The type-III interferon (IFN) family is composed of 3 molecules in humans: IFN-λ1 (interleukin-29 [IL-29]), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B), each of which signals through the same receptor complex. Plasmacytoid dendritic cells (pDCs) are major IFN-λ producers among peripheral lymphocytes. Recently, it has been shown that IFN-λ1 exerts a powerful inhibitory effect over the T-helper 2 (Th2) response by antagonizing the effect of IL-4 on CD4+ T cells and inhibiting the production of Th2-associated cytokines. Here, we asked whether Th2 cytokines exert reciprocal control over IFN-λ production. IL-4 treatment during stimulation of human peripheral lymphocytes significantly elevated IFN-λ1 transcription and secretion. However, pDCs were not directly responsive to IL-4. Using depletion and reconstitution experiments, we showed that IL-4–responsive monocytes are an intermediary cell, responding to IL-4 by elevating their secretion of IL-1 receptor antagonist (IL-Ra); this IL-1Ra acts on pDCs to elevate their IFN-λ1 output. Thus, our experiments revealed a novel mechanism for regulation of both IFN-λ1 production and pDC function, and suggests an expanded immunomodulatory role for Th2-associated cytokines.
Introduction
The 3 members of the interferon-λ (IFN-λ) family (λ1, λ2, and λ3) were originally described in the context of their similarity to both IFN-α/β and the IL-10 family of ligands.1,2 A recent report has confirmed that while IFN-λ exhibits IFN-like activity,3 it is structurally related to members of the IL-10 cytokine family, particularly IL-22.4 Early characterization of type-III IFN activity revealed that signaling is mediated through a heterodimeric receptor complex composed of the signaling subunit, IL-28Rα, and the nonsignaling partner IL-10Rβ; all 3 ligands signal through this receptor. Ligation of the IFN-λ receptor leads to the induction of signature molecules typically associated with antiviral immunity, such as 2′ to 5′ OAS and MxA.1,2 Although there is some overlap in the functions of type-I and type-III IFNs, there are clear differences in their biologic activities. The antiviral effects of IFN-λ are less robust compared with those of IFN-α,5,,–8 but the IFN-λ molecules remain able to induce antiviral activity both in vivo and in vitro,5,9,10 causing, for example, reduction of hepatitis B and C replication in hepatocytes, and of HSV-2 in the vaginal mucosa. These and other studies have highlighted the particularly powerful effect of IFN-λ1 on epithelial cells,11 which is mediated by the relatively high expression of IL-28Rα.12
In addition to their antiviral effects, type-III IFNs have been shown to play a critical role in regulating the adaptive immune response by acting directly on T cells to inhibit T-helper 2 (Th2) polarization and cytokine production.13,–15 Furthermore, treatment of peripheral blood mononuclear cells (PBMCs),16 and more specifically naive and memory CD4+ T cells,17 with IFN-λ1 results in marked reductions of IL-4, IL-13, and IL-5, and a down-regulation in both GATA3 and IL-4Rα expression.
An imbalance of Th1/Th2 regulation is a hallmark component of the pathophysiology of asthma and allergic inflammation. The chronic up-regulation of Th2 cytokines in patients with asthma drives inflammation and lymphocyte recruitment to the airway; high levels of IL-4 promote IgE class switching and mast cell activity, and excessive IL-13 contributes to airway remodeling.18,19 Recently, it was shown that patients with asthma are markedly deficient in IFN-λ1 production by both epithelial and brochoalveolar lavage cells.20 Many acute asthma exacerbations are a consequence of infection by respiratory viruses, most commonly rhinovirus. Primary bronchial epithelial cells from normal donors respond to rhinovirus (RV) stimulation by producing high levels of IFN-λ1, IFN-λ2, IFN-λ3, and IFN-β, and undergo rapid apoptosis, which prevents viral replication. However, patients with asthma do not produce sufficient amounts of type-I or type-III IFNs and thus yield high titers of infectious virus. The mechanisms by which deficient IFN-λ production supports asthma development are unknown (either through reduced ability to stifle viral replication or loss of control over elevated Th2 responses), but it is evident that IFN-λ is a pivotal element in this process.
