During antigen recognition by T cells, membrane receptors and cytoskeletal molecules form a specialized structure at the T cell–antigen-presenting cell junction called the immune synapse (IS). We report a role for the scaffolding protein A-kinase anchoring protein-450 (AKAP450), a member of the A-kinase anchoring protein family, in IS formation and T-cell signaling in antigen- and superantigen-dependent T-cell activation. Suppression of AKAP450 by overexpression of a dominant-negative form or siRNA knockdown disrupted the positioning and conformational activation of lymphocyte function-associated antigen 1 at the IS and impaired associated signaling events, including phosphorylation of phospholipase C-γ1 and protein kinase C-θ. AKAP450 was also required for correct activation and phosphorylation of CD3, LAT, and Vav1, key T-cell receptor-activated intracellular signaling molecules. Consistently, antigen-triggered reorientation of the microtubule-organizing center at the IS and interleukin-2 secretion were diminished in AKAP450-disrupted T cells. These results indicate key roles for AKAP450 in the organization and activation of receptor molecules at the IS during T-cell signaling events.
Introduction
During the process of antigen recognition by T cells, structural and spatial changes take place at the immune synapse (IS) formed at the contact with antigen-presenting cells (APCs), involving various recognition and signaling molecules as well as cytoskeletal components. In T cells, the IS contains a central supramolecular activation cluster (c-SMAC), which includes the T-cell receptor (TCR)/CD3 complex and various costimulatory receptors, and a peripheral ring (p-SMAC) where molecules, such as lymphocyte function-associated antigen 1 (LFA-1) and talin, localize.1,–3
The formation of the IS is associated with a substantial remodeling of the actin and tubulin cytoskeleton.4,5 An early event on antigen recognition is the translocation of the centrosome, or microtubule-organizing center (MTOC), and the associated Golgi apparatus (GA) and secretory vesicles toward the nascent IS, bringing them in close apposition with the APCs.6 This process provides the basis for polarized secretion of various molecules by the T cell, thus favoring controlled communication with the APCs.7,8 Moreover, during asymmetric T lymphocyte division, stable orientation of original MTOC is essential to initiate differentiation associated with the adaptive immune responses of T cells.9
The physical proximity of the MTOC and GA in mammalian cells is accompanied by a functional link between these organelles. The Golgi protein Golgi Matrix 130 (GM130) has recently been implicated in the regulation of MTOC morphology and function,10,11 and Golgi Reassembly Stacking protein 65 (GRASP65) is another Golgi-associated protein required for MTOC/GA remodeling and positioning.12 The scaffolding protein CG-NAP (centrosome and Golgi localized protein kinase N–associated protein), also known as A-kinase anchoring protein-9 (AKAP9), AKAP450, or AKAP350, is found at the MTOC in most cells but is not restricted to this location.13,14 This molecule is a member of the AKAP (A-kinase anchoring proteins) family and recruits numerous proteins involved in signal transduction. In addition to protein kinases, such as PKA, protein kinase N, or protein kinase C-ϵ (PKC-ϵ), AKAP450 also anchors phosphatases, including protein phosphatase 1 (PP1) and PP2A.13,15,16 Specific displacement of endogenous AKAP450 by expression of the AKAP450 C-terminus, which contains the MTOC-targeting domain but not its coiled-coil domains or binding sites for signaling molecules, disrupts centrosomal function and induces cell-cycle arrest at G1 in HeLa cells, showing defects in cytokinesis.17 In addition, Ran-GTP, involved in nuclear import and export proteins and microtubule nucleation, associates with AKAP450 at the MTOC.18 AKAP450 is also a component of the LFA-1–induced signaling complex involved in T-cell migration19 ; integrins, particularly LFA-1, are critical for leukocyte migration and for the correct formation of the IS.20 Furthermore, AKAP450 interacts with the dynein/dynactin complex, and this may be involved in the binding of the GA to the MTOC.21 Finally, AKAP450 has recently been shown to cooperate with GM130 in microtubule (MT) nucleation at the cis-side of the GA.22
The molecular mechanisms involved in the reorientation of the MTOC/GA toward the T-APC cell-cell contact remain poorly understood; however, MTOC repositioning after CD3 stimulation is known to be dependent on src kinases and immunoreceptor tyrosine-based activation motif phosphorylation,23 and the membrane-associated tyrosine kinase Fyn has been reported to play an important role in MTOC translocation on TCR engagement.24 How MTOC repositioning may influence surface receptor organization at the IS architecture and T-cell activation are additional important issues that deserve in-depth studies. Recently, it has been described that the function of Histone deacetylase 6 (HDAC6)25 and the microtubule motor complex dynein-dynactin26 are key for MTOC/GA relocation, acting as a mechanism to favor the formation of a multimolecular platform connecting TCRs and intracellular signaling proteins that controls T-cell activation.
To further explore the links between the IS formation, T-cell signaling, and the polarized positioning of the MTOC/GA, we have examined the role of AKAP450 in IS organization. Our results show that abolition of AKAP450 function during the formation of the IS disrupts LFA-1 organization and conformational activation into the p-SMAC and is associated with impaired activation of the CD3 complex, alterations to the intracellular signals induced through T-APC contact, and defects in MTOC translocation and interleukin-2 (IL-2) secretion.
Methods
Cells
Jurkat J77cl20 (J77) T cells and the lymphoblastoid B-cell lines Raji and HOM-2 were cultured in complete medium (RPMI 1640 and 10% fetal calf serum; Invitrogen). CH7C17 T cells (bearing a hemagglutinin [HA])–peptide-specific Vβ3 TCR)26 were cultured in complete medium supplemented with 400 μg/mL hygromicin B and 4 μg/mL puromycin. Peripheral blood lymphocytes were isolated from freshly prepared buffy coat from healthy donors, subjected to gradient centrifugation on Histopaque-1077 from Sigma-Aldrich, and followed by 2 rounds of plastic adherence. Human T lymphoblasts were obtained from 10-day culture Staphylococcal Enterotoxin E (SEE)–stimulated peripheral blood lymphocytes. CD4+ human primary T cells were isolated by negative selection using AutoMACS cell separation system, following the manufacturer's recommendations (Miltenyi Biotec). The purity of the obtained T cells was analyzed by flow cytometry.
Antibodies and reagents
The primary antibodies used were as follows: T3b (anti–human-CD3) and TP1/55 (anti–human CD69) were produced in the laboratory. A24 rabbit antibody, produced by Dr W. Kemmner (Munich, Germany), was raised against a His-tagged version of the clone 24 coding for chicken AKAP450 and recognizes exons 24 to 27 of the protein. The APA 1/1 and anti-CD3ζ monoclonal antibodies (mAbs) have been described previously.27 TS2/4 mAb has been described previously.28 m24 and Kim127 mAbs have been described previously.29 The 448 antibody (anti–human CD3) was a kind gift from Dr B. Alarcón (Centro de Biología Molecular). The anti–phosphoVav Y174 mAb was a kind gift from Dr X. Bustelo (Centro de Investigación del Cáncer). JL-8 anti–green fluorescent protein (GFP) and anti–human Vβ8 mAbs were from BD Biosciences. Unconjugated and fluorescein isothiocyanate–conjugated anti–α-tubulin, phalloidin, and anti–γ-tubulin mAbs were from Sigma-Aldrich. Anti-PKC-θ (Sc-13 and Sc-18) was from Santa Cruz Biotechnology. Anti-Vav, anti-LAT, anti–phospho-Erk1/2 (T202/Y204), and anti–phospho-LAT (Y191) were from Millipore. Anti–phospho-LAT (Y132) and anti-giantin were from Abcam. Anti–phospho-phospholipase C-γ1 (PLC-γ1) (Y783), anti–PLC-γ-1, and anti–phospho-PKC-θ (T538) were from Cell Signaling. Anti–phospho-CD3ζ (Y83) was from Epitomics.
