Abstract
The phase 3 trial HOVON-50 was designed to evaluate the effect of thalidomide during induction treatment and as maintenance in patients with multiple myeloma who were transplant candidates. A total of 556 patients was randomly assigned to arm A: 3 cycles of vincristine, adriamycin, and dexamethasone, or to arm B: thalidomide 200 mg orally, days 1 to 28 plus adriamycin and dexamethasone. After induction therapy and stem cell mobilization, patients were to receive high-dose melphalan, 200 mg/m2, followed by maintenance with α-interferon (arm A) or thalidomide 50 mg daily (arm B). Thalidomide significantly improved overall response rate as well as quality of the response before and after high dose melphalan. Best overall response rate on protocol was 88% and 79% (P = .005), at least very good partial remission 66% and 54% (P = .005), and complete remission 31% and 23% (P = .04), respectively, in favor of the thalidomide arm. Thalidomide also significantly improved event-free survival from median 22 months to 34 months (P < .001), and prolonged progression free from median 25 months to 34 months (P < .001). Median survival was longer in the thalidomide arm, 73 versus 60 months; however, this difference was not significant (P = .77). Patients randomized to thalidomide had strongly reduced survival after relapse. This trial was registered on www.controlled-trials.com as ISRCTN06413384.
Introduction
High-dose melphalan (HDM), followed by autologous stem cell support, has become standard of care for younger multiple myeloma (MM) patients. Impressive response rates including high percentages of complete remission (CR) and very good partial remissions (VGPR) after HDM have resulted in prolonged progression-free survival (PFS) and overall survival (OS) of high quality compared with conventional treatment.1-4 The achievement of CR plus VGPR after conventional and intensive therapy is an important prognostic factor for prolonged PFS and OS,5,6 so it is likely that more effective tumor reduction before HDM should translate into better quality of the response and improved survival after HDM. We recently published that the combination of thalidomide, adriamycin, and dexamethasone (TAD) compared with vincristine, adriamycin, and dexamethasone (VAD) induced a better response before HDM, which was maintained after intensification.7 However, the long-term effects on survival were not determined. Posttransplant therapy like thalidomide maintenance may further improve the quality of response rate after HDM. It is not clear whether this should be applied in all patients and for which period of time. It was shown that the beneficial effect on PFS and OS of maintenance thalidomide after HDM was mainly due to improved response in patients not already in CR and VGPR both in patients given thalidomide continuously and for a limited period.8 In our study, thalidomide as part of induction before and as maintenance after HDM improved response, event-free survival (EFS), and PFS, but not OS due to strongly reduced survival from relapse. These results may have implications for posttransplant therapies with thalidomide as well as for other novel agents like bortezomib and lenalidomide.
Methods
Patients with newly diagnosed MM, Salmon and Durie stage II or III, aged 18 to 65 years inclusive, were eligible for inclusion in the HOVON-50 study. The protocol was approved by the Research Ethics Board of each participating hospital, and the study was conductedin accordance with the Declaration of Helsinki (registered on www.controlled-trials.com as ISRCTN06413384). Patients underwent routine staging, including bone marrow investigations, to perform conventional karyotyping and fluorescence in situ hybridization (FISH) analysis of 13q abnormalities, as previously described,9 before start of therapy. After written informed consent was obtained, patients were randomly assigned to 3 cycles of VAD,10 vincristine (0.4 mg, intravenous [IV] rapid infusion on days 1-4), doxorubicin (9 mg/m2, IV rapid infusion on days 1-4), and dexamethasone (40 mg orally, days 1-4, 9-12, and 17-20), arm A, or to 200 to 400 mg of thalidomide orally daily, doxorubicin (9 mg/m2, IV rapid infusion on days 1-4), and dexamethasone (40 mg orally, days 1-4, 9-12, and 17-20; TAD), arm B. Thalidomide was continued from day 1 until 2 weeks before stem cell mobilization. Cycle 2 should start at day 29, and cycle 3 at day 57. The thalidomide dose could be escalated to maximally 400 mg in case of good tolerability. Patients randomized to thalidomide received thrombosis prophylaxis consisting of subcutaneously low-molecular-weight heparin nadroparine 2850 IE anti-Xa or 5700 anti-Xa in case of weight above 90 kg.11 Stem cells were mobilized using 1000 mg/m2 cyclophosphamide IV on day 1, 15 mg/m2 adriamycin IV rapid infusion on days 1 to 4, 40 mg of dexamethasone orally on days 1 to 4 (CAD), given at 4 to 6 weeks after induction treatment, plus 5 μg/kg granulocyte–colony-stimulating factor twice daily until collection. After stem cell harvest, patients received 1 or 2 courses of 200 mg/m2 HDM with autologous stem cell rescue. Centers committed to single or double HDM before start of the study. Patients achieving at least partial remission (PR) after HDM were eligible for maintenance. Patients randomized to arm A received maintenance therapy with α-interferon (3 × 106 IU, thrice weekly) starting between 2 and 3 months after HDM, and patients randomized to arm B received 50 mg of thalidomide daily starting between 2 and 3 months after HDM without venous thrombus embolism prophylaxis until relapse or progression. Patients with an human histocompatibility leukocyte antigen–identical sibling donor could proceed to nonmyeloablative allogeneic stem cell transplantation between 2 and 6 months after HDM, in the context of a separate HOVON-54 MM trial, evaluating a tandem autologous allogeneic approach.
Thalidomide for the trial was obtained from Grünenthal GmbH.
Assessments
Definition of end points
EFS was determined from the date of randomization until induction failure, progression, or death, whichever came first. Patients with an induction failure, that is, not at least a PR on protocol treatment, were considered a failure at 1 day after randomization. PFS was calculated from randomization until progression or relapse, whichever came first. OS was measured from randomization until death from any cause. Patients still alive at the date of last contact were censored.
Statistical considerations
The primary objective of the study was to compare EFS between the 2 treatment arms on an intention-to-treat basis, that is, patients were analyzed according to assignment to treatment arm A (no thalidomide) or B (with thalidomide). Patients who received an allogeneic transplantation (allogeneic stem cell transplantation [allo-SCT]) after HDM were censored for the primary end point EFS, at the date of allo-SCT. To stress the censoring, we will denote this end point as EFScens. To detect with a power of 80% a hazard ratio (HR) of 0.70, which corresponds to an increase of 3-year EFScens from 28% to 41%, and assuming approximately 10% allo-SCT, 450 patients had to be randomized and 252 events had to be observed.
Secondary end points were response (overall response rate, VGPR, and CR rate), PFS, and OS between the 2 treatment arms. Patient characteristics between the 2 treatment arms were compared using the Fisher exact test or the Pearson χ2 test in case of discrete variables, or the Wilcoxon rank sum test in case of continuous variables. The response end points were compared between the 2 treatment arms using logistic regression. Odds ratios (ORs) were calculated with a 95% confidence interval (CI).
EFScens, PFS, and OS were estimated by the Kaplan-Meier method, and 95% CIs were constructed. Survival analysis was performed using Cox regression to see whether there was a difference in survival between the 2 treatment arms. The HRs and corresponding 95% CIs were determined for all survival end points. Kaplan-Meier curves were generated to illustrate differences between the 2 treatment arms and compared using the log-rank test.
Logistic and Cox regression analysis were also used to evaluate the impact of treatment arm on response and survival when adjusted for other prognostic factors. The following baseline characteristics were included in the regression analyses: age, sex, World Health Organization performance status (0 vs 1 vs 2-3), stage according to Salmon and Durie (II vs III), immunoglobulin (Ig) isotype (IgA, IgG, other), lactate dehydrogenase (normal vs elevated), and international scoring system (ISS; 1 vs 2 vs 3).13 The MICE method of multiple imputations was used to cope with missing data on some of the baseline covariates14 to obtain 20 complete datasets. Each of those datasets was then analyzed separately, and estimates of the parameters of interest were averaged across the 20 copies to give a single estimate for the ORs and HRs with 95% CIs (see Carlin et al15 and references therein).
The impact of response at 1 year on outcome was analyzed using forest plots.16 Safety was analyzed using descriptive statistics to summarize the incidence of adverse events (AEs) and laboratory findings. Toxicity of the 2 regimens was assessed by laboratory evaluation, physical examination, vital signs, and AE assessments. AEs were scored using the National Cancer Institute common toxicity criteria, version 2.0.
All reported P values are 2-sided, and a significance level of α = .05 was used.
Results
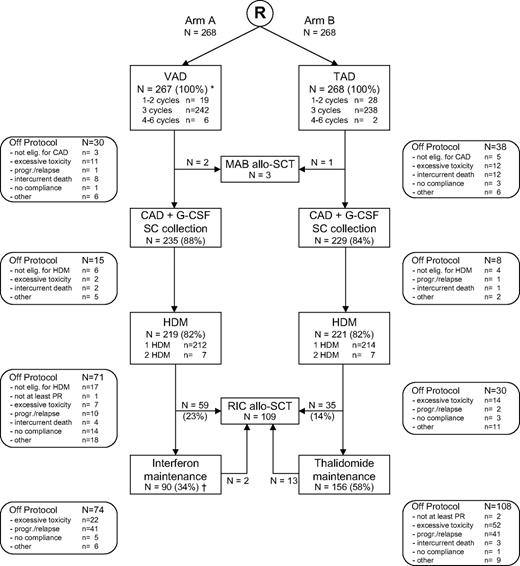
Between November 27, 2001 and May 31, 2005, 556 patients were randomized for study treatment. Twenty patients were not eligible because of stage I disease (n = 12), other cancer (n = 4), or other reasons (n = 4), and were excluded from the analysis. Of the remaining 536 patients, 268 were randomized to arm A (control arm) and 268 were randomized to arm B (treatment with thalidomide). Median age was 56 years (range, 30-65 years), 20% of patients were in Salmon and Durie stage II, and 80% of patients in stage III. Important patient characteristics and prognostic factors were equally divided in both arms (Table 1). Figure 1 shows the flow of the patients through the protocol, including reasons for going off treatment. A total of 82% of patients randomized to arm A and 82% of patients randomized to arm B received HDM and stem cell rescue. Further protocol treatment was administered in 149 of 219 patients (68%) in arm A (27% allo-SCT; 34% interferon maintenance) versus 191 of 221 patients (86%) in arm B (16% allo-SCT; 58% thalidomide maintenance).
Patient characteristics
| . | Arm A: VAD n (%) . | Arm B: TAD n (%) . | Totaln (%) . |
|---|---|---|---|
| Total | 268 | 268 | 536 |
| Sex | |||
| Male | 160 (60) | 177 (66) | 337 (63) |
| Female | 108 (40) | 91 (34) | 199 (37) |
| Age, y | |||
| Median | 56 | 57 | 56 |
| Range | 32-65 | 30-65 | 30-65 |
| Serum β2-microglobulin, mg/L | |||
| Median | 3.1 | 3.4 | 3.2 |
| Range | .0-34.8 | .1-35.4 | .0-35.4 |
| Number | 207 | 216 | 423 |
| Albumin, g/L | |||
| Median | 36.0 | 36.0 | 36.0 |
| Range | 17.1-59.1 | 4.2-57.4 | 4.2-59.1 |
| Number | 250 | 243 | 493 |
| Stage Salmon and Durie | |||
| IIA | 54 (20) | 54 (20) | 108 (20) |
| IIB | 1 (0) | 3 (1) | 4 (1) |
| IIIA | 179 (67) | 181 (68) | 360 (67) |
| IIIB | 34 (13) | 30 (11) | 64 (12) |
| M-protein | |||
| IgA | 53 (20) | 56 (21) | 109 (21) |
| IgG | 156 (59) | 161 (61) | 317 (60) |
| IgD | 3 (1) | 3 (1) | 6 (1) |
| LCD | 50 (19) | 43 (16) | 93 (18) |
| IgM | 1 (0) | — (0) | 1 (0) |
| Unknown* | 5 | 5 | 10 |
| ISS | |||
| I | 109 (56) | 98 (49) | 207 (52) |
| II | 40 (21) | 51 (25) | 91 (23) |
| III | 45 (23) | 52 (26) | 97 (25) |
| Unknown* | 74 | 67 | 141 |
| . | Arm A: VAD n (%) . | Arm B: TAD n (%) . | Totaln (%) . |
|---|---|---|---|
| Total | 268 | 268 | 536 |
| Sex | |||
| Male | 160 (60) | 177 (66) | 337 (63) |
| Female | 108 (40) | 91 (34) | 199 (37) |
| Age, y | |||
| Median | 56 | 57 | 56 |
| Range | 32-65 | 30-65 | 30-65 |
| Serum β2-microglobulin, mg/L | |||
| Median | 3.1 | 3.4 | 3.2 |
| Range | .0-34.8 | .1-35.4 | .0-35.4 |
| Number | 207 | 216 | 423 |
| Albumin, g/L | |||
| Median | 36.0 | 36.0 | 36.0 |
| Range | 17.1-59.1 | 4.2-57.4 | 4.2-59.1 |
| Number | 250 | 243 | 493 |
| Stage Salmon and Durie | |||
| IIA | 54 (20) | 54 (20) | 108 (20) |
| IIB | 1 (0) | 3 (1) | 4 (1) |
| IIIA | 179 (67) | 181 (68) | 360 (67) |
| IIIB | 34 (13) | 30 (11) | 64 (12) |
| M-protein | |||
| IgA | 53 (20) | 56 (21) | 109 (21) |
| IgG | 156 (59) | 161 (61) | 317 (60) |
| IgD | 3 (1) | 3 (1) | 6 (1) |
| LCD | 50 (19) | 43 (16) | 93 (18) |
| IgM | 1 (0) | — (0) | 1 (0) |
| Unknown* | 5 | 5 | 10 |
| ISS | |||
| I | 109 (56) | 98 (49) | 207 (52) |
| II | 40 (21) | 51 (25) | 91 (23) |
| III | 45 (23) | 52 (26) | 97 (25) |
| Unknown* | 74 | 67 | 141 |
VAD indicates vincristine, adriamycin, and dexamethasone; TAD, thalidomide, adriamycin, and dexamethasone; and LCD, light chain disease.
Data not included when calculating percentages.
Flow diagram of 536 adult patients with MM included in the HOVON-50 study by treatment arm. *One patient died 2 days after registration without any protocol treatment; †1 patient received interferon maintenance without prior HDM.
Flow diagram of 536 adult patients with MM included in the HOVON-50 study by treatment arm. *One patient died 2 days after registration without any protocol treatment; †1 patient received interferon maintenance without prior HDM.
Toxicity and compliance to the protocol
During TAD, 62% of patients were able to take the full dose of 200 mg of thalidomide daily during all cycles. Although allowed according to the study protocol, in none of the patient's dose was escalation beyond 200 mg of thalidomide daily performed. In 19% of patients, thalidomide was reduced; in 10% thalidomide was stopped, mainly due to (neuro)toxicity; and in 8% of patients randomized to TAD, thalidomide was not started. After 12, 24, and 36 months of maintenance, 68%, 47%, and 30% of patients were still taking thalidomide, whereas these percentages for interferon were 50%, 26%, and 20%, respectively. In 26% of patients, thalidomide was stopped due to progression. Thalidomide maintenance was stopped in 33% of patients due to toxicity. During follow-up, 54% of these patients showed progression. Side effects of common toxicity criteria grades 3 and 4 are summarized in Table 2. Neurologic side effects, mainly polyneuropathy (PNP), were significantly higher during induction in the thalidomide arm (grades 3-4, 15% vs 8%, P = .01; grades 2-4, 48% vs 29%, P = .007). During thalidomide maintenance (n = 156), neuropathy grade 1 was recorded in 21%, grade 2 in 33%, grade 3 in 9%, and grade 4 in 1% of patients, respectively. Deep venous thrombosis (DVT) occurred in 8% of patients during VAD and in 10% of patients during TAD. The cumulative incidence of DVT was 12% at 1 year.11
Number (%) of patients with adverse events CTC grade 3 to 4 (unless stated otherwise) during VAD/TAD cycles 1 to 3
| . | Arm A: VAD n = 267 (%) . | Arm B: TAD n = 268 (%) . | P . |
|---|---|---|---|
| Any adverse event | 100 (37) | 132 (49) | .006 |
| Cardiovascular function | 24 (9) | 25 (9) | .89 |
| Coagulation | 2 (1) | 9 (3) | .06 |
| Constitutional symptoms | 22 (8) | 25 (9) | .66 |
| Metabolic | 29 (11) | 24 (9) | .46 |
| Gastrointestinal | 14 (5) | 28 (10) | .03 |
| Pain | 16 (6) | 14 (5) | .70 |
| Neurology | 18 (7) | 35 (13) | .01 |
| Neurology CTC 2-4 | 56 (21) | 83 (31) | .008 |
| . | Arm A: VAD n = 267 (%) . | Arm B: TAD n = 268 (%) . | P . |
|---|---|---|---|
| Any adverse event | 100 (37) | 132 (49) | .006 |
| Cardiovascular function | 24 (9) | 25 (9) | .89 |
| Coagulation | 2 (1) | 9 (3) | .06 |
| Constitutional symptoms | 22 (8) | 25 (9) | .66 |
| Metabolic | 29 (11) | 24 (9) | .46 |
| Gastrointestinal | 14 (5) | 28 (10) | .03 |
| Pain | 16 (6) | 14 (5) | .70 |
| Neurology | 18 (7) | 35 (13) | .01 |
| Neurology CTC 2-4 | 56 (21) | 83 (31) | .008 |
Only adverse events with an incidence > 5% (except coagulation) are reported. Expected events such as diarrhea and mucositis were not recorded on the CRFs.
VAD indicates vincristine, adriamycin, and dexamethasone; TAD, thalidomide, adriamycin, and dexamethasone; and CTC, common toxicity criteria.
Response
Thalidomide induced a significantly higher response rate during induction before HDM, as well as a significantly higher response after HDM and “best” response on protocol. At least PR was achieved in 88% of patients randomized to arm B compared with 79% of patients randomized to arm A (OR = 1.92, 95% CI = 1.21-3.07, P = .005). Thalidomide also improved the quality of the response as determined by VGPR and CR percentages. At least VGPR on protocol was achieved in 54% of patients randomized to arm A compared with 66% of patients randomized to arm B (P = .005; Table 3). When adjusted for covariates, the differences of PR, VGPR, and CR rate remained statistically significant between the arms (Table 4).
Number (%) of patients with response during protocol treatment
| . | Arm A: VAD n = 268 (%) . | Arm B: TAD n = 268 (%) . | P . |
|---|---|---|---|
| Response on VAD/TAD | |||
| At least PR | 153 (57) | 189 (71) | .001 |
| At least VGPR | 49 (18) | 98 (37) | < .001 |
| CR | 6 (2) | 9 (3) | .43 |
| Response on VAD/TAD and HDM | |||
| At least PR | 204 (76) | 225 (84) | .02 |
| At least VGPR | 119 (44) | 145 (54) | .03 |
| CR | 31 (12) | 37 (14) | .44 |
| Response on protocol treatment | |||
| At least PR | 211 (79%) | 235 (88%) | .005 |
| At least VGPR | 144 (54%) | 176 (66%) | .005 |
| CR | 61 (23%) | 82 (31%) | .04 |
| . | Arm A: VAD n = 268 (%) . | Arm B: TAD n = 268 (%) . | P . |
|---|---|---|---|
| Response on VAD/TAD | |||
| At least PR | 153 (57) | 189 (71) | .001 |
| At least VGPR | 49 (18) | 98 (37) | < .001 |
| CR | 6 (2) | 9 (3) | .43 |
| Response on VAD/TAD and HDM | |||
| At least PR | 204 (76) | 225 (84) | .02 |
| At least VGPR | 119 (44) | 145 (54) | .03 |
| CR | 31 (12) | 37 (14) | .44 |
| Response on protocol treatment | |||
| At least PR | 211 (79%) | 235 (88%) | .005 |
| At least VGPR | 144 (54%) | 176 (66%) | .005 |
| CR | 61 (23%) | 82 (31%) | .04 |
VAD indicates vincristine, adriamycin, and dexamethasone; TAD, thalidomide, adriamycin, and dexamethasone; PR, partial remission; VGPR, very good partial remission; and CR, complete remission.
Prognostic value of treatment arm on survival end points
| . | Univariate analysis . | Adjusted for covariates . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| EFScens* | ||||||
| Arm B | .60 | .48-.75 | < .001 | .55 | .44-.69 | < .001 |
| Age | 1.01 | .99-1.02 | .42 | 1.01 | .99-1.02 | .39 |
| Female | .86 | .68-1.09 | .22 | .83 | .65-1.06 | .14 |
| WHO performance | 1.31 | 1.12-1.53 | .001 | 1.19 | 1.01-1.41 | .04 |
| S&D stage 3 | 1.41 | 1.06-1.88 | .02 | 1.21 | .90-1.64 | .21 |
| IgA | 1.20 | .92-1.57 | .17 | 1.28 | .90-1.80 | .17 |
| IgG | .91 | .73-1.14 | .42 | 1.04 | .77-1.39 | .80 |
| LDH > ULN | 1.81 | 1.38-2.38 | < .001 | 1.72 | 1.29-2.30 | < .001 |
| Log (β2m) | 1.31 | 1.11-1.54 | .001 | n.i. | — | — |
| Albumin | .98 | .96-1.00 | .02 | n.i. | — | — |
| ISS | 1.35 | 1.17-1.55 | < .001 | 1.27 | 1.09-1.48 | .002 |
| PFS | ||||||
| Arm B | .67 | .55-.82 | < .001 | .62 | .50-.76 | < .001 |
| Age | 1.02 | 1.00-1.03 | .04 | 1.02 | 1.00-1.03 | .02 |
| Female | .85 | .69-1.05 | .14 | .82 | .66-1.02 | .07 |
| WHO performance | 1.26 | 1.10-1.46 | .001 | 1.17 | 1.00-1.35 | .04 |
| S&D stage 3 | 1.33 | 1.03-1.72 | .03 | 1.18 | .90-1.55 | .22 |
| IgA | 1.28 | 1.01-1.63 | .04 | 1.37 | 1.00-1.87 | .05 |
| IgG | .90 | .73-1.10 | .32 | 1.06 | .80-1.39 | .68 |
| LDH > ULN | 1.83 | 1.42-2.36 | < .001 | 1.71 | 1.31-2.24 | < .001 |
| Log (β2m) | 1.28 | 1.09-1.49 | .002 | n.i. | — | — |
| Albumin | .98 | .97-1.00 | .02 | n.i. | — | — |
| ISS | 1.29 | 1.13-1.46 | < .001 | 1.23 | 1.07-1.41 | .003 |
| OS | ||||||
| Arm B | .96 | .74-1.25 | .77 | .92 | .71-1.20 | .54 |
| Age | 1.01 | .99-1.03 | .32 | 1.01 | .99-1.03 | .30 |
| Female | .87 | .66-1.14 | .31 | .90 | .68-1.20 | .48 |
| WHO performance | 1.27 | 1.06-1.52 | .009 | 1.16 | .96-1.40 | .13 |
| S&D stage 3 | 1.46 | 1.03-2.08 | .04 | 1.22 | .84-1.76 | .30 |
| IgA | 1.20 | .88-1.64 | .25 | 1.33 | .89-2.01 | .17 |
| IgG | .95 | .73-1.24 | .71 | 1.16 | .81-1.65 | .41 |
| LDH > ULN | 2.09 | 1.54-2.84 | < .001 | 1.83 | 1.33-2.51 | < .001 |
| Log (β2m) | 1.53 | 1.26-1.86 | < .001 | n.i. | — | — |
| Albumin | .97 | .96-.99 | .007 | n.i. | — | — |
| ISS | 1.43 | 1.21-1.69 | < .001 | 1.33 | 1.11-1.60 | .002 |
| . | Univariate analysis . | Adjusted for covariates . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| EFScens* | ||||||
| Arm B | .60 | .48-.75 | < .001 | .55 | .44-.69 | < .001 |
| Age | 1.01 | .99-1.02 | .42 | 1.01 | .99-1.02 | .39 |
| Female | .86 | .68-1.09 | .22 | .83 | .65-1.06 | .14 |
| WHO performance | 1.31 | 1.12-1.53 | .001 | 1.19 | 1.01-1.41 | .04 |
| S&D stage 3 | 1.41 | 1.06-1.88 | .02 | 1.21 | .90-1.64 | .21 |
| IgA | 1.20 | .92-1.57 | .17 | 1.28 | .90-1.80 | .17 |
| IgG | .91 | .73-1.14 | .42 | 1.04 | .77-1.39 | .80 |
| LDH > ULN | 1.81 | 1.38-2.38 | < .001 | 1.72 | 1.29-2.30 | < .001 |
| Log (β2m) | 1.31 | 1.11-1.54 | .001 | n.i. | — | — |
| Albumin | .98 | .96-1.00 | .02 | n.i. | — | — |
| ISS | 1.35 | 1.17-1.55 | < .001 | 1.27 | 1.09-1.48 | .002 |
| PFS | ||||||
| Arm B | .67 | .55-.82 | < .001 | .62 | .50-.76 | < .001 |
| Age | 1.02 | 1.00-1.03 | .04 | 1.02 | 1.00-1.03 | .02 |
| Female | .85 | .69-1.05 | .14 | .82 | .66-1.02 | .07 |
| WHO performance | 1.26 | 1.10-1.46 | .001 | 1.17 | 1.00-1.35 | .04 |
| S&D stage 3 | 1.33 | 1.03-1.72 | .03 | 1.18 | .90-1.55 | .22 |
| IgA | 1.28 | 1.01-1.63 | .04 | 1.37 | 1.00-1.87 | .05 |
| IgG | .90 | .73-1.10 | .32 | 1.06 | .80-1.39 | .68 |
| LDH > ULN | 1.83 | 1.42-2.36 | < .001 | 1.71 | 1.31-2.24 | < .001 |
| Log (β2m) | 1.28 | 1.09-1.49 | .002 | n.i. | — | — |
| Albumin | .98 | .97-1.00 | .02 | n.i. | — | — |
| ISS | 1.29 | 1.13-1.46 | < .001 | 1.23 | 1.07-1.41 | .003 |
| OS | ||||||
| Arm B | .96 | .74-1.25 | .77 | .92 | .71-1.20 | .54 |
| Age | 1.01 | .99-1.03 | .32 | 1.01 | .99-1.03 | .30 |
| Female | .87 | .66-1.14 | .31 | .90 | .68-1.20 | .48 |
| WHO performance | 1.27 | 1.06-1.52 | .009 | 1.16 | .96-1.40 | .13 |
| S&D stage 3 | 1.46 | 1.03-2.08 | .04 | 1.22 | .84-1.76 | .30 |
| IgA | 1.20 | .88-1.64 | .25 | 1.33 | .89-2.01 | .17 |
| IgG | .95 | .73-1.24 | .71 | 1.16 | .81-1.65 | .41 |
| LDH > ULN | 2.09 | 1.54-2.84 | < .001 | 1.83 | 1.33-2.51 | < .001 |
| Log (β2m) | 1.53 | 1.26-1.86 | < .001 | n.i. | — | — |
| Albumin | .97 | .96-.99 | .007 | n.i. | — | — |
| ISS | 1.43 | 1.21-1.69 | < .001 | 1.33 | 1.11-1.60 | .002 |
EFS indicates event-free survival; PFS, progression-free survival; OS, overall survival; WHO, World Health Organization; S&D, Salmon and Durie; ISS, International Scoring System; LDH, lactate dehydrogenase; ULN, upper limit of normal; β2m, β2-microglobulin; and n.i., not included in multivariate analysis (already used for ISS).
The subscript “cens” indicates that patients are censored at allo-SCT.
EFS, PFS, and OS
The survival end points are based on follow-up data available as of May 2009. The median follow-up of 309 patients still alive is 52 months (range, 2-86).
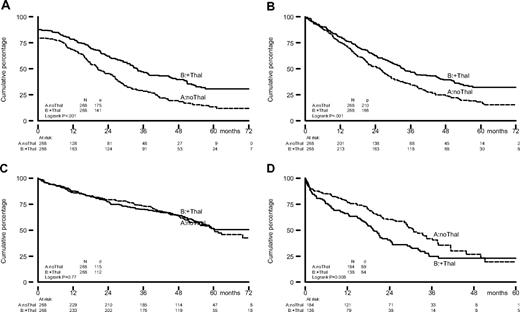
Patients randomized to thalidomide had a significantly prolonged EFScens (34 months vs 22 months; HR = 0.60, 95% CI = 0.48-0.75; P < .001) and PFS (34 months vs 25 months; HR = 0.67, 95% CI = 0.55-0.82; P < .001); see Table 4 and Figure 2. OS curves in both arms were comparable: median 60 months for the patients in the control arm and 73 months for the patients randomized to thalidomide, HR = 0.96, 95% CI = 0.74-1.25, P = .77 (Table 4 and Figure 2).
Kaplan-Meier estimates of EFScens, PFS, and OS from randomization, and OS from progression/relapse for patients who were randomized to TAD for induction to and thalidomide maintenance after HDM (solid line) or to VAD for induction to, and α-interferon maintenance after HDM (dotted line). For EFScens, estimate patients who received an allogeneic transplantation (allo-SCT) after HDM were censored and excluded at the date of allo-SCT. (A) EFScens, (B) PFS, (C) OS, and (D) OS from progression/relapse.
Kaplan-Meier estimates of EFScens, PFS, and OS from randomization, and OS from progression/relapse for patients who were randomized to TAD for induction to and thalidomide maintenance after HDM (solid line) or to VAD for induction to, and α-interferon maintenance after HDM (dotted line). For EFScens, estimate patients who received an allogeneic transplantation (allo-SCT) after HDM were censored and excluded at the date of allo-SCT. (A) EFScens, (B) PFS, (C) OS, and (D) OS from progression/relapse.
The HRs remained very similar when the analyses were adjusted for covariates, as can been seen in Table 4.
Median overall survival from progression/relapse was shorter for the patients randomized to thalidomide. Median OS was 20 months versus 31 months in arm A (HR = 1.50, 95% CI = 1.11-2.02, P = .009).
According to the protocol, PFS and OS have not been censored at allo-SCT. However, afterward, it was decided also to compare PFS and OS between the 2 arms with censoring at allo-SCT (PFScens and OScens). PFScens remained significantly improved in the thalidomide arm (HR = 0.59, 95% CI = 0.47-0.74, P < .001), whereas there was no significant impact on OScens (HR = 0.86, 95% CI = 0.64-1.14, P = .29).
Landmark analysis to determine impact of response
We have chosen for the landmark analysis to evaluate the prognostic impact of an early VGPR or CR, as maintenance was started very early after HDM, which excludes determining the response induced by HDM alone. Based on the best response achieved within 12 months, 373 patients were classified as CR (n = 60), VGPR (n = 176), or PR (n = 137). PFS and OS were calculated from 12 months. Patients achieving a CR within 12 months had improved PFS and OS, although this was not statistically significant; 68% of patients who were in CR at 12 months were still alive at 6 years.
Effect of thalidomide in different response categories
In a previous report, the prolonged PFS and OS induced by thalidomide maintenance was only apparent in patients who had not (yet) achieved a VGPR or CR after HDM.8 Also in our study, thalidomide treatment resulted in a prolonged PFS in the group of 373 patients with at least a PR at 12 months. However, in neither response category did the PFS prolongation result in an improved OS.
Impact of chromosome 13 abnormalities
Conventional karyotyping data were available in 421 (79%) patients. A chromosome 13 abnormality, defined as −13q or –13, was present in 79 (19%) patients, 18.5% in patients randomized to nonthalidomide, and 19.4% patients randomized to thalidomide. In univariate analysis, abnormalcy of chromosome 13 [abn (13)] had no statistically significant impact on PR, VGPR, CR, EFScens, PFS, or OS. There was also no statistically significant interaction between abn (13) and treatment arm, indicating that the effect of thalidomide was not significantly different in patients with or without abn (13).
FISH data regarding chromosome 13 abnormality were available for 294 (55%) patients. A chromosome 13 abnormality was present in 101 (34%) patients, 33.3% in patients randomized to nonthalidomide, and 34.8% of patients randomized to thalidomide. The presence of a FISH abn (13) had no significant impact on response and survival, except for PFS. Univariate analysis showed that patients with abn (13) had worse PFS (HR = 1.38, 95% CI = 1.04-1.84, P = .03). FISH analysis was not centralized and was performed on unpurified bone marrow samples.8 This may explain the rather low incidence of chromosome 13 abnormalities in our population.
Discussion
Our study was designed to evaluate whether thalidomide added to induction before and during maintenance after intensive therapy would result in a better outcome for newly diagnosed myeloma.17 We hypothesized that more effective tumor reduction before autologous stem cell transplantation using HDM should result into a higher and better quality of the response after intensification, which is probably the best situation to sustain the response by maintenance treatment.
Although thalidomide improved the response before and after HDM, prolonged EFS, and PFS, this benefit was not translated into a statistically significant lengthened survival. One explanation might be that 64% of relapsed patients from the nonthalidomide arm received thalidomide during salvage therapy compared with 38% of patients with relapse in the thalidomide arm. Alternatively, the reduced postrelapsed survival could be due to generation of aggressive drug-resistant clones that generate relapses after prolonged thalidomide exposure. Median survival from relapse was only 20 months for the patients randomized to thalidomide, although 51% of patients received bortezomib during salvage therapy compared with 39% of patients from the nonthalidomide arm. For lenalidomide, these percentages were 18% and 16%, and for a second autologous stem cell transplantation, 9% and 5%, respectively.
Our results are quite comparable with Total Therapy II, in which, as in our trial, in the thalidomide arm thalidomide was given both before and after HDM.18 The first results of this study, initially reported in 2006, also showed improved response and PFS, but no benefit in OS because of significantly shorter survival after relapse in the thalidomide group. Median overall survival from relapses was only 1.1 years for patients randomized to thalidomide versus 2.7 years for the nonthalidomide patients. Only after prolonged follow-up of median 72 months did a favorable effect of thalidomide survival become apparent for the one-third of patients with cytogenetic abnormalities.19 This might also become obvious with longer follow-up of our study, as we can observe a divergence in the OS curves at 5 years (Figure 2C), resulting in a prolongation of survival from 60 to 73 months for the thalidomide group. Although this difference is not (yet) statistically significant, this may still be of high clinical relevance from a patient's perspective.
The role, including positioning and duration of application, of thalidomide in combination with intensive therapy seems not yet defined. In the Intergroupe Francophone du Myélome (IFM) study with low risk patients defined by normal β2-microglobulin levels and no chromosome 13 abnormalities as determined by FISH, thalidomide maintenance 200 mg/daily after HDM prolonged EFS, PFS, and OS.8 In this study, thalidomide was not used before HDM. In the IFM trial, only patients not in VGPR and CR had a survival advantage from thalidomide, indicating that the beneficial effect of thalidomide was due to further tumor reduction rather than maintaining the achieved response. Most recent data are from an Australian study showing thalidomide consolidation for 12 months after autologous stem cell transplantation improved response, PFS, and OS.20 This improvement was not restricted to patients failing to achieve CR/VGPR. In this study, initial induction therapy was free and was mostly VAD-like, and thalidomide was combined with prednisone maintenance. The authors state that the positive outcome of their study is most likely due to the use of a sequential noncross-resistant antimyeloma approach.
These results seem to be in contrast with our study and the initial results of Total Therapy II. The HOVON and the Total Therapy II studies cannot be compared, as in the IFM and in the Australian studies, the only question was the role of thalidomide as maintenance after transplant. It is remarkable, however, that as well as in the HOVON-50 and Total II, survival from relapse was significantly shorter, HOVON-50 survival at 1 year 66% versus 77% and Total II 52% versus 76%, whereas 1-year survival from relapse was identical irrespective of thalidomide in the IFM study, 73% versus 78%, and in the Australian study, 79% and 77%, respectively.
Conflicting results have also been obtained with thalidomide in combination with melphalan and prednisone (MP) in elderly patients. In both IFM studies,21,22 the Italian23 and in the HOVON study,24 MP-thalidomide improved response and PFS compared with MP. A survival benefit, however, for MP-thalidomide was only observed in the IFM studies. An important difference in design between the studies was that thalidomide in the IFM studies was given for a limited period, and in the Italian and HOVON studies until relapse.
The impact of prior exposure and response to thalidomide (induction) therapy on its use as maintenance treatment is currently unknown. Taking all data together, a cautious conclusion might be that limited duration of postinduction/intensification therapy until maximal response is preferable to avoid resistant relapse and to minimize the side effects of prolonged thalidomide exposure. However, with the advent of many novel treatment options in relapsed multiple myeloma, PFS and EFS are probably better end points than OS, to assess the impact of a particular treatment regimen.
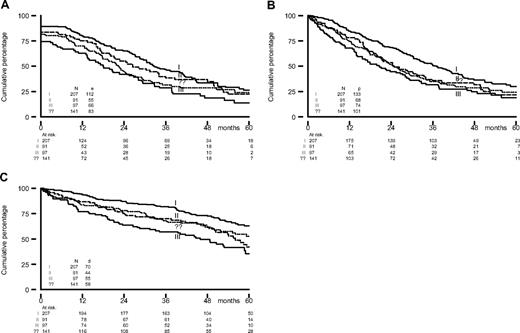
Elevated lactate dehydrogenase, the presence of IgA, Salmon and Durie stage III, and a higher ISS score predicted for inferior outcome on one or more end points (Table 4). It is important to notice how well the ISS stage differentiated between the different risk groups, which confirms the importance of this new prognostic staging system (Figure 3). Chromosome 13 abnormalities as determined by conventional karyotyping and FISH had no prognostic significance. Unfortunately, current well-known prognostic factors like 17p- and t(4;14) were not determined. Thalidomide did not reverse the impact of other unfavorable prognostic factors.
EFScens, PFS, and OS from randomization by ISS score. (A) EFScens, (B) PFS, and (C) OS.
EFScens, PFS, and OS from randomization by ISS score. (A) EFScens, (B) PFS, and (C) OS.
Neurotoxicity of thalidomide was high, although relatively low dosages during induction and during maintenance were used and recommendations for dose adjustments were given. PNP grades 2 to 4 developed in nearly 50% of patients, and in 58% of patients thalidomide was stopped or dose reduced. In the Total Therapy II trial, 30% of patients had to stop with thalidomide due to side effects in the first 2 years, also mainly because of PNP. The incidence of DVT was not higher in the thalidomide arm, probably as a result of the preventive effect of low-molecular-weight heparin for thalidomide-induced DVT.11 The number of patients who started maintenance with interferon was low, probably due to fear of patients and doctors for expected side effects. As there seemed to be also no favorable effect on PFS and EFS, our results also indicate that there is probably no role for interferon maintenance anymore.
The achievement of VGPR and CR has been shown to be a major prognostic factor for survival. This was partially confirmed by our landmark analysis for best response achieved within 12 months, which showed patients in CR had an impressive 68% survival at 6 years. It has already been shown that the application of the novel antimyeloma agents bortezomib and lenalidomide into front-line therapy may be even more effective with regard to improving the response before and after autologous stem cell transplantation.25-29 The combination of bortezomib with dexamethasone explored by the IFM in newly diagnosed patients induced at least PR in 80% of patients, including 47% of patients with at least VGPR and 21% of patients with CR/non-CR (near CR [nCR]).25 After HDM, the quality of response further improved to at least 62% VGPR, including 35% of patients with a CR/nCR. Even better results were obtained with the combination bortezomib/thalidomide/dexamethasone as induction therapy, resulting in at least 77% VGPR, including 54% of patients with a CR/nCR.26 In addition, lenalidomide combined with dexamethasone is highly effective, as shown by an overall response of more than 90%, including 57% of patients with CR/nCR.27 However, the long-term effect on PFS and OS of such (expensive) combination therapies as induction must be established before these can be recommended outside clinical trials. What also remains is how to implement the best maintenance strategy posttransplant, that is, which response category should receive maintenance, the duration of treatment, for a limited period as short consolidation or until relapse, and the analysis of the resistance profile at relapse. These are important issues that can only be addressed in randomized trials.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Presented in part at the 50th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 7, 2008.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.M.L. and P.S. designed and directed the study and edited the manuscript; S.Z., E.V., S.C., M.H.v.O., P.v.d.B, P.W., R.S., O.d.W., S.W., M.D., H.B., G.M.B., K.-S.G.J., H.S., M.v.M.-K., P.J., and M.C.M. and contributed patients to the study and reviewed the manuscript; B.v.d.H. participated in designing the research protocol, performed statistical analyses, and reviewed data and the manuscript; and R.v.A. reviewed data and the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of Dutch-Belgian HOVON participants, see the supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Henk M. Lokhorst, Department of Hematology, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; e-mail: h.lokhorst@umcutrecht.nl.