Dendritic cells (DCs) are known to be important producers of IFN-λ. IFN-λ mRNA is induced in monocyte-derived DCs (MDDCs) in response to influenza, poly I:C, and lipopolysaccharide (LPS),21,22 and in human plasmacytoid dendritic cells (pDCs) by influenza.21 IFN-λ protein production has been observed in MDDCs upon stimulation with LPS or CD40L plus IFN-γ,23 and recently in our own work, in pDCs in responses to HSV, CpG, and imiquimod.24 This is in line with robust type-I IFN responses typically observed in pDCs.25
Given the direct antagonism of IL-4 by IFN-λ in both T cells and myeloid/classical DCs (mDCs), we were prompted to ask whether Th2 cytokines act in a reciprocal fashion to regulate IFN-λ production. Our data show that in the presence of specific viral stimuli, IL-4 acts to enhance IFN-λ1 production from the PBMCs of healthy donors. Stepwise depletion and reconstitution experiments revealed that production of IL-1 receptor antagonist (IL-1Ra) from monocytes mediated this Th2-enhanced production of IFN-λ1 by human pDCs.
Methods
Isolation of human PBMCs
Buffy coats were obtained from the New Jersey Blood Bank, and PBMCs were isolated by density gradient separation over Ficoll-Paque (Sigma-Aldrich). Cells were then washed in RPMI 1640 (Invitrogen), resuspended in medium supplemented with 10% (vol/vol) fetal bovine Serum (FBS; Thermo Scientific), and enumerated by hemacytometer using trypan blue (30% vol/vol; Sigma-Aldrich). Cells were resuspended at a density of 1 × 106/mL and cocultured as indicated with HSV-1 (multiplicity of infection [MOI] = 1; originally a gift from Dr John Blaho, Medical Diagnostic Laboratories, Hamilton, NJ), in the presence or absence of IL-4 (50 ng/mL; R&D Systems).
IFN-λ1 quantitation
Levels of IFN-λ1 protein in culture supernatants were measured using the “Duoset” human IL-29 enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems according to the manufacturer's protocol. End point optical densities at 450 nm were determined using a VERSAmax spectrophotometer and compiled using SoftMax software (both Molecular Devices).
pDC isolation
pDCs were isolated from fresh PBMCs using the Human Plasmacytoid DC Negative Isolation Kit (StemCell Technologies) according to the manufacturer's protocol. The enriched cells were assessed for more than 95% purity using the following antibodies: anti-CD123–APC and anti–BDCA-2–FITC (Miltenyi Biotec) and anti–lineage cocktail–FITC and anti–HLA-DR–PerCP (Becton Dickinson–Pharmingen). Cells were blocked with 5% (vol/vol) heat-inactivated human AB serum and incubated with the appropriate antibodies in the dark at 4°C for 30 minutes, then washed twice in phosphate-buffered saline (PBS) and fixed in 1% paraformaldehyde in PBS before being acquired using a FACSCalibur flow cytometer (BD) and analyzed using CellQuest Pro (BD) and FlowJo (TreeStar) software packages.
PBMC depletions
PBMCs depleted of target subsets were generated from 1 × 108 PBMCs using the following positive isolation kits from StemCell Technologies according to the manufacturer's instructions: T cells, CD3+ EasySep positive selection kit; B cells, CD19+ EasySep positive selection kit; natural killer (NK) cells, CD56+ positive selection kit; and monocytes, CD14+ positive selection kit. pDCs were labeled as described using BDCA-2–PE and depleted using the PE Depletion kit. Both positively and negatively isolated populations were assayed for percentage of pDCs by flow cytometry, as well as for purity and recovery using the following antibodies: anti-CD3–FITC, anti-CD4–PeCy5, anti-CD14–PE, anti-CD16–FITC, anti-CD20–FITC, anti-CD56–PeCy5 (eBioscience), anti-CD14–PE, and anti-CD8–PeCy5 (BD Pharmingen).
Monocyte enrichment
Negatively isolated monocytes were selected using the Monocyte Enrichment kit; positively selected monocytes were obtained using the CD14+ Positive Selection kit, each from StemCell Technologies. The selected cells were harvested and washed twice in RPMI 1640. The resulting cell populations were assessed for purity and the presence of contaminating pDCs via flow cytometry. Neutralizing antibody to IL-1Ra (R&D Systems) was used at 10 μg/mL where indicated.
Results
pDCs are the principal IFN-λ1–secreting cells upon stimulation with HSV
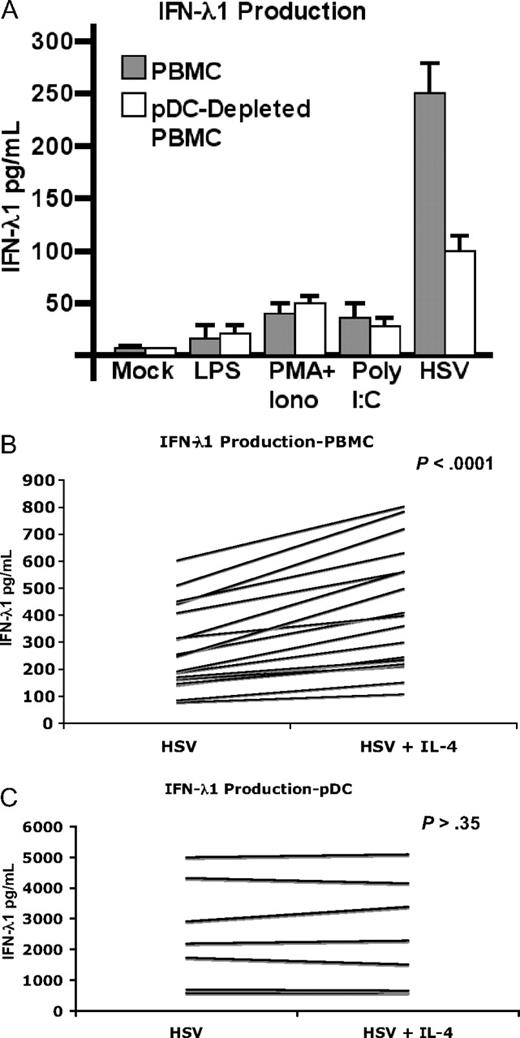
Human pDCs have been shown to produce IFN-λ1 in response to a variety of stimuli, including HSV. To verify whether pDCs are the principal IFN-λ1–secreting population in response to HSV, PBMCs were partially depleted of pDCs (mean reduction 42.5%) and cultured overnight in the presence of stimulatory poly I:C, LPS, PMA/ionomycin, or HSV. Compared with PBMCs, only HSV elicited a substantial production of IFN-λ1 (Figure 1A) that was decreased by approximately 60% in the pDC-depleted cells. Depleting pDCs did not affect secretion in response to any other stimulus. These findings confirmed previous observations that pDCs are the major PBMC population to produce IFN-λ1 in response to HSV,24 which is known to stimulate pDCs at least in part through Toll-like receptor 9 (TLR9)26,–28 and potentially through TLR7.29 Unlike myeloid/classical DCs (mDCs) or MDDCs,23 highly enriched pDCs did not produce appreciable amounts of IFN-λ1 in response to poly I:C or LPS, as has been previously observed.24 This is in agreement with their selective expression of TLR7 and TLR9, and paucity of expression and/or response to TLR3 (poly I:C) or TLR4 (LPS) agonists.30,31 Nonetheless, these stimuli elicit production from mDCs, which are present in PBMCs at nearly the same low frequency of pDCs (0.2%-0.5%).32
IL-4 enhances IFN-λ1 by PBMCs, but not purified pDCs. (A) Peripheral blood mononuclear cells (PBMCs) were partially depleted of plasmacytoid dendritic cells (pDCs) using magnetic beads. PBMCs or pDC-depleted PBMCs were incubated overnight with the indicated stimuli. Supernatants (SNs) were harvested and IFN-λ1 was measured by enzyme-linked immunosorbent assay (ELISA; n = 3; mean ± SD is shown). The effect of interleukin-4 (IL-4) on IFN-λ1 production by PBMCs (B) or pDCs (C) was determined by stimulating either population overnight with HSV in the presence or absence of IL-4. SNs were harvested and assayed as in panel A. IL-4 was shown to significantly increased IFN-λ1 production in HSV-stimulated PBMCs (n = 18; P < .001) but not pDCs (n = 7; P = .35) as determined by the Wilcoxon signed-rank test.
IL-4 enhances IFN-λ1 by PBMCs, but not purified pDCs. (A) Peripheral blood mononuclear cells (PBMCs) were partially depleted of plasmacytoid dendritic cells (pDCs) using magnetic beads. PBMCs or pDC-depleted PBMCs were incubated overnight with the indicated stimuli. Supernatants (SNs) were harvested and IFN-λ1 was measured by enzyme-linked immunosorbent assay (ELISA; n = 3; mean ± SD is shown). The effect of interleukin-4 (IL-4) on IFN-λ1 production by PBMCs (B) or pDCs (C) was determined by stimulating either population overnight with HSV in the presence or absence of IL-4. SNs were harvested and assayed as in panel A. IL-4 was shown to significantly increased IFN-λ1 production in HSV-stimulated PBMCs (n = 18; P < .001) but not pDCs (n = 7; P = .35) as determined by the Wilcoxon signed-rank test.
IL-4 modulates IFN-λ1 responses of PBMCs but not purified pDCs
Recent work in our laboratory has shown that IFN-λ1 exerts a significant negative regulatory effect on the production of Th2 cytokines by T cells. We hypothesized that type-III IFNs were a critical determinant during T-cell polarization and sought to understand what reciprocal effect, if any, Th2 cytokines had on IFN-λ1 production by PBMCs and/or pDCs. To that end, PBMCs were stimulated with HSV in the presence or absence of the Th2-associated cytokine IL-4 and analyzed for secretion of IFN-λ1.
A marked increase in IFN-λ1 production was observed when IL-4 was present during HSV stimulation (56.53%, SD ± 21.8; Figure 1B; P < .001). This effect was observed in 18 of 18 donors tested. To determine whether or not this was a direct effect of IL-4 on the principal IFN-λ1–producing cells, pDCs were isolated and stimulated in the same manner. However, IL-4 had no such effect on purified pDCs (Figure 1C; 0 of 7 donors tested). These data suggested that either a second cell type actively secreted IFN-λ1 in response to IL-4 and HSV (but to neither alone), or that an unidentified cell type was being stimulated to secrete one or more cytokines or additional factor(s) that in turn increased IFN-λ1 production by pDCs.
IL-4–responsive monocytes are permissive for enhanced IFN-λ1 production
To identify additional subpopulations that may contribute to IL-4–enhanced IFN-λ1 production, PBMCs were depleted of T cells, B cells, NK cells, or monocytes using antibody-conjugated magnetic beads. To assess the efficiency of each depletion, resultant populations were evaluated for the successful removal of target cells using flow cytometry (Figure 2A top panels; percentages shown indicate effective depletion of each subpopulation). The proportion of pDCs remaining in each subset-depleted population was also determined (Figure 2A bottom panels). A slight enrichment of pDCs can be observed in the T cell–depleted cells, due to the removal of the population that comprises a high percentage (∼ 75%) of PBMCs. B cells, NK cells, and monocytes combine to make up the majority of remaining portion, so their deletion did not result in the same elevation in pDC frequency.
Monocytes mediate IL-4–enhanced IFN-λ1 production. PBMCs were depleted of T cells, B cells, NK cells, or monocytes by magnetic bead separation. (A) Successful depletion of the indicated populations was verified using flow cytometry (percentage of remaining cells indicated; top panels). The percentage of pDCs present in each depleted population was also measured by flow cytometry (percentages indicated; bottom panels). Depleted populations were then stimulated with HSV in the presence or absence of IL-4 to determine the contribution of each cell type to enhanced IFN-λ1 production. (B) SNs were harvested after 24 hours and assayed for presence of IFN-λ1 by ELISAs (n = 4). The graph shows percentage change in HSV-induced IFN-λ1 production upon addition of IL-4. Means ± SE for the 4 donors are shown. *P < .05, determined using the Student t test.
Monocytes mediate IL-4–enhanced IFN-λ1 production. PBMCs were depleted of T cells, B cells, NK cells, or monocytes by magnetic bead separation. (A) Successful depletion of the indicated populations was verified using flow cytometry (percentage of remaining cells indicated; top panels). The percentage of pDCs present in each depleted population was also measured by flow cytometry (percentages indicated; bottom panels). Depleted populations were then stimulated with HSV in the presence or absence of IL-4 to determine the contribution of each cell type to enhanced IFN-λ1 production. (B) SNs were harvested after 24 hours and assayed for presence of IFN-λ1 by ELISAs (n = 4). The graph shows percentage change in HSV-induced IFN-λ1 production upon addition of IL-4. Means ± SE for the 4 donors are shown. *P < .05, determined using the Student t test.
Whole PBMCs and each of the depleted populations were then stimulated with IL-4, HSV, or both. IL-4–enhanced IFN-λ1 secretion continued to be observed after depletion of either T, B, or NK cells. In contrast, monocyte depletion prevented the expected IL-4–mediated enhancement of IFN-λ1 secretion (Figure 2B).
pDCs, not monocytes, secrete enhanced levels of IFN-λ1 in response to IL-4
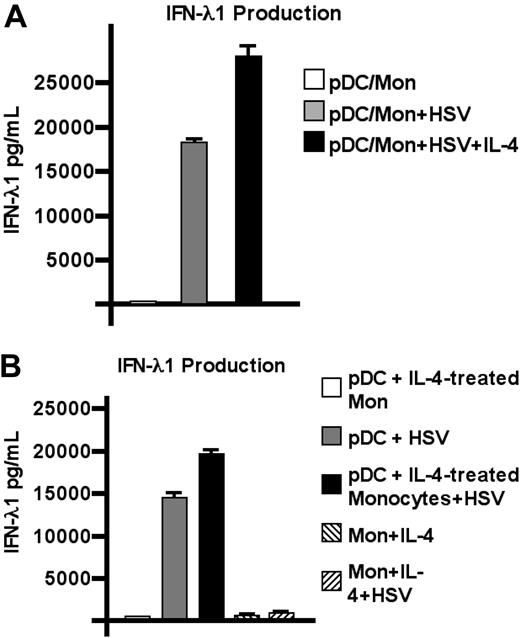
The results obtained from the depletion experiments suggested that monocytes are a critical component of IL-4–enhanced IFN-λ1 production. To determine whether pDCs work in concert with monocytes to achieve this, purified pDCs were cocultured with autologous monocytes at a ratio of 9:1 (pDC/monocyte). Supernatants (SNs) were harvested from these cultures after overnight incubation with HSV in the presence or absence of IL-4 (Figure 3A), and secreted IFN-λ1 was measured. As shown, the addition of monocytes to pDCs conferred the ability to produce elevated levels of IFN-λ1 upon viral stimulation, which does not occur in pDCs alone (Figure 1C).
pDCs are the cellular source of IL-4–enhanced IFN-λ1. (A) Purified pDCs were cocultured with autologous monocytes at a ratio of 9:1 (pDC/monocyte). SNs were harvested from these cultures after overnight incubation with HSV in the presence or absence of IL-4; IFN-λ1 levels were determined by ELISA. (B) Monocytes were stimulated with IL-4 (50 ng/mL) for 1 hour then washed. Cells were then cocultured with autologous pDCs and stimulated overnight with HSV in the presence or absence of IL-4 as shown. One representative experiment of 3 is shown; mean of triplicate wells ± SD.
pDCs are the cellular source of IL-4–enhanced IFN-λ1. (A) Purified pDCs were cocultured with autologous monocytes at a ratio of 9:1 (pDC/monocyte). SNs were harvested from these cultures after overnight incubation with HSV in the presence or absence of IL-4; IFN-λ1 levels were determined by ELISA. (B) Monocytes were stimulated with IL-4 (50 ng/mL) for 1 hour then washed. Cells were then cocultured with autologous pDCs and stimulated overnight with HSV in the presence or absence of IL-4 as shown. One representative experiment of 3 is shown; mean of triplicate wells ± SD.
Negatively isolated (ie, untouched) T cells, B cells, NK cells, and monocytes were also each assayed for their ability to produce IFN-λ1 in response to HSV. IL-4–enhanced IFN-λ1 production could not be detected in any of these isolated populations (data not shown). From these data, we hypothesized that in response to IL-4, monocytes induce pDCs to produce greater amounts of IFN-λ, either via secretion of a soluble factor or in a contact-dependent manner. To address this question, monocytes were treated with IL-4 for 1 hour, washed, then cocultured with pDCs. Monocytes alone or monocyte-pDC cocultures were then stimulated overnight with HSV (Figure 3B) and assessed for IFN-λ1 production by ELISA. IL-4–treated monocytes themselves did not produce IFN-λ1 (with or without viral stimulation). pDCs, however, once again exhibited increased IFN-λ1 production, dependent on IL-4–treated monocytes.
IL-4–treated monocytes up-regulate IFN-λ1 production by stimulated pDCs via IL-1Ra
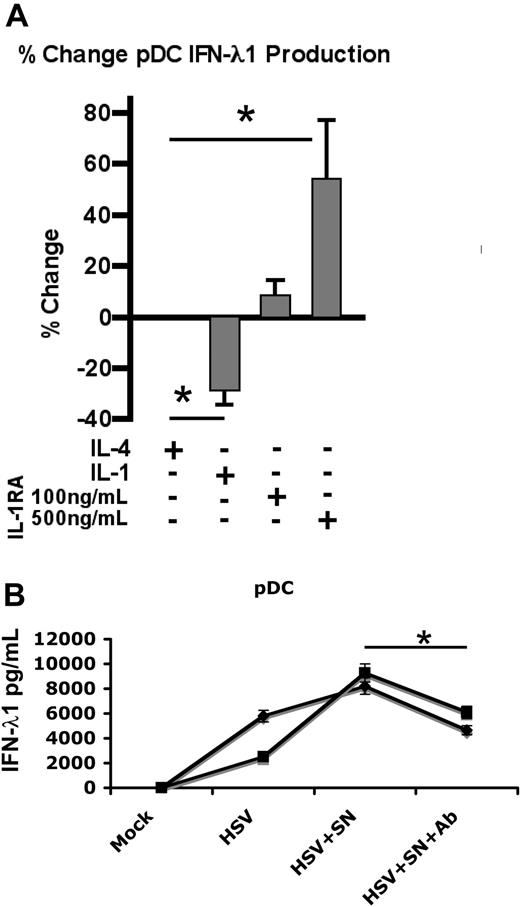
To identify factors which are secreted by monocytes and may mediate this phenomenon, positively selected monocytes were stimulated for 1 hour with IL-4, washed twice, and incubated overnight. Supernatants from IL-4–treated monocytes were analyzed using Cytokine Antibody Arrays from RayBiotech. This analysis identified the presence of IL-1Ra (which has been reported previously33 ). IL-1Ra is known to function as a soluble IL-1 antagonist by binding to the IL-1R with virtually equal avidity as IL-1, but failing to trigger receptor activation. To assess its effect on IFN-λ1 production, purified pDCs were exposed to HSV overnight in the presence of IL-4, IL-1β, or IL-1Ra (Figure 4A); supernatants were analyzed by ELISA. As observed previously, IL-4 did not alter the levels of IFN-λ1 secretion. IL-1β exerted an inhibitory effect on IFN-λ1 production, reducing expression by 26.6%. However, IFN-λ1 production was enhanced up to 57.5% (± 32.0%) upon addition of IL-1Ra, in a dose-responsive manner. To confirm that IL-1Ra was the agent mediating IL-4–enhanced, monocyte-dependent IFN-λ1 secretion, monocytes were cultured with IL-4 for 1 hour, washed twice, and incubated overnight; culture SNs were then harvested. pDCs were cultured in these supernatants and stimulated with HSV in the presence or absence of neutralizing antibody to IL-1Ra (Figure 4B). Supernatants from IL-4–treated monocytes were sufficient to mediate enhanced IFN-λ1 production in virally stimulated pDCs, demonstrating that the phenomenon is indeed contact-independent. Addition of neutralizing antibody to IL-1Ra blocked the enhanced IFN-λ1 secretion, thereby confirming the responsiveness of pDCs to IL-1Ra and its ability to modulate cytokine production.
IL-1Ra augments IFN-λ1 production by pDCs. (A) pDCs were stimulated overnight with HSV in the presence of IL-4 (50 ng/mL), IL-1 (100 ng/mL), or IL-1Ra as indicated; SNs were assayed by ELISA for IFN-λ1 (means ± SD from 3 independent experiments are shown). (B) Monocytes from 2 separate donors were incubated for 1 hour with IL-4, washed twice, and then cultured overnight. SNs were collected and used as culture medium for purified allogeneic pDCs, isolated from 2 independent donors. pDCs were incubated overnight in medium or SNs from IL-4–treated monocytes with or without neutralizing Ab to IL-1Ra, and stimulated with HSV. IFN-λ1 levels were measured by ELISA. A total of 2 independent experiments are shown (means ± SD). *P < .05 as determined by the Student t test.
IL-1Ra augments IFN-λ1 production by pDCs. (A) pDCs were stimulated overnight with HSV in the presence of IL-4 (50 ng/mL), IL-1 (100 ng/mL), or IL-1Ra as indicated; SNs were assayed by ELISA for IFN-λ1 (means ± SD from 3 independent experiments are shown). (B) Monocytes from 2 separate donors were incubated for 1 hour with IL-4, washed twice, and then cultured overnight. SNs were collected and used as culture medium for purified allogeneic pDCs, isolated from 2 independent donors. pDCs were incubated overnight in medium or SNs from IL-4–treated monocytes with or without neutralizing Ab to IL-1Ra, and stimulated with HSV. IFN-λ1 levels were measured by ELISA. A total of 2 independent experiments are shown (means ± SD). *P < .05 as determined by the Student t test.
Discussion
The Th1/Th2 paradigm was originally defined by Mosmann, Coffman, and colleagues.34 Recently, we have shown that IFN-λ1, a novel cytokine produced by pDCs24 and other immune cells,23 is a powerful inhibitor of human Th2-cell development.13,16,17
Here, we have shown that the Th2-related cytokine IL-4, through its activity on monocytes, serves to regulate the production of IFN-λ1 by human pDCs. This effect is specific to monocytes, as neither T, B, or NK cells responded to IL-4 in this way. Monocytes do not directly produce IFN-λ in response to IL-4, but rather enhance production by pDCs, through secretion of IL-1Ra; thus, we have defined a novel feedback loop between pDCs and Th2 cells. Although we have described the role of IL-4 here, it should be noted that we observed similar effects with IL-13; these effects were consistent and of a lesser magnitude than those observed with IL-4. This may be due to differences in expression of the IL-4/13 type-I/type-II receptors35 on pDCs, which we did not investigate here.
As we have shown, increased production of IFN-λ1 by pDCs is mediated principally by IL-1Ra, which attenuates the effect of IL-1 by competitively binding its receptor without activating the IL-1–signaling pathway.36,37 IL-1Ra was already known to be produced by monocytes in response to IL-4,33 and is shown here to enhance HSV-induced IFN-λ1 production by pDCs. Elevated serum levels of IL-1Ra have previously been observed in patients undergoing IL-4 cancer immunotherapy33 ; similarly, monocytes isolated from healthy individuals and treated with IL-4 in vitro also yield higher IL-1Ra levels.38 In studies reported here, we used increasing amounts of IL-1Ra (∼ 3- to 15-fold above systemic serum levels in patients) to show dose responsiveness on pDCs. For IL-1Ra to function as an enhancer of type-III cytokine production by pDCs, it would appear to counterbalance a repressive signal from IL-1, which our data also suggest. However, only sparse information exists about the response of pDCs to IL-1. Upon ligation of IL-1R by IL-1, the adaptor molecule myeloid differentiation factor 88 (MyD88) permits interaction with IL-1R–associated kinases (IRAKs) in a signaling cascade that results in activation of NF-κB.39,–41 TLR7 and TLR9, the predominant Toll-like receptors present in pDCs, both signal through MyD88- and IRAK-dependent pathways. Interestingly, in our experiments, agonists for each of these receptors (imiquimod and CpG, respectively) did not elicit the same high levels of IL-1Ra–enhanced IFN-λ1 production as HSV (data not shown). This may be because HSV can trigger a broad range of TLR-mediated pathways. There is some conjecture about the nature of HSV recognition by pDCs. Receptor binding to the mannose receptor and subsequent endocytosis were shown to be critical,42 and more recent studies suggest that not only TLR9,26 but also TLR729 and potentially TLR2,43 combine additively in signaling the detection and response to HSV by pDCs.
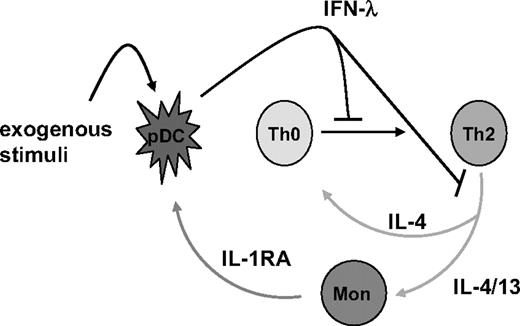
The contribution of IL-4 (and IL-13) to enhanced production of IFN-λ in this system is of specific interest in asthma, especially since IFN-λ1 functions as a potent inhibitor of Th2 cytokine production in both PBMCs and T cells.13,14,16,17 In T cells, this was preceded by a decrease in GATA3,17 the master regulator of Th2 polarization.44,–46 A major consequence of GATA3 deficiency is the loss of IL-4Rα from the cell surface. Left unable to respond to IL-4, developing T cells that have been treated with IFN-λ1 cannot sustain effective polarization toward a Th2 phenotype. In the present report, we show that the complementary result is also true, and define a novel feedback loop between pDCs and Th2 cells; Th2 cytokines, acting indirectly via monocytes, augment IFN-λ1 production in stimulated pDCs. In this way, IL-4 and IFN-λ1 comprise a feedback loop whereby Th2 cytokines induce their own repressor (Figure 5). Thus, IFN-λ is shown to not only inhibit the production of IL-4 and IL-13 by Th2-polarized cells, but also to suppress the differentiation toward a Th2 phenotype. In light of studies describing deficient viral-responsive IFN-λ production in patients with asthma, who display chronically elevated levels of IL-4 and IL-13,20 this feedback loop represents a natural checkpoint for control of Th2 cytokines. Whereas respiratory virus infection in healthy individuals triggers effective type-III IFN production, which may then act to dampen secretion of Th2 cytokines, asthmatics exhibit an impaired ability to secrete IFN-λ and may therefore be unable to repress these same cytokines.
Reciprocal control of IFN-λ1 and Th2-associated cytokines. An illustration representing our current thinking of the feedback loop between pDCs, Th2 cells, and monocytes is shown.
Reciprocal control of IFN-λ1 and Th2-associated cytokines. An illustration representing our current thinking of the feedback loop between pDCs, Th2 cells, and monocytes is shown.
Whether or not IFN-λ contributes to inhibition of inflammation in these patients is unknown. However, IFN-λ1 has been shown to increase production of IL-6, IL-8, and IL-10 by PBMCs, with no concomitant increase in IL-1 or TNF, suggesting it may not directly engender local tissue destruction, but could contribute to the inflammatory process.14 Irrespective of the pathways that govern this phenomenon, the reciprocal regulation of IFN-λ and IL-4 or IL-13 represents a previously undescribed channel through which a developing immune response may be regulated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was funded intramurally by HUMIGEN LLC.
Authorship
Contribution: N.J.M. designed and executed experiments, analyzed data, and wrote and revised the manuscript; G.E.G. contributed to experimental design, executed experiments, analyzed data, and proofread the manuscript; and G.G. designed experiments and analyzed data, and wrote, revised, and finalized the manuscript.
Conflict-of-interest statement: The authors are employees of HUMIGEN LLC, but otherwise declare no competing financial interests.
Correspondence: Grant Gallagher, HUMIGEN LLC, The Institute for Genetic Immunology, 2439 Kuser Rd, Hamilton, NJ 08690; e-mail: g.gallagher@humigen.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal