Abstract
Inflammation and coagulation are closely linked interdependent processes. Under physiologic conditions, the tissue microcirculation functions in anticoagulant and anti-inflammatory fashions. However, when inflammation occurs, coagulation is also set in motion and actively participates in enhancing inflammation. Recently, novel and unexpected roles of hemostasis in the humoral and cellular components of innate immunity have been described. In particular, the protein C system, besides its well-recognized role in anticoagulation, plays a crucial role in inflammation. Indeed, the protein C system is now emerging as a novel participant in the pathogenesis of acute and chronic inflammatory diseases, such as sepsis, asthma, inflammatory bowel disease, atherosclerosis, and lung and heart inflammation, and may emerge as unexpected therapeutic targets for intervention.
The PC pathway: its anticoagulant and cytoprotective functions
The PC pathway in hemostasis
Protein C (PC) is a vitamin K–dependent serine protease that is synthesized as a single polypeptide chain of 461 amino acids and is a natural anticoagulant protein.1-3 Whereas synthesis predominantly occurs in the liver, PC has also been identified in the epididymus, kidney, lung, brain, and male reproductive tissue.4 Cotranslational and posttranslational modifications of PC include β-hydroxylation, γ-carboxylation, and glycosylation; the γ-carboxylation is required for efficient secretion and for the anticoagulant activity of PC. PC is multimodular and contains structural elements similar to other vitamin K–dependent coagulation proteins. In human PC, the N-terminal region contains a γ-carboxyglutamic acid (Gla) domain, consisting of 9 Gla residues. In the presence of Ca2+, the Gla domain interacts with negatively charged phospholipids (phosphatidylserine/phosphatidylethanolamine) on cell surfaces and is a key site for interaction of PC with the endothelial PC receptor (EPCR).5 The Gla domain is followed by 2 epidermal growth factor–like modules: an activation peptide and a serine protease domain.
The conversion of PC to activated PC (aPC) results from cleavage of an Arg169-Leu170 peptide bond, which releases a dodecapeptide (residues 158-169) from the heavy chain. This reaction is facilitated by thrombin bound to thrombomodulin (TM)6 and is enhanced through interaction of PC with EPCR on the cell surface.7 Activation can also be triggered by thrombin alone at a less efficient rate8 and is probably not relevant in the circulation. The heavy chain of PC contains the catalytic triad His211, Asp257, and Ser360 (His57, Asp102, and Ser195, respectively, in chymotrypsin numbering). The function of aPC as an anticoagulant is manifested by its ability to inactivate 2 important cofactors of the coagulation cascade, factor (F) V/Va and FVIII/VIIIa. These events are enhanced by the presence of Ca2+, phospholipids, and cofactor protein S.9
Other functions of aPC in hemostasis are in keeping with its role of maintaining a fluid state of blood. By virtue of the ability of aPC to down-regulate thrombin, the activation of thrombin activatable fibrinolytic inhibitor (TAFI) is also suppressed, thus indirectly promoting fibrinolysis.10 Fibrinolysis is also stimulated by another activity of aPC, its ability to inhibit plasminogen activator inhibitor-1 (PAI-1).11
Both inherited and acquired, single and compound, and heterozygous or homozygous deficiencies of PC in humans have been described, and occur at numerous loci in the protein. Symptomatic heterozygous deficiencies can result in deep vein thrombosis and pulmonary embolism, and occur spontaneously or after challenge, for example, trauma. Homozygous PC deficiencies are rare and are associated with fatal systemic disseminated intravascular thrombosis, along with its cutaneous counterpart, purpura fulminans, in newborns,12 conditions that can be treated with administration of PC if detected early.
The anti-inflammatory role of the PC pathway
Although most aspects of the mechanisms underlying the role of aPC as an anticoagulant and profibrinolytic have been elucidated, studies have continually emerged implicating this protein in inflammation. Transcriptional profiling studies of human umbilical vein endothelial cells (HUVECs) have demonstrated that recombinant human aPC can regulate EC gene expression of proteins associated with inflammation and cell survival.13 Using HUVECs, aPC was found to suppress expression of p50 and p52 subunits of nuclear factor-κB (NF-κB), with or without stimulation of HUVECs with tumor necrosis factor-α (TNF-α) and, as a result, block expression of downstream NF-κB target genes. Among the TNF-α–stimulated proinflammatory genes, cell adhesion molecules—for example, E-selectin, ICAM-1, and VCAM-1—were down-regulated by aPC administration; concomitantly, TNF-α–induced binding of U937 monocytes with HUVEC was down-regulated by aPC.13 In addition, aPC was found to suppress lipopolysaccharide (LPS) and interferon-γ–stimulated production of several proinflammatory cytokines in monocytes,14,15 potentially by inhibiting Wnt5A expression,16 and to increase the production of anti-inflammatory cytokines, interleukin-10 (IL-10) and transforming growth factor-β.17 aPC also blocked neutrophil18 and eosinophil19 migration when stimulated by a number of chemoattractants in vitro. However, the mechanism underlying its anti-inflammatory function is poorly understood, particularly in regard to the involvement of its receptor, EPCR.
Anti-inflammatory functions of PC also include its ability to inhibit proinflammatory cytokine release in monocytes,14 cells that also express EPCR.20 Furthermore, aPC inhibits leukocyte adhesion to vascular endothelial cells, resulting in diminished accumulation of neutrophils in the lungs in an LPS-induced pulmonary vascular injury model in rats.21 aPC also diminishes neutrophil chemotaxis in response to IL-8, which can be neutralized by addition of antibodies to EPCR because neutrophils express EPCR mRNA and protein on the cell surface.18 Additional evidence has been obtained that demonstrates the involvement of EPCR in aPC-mediated attenuation of TNF-α–induced neutrophil adhesion to EC.22
The involvement of aPC in the inflammatory response in vivo was identified in mice. Transgenic mice expressing 1% to 18% PC in an endogenous PC−/− background have been generated. The onset and severity of PC-deficiency phenotypes in these mice varied significantly but were strongly dependent on their circulating levels of PC.23 LPS challenge studies in these mice demonstrated that genetic dosing of PC strongly correlated with survival.24 Mice expressing low levels of endogenous PC presented with enhanced inflammation relative to wild-type (WT) mice, and reconstitution of low-PC mice with aPC improved the disease status and survival outcome.
The antiapoptotic role of the PC pathway
The fact that aPC might serve a role as an antiapoptotic agent was first suggested by examining genes that were modulated by aPC in transcription profiling of HUVECs.13 In this case, aPC targeted apoptosis pathways, up-regulating the antiapoptotic protein, B-cell lymphoma-2, the endothelial survival factor eNOS, and the inhibitor of apoptosis gene, and suppressing genes associated with apoptosis, calreticulin, and TRMP-2. Moreover, treatment of cells with aPC blocked the induction of apoptosis in several cell lines by staurosporine13,22 and inhibited TNF-α–stimulated apoptosis of HUVECs by transcriptionally down-regulating the proapoptotic mediator, TNF-related apoptosis-inducing ligand.25 In brain microvascular EC, aPC, in an EPCR/PAR-1–dependent manner, blocked hypoxia-induced apoptosis of brain microvascular EC by inhibition of the up-regulation of p53 of these cells under hypoxic conditions, by suppression of the proapoptotic protein Bax-2, and by reduction of caspase-3 signaling.26
The cellular barrier protective role of aPC
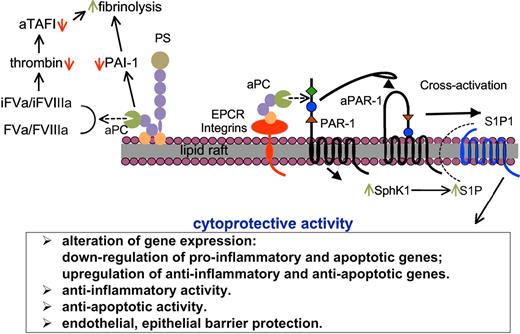
aPC also functions as a cell barrier protective protein, directly through colocalization of aPC/EPCR on lipid rafts, and activation of PAR-1. In turn, aPC/EPCR/PAR-1 cross-activates S1P122,27 and/or Tie228 signaling pathways. Indirectly, aPC, by down-regulating thrombin, inhibits thrombin/PAR-1–mediated vascular permeability,22,27,29 a process that involves, at least in dendritic cells, cross-activation of S1P3.30 Many of these observations were made in in vitro studies, but recently aPC/EPCR/PAR-1/S1P1 and thrombin/PAR-1/S1P3 pathways were shown to function in vivo in endotoxemia models of sepsis.30 Therefore, aPC and thrombin counterbalance vascular endothelial barrier functions, even though these functions are manifested through the same surface receptor, PAR-1. A summary of the cytoprotective functions of aPC is given in Figure 1.
Functions of aPC in humoral and cellular innate immunity. After activation of PC, a portion of aPC dissociates from EPCR and binds to phospholipid membranes, probably in lipid rafts, where it exerts anticoagulant effects directly via inactivation of FVa and FVIIIa. By down-regulating thrombin levels, aPC enhances fibrinolysis through down-regulation of activated TAFI, as well as by inactivating the fibrinolytic inhibitor PAI-1. PS is a cofactor for these humoral processes. aPC also binds to cellular receptors, depending on the cell type, for example, EPCR in endothelial cells, various integrins in macrophages and neutrophils, and LRP8 in platelets and monocytes; this complex activates PAR-1, which in turn can cross-activate S1P1, probably via intracellular up-regulation of SphK1 and outward migration of S1P. These steps cause a variety of cytoprotective cellular processes indicated in the box below the schematic.
Functions of aPC in humoral and cellular innate immunity. After activation of PC, a portion of aPC dissociates from EPCR and binds to phospholipid membranes, probably in lipid rafts, where it exerts anticoagulant effects directly via inactivation of FVa and FVIIIa. By down-regulating thrombin levels, aPC enhances fibrinolysis through down-regulation of activated TAFI, as well as by inactivating the fibrinolytic inhibitor PAI-1. PS is a cofactor for these humoral processes. aPC also binds to cellular receptors, depending on the cell type, for example, EPCR in endothelial cells, various integrins in macrophages and neutrophils, and LRP8 in platelets and monocytes; this complex activates PAR-1, which in turn can cross-activate S1P1, probably via intracellular up-regulation of SphK1 and outward migration of S1P. These steps cause a variety of cytoprotective cellular processes indicated in the box below the schematic.
Other PC/aPC receptors and ligand engagement-induced downstream effects
Although much information has been acquired concerning PC/EPCR/PAR-1 interactions and functions, recently a number of other potential physiologically relevant receptors for PC have been identified. PC/aPC has recently been identified as a ligand for platelet ApoER2, the only known member of the LRP family to be expressed by platelets, and glycosylphosphatidylinositol-bα (GPIbα).31 Studies have demonstrated that immobilized PC/aPC binds to platelets, leading to platelet activation, increased intracellular calcium influx, and platelet spreading. These events were conserved using an active site mutant of aPC (S360A) and an isoform of aPC that lacks Gla. Under shear conditions, platelets adhere and aggregate on immobilized PC/aPC, which is eliminated in the presence of an LRP antagonist receptor-associated protein (RAP), soluble ApoER2 containing only the low-density lipoprotein binding domain 1, or anti-GPIbα. Results obtained in these studies indicate that PC/aPC can induce platelet signaling through interaction with receptors ApoER2 and/or GPIbα. Because PC is present in plasma, the pathophysiologic role that aPC can play in platelet signaling is uncertain, especially considering the presence of other major participants in governing platelet signaling.
In studies using the monocytic cell line U937, aPC interacted with ApoER2 (apparent Kd ∼ 30nM), resulting in phosphorylation of Tyr220 in the adaptor protein disabled-1, Ser473 of Akt (survival pathway), and Ser9 of glycogen synthetase kinase 3β.32 These events were attenuated by RAP, but not by an antibody to EPCR that blocked its interaction with aPC, or by blocking PAR-1–dependent signaling. In addition, U937 adhesion to immobilized aPC was dependent on EPCR and a RAP-sensitive receptor, presumably ApoER2, both of which were required for aPC to inhibit the expression of procoagulant activity (tissue factor [TF]) after stimulation with LPS. Finally, recent work has identified aPC as a ligand for CD11b/CD18. These studies demonstrated that the anti-inflammatory effects of aPC on bone marrow–derived macrophages were dependent on CD11b/CD18 but were independent of EPCR. Further, CD11b/CD18 binds to aPC within specialized lipid microdomains and facilitates aPC-mediated activation of PAR-1 signaling, which leads to enhanced levels of sphingosine-1-phosphate and diminished proinflammatory responses by macrophages. Similar to ApoER2 and platelet activation, deletion of the Gla domain of aPC had no effect on these anti-inflammatory effects. To further support these observations, in vitro genetic inactivation of CD11b, PAR-1, or sphingosine kinase-1 blocked aPC from suppressing macrophage inflammatory responses after LPS stimulation. Finally, in a murine model of lethal endotoxemia, intravenous injection of aPC diminished mortality of WT mice but not CD11b−/− mice. Histologic analyses of these mice demonstrated an abundance of infiltrating leukocytes in lung tissue from WT mice, which was diminished in the mice that received aPC. However, in CD11b−/− mice, administration of aPC did not significantly reduce infiltration of leukocytes. These observations indicate the existence of a novel mechanism for aPC regulation of the inflammatory response.
Additional indirect evidence for the involvement of other aPC receptors in its cytoprotective functions is obtained from the data demonstrating that aPC-mediated down-regulation of TNF-related apoptosis-inducing ligand in TNF-α–induced apoptosis of HUVECs is dependent on aPC, PAR-1, and S1P signaling, but not on EPCR.25 The putative aPC receptor on HUVECs in this case has not been identified.
The role of the PC system in inflammatory diseases
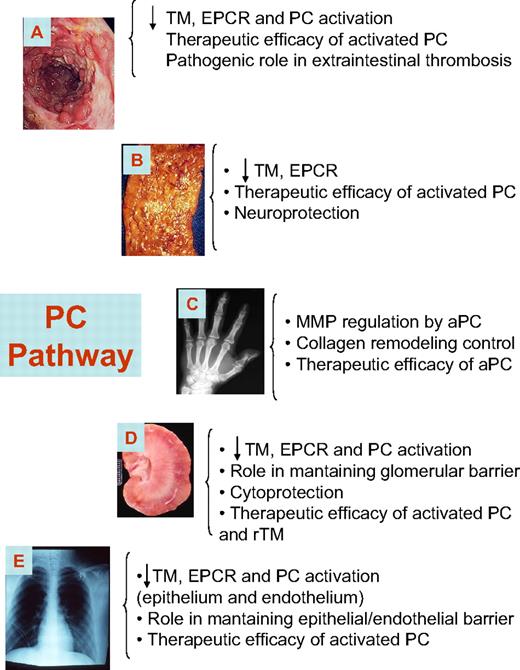
As reported in “The anti-inflammatory role of the PC pathway,” data strongly support the critical involvement of the PC pathway in inflammation, a growing body of evidence is emerging indicating that PC is actively involved in the pathogenesis of several human conditions, including sepsis, inflammatory bowel disease (IBD), airway inflammation, rheumatoid arthritis (RA), and chronic vascular inflammation (Figure 2).33-39 In the following paragraphs, we summarize the relevance of the PC pathway in inflammatory diseases, describing the evolving body of literature in this field.
The PC system in inflammatory disease. The PC pathway plays an important role in several human inflammatory conditions. Members of the PC pathway are down-regulated in patients with these diseases, and therapeutic efficacy has been observed for biologic therapeutics that modulate the PC pathway. (A) IBD. (B) Atherosclerosis. (C) RA. (D) Glomerulonephritis. (E) Asthma.
The PC system in inflammatory disease. The PC pathway plays an important role in several human inflammatory conditions. Members of the PC pathway are down-regulated in patients with these diseases, and therapeutic efficacy has been observed for biologic therapeutics that modulate the PC pathway. (A) IBD. (B) Atherosclerosis. (C) RA. (D) Glomerulonephritis. (E) Asthma.
The PC system in sepsis/endotoxemia
The role of the PC pathway in both human sepsis and in animal models of experimental sepsis has been widely investigated. Discussion of the PC pathway in sepsis is beyond the intent of this more general treatment, and several recent reviews on the topic of aPC in sepsis are available.40-43 However, some important lessons on the intricate relationships between hemostasis and inflammation have been learned from endotoxemia (LPS)–based animal models of sepsis, and a general summary of many important discoveries is presented in Figure 3. A very brief overview of the topic is presented here.
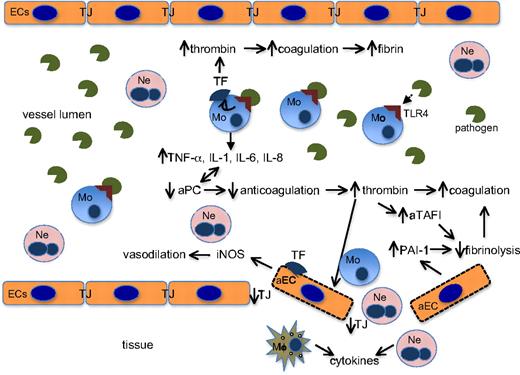
Links between hemostasis and innate immunity in sepsis. The invading pathogenic agent (in this example, LPS) binds to TLR4 on peripheral monocytes to initiate the hyperinflammatory and hypercoagulable cascades, as diagrammed. EC indicates endothelial cells; aEC, activated endothelial cells; TJ, intercellular tight junction proteins; Ne, neutrophils; Mo, monocytes; Mϕ, macrophages; aTAFI, activated thrombin activatable fibrinolytic inhibitor; and iNOS, inducible nitric acid synthetase. ↑ indicates up-regulation; ↓, down-regulation.
Links between hemostasis and innate immunity in sepsis. The invading pathogenic agent (in this example, LPS) binds to TLR4 on peripheral monocytes to initiate the hyperinflammatory and hypercoagulable cascades, as diagrammed. EC indicates endothelial cells; aEC, activated endothelial cells; TJ, intercellular tight junction proteins; Ne, neutrophils; Mo, monocytes; Mϕ, macrophages; aTAFI, activated thrombin activatable fibrinolytic inhibitor; and iNOS, inducible nitric acid synthetase. ↑ indicates up-regulation; ↓, down-regulation.
When a disseminated systemic pathogen, or an immunostimulatory pathogenic agent, for example, LPS from Gram-negative bacteria, engages its receptor, Toll-like receptor 4 (TLR4) on monocytes,44 TF is up-regulated,45 leading to a coagulation response, and cytokines, especially TNF-α, IL-1, IL-6, and IL-8, are released in an NF-κB–dependent nuclear translocation–dependent fashion.46 These cytokines, particularly TNF-α, down-regulate TM in EC and diminish the capacity of the endothelium to activate PC to aPC, thereby lowering aPC levels.47 In addition, TNF-α and IL-1 also down-regulate the mRNA for PC in liver and other organs.48 Thus, overexpression of cytokines in response to LPS has the overall effect of diminishing the anticoagulation pathway and further enhancing coagulation. Continuing this cycle, lowering of aPC by cytokine elaboration leads to further up-regulation of host cytokine production, and further diminishment of aPC.
aPC also regulates coagulation as a profibrinolytic agent, both directly by inactivating a fibrinolytic inhibitor, PAI-1,11 and indirectly via its inverse effect on thrombin levels. Thrombin activates a fibrinolytic inhibitor (TAFI), and lowering aPC, via inflammatory disease, not only leads to higher levels of available PAI-1, but increases thrombin levels, which in turn enhance activated TAFI levels, further inhibiting fibrinolysis (Figure 1).10 Thus, coagulation is additionally enhanced by diminishment of fibrinolysis under conditions of low aPC. Up-regulation of coagulation provides excess coagulation proteases, most notably thrombin, which not only influences platelet function, but also is a proinflammatory agent via signaling through protease-activated receptors.49
In addition to the role of the PC system in governing coagulation and inflammation in peripheral blood, the endothelium plays a critical role in the septic response. In one manner, by producing inflammatory mediators, such as TNF-α and thrombin in blood, downstream effects on the endothelium are quite profound. One activity manifest by thrombin is disruption of the endothelial barrier,50 probably through down-regulation of tight junction proteins and consequent rearrangement of the cytoskeleton.51 This activation of the endothelium produces a host of effects that regulate the septic response, including extravasation from blood into tissues of inflammatory leukocytes. Production and secretion of PAI-1 by the activated endothelium result in inhibition of fibrinolysis and up-regulation of coagulation. Shedding of TM and EPCR by activated (a)ECs occupies thrombin and PC in plasma, factors that down-regulate activation of PC, thereby attenuating anticoagulation. Further, aECs secrete inducible nitric acid synthetase,52 leading to the hypotension and hyperpolarization of cells in severe sepsis.
Many of these effects have been observed in mice with genetic deficiencies in PC that consequently produce very low levels of endogenous PC. These mice are hypercoagulable and hyperinflammatory,23 and have severe responses to LPS, including exacerbated hypotension, and many of these symptoms are alleviated by administration of aPC.24 In LPS-treated mice, the thrombin-mediated inflammatory response has been shown to be linked to dendritic cell signaling,30 thus opening new paradigms for possible intervention.
Because of the multiple potentially beneficial effects of aPC on the inflammation and coagulation in sepsis, aPC administration had a promising measure of success in a clinical trial (Protein C Worldwide Evaluation in Severe Sepsis [PROWESS]) for cases of severe sepsis, in which a statistically significant reduction of the 28-day mortality rate was achieved by aPC administration.53 However, questions linger as to usage of aPC because of the bleeding complications that can occur. Guidelines for patient selection and administration have been recommended.54
The PC system in IBD
Clinical experience and bench research have demonstrated that in the 2 major forms of IBD, Crohn disease and ulcerative colitis, a hypercoagulable and prothrombotic state exists.55,56 Cells and molecules classically implicated in the physiologic process of coagulation behave abnormally in IBD and may also play an active role in disease pathogenesis and/or progression. Although the close relationship between coagulation and inflammatory responses is well established, its relevance to the pathophysiology of IBD remains poorly understood; however, several observations point to a defect in the anticoagulant and anti-inflammatory functions of the PC pathway in IBD.
Recent data indicate that the PC pathway is defective in the endothelium of patients with IBD, whereas microvascular inflammation and thrombosis are well-documented events in these patients.57 Indeed, patients with Crohn disease and ulcerative colitis have elevated serum levels of TM and EPCR, implying increased shedding, and reduced expression in the inflamed colonic mucosal microvasculature.58-60 The resultant reduced potential for activation of PC may lead to microvascular thrombosis and mucosal ischemia, further contributing to mucosal injury. In addition, recent evidence implicates TF as an important mediator of microthrombi formation, as well as the inflammatory cell recruitment and mucosal injury that accompanies colitis.61 A recent study has shed further light on the role of the PC pathway in microvascular inflammation in IBD.60 It was found in isolated human intestinal microvascular endothelial cells that exposure to proinflammatory cytokines down-regulated the expression of TM and EPCR at the transcriptional level, inhibiting the conversion of PC into aPC. The addition of recombinant aPC had a potent anti-inflammatory effect in human intestinal microvascular endothelial cells, down-regulating cytokine-dependent CAM expression and chemokine production, as well as inhibiting leukocyte adhesion. Furthermore, mice with chemically induced colitis had a reduced capacity to convert PC into its active form. Administration of recombinant aPC was therapeutically effective in ameliorating colitis in these mice, without causing any bleeding events. aPC inhibited adhesion of leukocytes to inflamed intestinal vessels, which is an important pathogenic event in IBD that is currently being targeted with novel therapeutic agents.62,63
Besides its pathogenic role in intestinal inflammation, the association between the PC pathway and the risk for thromboembolism in IBD has been investigated. An investigation was undertaken aimed at determining whether the PC pathway contributes to the enhanced extraintestinal thrombosis that is observed in mice with dextran sodium sulfate–induced colitis.64 The thrombotic response was greatly attenuated in transgenic mice that overexpressed EPCR or by treatment of WT colitic mice with aPC, thus revealing that the PC pathway is a novel mediator of extraintestinal thrombosis.
Taken together, these data suggest that vascular inflammation is a key pathogenic component of chronic inflammatory disorders, such as IBD. In addition, they suggest that the PC pathway is critically involved in governing intestinal homeostasis mediated by the mucosal microvasculature, thus opening an entirely new therapeutic approach based on restoring the PC system.
The PC system in asthma
Asthma is a chronic inflammatory disease of the airway, an essential feature of which is structural alteration of the airway wall. This remodeling includes hypertrophy and hyperplasia of airway smooth muscle, mucus gland hyperplasia, thickening of the reticular basement membrane, and qualitative and quantitative changes of airway blood vessels.65 More recently, studies have reported that the PC pathway is involved in airway inflammation and remodeling in asthma.66,67 An accumulation of thrombin in the bronchial secretion was observed in patients with chronic bronchial asthma, which correlated with the degree of airway inflammation.38,68 In addition, increased thrombin in asthmatic patients induced abnormal production of mucin by epithelial cells, and stimulated the proliferation of smooth muscle cells.
Administration of aPC inhibited thrombin-induced goblet cell hyperplasia in rats, both in vitro and in vivo.69 Furthermore, administration of aPC was sufficient to suppress allergic responses in a dose-dependent manner in a murine ovalbumin-induced bronchial asthma model.66 Moreover, in the same experimental model, aPC, in its capacity as an endogenous anticoagulant, enhanced fibrinolysis and reduced obstruction caused by fibrin deposition. Together, these observations suggest that aPC is a key regulatory element in airway inflammation.
Although the mechanisms by which aPC exerts its regulatory functions have not yet been completely elucidated, emerging data indicate that the anti-inflammatory activity of aPC is partially the result of its ability to block the transcription of some inflammatory cytokines. In particular, NF-κB–dependent cytokines, including TNF-α, IL-4, IL-13, and IL-5, are blocked by a decrease in the nuclear translocation of signal transducer and activator of transcription 6, and inhibition of infiltration of leukocytes at sites of inflammation.66,70-73 Indeed, inhalation of aPC reduced nuclear translocation of NF-κB p50 and other members of the NF-κB/Rel family, and reduced IgE and eosinophilic infiltration in a murine bronchial asthma model.66 In support of this, mice deficient in NF-κB p50 lacked allergic airway inflammation, confirming that blockade of NF-κB is a crucial step in the resolution of inflammatory responses.74,75
The levels of aPC are decreased in human lung disease, which is associated with altered aPC/PC and aPC/thrombin ratios. Activation of PC is also reduced in the sputum of patients with bronchial asthma.38,69 This imbalance between increased thrombin and insufficient activation of the PC system appears to generate excessive coagulation and exacerbates airway inflammation. The conversion of PC to aPC is mediated by the thrombin/TM complex and enhanced by binding of PC to EPCR. Because of the unexpected demonstration that TM is expressed on bronchial epithelia and the efficient manner in which epithelial cells convert PC to aPC in the presence of thrombin,38 it has been postulated that airway epithelia provide an appropriate microenvironment for production of aPC. In vitro studies have revealed that lung epithelial cells express EPCR, whereas expression is down-regulated under conditions of inflammation by inflammatory stimuli, including TNF-α, RANTES, and eotaxin.38 Importantly, whereas expression of TM is lost from lung endothelial cells during inflammation secondary to total body irradiation and in asthmatic patients, soluble TM (sTM) is increased,76 confirming that inflammatory stimuli enhance degradation of cell membrane–bound TM on epithelial cells, which could in turn explain the decreased levels of aPC in asthmatic patients. Indeed, in rabbits with lung inflammation, administration of aPC attenuated the levels of sTM in the plasma and contributed to the resolution of fibrinolysis in the pulmonary compartment.77,78 Therefore, the capacity to produce aPC and its physiologic functionality in the alveolar compartment may be further decreased by inflammation-induced down-regulation of TM and EPCR expression by epithelial cells.38,79
TM on the cell surface plays an important role in the clearance of excessive production of thrombin from epithelial cells. It therefore seems probable that loss of this function would cause an imbalance between thrombin and aPC, resulting in excessive thrombin formation and fibrin deposition in asthma.80,81 On the other hand, given that aPC directly affects human pulmonary vascular permeability, conferring endothelial barrier protection via EPCR and S1P1,82 and that thrombin can affect airway permeability,83 the defective expression of EPCR on lung epithelium of asthmatic patients could alter the airway barrier, thereby exacerbating inflammation.
All of these findings have a special therapeutic relevance in humans. In a recent double-blind, placebo-controlled study in a human model of endotoxin-induced pulmonary inflammation, inhalation of aPC significantly reduced lung inflammation and leukocyte infiltration.84
The PC system in vascular inflammation
Atherosclerosis is the most common form of chronic vascular inflammation and leads to occlusive thrombosis. A logical determinant of such pathogenic phenomenon could be a local reduction of the anticoagulant properties exerted by the atherosclerotic endothelium. Laszik et al85 therefore investigated the expression of the PC pathway in coronary atherosclerotic vessels. Compared with control vessels, atherosclerotic vessels expressed significantly lower levels of EPCR and TM, thus supporting reduced anticoagulant and anti-inflammatory activity of local endothelial cells in which activation of PC is impaired.85 These observations have been confirmed and extended through use of an immunohistologic approach to demonstrate that expression of TM is also reduced on smooth muscle cells and foam macrophages in atherosclerotic plaques, supporting a pathologic role for the PC system in nonvascular cells.86 Consistent with this, atorvastatin, a statin used for hypercholesterolemia that has anti-inflammatory activities at the endothelium, inhibited TNF-α–dependent down-regulation of TM on human aortic endothelial cells in vitro, in an NF-κB–dependent manner, thus suggesting that statins may have anti-inflammatory and anticoagulant activity by attenuating inflammation-dependent down-regulation of TM.87
C-reactive protein, the classic mediator elevated in atherosclerosis and in vascular inflammation, decreases endothelial cell expression of TM and EPCR, thus reinforcing the notion that widespread inflammation may alter endothelial anticoagulant properties.88 In agreement with the demonstration of decreased expression in the atherosclerotic endothelium, patients with atherosclerosis or hypertension have elevated serum levels of sTM at the systemic level, a finding compatible with enhanced endothelial shedding resulting from inflammation.89,90
The PC system in neuropathies
Beyond its antithrombotic and anti-inflammatory properties, aPC is also neuroprotective in stroke.91-93 Indeed, in a murine model of focal cerebral ischemia, administration of aPC reduced cerebrovascular and brain damage by inhibiting infiltration of leukocytes in ischemic tissue.92 Furthermore, aPC controlled tissue-type plasminogen activator–mediated brain hemorrhage in ischemic brain endothelium, a serious complication of thrombolytic treatment in patients who had an ischemic stroke.94
Both in vivo and in vitro studies have provided further insight into the mechanisms underlying the cytoprotective effects of aPC. In addition to inhibition of production of TNF-α, and thus down-regulation of inflammation, aPC protected the brain from ischemic injury, exerting a dose-dependent cytoprotective effect directly on cells by preventing apoptosis. Indeed, under hypoxic conditions, aPC reduced the levels of proapoptotic Bax and p53 protein, and increased the levels of antiapoptotic B-cell lymphoma-2. These effects were mediated by binding of EPCR and activation of PAR-1,26 proteins that not only induce apoptotic signaling but also contribute to stabilization of the endothelial brain barrier.95 Interestingly, administration of aPC 6 or 24 hours after a transient ischemic attack improved the ischemic lesions, promoting neovascularization and neurogenesis.96
Similarly to stroke, a recent report has highlighted a therapeutic and protective role for administration of aPC in a model of myocardial ischemia-reperfusion injury, where myocardial damage was decreased in mice administered aPC, resulting from antiapoptotic and anti-inflammatory beneficial effects mediated by PAR-1 signaling.97 Taken together, these observations indicate that aPC has therapeutic potential in chronic inflammatory disorders, including atherosclerosis and stroke.
The importance of the neuroprotective role of aPC is further enhanced by the finding that aPC, in an EPCR-dependent manner, is able to cross the blood-brain barrier98 and blood–spinal cord barrier, where it attenuated expression of superoxidase dismutase-1 in microglial cells, microvessels, motor neurons, and cultured neuronal cells, via suppression of nuclear levels of the transcription factor, Sp1, through signaling mechanisms elaborated by aPC/EPCR/PAR-1(and/or PAR-3)/S1P1.99 These findings may be clinically translated to allow neuroprotection to be conferred by systemic administration of this protein. Indeed, in a murine model of amyotrophic lateral sclerosis–like disease, generated through expression of a mutant superoxidase dismutase-1,100 aPC administered intraperitoneally was found to slow disease expression and extend survival.99
The PC system in kidney inflammation
Coagulation and inflammation also play a major role in glomerulonephritis. For this reason, the PC system has been investigated in several forms of glomerular injury, including diabetic nephropathy. In the kidneys of patients with persistent hyperglycemia, reduced levels of TM were detected accompanied by impaired formation of aPC.101-103 Consistent with these findings, low levels of aPC in the glomeruli of animals with diabetes increased the activation of blood coagulation and renal fibrin deposition, compromising glomerular barrier function. aPC was protective in these mice, preventing apoptosis of glomerular cells and podocytes, thereby enhancing glomerular barrier functions and preventing hyperglycemia-induced renal dysfunction. These results indicate that maintaining the physiologic function of the PC pathway and high PC activation is protective for diabetic nephropathy.
Alterations to the PC pathway have been found in other forms of kidney injury. For instance, in patients with hemolytic uremic syndrome, a decrease of TM has been shown and consequently contributes to the procoagulant state of the endothelium, leading to glomerular injury.104 Similarly, in patients with renal insufficiency and in patients on peritoneal dialysis or hemodialysis, the concentration of sTM in plasma was significantly higher. This increase was accompanied by other markers of endothelial activation, such as increased levels of vascular cell adhesion molecule-1, suggesting involvement of the PC system in glomerular damage.105
In addition to investigation of the therapeutic efficacy of aPC, administration of sTM has been investigated and found to be highly effective. In experimental models of glomerulonephritis, sTM attenuated leukocyte infiltration and thrombotic glomerulonephritis.106 Similarly, in experimental models of ischemia-induced kidney dysfunction, sTM significantly improved tubular histologic damage, by decreasing leukocyte rolling and adhesion to the glomerular microvasculature and by reducing the number of TdT-mediated dUTP nick end labeling–positive apoptotic cells.
Taken together, these data support a role for the PC system in kidney inflammation and suggest that administration of aPC or sTM might be beneficial for tubular inflammation.
The PC system in RA
RA is characterized by persistent inflammation of multiple synovial joints that leads to progressive articular damage. The expression of PC and aPC was increased in RA synovial tissues and colocalized with MMP-2+ cells, such as endothelial and synovial cells, suggesting a potential role of the PC system in tissue remodeling.107 Indeed, fibroblasts and monocytes from patients with RA produced substantially more MMP-9 than those from control persons. Addition of recombinant aPC markedly reduced MMP-9 at the gene and protein levels and directly suppressed production of TNF-α and activation of NF-κB. These data unveil the PC system as an endogenous inhibitor of MMP-9 through blockade of TNF-α, given the initial evidence that aPC may be beneficial for the prevention of inflammation and joint destruction in patients with RA. These data have been further expanded by recent elucidation of the functional role of PC in cartilage degradation. Buisson-Legendre et al107 investigated expression of PC, EPCR, and TM by chondrocytes, as well as their level of colocalization with MMPs. APC was observed at sites of MMP activity in developing joints and in arthritic, but not normal, cartilage. Chondrocytes expressed the machinery of the PC pathway, and functional studies demonstrated that aPC augmented aggrecan release and initiated collagen breakdown in IL-1β– and TNF-α–treated cartilage. The authors therefore concluded that aPC may be a relevant activator of MMPs in cartilage and may play a role in progressive cartilage degradation in arthritis. These findings are consistent with the previous demonstration that successful treatment of RA is associated with a significant reduction of plasmin-generated sTM, suggesting that effective treatments deactivate the endothelium.
The PC system and pregnancy outcomes
Studies have shown that an intact protein C system is required for normal pregnancies to proceed to term and for survival of neonates. Initial studies demonstrated that breedings of PC+/− x PC+/− mice led to normal development and birth of PC−/− neonates, but none of these pups survived beyond 2 days after birth, resulting from severe intra-abdominal and intracranial bleeding.108 However, that maternal transfer of small amounts of PC could have been responsible for development of embryos to term could not be discounted. Some resolution of this issue was accomplished by development of very low PC-expressing mouse lines with PC expression only by a single allelic PC-cDNA transgene (tg) [(PC−/−(tg)], resulting in mice that expressed 1% to 18% of WT PC.23 Breedings of these mouse lines showed that mothers with no more than 3% of WT PC expression [PC−/−(tg785)] were fertile but could not carry pregnancies beyond 7.5 days postcoitum (dpc), resulting from thrombosis and inflammation in the ectoplacental cone region.23 However, female mice with 18% levels of WT PC expression [PC−/−(tg527)] delivered normal embryos that survived well into adulthood, with few neonatal gross abnormalities. These results were independent of the genotypes of the males because male PC−/−(tg785) mice (initially generated from breedings of PC+/− females with PC+/−(tg785) males) were able to successfully breed with PC−/−(tg527) females (initially generated by breeding PC+/− females to PC+/−(tg785) males). Currently, PC−/−(tg785) adult mice are routinely available for study from breedings of PC+/− female mice with PC−/−(tg785) males. Thus, it is clear that maternal PC expressing at least between 3% and 18% of WT PC levels was sufficient to sustain pregnancies. An extension of this work showed that when PC−/−(tg785) ovaries were transplanted in WT or PC−/−(tg527) females, and the recipient females mated with PC−/−(tg785) males, normal PC−/−(tg785) pups were delivered. These data showed that WT PC expression of no more than 3% of WT in the embryo throughout development was sufficient for normal birth.23 Thus, it seems doubtful that maternal transfer of PC was originally responsible for the development of PC−/− pups from matings of PC+/− parents. The basis of the effect appears to originate from the female requiring small amounts of PC to moderate thrombosis and inflammation in the ectoplacental cone region.
Further work on this topic confirmed that local maternal thrombosis accompanying a PC deficiency may be a mechanistic factor in adverse pregnancy outcomes in very low-PC mice. Specifically, doubly deficient mouse lines, with single allelic transgenes carrying expression of 1% of WT PC [PC−/−(Tg4)]23 and approximately 1% of TF [TF−/−(tg)],109 have been accomplished, leading to PC−/−(tg4)/TF−/−(tg) mice.110 Matings of PC−/−(tg4)/TF−/−(tg) males and females led to normal pregnancies and an improved percentage of PC−/−(tg4)/TF−/−(tg) pups that survive apparently normally into adulthood. These experiments show that the thrombosis and inflammatory cell recruitment that are detrimental to pregnancy outcomes in a low-PC condition probably involve TF-dependent fibrin formation and that a balance of TF-mediated thrombosis and aPC-mediated inflammatory cell recruitment must be maintained to facilitate proper embryonic implantation.110
Similar pregnancy outcomes accompany deficiencies of other components of the classic PC pathway. For example, breedings of EPCR+/− males and females do not lead to EPCR−/− pups, and their development appears to be arrested at approximately 10.5 dpc, resulting from placental thrombosis at the maternal-fetal interface because of a lack of EPCR in giant trophoblast cells of EPCR−/− embryos.111 That very little EPCR is needed in this regard was originally shown by development of hypomorphic EPCR mice (EPCR∂/∂), expressing less than 3% EPCR.112 Matings of (EPCR∂/∂) males and females proceed normally and the EPCR∂/∂ pups survive well into adulthood with few unchallenged abnormalities. A similar result was obtained with EPCRlox/lox mice, which express approximately 5% EPCR.113 Use of the EPCRlox/lox mice with a Cre recombinase that generates only extraembryonic EPCR expression, or combining a total EPCR deficiency with TF−/−(tg) mice, both rescue the embryonic lethality of EPCR−/− mice and allow unchallenged EPCR−/− mice to live into adulthood.113
As with EPCR, ablation of the TM gene leads to embryonic lethality of TM−/− pups at 8.5 dpc, before establishment of a functional cardiovascular system,114 and associated with a lack of TM in nonendothelial extraembryonic tissue.115 This lack of TM results in lack of control of thrombin functions produced by TF-mediated activation of the coagulation cascade at the maternal-fetal interface.116 Restoration of TM expression in nonendothelial cells of the placenta rescues the early embryonic lethality of TM−/− mice, but an embryonic endothelium-based lethal consumptive coagulopathy, resulting from excess thrombin activity, develops at 12.5 to 16.5 dpc.115 A single amino acid replacement in the TM gene leads to a mouse line (TMpro/pro) with a greatly reduced capacity to activate PC and to inhibit thrombin. Matings of male and female TMpro/pro mice result in pups that develop to term and survive into adulthood,117 suggesting that even a low level of aPC production is a dominant event in embryonic development, essentially reproducing results found in the case of very low-PC mice. Nonetheless, a PAR-4 deficiency in the mother and/or the absence of maternal platelets partly restores development in TM−/− embryos.118 Thus, it appears that elevated thrombin levels, via PAR-4, also play a role in fetal loss in TM−/− mice. These results suggest that a delicate balance between aPC and thrombin plays a profound effect in embryonic development.
Lastly, thrombophilia associated with a deficiency of the aPC cofactor, PS, underlies unfavorable pregnancy outcomes in humans.119,120 PS−/− offspring generated from matings of PS+/− males and females also die neonatally,121 thus offering another demonstration that defects in the natural anticoagulation system lead to adverse effects on pregnancy.
Conclusions and therapeutic implications
The evidence presented in these documented observations leaves no doubt that the PC pathway, once considered only as a component of the hemostasis system, has emerged as a crucial mediator of inflammation. It is now clear that the PC pathway is strategically located at the crossroads between coagulation and inflammation, where it exerts entirely unexpected roles in tissue injury and in the damage that occurs in chronic inflammatory conditions.
In sepsis, advances in the biology of the PC pathway have been accomplished, and it is now well established that PC plays a major role in controlling vascular inflammation and immune responses. Therefore, these pathophysiologic advances should be explored and eventually adapted in other forms of tissue inflammation where inflammatory mechanisms similar to sepsis are set in motion. Given this crucial role, the PC pathway is a logical therapeutic target for resolution of chronic inflammation and tissue repair. Because of its complex biology, therapeutic strategies may vary from restoring PC activation, either by administration of aPC or by restoring TM and EPCR, to targeting the cytoprotective effects and barrier function of PAR-1. In particular, targeting of PAR-1 is attractive in chronic diseases in which either the endothelial or the epithelial barrier is disrupted, such as chronic vascular inflammation, or IBD and asthma, respectively. In addition, the recent identification of other PC receptors is beginning to reveal PC-mediated activation of other signaling pathways that regulate cytoprotective function in an EPCR-independent manner.
Considering that the treatment of all the aforementioned chronic inflammatory diseases is still a major clinical challenge and that the heterogeneity of the patient response to therapy is probably the result of heterogeneity of the mechanisms of inflammation, understanding the pathogenic role of the PC pathway offers a very promising tool in the therapeutic arsenal against atherosclerosis, IBD, asthma, RA, and virtually any chronic inflammatory disease. It is therefore an important new avenue of exploration that would benefit from the use of translational research approaches.
Acknowledgments
This work was supported by the Broad Medical Research Program, the Italian Ministery of Health (Ricerca Finalizzata 2006, no.72, and the Bando Giovani Ricercatori 2007), Fondazione Cariplo (Milan, Italy), and the Italian Association for Cancer Research, AIRC, Milan, Italy (to S.D.) and by the National Institutes of Health (grant HL073750, to F.J.C.).
National Institutes of Health
Authorship
Contribution: All authors conducted a literature search and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Silvio Danese, Division of Gastroenterology, Laboratory of Immunology and Inflammation, Istituto Clinico Humanitas-IRCCS in Gastroenterology, Viale Manzoni 56, 20089, Rozzano, Milan, Italy; e-mail: sdanese@hotmail.com; or Francis J. Castellino, W. M. Keck Center for Transgene Research, 230 Raclin-Carmichael Hall, University of Notre Dame, Notre Dame, IN 46556; e-mail: fcastell@nd.edu.