate that different developmental stages of donor-derived cells of the various depleted populations account for the observed differences under competitive conditions.
Depletion of various precursors results in thymocytopoiesis progressing with different kinetics. CD4 versus CD8 profiles of experiments performed as described in Figure 2. (A) Analysis 2 weeks after transfer. One representative of 8 individual mice is shown. (Top panels) Competitor population. (Bottom panels) Test population. Numbers in quadrants indicate the percentage of cells. (B) Analysis 4 weeks after transfer. One representative of 8 individual mice is shown. (Top panels) Competitor population. (Bottom panels) Test population. Numbers in quadrants indicate the percentage of cells. (C) Proportions of DP (left panel) and SP (right panel) thymocytes within test (black bars) and competitor (gray bars) populations 4 weeks after transfer. Data are shown as mean ± SEM; *P < .05; ***P < .001; n = 8.
Depletion of various precursors results in thymocytopoiesis progressing with different kinetics. CD4 versus CD8 profiles of experiments performed as described in Figure 2. (A) Analysis 2 weeks after transfer. One representative of 8 individual mice is shown. (Top panels) Competitor population. (Bottom panels) Test population. Numbers in quadrants indicate the percentage of cells. (B) Analysis 4 weeks after transfer. One representative of 8 individual mice is shown. (Top panels) Competitor population. (Bottom panels) Test population. Numbers in quadrants indicate the percentage of cells. (C) Proportions of DP (left panel) and SP (right panel) thymocytes within test (black bars) and competitor (gray bars) populations 4 weeks after transfer. Data are shown as mean ± SEM; *P < .05; ***P < .001; n = 8.
Differential intrathymic differentiation kinetics of MPPs and CLPs do not depend on differential thymus-seeding capacity
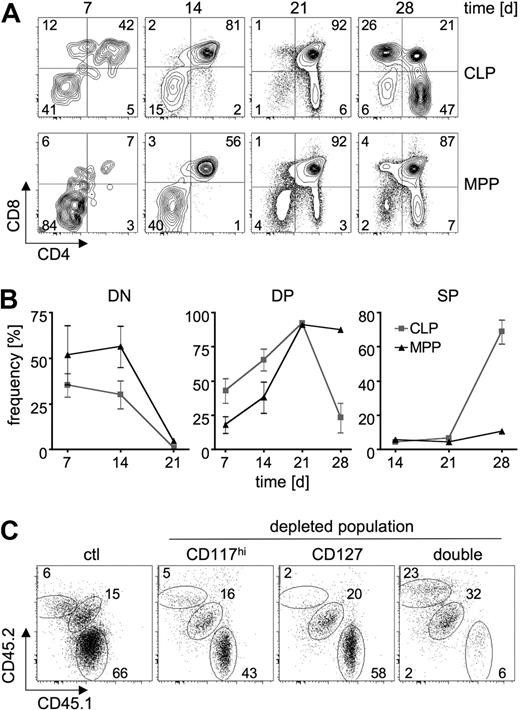
The kinetic differences in thymocyte differentiation after transfer of precursors devoid of CD117hi cells compared with precursors devoid of CD127+ cells could be due to cell-intrinsic differences. Alternatively, extended differentiation kinetics could depend on homing to other sites such as BM, differentiation into CD127+ (ie, CLP-like) cells, and thymus seeding occurring only after extrathymic differentiation events. To test these alternatives, we sorted lin−CD27+CD117hiSca-1+CD135+ MPPs and lin−CD27+CD117lo-Sca-1loCD127+CD135+ CLPs from B6 CD45.2 mice and transferred them intrathymically into B6 CD45.1 mice to bypass any potential additional requirements for homing. Differentiation of donor-derived thymocytes was monitored over the course of 28 days. Seven days after transfer, substantial amounts of donor-derived DP thymocytes were detected upon transfer of CLPs, but not MPPs (Figure 4A-B). After an additional 7 days, most CLP-derived thymocytes were in the DP stage and the first CD4 SP cells could be detected. In contrast, approximately half of the MPP-derived thymocytes still had a DN phenotype. After 21 days, both populations were largely DP. However, a larger proportion of CLP-derived thymocytes was SP compared with MPP-derived cells. Twenty-eight days after transfer, approximately 75% of CLP-derived thymocytes were CD4 or CD8 SP, whereas the majority of MPP-derived thymocytes were still at the DP stage. Taken together, these results indicate that the observed kinetic differences in development are indeed cell intrinsic and do not depend on additional extrathymic differentiation events. To more directly assess thymus seeding of CD117hi and CD127+ progenitor populations under competitive conditions, we used a short-term homing assay. Lin− BM cells from B6 CD45.1 mice were depleted of cells expressing high levels of CD117, or those positive for CD127. These cells were then mixed with complete lin− BM cells from B6 CD45.1/CD45.2 heterozygous mice and adoptively transferred into Il7ra-deficient mice. Five days after transfer, thymi were harvested and total thymocytes were subjected to in vitro differentiation on OP9-DL1 stroma cells for 14 days (Figure 4C). Donor-derived cells from both competitor and test populations were detected independent of depletion of CD117hi or CD127+ progenitors. These data suggest that both CLP-like and MPP-like cells can directly seed the thymus. However, it remains possible that certain progenitor populations re-enter BM, undergo differentiation, and are released as thymus-seeding progenitors of a different phenotype.
Differential intrathymic differentiation kinetics of MPPs and CLPs do not depend on differential thymus-seeding capacity. (A-B) Sorted lin−CD27+CD117hiSca-1+CD135+ MPPs and lin−CD27+CD117loSca-1loCD127+-CD135+ CLPs from B6 CD45.2 mice were intrathymically injected into B6 CD45.1 mice. Donor-derived cells were analyzed by FACS for the expression of CD4 and CD8 after 7, 14, 21, and 28 days. (A) Representative FACS plots of donor-derived (CD45.1+) thymocytes. Numbers in quadrants indicate frequency of donor-derived cells. (B) Analysis of donor-derived thymic subsets of at least 3 mice per group (DN: CD4−CD8−; DP: CD4+CD8+; SP: CD4+CD8− and CD4−CD8+). (C) Lin− BM cells from B6 CD45.1 mice (test) were depleted of CD117hi cells, CD127+ cells, or both (double), mixed with lin− BM cells from B6 CD45.1/CD45.2 mice (competitor), and transferred intravenously into Il7ra-deficient hosts. Ctl indicates mixture of nondepleted B6 CD45.1 lin− BM cells with B6 CD45.1/CD45.2 lin− BM cells. Five days after transfer thymocytes were harvested, cultured on OP9-DL1 cells for an additional 14 days, and analyzed for the expression of CD45.1 and CD45.2. Numbers indicate frequencies of cells in adjacent gates.
Differential intrathymic differentiation kinetics of MPPs and CLPs do not depend on differential thymus-seeding capacity. (A-B) Sorted lin−CD27+CD117hiSca-1+CD135+ MPPs and lin−CD27+CD117loSca-1loCD127+-CD135+ CLPs from B6 CD45.2 mice were intrathymically injected into B6 CD45.1 mice. Donor-derived cells were analyzed by FACS for the expression of CD4 and CD8 after 7, 14, 21, and 28 days. (A) Representative FACS plots of donor-derived (CD45.1+) thymocytes. Numbers in quadrants indicate frequency of donor-derived cells. (B) Analysis of donor-derived thymic subsets of at least 3 mice per group (DN: CD4−CD8−; DP: CD4+CD8+; SP: CD4+CD8− and CD4−CD8+). (C) Lin− BM cells from B6 CD45.1 mice (test) were depleted of CD117hi cells, CD127+ cells, or both (double), mixed with lin− BM cells from B6 CD45.1/CD45.2 mice (competitor), and transferred intravenously into Il7ra-deficient hosts. Ctl indicates mixture of nondepleted B6 CD45.1 lin− BM cells with B6 CD45.1/CD45.2 lin− BM cells. Five days after transfer thymocytes were harvested, cultured on OP9-DL1 cells for an additional 14 days, and analyzed for the expression of CD45.1 and CD45.2. Numbers indicate frequencies of cells in adjacent gates.
Depletion of circulating CD117hi or CD127+ precursors does not abrogate T-cell differentiation
To reach the thymus, precursors must enter the circulation. Thus, it cannot be excluded that BM-derived precursor populations might contain cells that, under physiologic conditions, might never enter the thymus because of being confined to BM. Such precursors might out-compete physiologic T-cell precursors when ectopically put into circulation by intravenous injection. Therefore, we assessed T-lineage potential of circulating precursor populations using a similar competitive depletion approach as described for BM-derived precursors. In a first experiment, we analyzed BM and blood for frequencies and ratios of CD27+CD135+ MPP, CLP, and CD90 populations. The frequency of CD27+CD135+ cells within the lin− subset was much lower in blood compared with BM (Figure 5A). However, analysis of CLPs and MPPs within lin−CD27+CD135+ cells showed that they were present at largely similar ratios in blood and BM with slightly reduced frequencies of CD127+CD117lo CLPs. Notably, frequencies of lin−CD27+-CD135+CD90+ cells were higher in blood compared with BM. However, it remains unclear how many of these cells contain T-precursor potential, as CTPs can so far be reliably identified only in mice transgenic for a Ptcra reporter gene.11 In conclusion, these data show that the fraction of putative T-cell precursors in blood (lin−CD27+CD135+) contains phenotypically similar cells compared with the corresponding BM subset.
Depletion of circulating CD117hi or CD127+ precursors does not abrogate T-cell differentiation. (A) Flow cytometric analysis of lin− cells from BM (top panels) and blood (bottom panels). Cells were stained with antibodies against lineage markers CD90, CD117, CD127, CD27, Sca-1, and CD135. Numbers indicate frequencies of cells within gates. BM cells from 5 mice and blood cells from 10 mice were pooled for analysis. Plots are representative of 3 independent experiments. (B-C) Lin− blood cells from 20 B6 CD45.2 mice (blood) were depleted of CD117hi cells or CD127+ cells, mixed with lin− BM cells from B6 CD45.1 mice (BM), and transferred intravenously into sublethally irradiated Il7ra-deficient hosts. Ctl indicates mixture of nondepleted B6 CD45.2 lin− blood cells with B6 CD45.1 lin− BM cells. Donor-derived thymocytopoiesis was analyzed flow cytometrically by staining for CD45.1 and CD45.2 (B) as well as CD4 and CD8 (C). Numbers indicate frequencies of cells within gates or quadrants. Data are representative of 2 independent experiments with 2 to 3 recipients per group.
Depletion of circulating CD117hi or CD127+ precursors does not abrogate T-cell differentiation. (A) Flow cytometric analysis of lin− cells from BM (top panels) and blood (bottom panels). Cells were stained with antibodies against lineage markers CD90, CD117, CD127, CD27, Sca-1, and CD135. Numbers indicate frequencies of cells within gates. BM cells from 5 mice and blood cells from 10 mice were pooled for analysis. Plots are representative of 3 independent experiments. (B-C) Lin− blood cells from 20 B6 CD45.2 mice (blood) were depleted of CD117hi cells or CD127+ cells, mixed with lin− BM cells from B6 CD45.1 mice (BM), and transferred intravenously into sublethally irradiated Il7ra-deficient hosts. Ctl indicates mixture of nondepleted B6 CD45.2 lin− blood cells with B6 CD45.1 lin− BM cells. Donor-derived thymocytopoiesis was analyzed flow cytometrically by staining for CD45.1 and CD45.2 (B) as well as CD4 and CD8 (C). Numbers indicate frequencies of cells within gates or quadrants. Data are representative of 2 independent experiments with 2 to 3 recipients per group.
Phenotypic analysis suggested that also in blood multiple T-lineage precursors exist. To directly test this hypothesis, we modified the competitive depletion approach using lin− blood cells as test populations. Lin− blood cells from B6 CD45.2 mice were depleted of cells expressing high levels of CD117, or those positive for CD127. These cells were then mixed with complete lin− BM cells from B6 CD45.1 mice and adoptively transferred into sublethally irradiated Il7ra-deficient mice (CD45.1). Analysis of the contribution of test and competitor populations to thymocytopoiesis revealed that all blood-derived test populations were able to contribute to T-lineage differentiation irrespective of depletion of CD117hi MPP-like or CD127+ CLP-like subsets (Figure 5B). Nevertheless, depletion of certain progenitor populations resulted in a somewhat reduced contribution to thymocytopoiesis, whereas no major differences were found with respect to the differentiation status of thymocytes derived from the various test populations (Figure 5C). Taken together, these data indicate that not only in BM, but also in circulation, multiple precursors are able to contribute to thymocytopoiesis under competitive conditions.
Characterization of putative lin−CD117−/loCD127−CD90− BM-derived T-cell precursors
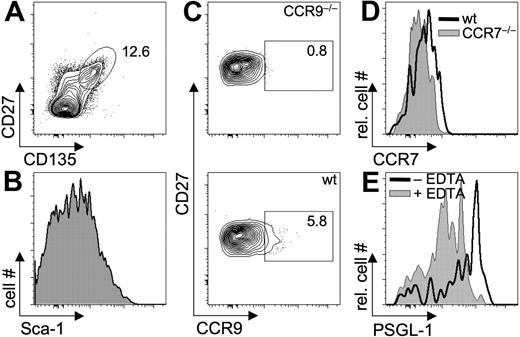
Depletion of CD117hi, CD127+, and CD90+ BM-derived precursor populations together did not result in complete abrogation of T-lineage reconstitution by the remaining cells under competitive conditions, suggesting that additional T-lineage precursors exist (Figure 2). This prompted us to phenotypically characterize the lin−CD117−/loCD127−CD90− fraction of BM cells for surface molecules implied as surface markers of thymus-seeding progenitors, such as CD27 and CD135 as well as molecules implied in migration of T-cell precursors to the thymus, such as P-selectin ligand-1 (PSGL-1), CCR9, and CCR7.18,19,26 Approximately 12% of lin−CD117−/loCD127−CD90− BM cells expressed CD27 and CD135 and, thus, constitute potential T-lineage precursors (Figure 6A). These cells displayed heterogeneous expression of Sca-1 (Figure 6B). Analysis of chemokine receptor expression revealed that only a subset of lin−CD117−/loCD127−CD90−CD27+CD135+ cells was positive for CCR9 (Figure 6C) and CCR7 expression was low (Figure 6D). In addition, most lin−CD117−/loCD127−CD90−CD27+CD135+ cells were positive for PSGL-1. Taken together, these data indicate that the remaining lin−CD117−/loCD127−CD90− fraction of BM cells is heterogeneous with one or multiple subsets expressing surface markers compatible with potential T-cell precursors. These findings further support the notion that multiple T-lineage progenitors exist, some of which still warrant extensive characterization.
Characterization of putative lin−CD117−/loCD127−CD90− BM-derived T-cell precursors. Flow cytometric analysis of lin−CD117−/loCD127−CD90− cells from BM. Cells were stained with antibodies against lineage markers CD90, CD117, CD127, CD27, and CD135 (A), and Sca-1 (B), CCR9 (C), CCR7 (D), or with P-selectin Ig to reveal expression of PSGL-1 (E). Cells from CCR9-deficient and CCR7-deficient mice were used as controls in panels C and D, respectively. For staining of PSGL-1, P-selectin-Ig was used in the presence (negative control) and absence of 10 mM EDTA (ethylenediaminetetraacetic acid). Numbers indicate frequencies of cells within gates. Data are representative of 2 independent experiments.
Characterization of putative lin−CD117−/loCD127−CD90− BM-derived T-cell precursors. Flow cytometric analysis of lin−CD117−/loCD127−CD90− cells from BM. Cells were stained with antibodies against lineage markers CD90, CD117, CD127, CD27, and CD135 (A), and Sca-1 (B), CCR9 (C), CCR7 (D), or with P-selectin Ig to reveal expression of PSGL-1 (E). Cells from CCR9-deficient and CCR7-deficient mice were used as controls in panels C and D, respectively. For staining of PSGL-1, P-selectin-Ig was used in the presence (negative control) and absence of 10 mM EDTA (ethylenediaminetetraacetic acid). Numbers indicate frequencies of cells within gates. Data are representative of 2 independent experiments.
Discussion
The nature of BM-derived precursors seeding the thymus and giving rise to T cells remains elusive. Furthermore, it is not known whether there is only one physiologic T-cell precursor or many. To address these questions we have used competitive adoptive transfer experiments of BM cells that were depleted of rather than enriched for certain precursor populations. Thus, physiologic precursor ratios were only minimally altered with a 50% reduction in frequency of the population of interest. Importantly, in contrast to experimental setups relying on the analysis of highly purified precursors, our experimental approach does not depend on near-absolute purity of the populations of interest, because the basis of the analysis here lies in shifting the ratios of candidate precursor populations rather than completely eliminating certain populations.
We found that, under these conditions, BM-derived precursors are virtually exclusively confined to CD135+CD27+ populations, which contain MPP and CLP populations, as has previously been suggested.17,18 However, neither depletion of CD117hi cells containing various MPP populations nor depletion of CD127+ cells containing CLP cells abrogated T-cell differentiation, suggesting that both MPP-like and CLP-like cells contribute to T-cell development under competitive conditions. Depletion of both cell types as well as CD90+ cells that could represent precursors of CTPs resulted in reduced, but not absent, T-lineage reconstitution and phenotypic analysis revealed that the remaining BM-derived cells contained one or more populations expressing surface markers compatible with a role as T-cell precursors. Therefore, it is likely that additional, non-MPP, non-CLP CD135+CD27+ precursor populations exist. Recently, Umland et al identified a CD127−/lo population with otherwise CLP-like characteristics that would not be included in our depletion approach and may constitute a physiologic T-lineage precursor.27
To reach the thymus, precursors must enter the circulation. Our study revealed that in both BM and blood multiple progenitor populations capable of thymus seeding and T-lineage reconstitution exist. Our phenotypic analysis of blood-derived precursor populations is in agreement with previous work, indicating that most BM-derived T-cell precursors such as MPPs and CLPs as well as CLP-like cells have been found in blood.27 However, CLP-2 cells remain yet to be identified above the limit of detection and a direct BM-resident precursor of CTPs remains elusive.11 Comparison of phenotypically matched precursors in BM and blood revealed virtually no differences with respect to lineage potential and differentiation kinetics.4,27 Interestingly, when assessing T-lineage potential of blood-derived precursors under competitive conditions, we observed T-lineage reconstitution only after transfer into sublethally irradiated, but not nonirradiated hosts (data not shown), whereas BM-derived precursors readily settled nonirradiated thymi as well. On the one hand, this could be due to limitations of the experimental system, as blood contains very low numbers of precursors.11,14 On the other hand, this observation might reflect an intrinsic characteristic of blood-derived precursors, such as a prolonged lack of supportive signals from a specific BM niche. Nevertheless, previous work and our data suggest that thymus entry rather than release from BM is critical for defining physiologic T-cell precursors.
Our analysis revealed that depletion of various precursor populations resulted in different contribution to thymocytopoiesis over time. Depletion of MPP-like CD117hi cells resulted in a competitive disadvantage, whereas depletion of CLP-like CD127+ cells led to an increased contribution of cells lacking this population. Intrathymic transfers and short-term homing assays suggested that the capacity of different precursors to sustain thymocytopoiesis over an extended period of time is a cell-intrinsic property, reflecting the maturation status before thymus seeding, rather than a different capacity to seed the thymus. On the one hand, MPP-like cells represent a more immature subset and its depletion results in reduced long-term reconstitution. On the other hand, CLP-like cells represent a more mature subset and its depletion promotes long-term reconstitution by less mature subsets. These data are in line with both data obtained from in vitro culture experiments20 as well as in vivo data obtained from experiments not including the CD27 marker as indicator of cells with thymus-seeding capacity.17 Short-term homing assays over 5 days suggest that both MPP-like and CLP-like cells can directly seed the thymus. Nevertheless, our data do not formally exclude the possibility of an additional differentiation step of MPPs into CLPs within BM or even peripheral blood as suggested by Serwold et al.18 However, they do demonstrate that such a detour is not required for thymocytopoiesis from MPPs.
Of note, we did not observe a reversal of competitive advantages and disadvantages between various populations after 2 and 4 weeks, but virtually equal contribution to thymocytopoiesis after 2 weeks. This might be because CD117hiCD135+ MPPs still constitute a heterogeneous population, which can be further subdivided into more lymphoid primed Rag+ ELPs or, based on vascular cell adhesion molecule 1 (VCAM-1) expression, into more or less mature MPPs.28,29 VCAM-1− MPPs display more rapid T-lineage differentiation compared with VCAM-1+ MPPs. Thus, after depletion of both CD117hi and CD127+ cells, precursors with short differentiation kinetics are likely to be retained, whereas precursors with prolonged differentiation kinetics appear to be confined to the CD117hi phenotype.
In conclusion, we showed that in the presence of physiologic ratios T-cell precursors are confined to the CD27+CD135+ subsets. However, depletion of neither CD117hi, CD127+, nor CD90+ cells completely abrogates T-lineage differentiation, indicating that multiple precursors, of both MPP-like and CLP-like phenotype, are able to sustain T-cell development under these conditions. Thymus seeding has been suggested to be dependent on the periodic opening of a very limited number of progenitor niches about every 4 weeks.30 Our data suggest that these niches are able to sustain T-lineage differentiation of multiple precursors of various maturities and commitment statuses. Thus, the thymus appears to be capable of efficiently exploiting the scarce numbers of progenitors circulating in peripheral blood.11,14,17 Of note, depletion of different precursor populations resulted in changes of developmental kinetics of test versus competitor populations. This finding provides a possible explanation for the fact that, although thymus seeding appears to be a discontinuous event dependent on periodic niche opening,30,31 the steady-state thymus is continuously producing an output of mature T cells likely to be derived from various progenitor populations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Jasmin Bölter, Stefanie Willenzon, and Mathias Herberg for technical assistance and animal care, and to Christina Reimer and Mathias Rhein for help with cell sorting. We thank Juan Carlos Zúñiga-Pflücker for providing OP9-DL1 cells and Immo Prinz and Reinhold Förster for helpful discussions and critical reading of the paper.
This work was supported by grants from the German Research Foundation (DFG, Emmy-Noether Program, KR2320/2-1 and EXC62, “Rebirth”; A.K.) and the National Institutes of Health (5R01 AI051378; H.v.B.).
National Institutes of Health
Authorship
Contribution: H.v.B. and A.K. designed research; N.S., M.L., J.P., K.W., R.V., M.B., and A.K. performed research; N.S., H.v.B., and A.K. analyzed data; N.S. and A.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests
Correspondence: Andreas Krueger, Institute for Immunology, OE5240, Hannover Medical School, Carl-Neuberg-Str 1, 30625 Hannover, Germany; e-mail: krueger.andreas@mh-hannover.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal