Abstract
Few treatment options exist for patients with myelofibrosis (MF), and their survival is significantly shortened. Activating mutation of the JAK2 tyrosine kinase (JAK2V617F) is found in approximately 50% of MF patients. CEP-701 is a tyrosine kinase inhibitor that inhibits JAK2 in in vitro and in vivo experiments. We conducted a phase 2 clinical study of CEP-701 in 22 JAK2V617F-positive MF patients (80 mg orally twice daily), and 6 (27%) responded by International Working Group criteria (clinical improvement in all cases): reduction in spleen size only (n = 3), transfusion independency (n = 2), and reduction in spleen size with improvement in cytopenias (n = 1). Median time to response was 3 months, and duration of response was more than or equal to 14 months. No improvement was seen in bone marrow fibrosis or JAK2V617F allele burden. Phosphorylated STAT3 levels decreased from baseline in responders while on therapy. Eight patients (36%) experienced grade 3 or 4 toxicity, and 6 (27%) required dose reduction. Main side effects were myelosuppression (grade 3 or 4 anemia, 14%; and thrombocytopenia, 23%) and gastrointestinal disturbances (diarrhea, any grade, 72%; grade 3 or 4, 9%; nausea, grade 1 or 2 only, 50%; vomiting, grade 1 or 2 only, 27%). In conclusion, CEP-701 resulted in modest efficacy and mild but frequent gastrointestinal toxicity in MF patients. The study was registered at http://clinicaltrials.gov as NCT00494585.
Introduction
Primary myelofibrosis (MF) is one of the Philadelphia chromosome (Ph)–negative myeloproliferative neoplasms (MPNs).1,2 It is a clonal stem cell disorder characterized by bone marrow fibrosis, extramedullary hematopoiesis with splenomegaly, anemia, and a peripheral blood smear showing teardrop red cells and leukoerythroblastosis.1-3 MF also occurs as an end-stage manifestation of polycythemia vera (post-PV MF) or essential thrombocythemia (post-ET MF).3-5 Median survival of MF patients varies and ranges from 2 years to more than 10 years, which depends on the presence of “risk factors” at the time of diagnosis, including advanced age, anemia, leukocytosis, cytogenetic abnormalities, constitutional symptoms, circulating blasts, and others.6-13 There are no therapies approved specifically for MF, so that patient care is symptom-directed and palliative in nature.3 Hematopoietic stem cell transplantation (SCT) is potentially curative, but it is applicable for a minority of patients.14
Janus kinase 2 (JAK2) is a nonreceptor tyrosine kinase associated with receptors for cytokines, such as erythropoietin, granulocyte-colony stimulating factor and thrombopoietin.15-17 On ligand binding to a receptor and phosphorylation of JAK2, it activates downstream transcription factors, such as STAT3 and STAT5.15,18 A dominant gain-of-function mutation G → T in nucleotide 1849 that leads to a substitution of valine for phenylalanine (JAK2V617F) has been described in patients with Ph− MPN, mainly in PV (∼ 95%), but also in ET (∼ 50%) and primary myelofibrosis (∼ 50%).19-22 It is the first somatic mutation to be described in patients with Ph− MPN. The V617F mutation occurs in the JAK2 pseudokinase domain and generates a constitutively active molecule resulting from a loss of the autoinhibitory effect of the pseudokinase domain on the kinase domain. Cells expressing JAK2V617F acquire cytokine-independent growth ability and/or cytokine hyper-responsiveness.19,20 The expression of JAK2V617F in mouse models leads to the development of a disease with a similar phenotype to PV, with eventual progression to MF, underscoring the central role of this mutation in the pathogenesis of MPN.23,24 Therefore, there is a strong rationale for the development of JAK2 tyrosine kinase inhibitors as therapy for Ph− MPN.
CEP-701 (also known as lestaurtinib) is an orally available tyrosine kinase inhibitor derived from K252a, a fermentation product from the bacteria Nonomurea longicatena, and belongs to the chemical class of indocarbazole alkaloids. CEP-701 is a known inhibitor of Fms-like tyrosine kinase 3 (FLT3) and is being investigated in clinical trials for patients with acute myeloid leukemia (AML) with FLT3-activating mutations.25,26 CEP-701 also inhibits both wild-type and mutant JAK2 in an in vitro kinase assay.27 At nanomolar concentrations, it markedly inhibits the proliferation of primary erythroid cells from PV patients, inhibiting phosphorylation of JAK2 and components of the downstream signaling cascade, including STAT5, AKT, and ERK.27 CEP-701 inhibits the proliferation of the JAK2V617F-carrying HEL92.1.7 cells xenografted in nude mice.27 In clinical studies so far, CEP-701 has been relatively well tolerated, with the most common toxicities being nausea, vomiting, anorexia, and diarrhea.26,28 The dose of 80 mg twice a day by mouth was recommended based on a phase 2 clinical study in AML, based on tolerability and inhibition of target kinase, and was the starting dose in our trial.26 We report the results of the first clinical trial of CEP-701 when used as a JAK2 inhibitor, in patients with MF.
Methods
Eligibility criteria
Patients at least 18 years of age with a diagnosis of JAK2V617F-positive MF (according to the World Health Organization criteria, revised in 2001) requiring therapy, including previously treated patients who were relapsed, intolerant, or refractory to therapy or, if newly diagnosed, then with intermediate or high risk according to the Lille scoring system13 (adverse prognostic factors: hemoglobin < 10 g/dL and white blood cell count < 4 or > 30 × 109/L; risk groups: 0 = low, 1 = intermediate, 2 = high), or with a symptomatic spleen that is 10 cm or more below costal margin. However, patients with asymptomatic intermediate risk disease were not eligible. Other eligibility criteria included (1) Eastern Cooperative Oncology Group performance status of 0 to 2; (2) total bilirubin less than or equal to 2.0 mg/dL, alanine aminotransferase less than or equal to 2.0× upper limit of normal; (3) creatinine less than or equal to 2.0 mg/dL. In addition, (4) patients needed to be off for at least 2 weeks any prior chemotherapy, biologic therapy, or other anticancer therapy that was considered MF-directed, and to have recovered from prior toxicities (however, concurrent therapy with hydroxyurea and anagrelide was allowed to control elevated white blood cell or platelet counts); and (5) all men of reproductive potential and women of child-bearing potential had to practice effective contraception. Excluded from the study were (1) pregnant or nursing women; (2) patients diagnosed with another malignancy unless they were disease-free for at least 3 years or had a diagnosis of nonmelanoma skin cancer or cervical intraepithelial neoplasia; (3) HIV-positive or hepatitis type A-, B-, or C-positive patients; (4) patients with any condition or medication that increased the risk of gastrointestinal (GI) bleeding (eg, coumadin) or history of any upper or lower GI bleeding within 6 months before enrollment; and (5) patients with elevated international normalized ratio or partial thromboplastin time. This study was approved by the M. D. Anderson Institutional Review Board and conducted in accordance with the principles of the Declaration of Helsinki. All patients gave written informed consent before study entry.
Treatment plan
CEP-701 was supplied by Cephalon as a clear yellow solution in amber glass bottles to be diluted with juice and taken by mouth at the dose of 80 mg twice a day. Patients received CEP-701 continuously from day 1. Premedication with antinausea medication was recommended for patients who developed therapy-related nausea/vomiting. One month (30 days) was considered 1 cycle of therapy. Response assessments were done after each 3 cycles of therapy. Patients were treated for at least 6 cycles unless severe toxicity ensued that precluded treatment. Patients without a response after 6 to 12 months of treatment were taken off study. Responders were to continue therapy for 5 years unless progression of disease or treatment toxicity warranting discontinuation of therapy was observed. Dose modifications were allowed in case of toxicity or lack of response. Patients who developed grade 3 or 4 hematologic toxicity had to discontinue therapy with CEP-701 until counts recovered to grade less than or equal to 2, and CEP-701 was restarted at 60 mg twice daily. Use of growth factors was allowed in cases of drug-induced myelosuppression. Patients who developed grade 3 or 4 nonhematologic toxicity had to discontinue therapy with CEP-701 until adverse event recovered to grade less than or equal to 1, and CEP-701 was restarted at 60 mg twice daily. If toxicity did not resolve within 2 months, therapy was discontinued. Dose escalation to 100 mg twice daily was allowed for patients who did not respond after the initial 3 cycles of therapy. Only patients who did not develop drug-related grade 3 or 4 toxicities could be dose escalated. Toxicities were evaluated using the National Cancer Institute Common Toxicity Criteria, Version 2.0.
Patient evaluation and response criteria
Baseline studies included a complete physical examination, complete blood count, comprehensive biochemistry panel (including liver function tests), CD34+ cell count in blood and bone marrow aspirate with cytogenetics, and molecular test for JAK2V617F mutation. JAK2V617F mutation status and JAK2V617F allelic burden (ratio between mutated and total JAK2 DNA) were determined based on a previously published method.29 Patients were seen monthly for the first 6 months, then every 3 months unless grade 3 or 4 toxicity had ensued, in which case a monthly telephone assessment with review of blood tests was done. A complete blood count and comprehensive biochemistry panel were obtained every 2 weeks for the first 3 months, and then monthly while on study. A CD34+ cell count and JAK2 mutational analysis in peripheral blood were obtained monthly for the first 3 months and then every 3 months. Bone marrow biopsy with staining for fibrosis, cytogenetic analysis (if abnormal before therapy), and JAK2 mutational analysis on bone marrow sample were done every 3 months.
Response criteria used in this study were developed by the International Working Group on Myelofibrosis Research and Treatment (IWG-MRT).30 Even though the study allowed for the use of hydroxyurea and anagrelide, patients had to be off hydroxyurea, anagrelide, growth factors, and/or transfusions for at least 8 weeks for a particular response to be evaluable.
Bio-Plex assay for measurement of cytokines
Proinflammatory and angiogenic cytokines are expressed at high levels in MF. Therefore, measurements of 27 cytokine levels in peripheral blood plasma were carried out using Bio-Plex human cytokine 27-plex panel assay (171-A11127; Bio-Rad); samples were collected before treatment and at 3, 6, 9, 12, and 15 months during therapy and were used for simultaneous quantitation of 27 cytokines according to the manufacturer's instructions.
Western blot analysis
Peripheral blood mononuclear cells were separated by Histopaque (density 1.077) gradient centrifugation, washed with phosphate buffer, and frozen at −80°C until use in experiments. Cellular lysates from mononuclear cells were prepared by resuspending the cells in lysis buffer (10mM sodium phosphate, pH 7.2, containing 100mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 0.5% deoxycholate, 5mM ethylenediaminetetraacetic acid, 1mM phenylmethylsulfonyl fluoride, 1mM sodium orthovanadate, and 1× Roche complete mini-protease-inhibitor cocktail 1× Roche PhosStop inhibitor (04906845001; Roche Diagnostics). After centrifugation at 14 000g for 30 minutes at 4°C, the supernatant was removed and protein concentration estimated using the Bradford reagent (Bio-Rad). Extracted proteins (50 μg) were denatured and separated on NuPAGE 4% to 12% Bis-Tris gel (Invitrogen). After transferring, the nitrocellulose membrane was blocked with 5% nonfat milk in phosphate-buffered saline/0.1% Tween-20 for 3 hours, and incubated with different antibodies: mouse antiphosphorylated STAT3 (05-485) and rabbit anti–total STAT3 (06-596) from Upstate Biotechnology; and mouse anti–β-actin (A5441; Sigma-Aldrich). Each antibody was diluted in 5% nonfat milk and incubated overnight at 4°C. Active bands were detected using conjugated horseradish peroxidase–sheep anti–mouse or horseradish peroxidase–donkey anti–rabbit antibody. Detection was performed by enhanced chemiluminescence as specified by the manufacturer (GE Healthcare).
Study design
The primary objective of the study was to assess objective response rate (complete response, partial response, clinical improvement [CI]) according to IWG-MRT. The MinMax 2-stage design proposed by Simon was implemented.31 The target response rate was 35%. A response rate of 20% or less was considered unacceptable. Given these response rates, if the probability of inappropriately accepting a poor therapy is 10%, a total sample size of 41 patients results in 80% power. In the first stage of the design, a total of 22 patients would be enrolled and the study put on hold to assess response. If 4 or fewer patients responded to the therapy, after being treated for 6 months, then the study would be terminated and the therapy will be declared ineffective. However, as soon as 5 or more patients respond to the CEP-701, an additional 19 patients would be enrolled to complete the study. Safety of the therapy was monitored by the principal investigator and his staff. Although no formal stopping rule was designed, standard good quality clinical practice allowed for early termination of the study in a case of an excess toxicity or unsatisfactory risk/benefit ratio of the therapy, as judged by study staff and involved treating physicians. After the enrollment of the 22nd patient in the first stage of the trial, it was determined that the risk/benefit ratio of the therapy did not justify continuation of the study using the liquid formulation of the drug, and an additional 19 patients were not enrolled.
Descriptive statistics were used to assess response, time to response, and response duration. The Mann-Whitney U test and Wilcoxon signed rank test were used for comparison of cytokine levels.32 Responses were categorized as the best response achieved during the course of the study. Time to response was defined as the time from start of therapy until the response criteria were fulfilled. Response duration was defined as the time from response until loss of response or death and was estimated by the Kaplan-Meier method.33 Calculations were done in Statistica, Version 6.1 (StatSoft).
Results
Patient characteristics
Patients' baseline clinical features are summarized in Table 1. Twenty-two patients (16 males and 6 females) were enrolled between June and October 2007. Before CEP-701, patients had received a median of 3 therapies (range, 0-6), including hydroxyurea (n = 14), erythropoietin (n = 10), lenalidomide (n = 8), anagrelide (n = 6), thalidomide (n = 6), prednisone (n = 4), sunitinib (n = 3), and interferon-α (n = 3). Only 2 patients were previously untreated. Fourteen patients presented with cytogenetic abnormalities at the time of enrollment, including del(20q) (n = 4), del(13q) (n = 3), and del(7q) (n = 2).
Baseline patient characteristics
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 61 (38-83) |
| Male sex, no. (%) | 16 (73) |
| MF type, no. (%) | |
| Primary | 15 (68) |
| Post-PV | 4 (18) |
| Post-ET | 3 (14) |
| Time from diagnosis, mo | 28 (2-184) |
| No. of prior therapies | 3 (0-6) |
| Patients previously untreated | 2 (9) |
| Hemoglobin, g/dL | 9.7 (6.9-15.8) |
| WBC, × 109/L | 13.3 (1.3-62.2) |
| Platelets, × 109/L | 135 (14-1328) |
| Transfusion dependency | 8 (36) |
| Splenomegaly | 18 (90)* |
| Spleen size, cm from left costal margin | 19 (0-30) |
| JAK2V617F/total JAK2 ratio, percentage | 53.5 (13.5-96.6) |
| Abnormal cytogenetics | 14 (64) |
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 61 (38-83) |
| Male sex, no. (%) | 16 (73) |
| MF type, no. (%) | |
| Primary | 15 (68) |
| Post-PV | 4 (18) |
| Post-ET | 3 (14) |
| Time from diagnosis, mo | 28 (2-184) |
| No. of prior therapies | 3 (0-6) |
| Patients previously untreated | 2 (9) |
| Hemoglobin, g/dL | 9.7 (6.9-15.8) |
| WBC, × 109/L | 13.3 (1.3-62.2) |
| Platelets, × 109/L | 135 (14-1328) |
| Transfusion dependency | 8 (36) |
| Splenomegaly | 18 (90)* |
| Spleen size, cm from left costal margin | 19 (0-30) |
| JAK2V617F/total JAK2 ratio, percentage | 53.5 (13.5-96.6) |
| Abnormal cytogenetics | 14 (64) |
Two patients had prior splenectomy.
Responses and outcomes
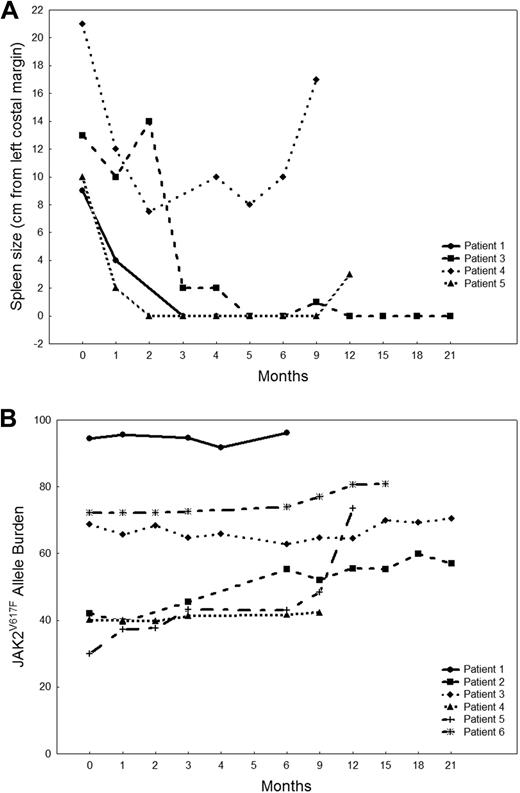
Six patients had a response by IWG-MRT criteria, for an overall response rate of 27% (Table 2): their median follow-up is16.5 months (range, 6-23+ months) and median time to response 3 months (range, 1-9 months). All responses were CI: 3 patients had a decrease in spleen size greater than 50%, 2 became transfusion independent, and one had decrease in spleen size greater than 50%, with an increase of more than 100% in platelets and absolute neutrophil count. No patient had improvement in bone marrow fibrosis. All responders were previously treated: 3 had primary MF, 2 post-PV MF, and one post-ET MF. Three responders had an abnormal karyotype, but none had a cytogenetic response. The median duration of response is 14+ months (range, 3-22+ months). Patient 1 achieved a response that was maintained for 3 months but then was taken off protocol per her request (relocated out of the country) and was lost to follow-up. Patients 2 and 3 are still on the study and have maintained their response for 22+ and 19+ months, respectively. Patient 4 lost response after 7 months, and patient 5 maintained a response for 14 months but then died of sepsis. Patient 6 had a mixed response, for he achieved transfusion independence but, at the same time, had an increase in the size of his spleen from 0 to 14 cm, so he stopped therapy and underwent SCT. Figure 1A shows changes in spleen size in the 4 patients (1, 3, 4, and 5) who had a decrease in splenomegaly. The median JAK2V617F allelic burden at baseline in responders was 55.4% (range, 30%-94.5%), compared with 53.3% (range, 13.5%-96.6%) in nonresponders (P = .97). Therapy with CEP-701 did not affect JAK2V617F allelic burden in responding patients (Figure 1B).
Clinical characteristics of patients with MF who responded to CEP-701
| Patient no. . | Sex/age, y . | Prior treatment . | Hemoglobin (g/dL) . | WBC (× 109/L) . | Platelets (× 109/L) . | Spleen, cm . | JAK2V617F allele burden, percentage . | Karyotype . | Cycles to response, mo . | Response and response duration, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female/61 | Hydroxyurea, anagrelide | 13.3 | 39.2 | 457 | 9 | 94.5 | Diploid | 3 | CI (spleen) 3+ |
| 2 | Male/59 | Erythropoietin | 8.9* | 3.8 | 66 | 0 | 42.1 | Insufficient yield | 1 | CI (hemoglobin) 22+ |
| 3 | Male/56 | Lenalidomide | 13 | 19.7 | 223 | 13 | 68.7 | 46, XY, del 13(q12;q22)[3] | 3 | CI (spleen) 19+ |
| 4 | Female/70 | IFN, thalidomide, lenalidomide, erythropoietin, prednisone | 10 | 14.9 | 70 | 21 | 40.2 | 46, X, t(X;1)(q11;p31), del(13)(q12q22)[11]; 46,XX,del(20) (q11q13)[9] | 4 | CI (spleen) 5 |
| 5 | Female/71 | Chlorambucil, hydroxyurea, thalidomide, lenalidomide, anagrelide, erythropoietin | 8.4* | 1.6 | 22 | 10 | 30 | 46,XX,del(4) (q21q33) [7]; 46,X,del(X) (q22q28) [6]; 46,XX,add(3) (q27), del(3) (q21q26.2), del(5) (q31q35), del(6) (p21p23), add(20) (q13) [2]; 46,XX,del(2) (p11p25) [1] | 1 | CI (absolute neutrophil count, spleen, platelets) 14 |
| 6 | Male/60 | Hydroxyurea, anagrelide, darbepoietin | 9.5* | 11.7 | 216 | 0 | 72.2 | Diploid | 9 | CI (hemoglobin) 12 |
| Patient no. . | Sex/age, y . | Prior treatment . | Hemoglobin (g/dL) . | WBC (× 109/L) . | Platelets (× 109/L) . | Spleen, cm . | JAK2V617F allele burden, percentage . | Karyotype . | Cycles to response, mo . | Response and response duration, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female/61 | Hydroxyurea, anagrelide | 13.3 | 39.2 | 457 | 9 | 94.5 | Diploid | 3 | CI (spleen) 3+ |
| 2 | Male/59 | Erythropoietin | 8.9* | 3.8 | 66 | 0 | 42.1 | Insufficient yield | 1 | CI (hemoglobin) 22+ |
| 3 | Male/56 | Lenalidomide | 13 | 19.7 | 223 | 13 | 68.7 | 46, XY, del 13(q12;q22)[3] | 3 | CI (spleen) 19+ |
| 4 | Female/70 | IFN, thalidomide, lenalidomide, erythropoietin, prednisone | 10 | 14.9 | 70 | 21 | 40.2 | 46, X, t(X;1)(q11;p31), del(13)(q12q22)[11]; 46,XX,del(20) (q11q13)[9] | 4 | CI (spleen) 5 |
| 5 | Female/71 | Chlorambucil, hydroxyurea, thalidomide, lenalidomide, anagrelide, erythropoietin | 8.4* | 1.6 | 22 | 10 | 30 | 46,XX,del(4) (q21q33) [7]; 46,X,del(X) (q22q28) [6]; 46,XX,add(3) (q27), del(3) (q21q26.2), del(5) (q31q35), del(6) (p21p23), add(20) (q13) [2]; 46,XX,del(2) (p11p25) [1] | 1 | CI (absolute neutrophil count, spleen, platelets) 14 |
| 6 | Male/60 | Hydroxyurea, anagrelide, darbepoietin | 9.5* | 11.7 | 216 | 0 | 72.2 | Diploid | 9 | CI (hemoglobin) 12 |
CI indicates clinical improvement.
Patients were transfusion-dependent.
MF patients who responded to CEP-701. (A) Spleen size measurements in MF patients who responded to CEP-701. Data are shown for 4 patients who had a decrease in spleen size on therapy. (B) JAK2V617F allele burden in MF patients who responded to CEP-701. Data are shown for all 6 clinical responders.
MF patients who responded to CEP-701. (A) Spleen size measurements in MF patients who responded to CEP-701. Data are shown for 4 patients who had a decrease in spleen size on therapy. (B) JAK2V617F allele burden in MF patients who responded to CEP-701. Data are shown for all 6 clinical responders.
Toxicity
Main toxicities are shown in Table 3. Eight patients (36%) experienced 10 episodes of grade 3 or 4 toxicities, including anemia (14%), thrombocytopenia (23%), and diarrhea (9%). The most common toxicities affected the GI tract and included diarrhea (any grade, 73%), nausea (grade 1 or 2 only, 50%), vomiting (grade 1 or 2 only, 27%), and flatulence (grade 1 or 2 only, 23%). Six patients (27%) required CEP-701 dose reductions, and the median time to dose reduction was 3 months (range, 1-6 months). Causes for dose reduction were thrombocytopenia only (n = 3), diarrhea only (n = 2), and both thrombocytopenia and diarrhea (n = 1). One responder (patient 5) had the dose of CEP-701 reduced because of diarrhea to 60 mg twice daily 3 months after starting therapy and 2 months after having achieved a response. This patient maintained clinical response with the lower dose of CEP-701 until dying from septic shock 15 months after starting therapy. Eight patients (36%) had their dose of CEP-701 escalated to 100 mg twice daily, maximum dose allowed by the protocol. Only one achieved a response after increasing the dose (patient 6). Twenty patients (91%) discontinued therapy with CEP-701: lack of response (n = 13), death (n = 2), patient's request (n = 2), decision to proceed to SCT (n = 1), loss of response (n = 1), and progression to AML (n = 1). One patient discontinued therapy with CEP-701 after 10 days of treatment at his own request and went to hospice. Six patients died after starting the study: 2 while receiving therapy (one from septic shock and the other from head trauma/intracranial bleed); one patient progressed to JAK2V617F-negative AML 2 months after starting therapy with CEP-701 and died 3 months later; 2 patients died from MF progression; and one died of unrelated medical problems.
Recorded side effects of CEP-701 therapy
| Toxicity . | No. (%) of patients . | ||
|---|---|---|---|
| Any grade . | Grade 3 or 4 . | ||
| Hematologic | |||
| Anemia | 6 (27) | 3 (14) | |
| Thrombocytopenia | 5 (23) | 5 (23) | |
| Nonhematologic | |||
| Diarrhea | 16 (73) | 2 (9) | |
| Nausea | 11 (50) | — | |
| Headache | 7 (32) | — | |
| Vomiting | 6 (27) | — | |
| Flatulence | 5 (23) | — | |
| Heartburn | 4 (18) | — | |
| Mucositis | 3 (14) | — | |
| Peripheral neuropathy | 3 (14) | — | |
| Anorexia | 2 (9) | — | |
| Fatigue | 2 (9) | — | |
| Laboratory | |||
| Liver enzymes (AST, ALT) | 6 (27) | — | |
| Alkaline phosphatase | 2 (9) | — | |
| Bilirubin | 2 (9) | — | |
| Toxicity . | No. (%) of patients . | ||
|---|---|---|---|
| Any grade . | Grade 3 or 4 . | ||
| Hematologic | |||
| Anemia | 6 (27) | 3 (14) | |
| Thrombocytopenia | 5 (23) | 5 (23) | |
| Nonhematologic | |||
| Diarrhea | 16 (73) | 2 (9) | |
| Nausea | 11 (50) | — | |
| Headache | 7 (32) | — | |
| Vomiting | 6 (27) | — | |
| Flatulence | 5 (23) | — | |
| Heartburn | 4 (18) | — | |
| Mucositis | 3 (14) | — | |
| Peripheral neuropathy | 3 (14) | — | |
| Anorexia | 2 (9) | — | |
| Fatigue | 2 (9) | — | |
| Laboratory | |||
| Liver enzymes (AST, ALT) | 6 (27) | — | |
| Alkaline phosphatase | 2 (9) | — | |
| Bilirubin | 2 (9) | — | |
AST indicates aspartate aminotransferase; ALT, alanine aminotransferase; and —, not applicable.
Cytokine analysis
Proinflammatory and angiogenic cytokines are expressed at high levels in MF. Therefore, we measured the levels of several cytokines in patients samples while on the study drug. The median baseline levels of 19 different cytokines (interleukin-1B [IL-1B], IL-1ra, IL-2, IL-6, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, fibroblast growth factor basic, granulocyte-macrophage colony-stimulating factor, interferon-γ, IP-10, macrophage inflammatory protein-1α, macrophage inflammatory protein-1β, RANTES, tumor necrosis factor-α, and vascular endothelial growth factor) were significantly elevated in MF patients compared with controls, but there was no significant difference in the baseline level of any cytokine between responders and nonresponders (data not shown). Similarly, there was no significant change from baseline in cytokine levels measured in patients while on therapy, including no difference between responders and nonresponders (data not shown).
Western blot analysis of STAT3 phosphorylation
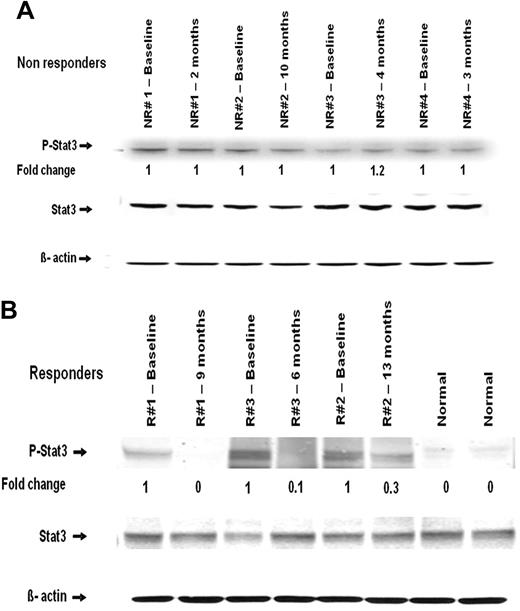
Protein extracts from peripheral blood mononuclear cells of 2 healthy controls and several MF patients with available paired samples from before and during therapy with CEP-701 were examined for STAT3 phosphorylation. There was no change in the phosphorylation of STAT3 (P-STAT3) in the nonresponders during therapy (Figure 2A). In responders, however, a significant reduction in P-STAT3 levels was found in samples obtained while they were on therapy (Figure 2B).
Western blot analysis of STAT3 and P-STAT3 in mononuclear cells obtained from patients before and during therapy. (A) Nonresponders. (B) Responders. Time point at which samples were obtained is indicated. NR indicates nonresponder; and R, responder.
Western blot analysis of STAT3 and P-STAT3 in mononuclear cells obtained from patients before and during therapy. (A) Nonresponders. (B) Responders. Time point at which samples were obtained is indicated. NR indicates nonresponder; and R, responder.
Discussion
The current JAK2 inhibitors, including CEP-701, inhibit both wild-type and mutated JAK2 enzyme,27,34 and in vitro data have revealed that they suppress hematopoietic colony growth not only of cells harboring JAK2V617F but also of those with wild-type JAK2.27 These data support the notion that most, if not all, MPN patients may benefit from current JAK2 inhibitor therapies, regardless of their mutational status. There might be a preferential effect on the cells carrying JAK2V617F mutation, however, as their survival depends, to a great extent, on the constitutively active mutated enzyme.34 This is where a possible therapeutic window of an opportunity lies, and is the reason why this study has enrolled only patients with JAK2V617F mutation, with a hope that therapy would induce a significant decrease in the JAK2V617F allelic burden. We found CEP-701 to have, however, a modest efficacy in our patients, and most responses consisted of improvement in the spleen size. Although early in clinical development, several other JAK2 inhibitors have also shown promise in decreasing myeloproliferation and ameliorating MF-related signs and manifestations (ie, degree of splenomegaly), but with higher response rates.35,36 One of the reasons for only modest results might be the toxicity profile of CEP-701. Overall, therapy with CEP-701 was reasonably well tolerated, and the most common grade 3 or 4 toxicity was anemia and thrombocytopenia, similar to what has been reported with other JAK2 inhibitors.35,36 This is an expected side effect, and it is directly linked to the importance of wild-type JAK2 in normal hematopoiesis. As expected from previous CEP-701 clinical studies, GI disturbances happened often and were mild in most instances. However, the percentage of patients with GI problems was much higher than in previously reported studies in AML, which may be related to the age of MF patients (older) and to their already impaired GI function because of enlarged spleen and liver. The dose of CEP-701 used in our trial was based on a previous report in patients with FLT3+ AML.26 Pharmacokinetic analysis conducted in AML studies revealed a mean trough level of 4.4μM in patients receiving a dose of 60 mg twice daily, well above the IC50 for inhibition of JAK2 by CEP-701.26 In the current study, the plasma levels of CEP-701 were not measured to know whether the GI uptake of the drug was optimal in our patients. The dose of 80 mg twice daily does not fulfill criteria for maximum tolerated dose, and it is possible that higher doses might increase its efficacy in MF. However, we did not observe an increased response rate in patients who received 100 mg twice daily. We used a liquid formulation of CEP-701, as it was used in patients with AML. It is possible that other formulations could improve tolerability and drug exposure.
Although JAK2V617F allelic burden did not decrease in responders to CEP-701, we observed a significant decrease in the level of P-STAT3 in these patients. STAT3 is a latent transcription factor that is activated in the presence of inflammatory cytokines and is a substrate of JAK2.37 STAT3 increases the expression of several genes that contribute to the neoplastic phenotype (eg, BCL2L1, MCL1, and VEGF),37 and deregulated activation of STAT3 is found in several types of cancer, including MF.38,39 Therefore, decreased phosphorylation of STAT3 in responders is highly suggestive that in these patients CEP-701 did indeed affect the JAK2 tyrosine kinase. In our study, 2 of 8 patients became transfusion independent. Although the small number of patients precludes a conclusion about the efficacy in improving transfusion dependency and blood counts, most clinical trials with JAK2 inhibitors report low rates of transfusion independence.35,36 It seems that CEP-701, like other current JAK2 inhibitors, is able to control the myeloproliferative component of the disease in some patients but is unlikely to change its biology (eg, no change in bone marrow findings, JAK2 allele burden, and level of cytokines).
Another possible reason for the modest results observed in this trial might be a known complex biology of MF. Other molecules play an important role in the pathogenesis of MF, such as the recently described TET2.40 Cytogenetic abnormalities are found in approximately 40% of patients with MF, and patients may harbor several distinct subclones carrying additional karyotypic abnormalities.41,42 Thus, it might be that targeting a single mutated kinase might not be enough for achieving clonal eradication in MF.
In conclusion, this is the first report of a JAK2 inhibitor in treatment of MF. Therapy with CEP-701 had modest efficacy, with most responses consisting of reduction of spleen size. There was no improvement in the JAK2V617F allelic burden. Future studies will help define the role of CEP-701 in the management of patients with MF.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Chambers Medical Foundation, the Joe W. and Dorothy Dorsett Brown Foundation, and the Marshall Heritage Foundation (all S.V.).
Authorship
Contribution: F.P.S.S. collected data, analyzed data, and wrote the paper; N.J. collected data and reviewed the paper; T.M. did laboratory studies; D.K. designed research and reviewed the paper; S.V. designed research, provided patient care, analyzed data, and wrote the paper; and H.M.K., G.G.-M., D.A.T., Z.E., and J.C. provided patient care and reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Srdan Verstovsek, Department of Leukemia, University of Texas, M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0428, Houston, TX 77030; e-mail: sverstov@mdanderson.org.