Human fibronectin (FN) and poly-L-lysine (PLL) were from Sigma-Aldrich, SEE from Toxin Technology, puromycin from InvivoGen, and hygromycin B from Roche Diagnostics. Anti-Erk 1/2 and the fluorescent secondary antibodies (Alexa 568 and 647, and rhodamine red X) and cell trackers (7-amino-4-chloromethylcoumarin [CMAC], 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine) were obtained from Invitrogen. Propidium iodide was from Sigma-Aldrich. All other reagents were of the purest grade available.
Recombinant DNA constructs and cell transfection
The cDNA encoding the C-terminal GFP-linked CG-NAP/AKAP450 construct has been described.19 The indicated cDNAs were transiently transfected into T cells (2 × 107) with the Bio-Rad Gene Pulser II electroporation system. At 16 hours after transfection, viable J77 or CH7C17 cells were isolated by centrifugation on a Ficoll-Hypaque gradient. Primary T cells and human T lymphoblasts were transfected with Nucleofector electroporation-based system from Amaxa Biosystems following the manufacturer's recommendations.
siRNA experiments
J77 or CH7C17 T cells were electroporated twice (at 0 and 72 hours) with a double-stranded siRNA (21 bp) against AKAP450 (5′-AACUUUGAAGUUAACUAUCAA-3′; Eurogentec) at a final concentration of 20μM per sample. Cells were then collected, and the efficiency of gene silencing was assessed by Western blot. Control-transfected cells (siRNA-negative; Eurogentec) were used as a negative control. Cells were used for experiments on day 6.
Cell conjugate formation and immunofluorescence analysis
CMAC-loaded Raji B cells were incubated in Hanks balanced salt solution for 30 minutes with or without 0.5 μg/mL SEE, centrifuged at low speed, and allowed to form conjugates with J77 Jurkat cells or SEE-CD4 human primary T cells for 20 minutes at 37°C. HOM-2 cells were also loaded with CMAC, incubated for 2 hours with 50 μg/mL HA peptide, and allowed to form conjugates with CH7C17 cells. In these assays, the T cells (2 × 105) were mixed with an equal number of APCs in a final volume of 80 μL, gently resuspended, and plated onto slides coated with PLL (Jurkat) or FN (CH7C17). Cells were fixed with 4% paraformaldehyde in PHEM/saccharose and permeabilized when needed for 5 minutes in 2% paraformaldehyde and 0.2% Triton X-100 in PHEM/saccharose, blocked, and stained with the indicated Abs.26 Stained cells were mounted in a Mowiol-based mounting solution (ProLong Gold antifade reagent; Invitrogen) and observed at room temperature under Leica DMR photomicroscope fitted with an HCX PL APO 63×/1.32-0.6 oil objective (Leica Microsystems) coupled to a COHU 4912-5010 charge-coupled camera. The acquisition software was QFISH V2.1 (Leica), and images were processed with Photoshop CS (Adobe Systems). Alternatively, cells were analyzed at 18°C with a confocal laser scanning unit (TCS SP5; Leica) attached to an inverted epifluorescence microscope (DMI6000; Leica) fitted with an HCX PL APO 63×/1.40-0.6 oil objective. Images were acquired and processed with accompanying confocal software (LCS; Leica) or WCIF ImageJ (http://rsbweb.nih.gov/ij/). Three-dimensional maximal projections of the T-cell/APC contact area were generated with the ImageJ to obtain a z stack projection. Figures were composed with Photoshop CS.
Analysis of active LFA-1, CD3, and PKC-θ accumulation at the T-APC contact area
T-APC conjugates were formed between GFP or C-term AKAP450-GFP T cells and HA- or SEE- loaded APCs, and processed with ImageJ software. Using region of interest of the same area for all measurements, we quantified the signal generated by the T-APC interaction at the contact area (IS), the signal at the area of APC membrane not in contact with the T cell (B), at the area of T-cell membrane not in contact with the APC (T), and the background (Bg). We first subtracted the Bg signal from the other measurements and then obtained ratio of the corresponding protein at the IS in relation to the rest of the T cell, according to the formula (IS − B)/T. We assumed that protein accumulation was homogeneous in the APCs, with no additional accumulation at the contact zone with the T cell.
Time-lapse fluorescence confocal microscopy
Raji APCs (5 × 105; SEE-pulsed or controls) were allowed to adhere on FN-coated coverslips. The cells were maintained in 1 mL of Hanks balanced salt solution (2% bovine serum albumin) in Attofluor open chambers (Invitrogen) at 37°C in a 5% CO2 atmosphere, and placed on the microscope stage. Jurkat T cells (5 × 105) were added, and a series of fluorescence and differential interference contrast frames were captured simultaneously every 30 seconds using a TCS SP5 confocal laser scanning unit attached to an inverted epifluorescence microscope (DMI6000) with an HCX PL APO 63×/1.40-0.60 oil objective. Images were acquired with the accompanying confocal software (LCS; Leica) and processed with ImageJ software. Premiere 6.0 software (Adobe) was used for generating QuickTime videos (Apple).
MTOC translocation and functional assays
T cells were mixed with Raji or HOM-2 cells at a 1:1 ratio for 15 minutes at 37°C. Cell conjugates were gently resuspended, plated onto PLL- or FN-coated coverslips for an additional 15 minutes, and processed for immunofluorescence. The MTOC was scored as reoriented when it was located in close proximity to the T-cell plasma membrane at the contact area with the APCs. At least 300 cell conjugates were scored in each experiment. Results are expressed as the percentage of conjugates with MTOC reoriented to the contact area.
For IL-2 production and CD69 expression assays, conjugates were formed and cultured in flat-bottom, 96-well plates. Culture supernatant was harvested after 16-hour culture and analyzed for IL-2 concentration by enzyme-linked immunosorbent assay (Diaclone, Gen-Probe-TDI). T cells from these cultures were analyzed for CD69 expression by flow cytometry using a FACSCalibur flow cytometer and were processed with CellQuest Pro 4.0.2 software (BD Biosciences).
Immunoblotting
For analysis of protein phosphorylation during formation of the IS, Raji or HOM-2 cells (1 × 106) were preloaded with 0.5 μg/mL SEE (30 minutes) or 50 μg/mL HA peptide (2 hours) at 37°C and mixed with 5 × 106 Jurkat or CH7C17 cells at 37°C, respectively. Cells were lysed at 4°C (40 minutes) in 50mM Tris-HCl, pH 7.5, containing 1% NP-40, 0.2% Triton X-100, 150mM NaCl, and phosphatase and protease inhibitors. Cell lysates were spun at 2500g (10 minutes) to remove cell debris and nuclei. Whole lysates were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, and probed with the indicated antibodies in Tris-buffered saline–Tween 20. Bound antibodies were reacted with horseradish peroxidase secondary antibodies, and membranes were developed by enhanced chemiluminescence with SuperSignal West Pico or Femto chemiluminescent substrate (Pierce Chemical). Densitometric analyses were performed with ImageGauge 3.46 software (Fujifilm).
Statistical analysis
Data were tested for normality using the D'Agostino-Pearson omnibus normality test. Differences between means were tested by Student t test for normal data, whereas no normal data were analyzed using the Mann-Whitney test. Significant differences are considered with a P value of less than .05.
Results
AKAP450 is required for the positioning and activation of LFA-1 at p-SMAC of IS
The formation and maturation of the IS involve the respective clustering of CD3 and LFA-1 at the c-SMAC and p-SMAC.1 To assess the possible role of AKAP450 in SMAC organization, we overexpressed a dominant-negative form of AKAP450 in T cells. The dominant-negative form, composing the GFP-tagged AKAP450 C-terminus, affects neither the expression of endogenous AKAP450 (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) nor the cell cycle of the T cells (supplemental Figure 1B) and colocalizes with γ-tubulin in MTOC (supplemental Figure 1C). CD3 and LFA-1 localization in T cell–APC conjugates was analyzed by confocal fluorescence microscopy. The number of total cell conjugates formed was quantified. No significant differences were found between GFP and C-term AKAP450-GFP–expressing cells (supplemental Figure 1D). CD3 was apparently localized at the central area of the IS in both control GFP and C-term AKAP450-GFP cells (Figure 1A). Analysis of LFA-1 showed that it was not clearly separated from the c-SMAC in cells expressing C-term AKAP450-GFP, and 3-dimensional reconstruction confirmed that the LFA-1 p-SMAC ring was deformed in these cells (Figure 1A). Fluorescence histograms confirmed that LFA-1 overlapped with CD3 at the c-SMAC in C-term AKAP450-GFP cells (histograms in Figure 1A), consistent with the view that AKAP450 functionality might be necessary for correct segregation of this integrin to the p-SMAC. Quantitative analysis of LFA-1 alteration at the IS is shown (graph in Figure 1A). The expression levels of LFA-1, β1 integrins, TCR/CD3, CD4, and other IS markers were unaffected by C-term overexpression (data not shown). Further studies with conjugates of human T lymphoblasts and SEE-pulsed Raji cells (APCs) confirmed that the LFA-1 ring was also altered after C-term expression (Figure 1B; the effect was quantified and results are displayed in the graph).
AKAP450 is required for the correct configuration and activation of LFA-1 at the IS. (A) J77 cells expressing GFP or C-term AKAP450-GFP were incubated with SEE-pulsed Raji B cells. Conjugates were stained with antibodies to LFA-1 (blue) and CD3ζ (red). Confocal z slices and corresponding 3-dimensional reconstruction of SMAC area (right) are shown. Histograms show the fluorescence intensity profiles of staining for CD3 (red) and LFA-1 (blue) along the diagonal black lines in the DIC images. Graph represents quantification of immune synapse with LFA-1 ring showing correct architecture. Data are the arithmetic mean ± SD of correct LFA-1 segregation. *P < .05 compared with J77 cells transfected with GFP (Student t test). (B) T lymphoblasts expressing GFP or C-term AKAP450-GFP were incubated with SEE-pulsed Raji B cells. Conjugates were stained with antibodies to LFA-1 (red). Confocal z slices and corresponding 3-dimensional reconstruction of SMAC area (right) are shown. Scale bars represent 10 μm. Graph represents quantification of immune synapse with LFA-1 ring showing correct architecture. Data are the arithmetic mean ± SD of correct LFA-1 segregation. *P < .05 compared with J77 cells transfected with GFP (Student t test). (C) Cell conjugates were formed between CH7 cells expressing GFP or C-term AKAP450-GFP and HA-stimulated HOM-2 APCs. Cells were stained with the antibodies to active conformation of LFA-1 (red), m24 (top panel), and KIM127 (bottom panel). A confocal z section of conjugates formed and processed as in panel A is shown (3-dimensional). Quantification of the active LFA-1 accumulation (m24, top panel; KIM127, bottom panel) at the IS. A total of 50 conjugates were counted for each condition. *P < .01 compared with CH7C17 cells transfected with GFP control (Mann-Whitney test). (A-C) Arrowheads in DIC images indicate the cell-cell junction selected for 3-dimensional projection. Yellow arrows indicate the positions of immune synapses; blue arrow, the position of the mislocalized MTOC in cells overexpressing C-term AKAP450-GFP. Asterisks in DIC images identify CMAC-loaded Raji APCs. Scale bars represent 10 μm.
AKAP450 is required for the correct configuration and activation of LFA-1 at the IS. (A) J77 cells expressing GFP or C-term AKAP450-GFP were incubated with SEE-pulsed Raji B cells. Conjugates were stained with antibodies to LFA-1 (blue) and CD3ζ (red). Confocal z slices and corresponding 3-dimensional reconstruction of SMAC area (right) are shown. Histograms show the fluorescence intensity profiles of staining for CD3 (red) and LFA-1 (blue) along the diagonal black lines in the DIC images. Graph represents quantification of immune synapse with LFA-1 ring showing correct architecture. Data are the arithmetic mean ± SD of correct LFA-1 segregation. *P < .05 compared with J77 cells transfected with GFP (Student t test). (B) T lymphoblasts expressing GFP or C-term AKAP450-GFP were incubated with SEE-pulsed Raji B cells. Conjugates were stained with antibodies to LFA-1 (red). Confocal z slices and corresponding 3-dimensional reconstruction of SMAC area (right) are shown. Scale bars represent 10 μm. Graph represents quantification of immune synapse with LFA-1 ring showing correct architecture. Data are the arithmetic mean ± SD of correct LFA-1 segregation. *P < .05 compared with J77 cells transfected with GFP (Student t test). (C) Cell conjugates were formed between CH7 cells expressing GFP or C-term AKAP450-GFP and HA-stimulated HOM-2 APCs. Cells were stained with the antibodies to active conformation of LFA-1 (red), m24 (top panel), and KIM127 (bottom panel). A confocal z section of conjugates formed and processed as in panel A is shown (3-dimensional). Quantification of the active LFA-1 accumulation (m24, top panel; KIM127, bottom panel) at the IS. A total of 50 conjugates were counted for each condition. *P < .01 compared with CH7C17 cells transfected with GFP control (Mann-Whitney test). (A-C) Arrowheads in DIC images indicate the cell-cell junction selected for 3-dimensional projection. Yellow arrows indicate the positions of immune synapses; blue arrow, the position of the mislocalized MTOC in cells overexpressing C-term AKAP450-GFP. Asterisks in DIC images identify CMAC-loaded Raji APCs. Scale bars represent 10 μm.
To further characterize the role of AKAP450 in the correct configuration of p-SMAC, we next analyzed the level of activation of the LFA-1 molecule in T cell–APC conjugates by confocal microscopy. Binding of ligands to the extracellular domains of integrins initiates intracellular signaling (outside-in signal) and induces conformational changes in both the extracellular and the cytoplasmic domains. m24 and KIM127 are 2 mAbs that recognize the activated β2 I-like domain and the EGF2 domain in the leg of the β2 subunit,29,30 respectively, and they recognize both ligand-bound and -unbound active conformations of LFA-1. Immunofluorescence analysis of C-term AKAP450 T cell–APC conjugates showed a reduction in the level of activated LFA-1 (m24 and KIM127) compared with controls, which correlates with the defective segregation of LFA-1 at the p-SMAC ring (Figure 1C). Quantitative analysis of control CH7C17-HOM-2 conjugates showed that stimulation of T cells caused a 2.4-fold increase in m24 levels compared with a 1.1-fold increase in C-term AKAP450-GFP conjugates (graph in Figure 1C); similar results were obtained in KIM127 staining (3.2-fold increase in control cells compared with 1.4-fold increase in C-term AKAP450-GFP cells; graph in Figure 1C). Together, these data suggest the implication of AKAP450 in the correct activation of LFA-1 at IS.
Suppression of AKAP450 function impairs the signaling events related with the activation of LFA-1
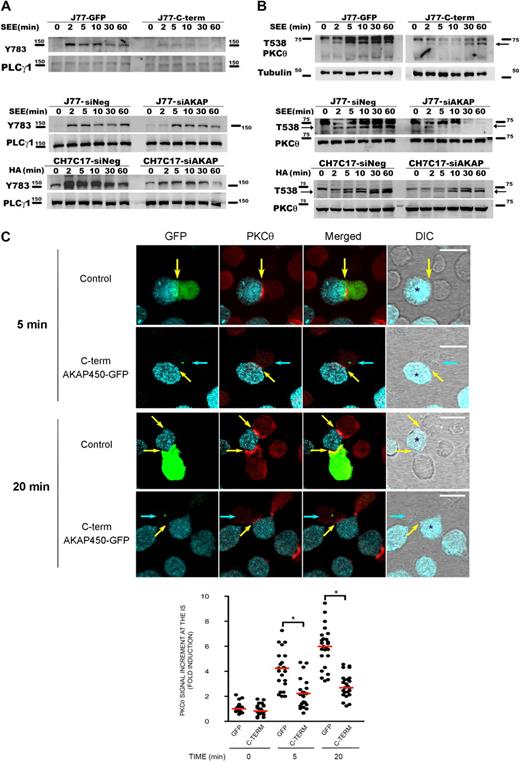
It has been described that LFA-1 signaling includes many events, such as phosphorylation of PLC-γ1 and activation of different kinases, such as isoenzymes of PKC and tyrosine kinases.31,–33 To examine the potential role of AKAP450 in these signaling events, we assessed intracellular pathways during T-APC conjugate formation on antigen stimulation. Time-course activation experiments revealed that the phosphorylation of PLC-γ1 was significantly diminished in C-term AKAP450 J77-Raji APC conjugates (Figure 2A).
Disruption of AKAP450 function impairs the phosphorylation of different molecules associated with the activation of LFA-1. (A) Top blot: J77 expressing GFP (J77-GFP) or C-term AKAP450-GFP (J77-C-term) were conjugated with SEE-pulsed Raji cell for the times indicated, and a representative Western blot of phospho-PLC-γ1 (Y783) is shown. Total PLC-γ1 expression was detected as a loading control. Bottom blots: J77/SEE-pulsed Raji cell conjugates and CH7C17/HOM-2 conjugates expressing control siRNA (siNeg) or AKAP450 siRNA (siAKAP). Control and AKAP450 knockdown T cells were conjugated as in panel A, and representative Western blots of phospho-PLC-γ1 (Y783) are shown. Total PLC-γ1 expression was detected as a loading control. One of 4 representative experiments is shown. (B) Top blot: J77 expressing GFP (J77-GFP) or C-term AKAP450-GFP (J77-C-term) were conjugated as in panel A, and a representative Western blot of phospho-PKC-θ (T538) is shown. Total PKC-θ expression was detected as a loading control. Bottom blots: J77/SEE-pulsed Raji cell conjugates and CH7C17/HOM-2 conjugates expressing control siRNA (siNeg) or AKAP450 siRNA (siAKAP). Control and AKAP450 knockdown T cells were conjugated as in panel A, and representative Western blots of phospho-PKC-θ (T538) are shown. Total PKC-θ expression was detected as a loading control. One of 4 representative experiments is shown. (C) Conjugates were formed between J77 cells expressing GFP or C-term AKAP450-GFP and SEE-pulsed Raji B cells for the different times (5 minutes, top panel; 20 minutes, bottom panel), and were stained for PKC-θ (red). Yellow arrows indicate the positions of immune synapses; blue arrow, position of the mislocalized MTOC in cells overexpressing C-term AKAP450-GFP. Asterisks in DIC images identify CMAC-loaded Raji APCs. Scale bars represent 10 μm. The graph shows the quantification of the total PKC-θ accumulation for the times indicated at the IS. A total of 25 conjugates were counted for each time and condition. Data are the arithmetic mean ± SD of PKC-θ accumulation. *P < .05 compared with J77 cells transfected with GFP (Student t test).
Disruption of AKAP450 function impairs the phosphorylation of different molecules associated with the activation of LFA-1. (A) Top blot: J77 expressing GFP (J77-GFP) or C-term AKAP450-GFP (J77-C-term) were conjugated with SEE-pulsed Raji cell for the times indicated, and a representative Western blot of phospho-PLC-γ1 (Y783) is shown. Total PLC-γ1 expression was detected as a loading control. Bottom blots: J77/SEE-pulsed Raji cell conjugates and CH7C17/HOM-2 conjugates expressing control siRNA (siNeg) or AKAP450 siRNA (siAKAP). Control and AKAP450 knockdown T cells were conjugated as in panel A, and representative Western blots of phospho-PLC-γ1 (Y783) are shown. Total PLC-γ1 expression was detected as a loading control. One of 4 representative experiments is shown. (B) Top blot: J77 expressing GFP (J77-GFP) or C-term AKAP450-GFP (J77-C-term) were conjugated as in panel A, and a representative Western blot of phospho-PKC-θ (T538) is shown. Total PKC-θ expression was detected as a loading control. Bottom blots: J77/SEE-pulsed Raji cell conjugates and CH7C17/HOM-2 conjugates expressing control siRNA (siNeg) or AKAP450 siRNA (siAKAP). Control and AKAP450 knockdown T cells were conjugated as in panel A, and representative Western blots of phospho-PKC-θ (T538) are shown. Total PKC-θ expression was detected as a loading control. One of 4 representative experiments is shown. (C) Conjugates were formed between J77 cells expressing GFP or C-term AKAP450-GFP and SEE-pulsed Raji B cells for the different times (5 minutes, top panel; 20 minutes, bottom panel), and were stained for PKC-θ (red). Yellow arrows indicate the positions of immune synapses; blue arrow, position of the mislocalized MTOC in cells overexpressing C-term AKAP450-GFP. Asterisks in DIC images identify CMAC-loaded Raji APCs. Scale bars represent 10 μm. The graph shows the quantification of the total PKC-θ accumulation for the times indicated at the IS. A total of 25 conjugates were counted for each time and condition. Data are the arithmetic mean ± SD of PKC-θ accumulation. *P < .05 compared with J77 cells transfected with GFP (Student t test).
To further explore the role of AKAP450 in LFA-1 signaling, its expression in J77 and CH7C17 cells was suppressed by siRNA knockdown. AKAP450-interfered T cells showed a significant (65%-75%) reduction in AKAP450 expression (supplemental Figure 2A-B). In this case, we have analyzed the phosphorylation of PLC-γ1 at Y783, and similar results were obtained in AKAP450-interfered J77 and CH7C17 cells stimulated with SEE-pulsed Raji and HA-pulsed HOM-2 APCs, respectively (Figure 2A). Subsequently, we analyzed the activation of PKC-θ, and the phosphorylation of this protein in AKAP450-targeted T cells was decreased compared with the control cells (Figure 2B). Moreover, PKC-θ recruitment to the IS was also impaired by C-term AKAP450-GFP overexpression (Figure 2C). The analysis of conjugates at early times on cell-cell contact (5 minutes) showed that the recruitment of PKC-θ is defective from the initiation of IS formation (Figure 2C). This effect was also observed at later times (20 minutes; Figure 2C), correlating with the observed reduction in the phosphorylation at PKC-θ Thr538 (Figure 2B). The defective phosphorylation of PLC-γ1 and PKC-θ observed in AKAP450-disrupted cells suggests a defect in costimulation and integrin signaling at the IS, in accordance with the defective activated conformation and architecture of LFA-1 at the p-SMAC.
Dominant-negative AKAP450 impairs activation of the TCR-CD3 complex and alters signaling downstream of the TCR
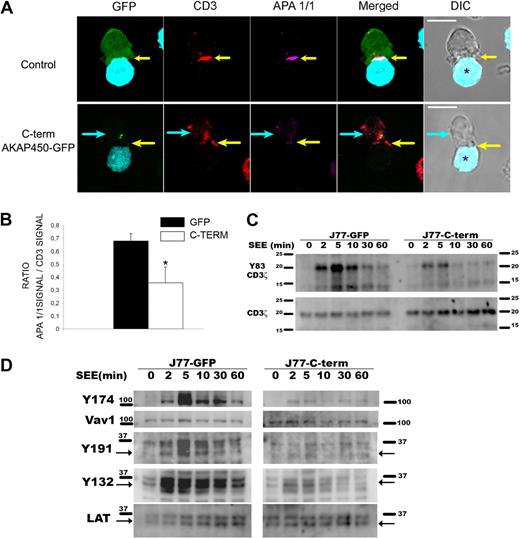
It has been described that adhesion molecules, such as LFA-1 and ICAM-1, initially localize to the central contact area in T-APC conjugates preceding the TCR/CD3 complex, and then dynamically relocates to the p-SMAC.2 Because we found an abnormal LFA-1 configuration at the p-SMAC, we decided to study the activation of the TCR/CD3 complex at the c-SMAC. When T cells specifically interact with APCs, the CD3ϵ chain undergoes a conformational change that can be detected with the APA1/1 mAb.27 Immunofluorescence analysis of C-term AKAP450 T cell-APC conjugates showed a clear reduction in the staining for activated CD3ϵ compared with controls, in which there was a complete colocalization of total CD3 and activated CD3ϵ staining (Figure 3A). To quantify this effect, we measured the ratio between APA 1/1 and CD3 signal detected in the contact area in T-APC conjugates by confocal microscopy, showing a clear reduction in the APA 1/1 signal in C-term AKAP450-GFP cells with respect to CD3 signal (Figure 3B). Consistently, C-term AKAP450-GFP overexpression impaired tyrosine phosphorylation of CD3ζ (Y83) in the TCR/CD3 complex of SEE-stimulated Jurkat cells (Figure 3C).
Overexpression of C-term AKAP450-GFP impairs the activation of the TCR/CD3 complex and T-cell signaling molecules. (A) Conjugates were formed between J77 cells expressing GFP or C-term AKAP450-GFP and SEE-pulsed Raji B cells, and were stained for CD3ζ (red) and activated CD3ϵ (APA1/1: magenta). Yellow arrows indicate the positions of immune synapses; blue arrow, position of the mislocalized MTOC in cells overexpressing C-term AKAP450-GFP. Asterisks in DIC images identify CMAC-loaded Raji APCs. Scale bars represent 10 μm. (B) Histogram shows quantification of the relation between APA1/1 signal and CD3 signal in conjugates formed as in panel A. A total of 50 conjugates were counted for each condition. Results are the arithmetic mean ± SD. *P < .05 compared with J77 cells transfected with GFP control (Student t test). (C) Representative Western analysis of phospho-CD3ζ (Y83) in J77/SEE-pulsed Raji cell conjugates as in panel A, formed for the indicated times. Total CD3 expression was detected as a loading control. One of 4 representative experiments is shown. (D) J77 expressing GFP (J77-GFP) or C-term AKAP450-GFP (J77-C-term) were conjugated with SEE-pulsed Raji cell for the times indicated, and cell proteins were separated by SDS-PAGE and processed for Western blotting. Blots show the phosphorylation of specific sites on Vav1 (Y174) and LAT (Y191, Y132) together with the total expression of these proteins. Arrows indicate target band sizes. One of 4 experiments is shown.
Overexpression of C-term AKAP450-GFP impairs the activation of the TCR/CD3 complex and T-cell signaling molecules. (A) Conjugates were formed between J77 cells expressing GFP or C-term AKAP450-GFP and SEE-pulsed Raji B cells, and were stained for CD3ζ (red) and activated CD3ϵ (APA1/1: magenta). Yellow arrows indicate the positions of immune synapses; blue arrow, position of the mislocalized MTOC in cells overexpressing C-term AKAP450-GFP. Asterisks in DIC images identify CMAC-loaded Raji APCs. Scale bars represent 10 μm. (B) Histogram shows quantification of the relation between APA1/1 signal and CD3 signal in conjugates formed as in panel A. A total of 50 conjugates were counted for each condition. Results are the arithmetic mean ± SD. *P < .05 compared with J77 cells transfected with GFP control (Student t test). (C) Representative Western analysis of phospho-CD3ζ (Y83) in J77/SEE-pulsed Raji cell conjugates as in panel A, formed for the indicated times. Total CD3 expression was detected as a loading control. One of 4 representative experiments is shown. (D) J77 expressing GFP (J77-GFP) or C-term AKAP450-GFP (J77-C-term) were conjugated with SEE-pulsed Raji cell for the times indicated, and cell proteins were separated by SDS-PAGE and processed for Western blotting. Blots show the phosphorylation of specific sites on Vav1 (Y174) and LAT (Y191, Y132) together with the total expression of these proteins. Arrows indicate target band sizes. One of 4 experiments is shown.
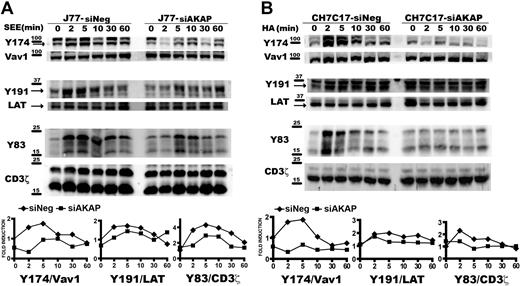
These results prompted us to study how different TCR/CD3-emanating signaling pathways are affected by disruption of the AKAP450 function. We next examined the specific phosphorylation of molecules known to be important for T-cell activation. Phosphorylation of LAT was observed initially after SEE stimulation but, in contrast to control cells, was not sustained for longer periods and significantly diminished in C-term AKAP450 and AKAP450-interfered SEE-specific J77-Raji APC conjugates (Figures 3D,4A). We also analyzed the activation of Vav1, which is linked to LAT phosphorylation at Y191.34 Phosphorylation of this signaling protein was reduced in AKAP450-disrupted cells, correlating with the timing of LAT phosphorylation (Figures 3D,4A). Subsequently, F-actin content was analyzed in T cells by flow cytometry and confocal microscopy. In C-term AKAP450 Jurkat and CD4 primary human T cells, a defective actin polymerization response to TCR/CD3 crosslinking was observed (supplemental Figure 3A), and immunofluorescence analysis of C-term AKAP450 T cell–APC conjugates showed a reduction in F-actin levels compared with controls (supplemental Figure 3B), which correlates with the defective activation of Vav1. Defective phosphorylation of CD3ζ (Y83), LAT (Y191), and Vav1 (Y174) was also detected in AKAP450-interfered CH7C17 cells stimulated with HA-pulsed HOM-2 APCs (Figure 4B), compared with the control cells, which is parallel to what occurred in C-term AKAP450-GFP cells (Figure 3D).
AKAP450 knockdown impairs the activation of T-cell signaling molecules. J77/SEE-pulsed Raji cell conjugates (A) and CH7C17/HOM-2 conjugates (B) expressing control siRNA (siNeg) or AKAP450 siRNA (siAKAP). Control and AKAP450 knockdown T cells were conjugated as in Figure 3D. Cell proteins were separated by SDS-PAGE and processed for Western blotting. Blots show the phosphorylation of specific sites on Vav1 (Y174), LAT (Y191), and CD3ζ (Y83) together with the total expression of these proteins. Arrows indicate target band sizes. Graphs correspond to the densitometric analysis of the Western blots in panels A and B. One of 4 experiments is shown.
AKAP450 knockdown impairs the activation of T-cell signaling molecules. J77/SEE-pulsed Raji cell conjugates (A) and CH7C17/HOM-2 conjugates (B) expressing control siRNA (siNeg) or AKAP450 siRNA (siAKAP). Control and AKAP450 knockdown T cells were conjugated as in Figure 3D. Cell proteins were separated by SDS-PAGE and processed for Western blotting. Blots show the phosphorylation of specific sites on Vav1 (Y174), LAT (Y191), and CD3ζ (Y83) together with the total expression of these proteins. Arrows indicate target band sizes. Graphs correspond to the densitometric analysis of the Western blots in panels A and B. One of 4 experiments is shown.
Suppression of AKAP450 function prevents MTOC translocation toward the IS
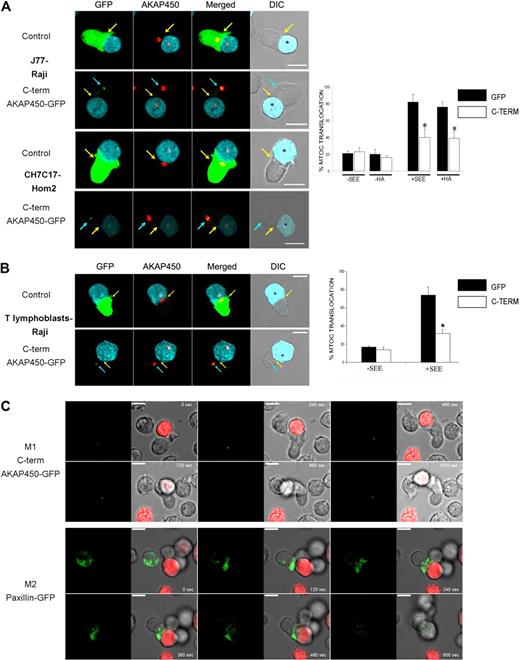
AKAP450 is located at the GA and MTOC and controls centrosome function during cell cycle,17 Golgi organization,35 and microtubule nucleation22 in adherent epithelial cells and fibroblasts. However, in T lymphocytes, AKAP450 targeting with the C-term AKAP450-GFP construct did not significantly affect the cell-cycle progression and Golgi structure/organization by confocal level (supplemental Figures 1B, 4). We next assessed the possible role of AKAP450 in the polarization of the MTOC to the contact between the T cell and APC. Overexpression of C-term AKAP450 delocalized the MTOC from the contact area between J77 cells and SEE-preloaded Raji B cells (Figure 5A top panel). C-term AKAP450 had a similar effect in an antigen peptide-specific model of the IS, in which CH7C17 T cells specifically recognize HA-pulsed HOM-2 APCs (Figure 5A bottom panel). Quantitative analysis of SEE-stimulated Jurkat cells showed that only 39% of C-term AKAP450-GFP expressing cells displayed their MTOC at the IS, compared with nearly 82% of GFP-transfected control cells (graph in Figure 5A). Similar results were obtained in T cells conjugated with HA-pulsed HOM-2 APCs, with MTOC orientation detected in only 37% of CH7C17 cells expressing C-term AKAP450-GFP, compared with 78% of GFP-expressing controls (graph in Figure 5A). In the case of human T lymphoblasts, the translocation of the MTOC toward the contact area with the APCs was also affected by C-term AKAP450-GFP expression (Figure 5B). To more precisely investigate this, we monitored the MTOC movement in Jurkat cells by time-lapse confocal videomicroscopy. The MTOC translocation in C-term AKAP450-GFP expressing cells was not evident, showing no effective rearrangement toward the IS (Figure 5C, M1; and supplemental Video 1). In contrast, in the case of control cells overexpressing paxillin-GFP,36 the MTOC translocated directly to the IS within the first 5 minutes (Figure 5C, M2; and supplemental Video 2). In accordance, the GA was similarly not reoriented toward the IS in T cells expressing C-term AKAP450, and remained well organized around the MTOC (supplemental Figure 4).
MTOC polarization is prevented by C-term AKAP450-GFP expression. (A) Top panel: Cell conjugates were formed between J77 cells expressing GFP (control) or C-term AKAP450-GFP and SEE-stimulated Raji B cells. Cells were stained for endogenous AKAP450 (red). Bottom panel: Cell conjugates were formed between CH7C17 cells expressing GFP (control) or C-term AKAP450-GFP and HA-stimulated HOM-2 APC. Cells were also stained for endogenous AKAP450. Yellow arrows indicate the positions of immune synapses; blue arrow, position of the mislocalized MTOC in cells overexpressing C-term AKAP450-GFP. Asterisks in DIC images identify CMAC-loaded Raji APCs. Scale bars represent 10 μm. Quantification of MTOC translocation in conjugates formed is shown in the graph. More than 300 conjugates were counted for each condition. Data are the arithmetic mean ± SD of MTOC translocation. *P < .05 compared with J77 or CH7C17 cells transfected with GFP (Student t test). (B) T lymphoblasts were incubated with SEE-pulsed Raji cells loaded with CMAC cell tracker (cyan), fixed, and stained for endogenous AKAP450 (red) as in panel A. Top panel: GFP-expressing T lymphoblasts. Bottom panel: C-term AKAP450-GFP expressing T lymphoblasts. Yellow arrows indicate the positions of immune synapses; blue arrow, position of the mislocalized MTOC in cells overexpressing C-term AKAP450-GFP. Asterisks in DIC images identify CMAC-loaded Raji APCs. The graph shows the quantification of MTOC translocation in conjugates. More than 50 conjugates were counted for each condition. Data are the arithmetic mean ± SD of MTOC translocation. *P < .05 compared with T lymphoblast cells transfected with GFP (Student t test). (C) J77 cells expressing C-term AKA450-GFP (M1) or paxillin-GFP (M2) were incubated with SEE-pulsed Raji B cells loaded with 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine cell tracker (red) and time-lapse confocal video microscopy sequences are shown at the time indicated. Images were captured at 37°C. Time is shown in seconds. M indicates movie. Scale bars represent 10 μm.
MTOC polarization is prevented by C-term AKAP450-GFP expression. (A) Top panel: Cell conjugates were formed between J77 cells expressing GFP (control) or C-term AKAP450-GFP and SEE-stimulated Raji B cells. Cells were stained for endogenous AKAP450 (red). Bottom panel: Cell conjugates were formed between CH7C17 cells expressing GFP (control) or C-term AKAP450-GFP and HA-stimulated HOM-2 APC. Cells were also stained for endogenous AKAP450. Yellow arrows indicate the positions of immune synapses; blue arrow, position of the mislocalized MTOC in cells overexpressing C-term AKAP450-GFP. Asterisks in DIC images identify CMAC-loaded Raji APCs. Scale bars represent 10 μm. Quantification of MTOC translocation in conjugates formed is shown in the graph. More than 300 conjugates were counted for each condition. Data are the arithmetic mean ± SD of MTOC translocation. *P < .05 compared with J77 or CH7C17 cells transfected with GFP (Student t test). (B) T lymphoblasts were incubated with SEE-pulsed Raji cells loaded with CMAC cell tracker (cyan), fixed, and stained for endogenous AKAP450 (red) as in panel A. Top panel: GFP-expressing T lymphoblasts. Bottom panel: C-term AKAP450-GFP expressing T lymphoblasts. Yellow arrows indicate the positions of immune synapses; blue arrow, position of the mislocalized MTOC in cells overexpressing C-term AKAP450-GFP. Asterisks in DIC images identify CMAC-loaded Raji APCs. The graph shows the quantification of MTOC translocation in conjugates. More than 50 conjugates were counted for each condition. Data are the arithmetic mean ± SD of MTOC translocation. *P < .05 compared with T lymphoblast cells transfected with GFP (Student t test). (C) J77 cells expressing C-term AKA450-GFP (M1) or paxillin-GFP (M2) were incubated with SEE-pulsed Raji B cells loaded with 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine cell tracker (red) and time-lapse confocal video microscopy sequences are shown at the time indicated. Images were captured at 37°C. Time is shown in seconds. M indicates movie. Scale bars represent 10 μm.
Furthermore, analysis of AKAP450-interfered T cell–APC conjugates revealed an inhibition of MTOC translocation comparable with that observed with the dominant-negative C-term AKAP450 (Figure 6). Together, these data show that AKAP450 is required for the APC-induced polarization of the T-cell MTOC.
Defective MTOC polarization in AKAP450 knockdown cells. J77 cells (A) and CH7C17 cells (B) were transfected with a control siRNA or AKAP450 siRNA, and cell conjugates were formed as in Figure 5A. Conjugates were stained for endogenous AKAP450 (red) and α-tubulin (green). Yellow arrows indicate the positions of immune synapses; blue arrows, position of the mislocalized MTOC in AKAP450 knockdown cells. CMAC-labeled SEE-pulsed Raji and HA-pulsed HOM-2 APCs are distinguished by cyan fluorescence in DIC images (*). Scale bars represent 10 μm. (A-B) Histograms show quantification of MTOC translocation in (A) J77 and (B) CH7C17 cells. More than 200 conjugates were counted for each condition. Results are the arithmetic mean ± SD of MTOC translocation. Control incubation with uploaded APCs represented as −SEE and −HA in the graphs. *P < .05 compared with T cells transfected with control siRNA (Student t test).
Defective MTOC polarization in AKAP450 knockdown cells. J77 cells (A) and CH7C17 cells (B) were transfected with a control siRNA or AKAP450 siRNA, and cell conjugates were formed as in Figure 5A. Conjugates were stained for endogenous AKAP450 (red) and α-tubulin (green). Yellow arrows indicate the positions of immune synapses; blue arrows, position of the mislocalized MTOC in AKAP450 knockdown cells. CMAC-labeled SEE-pulsed Raji and HA-pulsed HOM-2 APCs are distinguished by cyan fluorescence in DIC images (*). Scale bars represent 10 μm. (A-B) Histograms show quantification of MTOC translocation in (A) J77 and (B) CH7C17 cells. More than 200 conjugates were counted for each condition. Results are the arithmetic mean ± SD of MTOC translocation. Control incubation with uploaded APCs represented as −SEE and −HA in the graphs. *P < .05 compared with T cells transfected with control siRNA (Student t test).
Suppression of AKAP450 function impairs IL-2 release during the formation of the IS
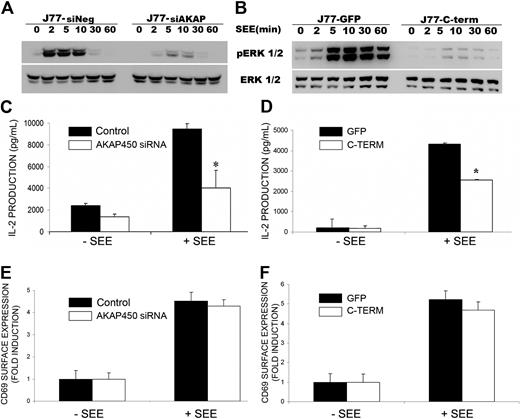
One of the first events on TCR activation is a polarized release of IL-2 by T lymphoblasts.37 The production of IL-2 is dependent on the activation of the mitogen-activated protein kinase (Erk 1/2) pathway.5 As a measure of the sustained activation of T cells, phosphorylation of ERK 1/2 was assessed in cell extracts from AKAP450-disrupted cells. A strong reduction in protein phosphorylation was observed in these cells (Figure 7A-B). In accord, both AKAP450 knockdown and C-term AKAP450-GFP overexpression considerably impaired IL-2 release, detectable as early as 16 hours after cell conjugate formation (Figure 7C-D). In contrast, CD69 surface expression was not significantly affected (Figure 7E-F).
IL-2 production is impaired by C-term AKAP450-GFP overexpression and AKAP450 depletion. (A-B) J77/SEE-pulsed Raji cell conjugates of cells expressing (A) control siRNA (siNeg) or AKAP450 siRNA (siAKAP) and (B) GFP (J77-GFP) or C-term AKAP450-GFP (J77-C-term) were incubated for the times indicated, and a representative Western blot of phospho-Erk1/2 (T202/Y204) is shown. Total Erk1/2 expression was detected as loading control. One of 3 representative experiments is shown. (C-D) IL-2 release was detected by enzyme-linked immunosorbent assay in the culture supernatants of conjugates formed between SEE-pulsed Raji cells and J77 cells transfected with control or AKAP450 siRNA (C) and overexpressing GFPs or C-term AKAP450 GFPs (D). Data from control incubations with unloaded APCs (−SEE). (E-F) Relative surface expression of CD69 in conjugates formed as in panels C and D, detected by flow cytometry. Data are the arithmetic mean ± SD of 3 independent experiments performed in triplicate. *P < .05 (Student t test).
IL-2 production is impaired by C-term AKAP450-GFP overexpression and AKAP450 depletion. (A-B) J77/SEE-pulsed Raji cell conjugates of cells expressing (A) control siRNA (siNeg) or AKAP450 siRNA (siAKAP) and (B) GFP (J77-GFP) or C-term AKAP450-GFP (J77-C-term) were incubated for the times indicated, and a representative Western blot of phospho-Erk1/2 (T202/Y204) is shown. Total Erk1/2 expression was detected as loading control. One of 3 representative experiments is shown. (C-D) IL-2 release was detected by enzyme-linked immunosorbent assay in the culture supernatants of conjugates formed between SEE-pulsed Raji cells and J77 cells transfected with control or AKAP450 siRNA (C) and overexpressing GFPs or C-term AKAP450 GFPs (D). Data from control incubations with unloaded APCs (−SEE). (E-F) Relative surface expression of CD69 in conjugates formed as in panels C and D, detected by flow cytometry. Data are the arithmetic mean ± SD of 3 independent experiments performed in triplicate. *P < .05 (Student t test).
Discussion
Several scaffolding proteins have been documented to play important roles in orchestrating the molecular reorganization at the T-cell side of the IS, either through the spatiotemporal targeting of effector kinases and phosphatases or by providing sites for the docking of cell surface proteins to cytoskeletal components.38 Among these scaffolding proteins, AKAP proteins have been shown to be important for the maintenance of T-cell homeostasis.39 In this study, we examined the potential role of AKAP450, which links the GA to the MTOC, in integrin and TCR/CD3 molecular reorganization and signaling leading to MTOC translocation to the IS during T cell–APC interaction. Our results show that conformational activated states of LFA-1 and TCR/CD3 molecules as well as the subsequent signaling events are impaired by AKAP450 targeting. As consequence, MTOC translocation is prevented by siRNA suppression of AKAP450 expression or by displacement of the endogenous protein with a dominant-negative mutant form.
In migrating T cells, AKAP450 nucleates several proteins at LFA-1 clusters, such as PKC-β and PKC-δ.19 Our data demonstrate that AKAP450 supports LFA-1 organization and activation at the IS. The p-SMAC is not well organized in a ring-shaped cluster but instead appears as poorly stained with irregular aggregation of integrin molecules at the T cell–APC interface. Together, these data indicate that AKAP450 plays a role in IS formation, the consequence of which is the observed defect in MTOC translocation. The abnormal configuration of the p-SMAC on disruption of AKAP450 function might prevent MTOC anchorage at the IS, thereby delocalizing it from the T cell–APC contact area. Considering that the integrin- and actin-enriched p-SMAC associates with microtubules during IS formation,40 the possibility that LFA-1 and other p-SMAC–localized integrins, such as VLA-4,41 might act as MTOC-docking sites is an attractive hypothesis that warrants investigation. Accordingly, our data show a reduction in the staining with the m24 and KIM127 mAbs, 2 antibodies that recognize active conformation of LFA-1.29,30 Together with the observed defect in MTOC translocation, we have found a defect in actin polymerization on TCR/CD3 stimulation, which correlates with the lack of integrin and CD3 activation in AKAP450 disrupted cells. This experimental evidence underscores the role of AKAP450 in the initial activation of integrin receptors and the remodeling of the cytoskeleton at the IS, necessary for late events in T-cell activation, such as the IL-2 production.37
The defective phosphorylation of PLC-γ1 and PKC-θ in AKAP450-disrupted cells suggests a defect in costimulation and integrin signaling at the IS, which agrees with the defective architecture of LFA-1 at the p-SMAC we observed. The defective recruitment of PKC-θ to the IS in AKAP450-disrupted cells might indicate direct interaction between these molecules, as previously observed for other members of the PKC family,16 or alternatively might result from the defective clustering and activation of CD3. The translocation of PKC-θ toward the IS is regulated by a PI3-K– and Vav-dependent pathway,42 and the diminished phosphorylation of Vav1 observed in AKAP450-disrupted cells could explain the defective recruitment of PKC-θ. In addition, AKAP450 acts as a platform for PKA signaling and harbors binding sites for protein phosphatases PP1 and PP2 and for phosphodiesterases.13,43 Interestingly, PKA has been described as a negative regulator of integrin adhesion strength during leukocyte chemotaxis.44 AKAP450 might thus act at different stages of T-cell activation, initially sustaining integrin activation and MTOC translocation and later contributing to the disengagement of the IS. In addition to its role in IS formation, AKAP450 might also regulate GA function. AKAP450 is localized at the Golgi complex,21,35 and its role in nucleating microtubules at the cis face of the Golgi might be important for MTOC/GA translocation and function.22 The diminished release of IL-2 by AKAP450-disrupted T cells during the formation of the IS could therefore be the result of the defective MTOC/GA translocation observed in these cells.
A large number of molecules are recruited to the T lymphocyte side of the IS via the MTOC, the associated Golgi, and other organelles, such as mitochondria.45,46 Recruitment is directed by dynein/dynactin motor complex26 and is tightly regulated by numerous signaling and adapter molecules. ZAP-70 is required for MTOC polarization toward the IS,47 and it has recently been reported that MTOC polarization is also modulated by LAT and Fyn.24,45 Our study shows that AKAP450 exerts a similar effect on MTOC polarization. Moreover, suppression of AKAP450 function had a negative effect on the activation of CD3 and PKC-θ at the IS and on the activation of PLC-γ1, Vav1, PKC-θ, and LAT, suggesting that AKAP450 is also involved in the polarization of signaling proteins essential for TCR-induced activation. Recently, it has been shown that local TCR activation promotes the accumulation of diacylglycerol at the T-cell membrane, which concomitantly drives the gathering of dynein and MTOC movement toward this position.48 Therefore, a defect in PLC-γ1 activation would lead to a defect in diacylglycerol production, thus preventing dynein function and MTOC translocation. The sustained activation of the T cell could then be compromised as described.26 In this sense, the expression of a nonphosphorylatable mutant form of LAT has been reported to inhibit further recruitment of LAT to the IS,45 and we previously found that LAT sustained phosphorylation is defective on impairment of MTOC translocation to the IS, although very-early phosphorylation was preserved.26 In the present study, we found that MTOC delocalization in TCR-engaged AKAP450-targeted cells was accompanied by decreased LAT phosphorylation. Together, these data suggest that the effect of AKAP450 disruption on LAT-sustained activation might be the result of deficient recruitment of LAT from its intracellular pool toward the IS,45 as a consequence of the inhibition of translocation of the MTOC and GA. However, disruption of AKAP450 function also altered TCR/CD3 activation state at the nascent c-SMAC, supporting the role of AKAP450 in the proper formation of the SMACs. This is consistent with the diminished activation of CD3 observed in C-term AKAP450-GFP T cells, indicated by the reductions in the staining with the CD3/TCR-activation-reporter APA1/1 mAb and in the phosphorylation of CD3ζ. Nevertheless, we have observed that TCR-mediated signaling was not abrogated completely by AKAP450 disruption, and it is conceivable that the remaining initial activation of TCR/CD3 would be sufficient to promote a partial MTOC translocation toward the IS, which could explain the small percentage of C-term–expressing or AKAP450 knockdown cells that still translocate their MTOC toward the IS.
In conclusion, our data suggest that AKAP450 is necessary for T-cell activation by regulating the conformational activation of LFA-1 and TCR/CD3 molecules at the immune synapse and MTOC translocation. The involvement of the MTOC/GA in the recruitment of intracellular pools of signaling molecules (LAT, Fyn, Pyk245,49 ) toward the IS seems to play an important role in the amplification of intracellular signals and in cytokine secretion by T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr R. Gonzalez-Amaro and S. Barlett for critical reading and editorial support of the manuscript and Drs Hortensia de la Fuente and Esteban Veiga for technical advice and generous help.
This work was supported by Ministerio de Educación y Ciencia of Spain (FPU fellowship) (J.R.V.), postdoctorate position (N.B.M.C.; and SAF2008-02635), Comunidad de Madrid (INSINET 0159/2006), Red RECAVA (RD06/0014-0030), and Fundación CNIC from Instituto Carlos III (Ministerio de Educación y Ciencia of Spain).
Authorship
Contribution: J.R.-V., N.B.M.-C., and F.S.-M. designed experimentation and analyzed results; J.R.-V., N.B.M.-C., and A.L. collected and analyzed the data; J.R.-V. and N.B.M.-C. made the figures; S.M. and Y.V. provided valuable reagents and performed critical reading of the manuscript; and J.R.-V. wrote the manuscript with input from N.B.M.-C. and F.S.-M.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francisco Sánchez-Madrid, Servicio de Inmunlogía, Hospital de la Princesa, Diego de León, 62, 28006, Madrid, Spain; e-mail: fsanchez.hlpr@salud.madrid.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal