Abstract
Idiopathic CD4+ T-cell lymphocytopenia (ICL) is a rare acquired T-cell immunodeficiency of unknown pathogenic basis. Six adults with ICL who developed opportunistic infections were investigated using extensive immunophenotyping analysis and functional evaluation of the chemokine receptor CXCR4. For all 6 patients studied, a profound defect in CXCR4 expression was detected at the surface of CD4+ T lymphocytes, in association with an abnormal intracellular accumulation of CXCR4 and of its natural ligand, the chemokine CXCL12. For all patients studied, CD4+ T-cell chemotactic response toward CXCL12 was decreased, whereas sensitivity to CXCL8 was preserved. CXCR4 recovery after ligand-induced endocytosis was impaired in ICL CD4+ T cells. Upon in vitro addition of interleukin-2 (IL-2), membrane expression of CXCR4 returned to normal levels in 5 of 6 patients, whereas intracellular accumulation of CXCR4 and CXCL12 disappeared. Upon therapeutic administration of IL-2, CD4+ T-cell count and membrane CXCR4 expression and function improved over time in 3 of 4 patients treated. Therefore, our data indicate that ICL is associated with defective surface expression of CXCR4, which may be reversed by IL-2.
Introduction
Idiopathic CD4+ T-cell lymphocytopenia (ICL) is characterized by a profound and persistent CD4+ T-cell defect that predisposes to opportunistic infections.1-5 ICL definition includes an absolute CD4+ T-cell count less than 300 cells/mm3 or less than 20% of CD4+ T cells on more than one occasion at least 6 weeks apart, together with lack of other known immune defects.6 There is no clear evidence for an infectious or environmental cause of the disease.3,7 Heterogeneous immunologic profiles have been reported so far in ICL patients.1,8,9 Interleukin-2 (IL-2) has been reported to increase CD4+ T-lymphocyte count and improve the outcome in a few ICL patients.10,11
Mechanistic studies of T-cell function in ICL remain scarce. Decreased T-cell responses as well as increased T-cell activation have been reported.7,12 CD8+ T-cell counts remain in the normal range or are often decreased in ICL.13 Functional investigations have revealed an increased propensity of ICL T cells to undergo apoptosis, a process partially dependent on Fas expression.14,15 Markers for activation and turnover are increased in CD4+ T cells but not in CD8+ T cells, pointing at a specific alteration of the CD4+ T-cell compartment.13 Another factor that may contribute to the CD4+ T-cell defect is a decreased clonogenic capacity of the bone marrow (BM) in ICL patients.16 A frequent alteration observed in ICL consists is increased levels of IL-7 in peripheral blood, consistent with the triggering of a homeostatic response to restore normal CD4+ T-cell counts.9,17
Chemokines are secreted proteins that govern the migration of leukocyte subsets to their specific niches within lymphoid organs and inflammatory sites.18,19 Chemokines mediate their functions by binding to chemokine receptors, which belong to the heptahelical G protein–coupled receptor (GPCR) family.20 The chemokine stromal cell–derived factor-1 (SDF-1/CXCL12), the sole natural ligand for the broadly expressed GPCR CXCR4, acts as a chemoattractant for hematopoietic progenitor cells (HPCs) and leukocytes (reviewed in Lataillade et al21 ). CXCL12 is constitutively produced by stromal, epithelial, and endothelial cells, notably in lymphoid organs. In postnatal life, CXCL12/CXCR4 interactions regulate the BM homing and retention of HPCs, the transendothelial migration of leukocytes, as well as their lymphoid and peripheral trafficking.22-25 In the thymus, CXCL12 expression by epithelial cells is required for the settling, survival/expansion, and subsequent differentiation of CXCR4+ early T-lymphoid progenitors (reviewed in Takahama26 ). In the periphery, the CXCL12/CXCR4 axis contributes to the homing, positioning, and activation of T cells within secondary lymphoid tissues (reviewed in Bromley et al27 and Patrussi and Baldari28 ). Because of these pleiotropic effects, alterations of CXCR4 expression or activity are likely to severely impact T-cell differentiation and trafficking. Supporting this notion, mice reconstituted with progenitor cells expressing a CXCL12 intrakine, which binds intracellularly to CXCR4 and prevents its membrane expression, display a T-cell lymphopenia together with an impaired intrathymic maturation.29 Conversely, transgenic mice overexpressing a human CXCR4 gene in CD4+ T cells harbor a severe CD4+ T-cell depletion in peripheral blood, with a concomitant increased homing of these cells in the BM.30 These studies clearly indicate that modulation of CXCR4 expression or function disturbs CD4+ T-cell trafficking within tissues.
The molecular basis of ICL is still unknown. We hypothesized that expression or function of CXCR4 could be altered in ICL CD4+ T cells. We provide evidence for defective CXCR4 expression and function in circulating T lymphocytes from 6 patients with ICL, and analyze the restoration of these defects by IL-2 therapy.
Methods
Subjects
Six adult patients were referred to us for investigation of a first opportunistic infection occurring in the setting of CD4+ T-cell counts persistently less than 300/mm3 (Table 1). All patients were seronegative for HIV-1 and -2, human T-lymphotropic virus-1 and -2, hepatitis B virus, hepatitis C virus, and human herpesvirus 8. HIV load was undetectable and patients had no clinical or biologic evidence of active Epstein-Barr virus or cytomegalovirus infections, systemic lupus erythematosus, or sarcoidosis. All patients met the definition of ICL,6 and none mentioned a familial history of severe infections. We obtained authorization from the French governmental agency (Agence Française de Sécurité Sanitaire des Produits de Santé), which delivers authorization for drug use beyond approved usages, to treat 4 of the 6 patients (P1, P2, P3, and P6) with recombinant IL-2 (one course consisting in 4.5 millions units twice a day subcutaneously for 5 days, every 6 weeks) for at least 8 cycles.31 Tolerance of IL-2 was considered acceptable enough to repeat therapeutic cycles, although all patients experienced some side effects.32 The patients' informed consent was obtained for molecular and immunologic investigations and IL-2 therapy in accordance with the Declaration of Helsinki. The control group included 12 healthy volunteers aged 18 to 60 years of both sexes, who were nonsmokers, seronegative for HIV, hepatitis B virus, or hepatitis C virus, without evidence of cancer, congenital heart disease, or connective tissue disorder, and who had all received Bacille Calmette-Guérin vaccination.
Clinical and immunologic profiles of 6 patients with ICL compared with healthy controls
| Characteristic . | ICL patient no. . | Healthy controls . | |||||
|---|---|---|---|---|---|---|---|
| P1 . | P2 . | P3 . | P4 . | P5 . | P6 . | ||
| Age, y | |||||||
| At time of study | 43 | 51 | 51 | 31 | 35 | 75 | 18-60 |
| At time of first infection | 42 | 50 | 48 | 30 | 33 | 72 | |
| Opportunistic infection | |||||||
| Cryptococcus neoformans | Disseminated | Meningeal | Bone | ||||
| Alternaria sp | Skin (recurrent) | ||||||
| Nocardia brasiliensis | Skin + lung | ||||||
| Mycobacterium tuberculosis | Lung | ||||||
| M kansasii | Disseminated | ||||||
| Pneumocystis jirovecii | Lung | ||||||
| IgG, g/L | 7.9 | 12.0 | 7.5 | 12.0 | 11.1 | 6.2 | 7-14 |
| IgM, g/L | 0.7 | 0.6 | 1.6 | 1.7 | 1.1 | 0.4 | 0.4-2.4 |
| IgA, g/L | 1.3 | 1.9 | 2.0 | 1.0 | 1.5 | 0.9 | 0.7-3.7 |
| Pre/post IL-2 values | |||||||
| Total Ly/mm3 | 653/857 | 390/1340 | 165/200 | 799/ND | 450/ND | 979/990 | 1742 ± 429 |
| CD4+ T Ly/mm3 (% Ly) | 20(3)/215(15) | 120(31)/582(43) | 6(4)/10(5) | 208(26)/ND | 185(41)/ND | 137(14)/247(25) | 44 ± 11 |
| CD8+ T Ly/mm3 (% Ly) | 418(64)/377(44) | 148(38)/432(32) | 2(1)/5(2) | 228(28)/ND | 81(18)/ND | 206(21)/178(18) | 28 ± 7 |
| B Ly/mm3 (% Ly) | < 1/< 1 | 27(7)/ND | < 1/< 1 | 40(5)/ND | 9(2)/ND | 176(18)/198(20) | 17 ± 9 |
| γδ T Ly/mm3 (% Ly) | 144(22)/214(25) | 47(12)/ND | 46(28)/ND | 119(15)/ND | 71(15)/ND | 117(12)/40(4) | 3.5 ± 2 |
| NK Ly/mm3 (% Ly) | 79(12)/215(15) | 63(16)/ND | 102(60)/ND | 144(18)/ND | 104(22)/ND | 343(35)/327(33) | 9.5 ± 4.8 |
| CD4+ T Ly surface levels, % | |||||||
| CXCR4 | < 3 | 12 | < 3 | 8 | < 3 | 4.3 | 25 ± 5 |
| CCR5 | 15 | 8 | 22 | 17 | 12 | 17.5 | 16 ± 8 |
| CD8+ T Ly surface levels, % | |||||||
| CXCR4 | 4 | 9.7 | 3 | 10.4 | 1 | 10.5 | 19 ± 8 |
| CCR5 | 11 | 8 | 18 | 21 | 8 | 22 | 12 ± 15 |
| CD4+ T Ly intracytoplasmic levels, % | |||||||
| CXCR4 | 56 | 35 | 47 | 32 | 52 | 29 | 2.0 ± 0.6 |
| CCR5 | 3 | 7 | 5 | 2 | 1 | 3 | 0 |
| CXCL12 | 69 | 38 | 42 | 22 | 34 | 32 | 0 |
| CD8+ T Ly intracytoplasmic levels, % | |||||||
| CXCR4 | 9 | 12 | < 1 | 2 | 8 | 3 | < 1.0 |
| CCR5 | 1 | 2 | < 1 | 0 | 0 | 0 | 0 |
| CXCL12 | 1 | 2 | 2 | 1 | 1 | 1 | 0 |
| Characteristic . | ICL patient no. . | Healthy controls . | |||||
|---|---|---|---|---|---|---|---|
| P1 . | P2 . | P3 . | P4 . | P5 . | P6 . | ||
| Age, y | |||||||
| At time of study | 43 | 51 | 51 | 31 | 35 | 75 | 18-60 |
| At time of first infection | 42 | 50 | 48 | 30 | 33 | 72 | |
| Opportunistic infection | |||||||
| Cryptococcus neoformans | Disseminated | Meningeal | Bone | ||||
| Alternaria sp | Skin (recurrent) | ||||||
| Nocardia brasiliensis | Skin + lung | ||||||
| Mycobacterium tuberculosis | Lung | ||||||
| M kansasii | Disseminated | ||||||
| Pneumocystis jirovecii | Lung | ||||||
| IgG, g/L | 7.9 | 12.0 | 7.5 | 12.0 | 11.1 | 6.2 | 7-14 |
| IgM, g/L | 0.7 | 0.6 | 1.6 | 1.7 | 1.1 | 0.4 | 0.4-2.4 |
| IgA, g/L | 1.3 | 1.9 | 2.0 | 1.0 | 1.5 | 0.9 | 0.7-3.7 |
| Pre/post IL-2 values | |||||||
| Total Ly/mm3 | 653/857 | 390/1340 | 165/200 | 799/ND | 450/ND | 979/990 | 1742 ± 429 |
| CD4+ T Ly/mm3 (% Ly) | 20(3)/215(15) | 120(31)/582(43) | 6(4)/10(5) | 208(26)/ND | 185(41)/ND | 137(14)/247(25) | 44 ± 11 |
| CD8+ T Ly/mm3 (% Ly) | 418(64)/377(44) | 148(38)/432(32) | 2(1)/5(2) | 228(28)/ND | 81(18)/ND | 206(21)/178(18) | 28 ± 7 |
| B Ly/mm3 (% Ly) | < 1/< 1 | 27(7)/ND | < 1/< 1 | 40(5)/ND | 9(2)/ND | 176(18)/198(20) | 17 ± 9 |
| γδ T Ly/mm3 (% Ly) | 144(22)/214(25) | 47(12)/ND | 46(28)/ND | 119(15)/ND | 71(15)/ND | 117(12)/40(4) | 3.5 ± 2 |
| NK Ly/mm3 (% Ly) | 79(12)/215(15) | 63(16)/ND | 102(60)/ND | 144(18)/ND | 104(22)/ND | 343(35)/327(33) | 9.5 ± 4.8 |
| CD4+ T Ly surface levels, % | |||||||
| CXCR4 | < 3 | 12 | < 3 | 8 | < 3 | 4.3 | 25 ± 5 |
| CCR5 | 15 | 8 | 22 | 17 | 12 | 17.5 | 16 ± 8 |
| CD8+ T Ly surface levels, % | |||||||
| CXCR4 | 4 | 9.7 | 3 | 10.4 | 1 | 10.5 | 19 ± 8 |
| CCR5 | 11 | 8 | 18 | 21 | 8 | 22 | 12 ± 15 |
| CD4+ T Ly intracytoplasmic levels, % | |||||||
| CXCR4 | 56 | 35 | 47 | 32 | 52 | 29 | 2.0 ± 0.6 |
| CCR5 | 3 | 7 | 5 | 2 | 1 | 3 | 0 |
| CXCL12 | 69 | 38 | 42 | 22 | 34 | 32 | 0 |
| CD8+ T Ly intracytoplasmic levels, % | |||||||
| CXCR4 | 9 | 12 | < 1 | 2 | 8 | 3 | < 1.0 |
| CCR5 | 1 | 2 | < 1 | 0 | 0 | 0 | 0 |
| CXCL12 | 1 | 2 | 2 | 1 | 1 | 1 | 0 |
BM biopsies showed myelodysplasia and myelofibrosis in P3 and no abnormality in the 5 other patients.
ND indicates not done; and Ly, lymphocyte.
Sample processing and cell culture
Blood samples were drawn several months after effective cure to investigate the immune defect and the molecular basis of ICL. All experiments were performed within 3 hours after blood sampling. Samples from healthy controls were processed in parallel. Peripheral blood mononuclear cells (PBMCs) were isolated from heparin-treated blood samples using Ficoll-Paque Plus (Amersham Biosciences) density gradient centrifugation. Whole blood and PBMCs were used either right after collection or after overnight incubation at 37°C with 9% CO2 in RPMI 1640 culture medium supplemented with 10% heat-inactivated human AB serum (Valbiotech) or fetal calf serum (FCS) in the presence or absence of recombinant IL-2 (10 UI/mL; Peprotech Inc). Because of a complex experimental design (patients living in remote areas requesting to combine outpatient visit, sampling, and experiments), the number and identity of the patients varied for some of the analyses (specified in the corresponding sections).
Immunophenotypic analyses
Absolute counts were determined by 4-color flow cytometry analyses (EPICS-XL MCL flow cytometer; Coulter Electronics Inc) on whole blood samples with fluorescent beads used as an internal standard.33 Seven antibody (Ab) combinations were used for analysis of leukocyte subsets: (1) CD45–fluorescein isothiocyanate (FITC), CD3–phycoerythrin (PE)–cyanin 5 (Cy5), CD4-RD1, CD8-phycoerythrin–Texas Red (ECD); (2) CD4-ECD, CD8-PE-Cy5, CD38-RD1, HLA-DR–FITC; (3) CD4-ECD, CD8-PE-Cy5, CD25-RD1, CD28-FITC; (4) CD4-ECD, CD8-PE-Cy5, CD62L-RD1, CD45RA-FITC; (5) CD19-ECD, CD8-PE-Cy5, CD56-RD1, CD16-FITC; (6) CD4-ECD, CD8-PE-Cy5, CXCR4-PE, CCR5-FITC; and (7) CD3-ECD, CD8-PE-Cy5, T-cell receptor αβ–RD1, T-cell receptor γδ–FITC. All monoclonal Abs (mAbs) were from Beckman Coulter except those specific for CXCR4 (clone 12G5) or CCR5 (clone 2D7; BD Biosciences). Naive T cells were defined by the coexpression of CD45RA and CD62L markers. Cells not coexpressing CD45RA and CD62L markers were considered as memory T cells and those expressing CD38 and/or HLA-DR, as activated T cells.
Intracellular staining was performed in PBMCs to detect CXCR4, CCR5, or CXCL12. After incubation for 30 minutes with saturating amounts of nonconjugated Abs against CXCR4 and CCR5, cells were washed twice in phosphate-buffered saline, fixed with 2% formaldehyde solution, and then treated for 10 minutes at 25°C with a permeabilization buffer (0.5% saponin, 5% FCS in phosphate-buffered saline). CCR5 (FITC)– and CXCR4 (PE)–conjugated mAbs or the K15C anti-CXCL12 mAb,34 followed by an anti–mouse FITC-conjugated donkey polyclonal Ab, were then added for 30 minutes at 25°C. Cells were analyzed after further washes. Leukocytes from 12 healthy donors were used for the control group.
In vitro lymphocyte proliferation assays
PBMCs grown in 96-well culture plates at 1 × 105 cells per well were stimulated with 0.5 μg/mL Staphylococcus aureus enterotoxin B (SEB; Sigma), as a positive control, tetanus anatoxin (TT, 10 μg/mL), candidin (20 μg/mL), purified protein derivative from Mycobacterium tuberculosis (PPD, 5 μg/mL; Statens Institut),33 and the mannoprotein antigen from Cryptococcus neoformans (MANO, 20 μg/mL; a gift from S. M. Levitz, University of Massachusetts Medical School). Proliferation was quantified by flow cytometry, after labeling cells with 10μM 5(6)-carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) before culture. Fluorescence intensity loss due to division cycles was determined after 6 days of culture (% CFSElow cells). Background proliferation was less than 3%. Control values were obtained from 25 healthy volunteers, except for MANO stimulation (5 subjects).
Cytokine secretion assay
Interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) secretion were measured in PBMC-derived supernatants after 36 hours of culture in the presence of SEB, TT, candidin, PPD, or MANO. Cytokines were detected by flow cytometry using the cytometric bead array technique (BD Biosciences). All analyses were performed with the Statistica 8.0 software, using the Mann-Whitney U nonparametric statistical test. Significant differences between groups (P < .05) were reported on data plots.
Chemotaxis assays
Chemotaxis was performed using 24-well chemotaxis chambers with polycarbonate filters (3-μm pore size) as described.35,36 Briefly, diluted recombinant CXCL12 (chemically synthesized and provided by F. Baleux, Unité de Chimie Organique, Institut Pasteur) at various concentrations or recombinant CXCL8 (R&D Systems) at 30nM was added to the lower chamber. PBMCs that migrated to the lower chamber after 2-hour incubation were collected, stained with CD45, CD4, CD8, and CD3 mAbs, and counted by flow cytometry. Cells migrating across the membrane were calculated as follows: {[(number of cells migrating to the lower chamber in response to the chemokine) − (number of cells migrating spontaneously)]/number of cells added to the upper chamber at the start of the assay} ×100.
CXCR4 internalization and recycling assays
CXCR4 internalization was studied as described.36,37 Briefly, PBMCs (2 × 106 cells/mL) were incubated for 40 minutes at 37°C with 200nM CXCL12 in the presence of 50 μg/mL cycloheximide (CHX; Sigma). For CXCR4 recycling assays, PBMCs were washed in acidic glycine buffer (pH = 2.7) and further cultured for up to 120 minutes at 37°C with CHX in the absence of CXCL12. Levels of membrane CXCR4 expression were determined by flow cytometry (FACSCalibur; BD Biosciences) using the PE-conjugated anti–human CXCR4 (clone 12G5) or the corresponding isotype control Ab in combination with FITC-conjugated anti–human CD3 (clone SK7) and allophycocyanin-conjugated anti–human CD4 (clone RPA-T4) mAbs (BD Biosciences). CXCR4 expression in stimulated cells was calculated as follows: (CXCR4 geometric mean fluorescence intensity [MFI] of treated cells/CXCR4 geometric MFI of unstimulated cells) ×100.
Results
Immunologic features of patients with ICL
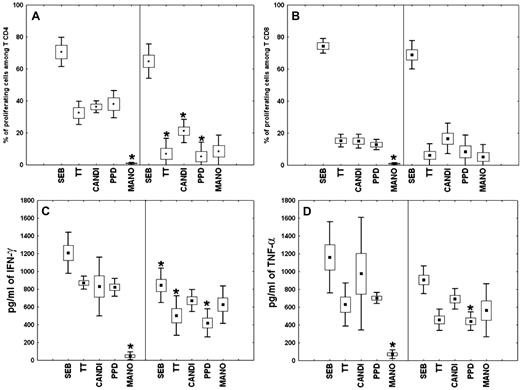
All patients presented with CD4+ T-cell lymphocytopenia, an increase in natural killer (NK)– and γδT-cell populations, and a decrease in B-cell population, which reached undetectable levels for 2 patients (P1 and P3; Table 1). For all patients, serum levels of IgG, IgM, and IgA were within the normal range (Table 1). Of note, CD4+ T-cell depletion has persisted for more than 18 months after the diagnosis of lymph node tuberculosis for P5. Proportions of ICL-naive CD4+ (35.3% ± 27.3%) and CD8+ (49.8% ± 22.8%) T cells did not differ significantly from those of controls (41.4% ± 19.5% and 46.3% ± 8.4%, respectively). In ICL, 17.3% (± 5.6%) of total lymphocytes, 41.2% (± 15.9%) of CD4+ T cells, and 3.2% (± 1.4%) of CD8+ T cells expressed the CD25 marker, whereas the activation markers CD38 and/or HLA-DR were expressed in all populations at normal levels (data not shown). Proliferation of CD4+ and CD8+ T cells in the presence of TT, candidin, and PPD was consistently lower in ICL patients than in controls, whereas proliferation to the superantigen SEB was normal (Figure 1A-B). As expected, MANO proliferation was observed only in ICL T cells. After specific stimulations, a decrease in the production of IFN-γ and TNF-α by ICL PBMCs was observed (Figure 1C-D). These findings provide evidence for severely compromised T-cell responses but normal immunoglobulin production in ICL patients.
Proliferative response and cytokine production by ICL PBMCs. (A-B) Proliferation of CD4+ (A) or CD8+ (B) T cells was assessed in the presence of Staphylococcus aureus enterotoxin B (SEB), tetanus toxin (TT), candidin (CANDI), purified protein derivative from Mycobacterium tuberculosis (PPD), or the mannoprotein antigen from Cryptococcus neoformans (MANO) among PBMCs from healthy subjects (left of each panel) and ICL patients (right of each panel). Results are expressed as percentages of proliferating CD4+ and CD8+ T lymphocytes (means ± SEM and percentiles) after antigenic stimulation, as measured by the fraction of cells with decreased CFSE staining. (C-D) IFN-γ (C) or TNF-α (D) production by PBMCs from healthy persons and ICL patients after in vitro stimulation. Supernatants were harvested 36 hours after stimulation and measured for cytokine levels by the cytometric bead array method. Values represent means ± SEM and percentile of triplicate supernatant samples. *P < .001 compared with control or ICL cells.
Proliferative response and cytokine production by ICL PBMCs. (A-B) Proliferation of CD4+ (A) or CD8+ (B) T cells was assessed in the presence of Staphylococcus aureus enterotoxin B (SEB), tetanus toxin (TT), candidin (CANDI), purified protein derivative from Mycobacterium tuberculosis (PPD), or the mannoprotein antigen from Cryptococcus neoformans (MANO) among PBMCs from healthy subjects (left of each panel) and ICL patients (right of each panel). Results are expressed as percentages of proliferating CD4+ and CD8+ T lymphocytes (means ± SEM and percentiles) after antigenic stimulation, as measured by the fraction of cells with decreased CFSE staining. (C-D) IFN-γ (C) or TNF-α (D) production by PBMCs from healthy persons and ICL patients after in vitro stimulation. Supernatants were harvested 36 hours after stimulation and measured for cytokine levels by the cytometric bead array method. Values represent means ± SEM and percentile of triplicate supernatant samples. *P < .001 compared with control or ICL cells.
Abnormal CXCR4 and CXCL12 detection in ICL CD4+ T cells
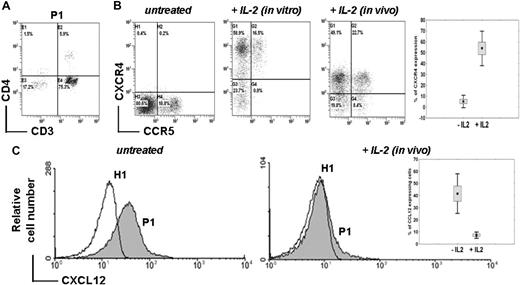
Membrane CXCR4 expression was analyzed in whole blood samples. For all patients, CD4+ T-cell lymphocytopenia (Figure 2A) was associated with a profound decrease in CXCR4 expression at the surface of CD4+ T cells (5.7% ± 3.6% vs 25.0% ± 5.0% in controls, P < .001) and CD8+ T cells (6.4% ± 3.8% vs 19.0% ± 8.0% in controls, P < .001; Table 1 and Figure 2B). Of note, CXCR4 down-regulation was more pronounced within the CD4+ than the CD8+ T-cell subset. Membrane CXCR4 expression was found to be markedly decreased in both naive and memory CD4+ and CD8+ T-cell subsets (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In contrast, CXCR4 expression was preserved at the surface of monocytes (data not shown), suggesting a T cell–specific defect. We found that lack of CXCR4 expression was not due to masking by a ligand, as cell washing in acidic glycine buffer (pH = 2.7) did not change CXCR4 expression levels (data not shown). Expression levels of CCR5 in ICL CD4+ and CD8+ T cells were in the normal range or even increased (Table 1, Figure 2B, and supplemental Figure 1), indicating a CXCR4-specific rather than a global chemokine receptor defect. After overnight incubation with recombinant IL-2, expression levels of CXCR4 at the surface of CD4+ T cells from 5 of 6 patients were similar to those detected in control cells (Figure 2B). For the last patient (P3), CXCR4 remained barely detectable at the CD4+ T-cell surface after in vitro IL-2 treatment.
Abnormal CXCR4 expression and intracellular detection of CXCL12 in ICL CD4+ T lymphocytes. (A) Proportion of CD3+ CD4+ T cells in whole blood sample from a representative ICL patient (P1) as determined by flow cytometry. (B) Membrane expression levels of endogenous CCR5 and CXCR4 in CD4+ T cells from P1 (expressed as %). Whole blood recovered before (untreated) or after (in vivo) 5 days of therapeutic administration of IL-2 (4.5 million units twice a day subcutaneously) or PBMCs incubated overnight with 10 UI/mL IL-2 (in vitro) were labeled using FITC-conjugated CCR5 and PE-conjugated CXCR4 mAbs. Background fluorescence was measured using the corresponding isotype control Ab. The inset summarizes the proportion of CXCR4+ CD4+ T cells in the 3 patients (P1, P2, and P6) before and after IL-2 therapy. Mean CXCR4 expression is reported before and after the first 5-day cycle of IL-2 (P < .001). (C) Intracellular staining of CXCL12 in CD4+-gated T cells from PBMCs of a healthy (H1) or ICL (P1) subject obtained before (untreated) or after (in vivo) 5 days of IL-2 administration. Staining was done using an anti–human CXCL12 mAb followed by a FITC-conjugated anti–mouse Ab. The inset summarizes the proportion of CXCL12-containing CD4+ T cells in the 3 patients before and after administration of the first cycle of IL-2.
Abnormal CXCR4 expression and intracellular detection of CXCL12 in ICL CD4+ T lymphocytes. (A) Proportion of CD3+ CD4+ T cells in whole blood sample from a representative ICL patient (P1) as determined by flow cytometry. (B) Membrane expression levels of endogenous CCR5 and CXCR4 in CD4+ T cells from P1 (expressed as %). Whole blood recovered before (untreated) or after (in vivo) 5 days of therapeutic administration of IL-2 (4.5 million units twice a day subcutaneously) or PBMCs incubated overnight with 10 UI/mL IL-2 (in vitro) were labeled using FITC-conjugated CCR5 and PE-conjugated CXCR4 mAbs. Background fluorescence was measured using the corresponding isotype control Ab. The inset summarizes the proportion of CXCR4+ CD4+ T cells in the 3 patients (P1, P2, and P6) before and after IL-2 therapy. Mean CXCR4 expression is reported before and after the first 5-day cycle of IL-2 (P < .001). (C) Intracellular staining of CXCL12 in CD4+-gated T cells from PBMCs of a healthy (H1) or ICL (P1) subject obtained before (untreated) or after (in vivo) 5 days of IL-2 administration. Staining was done using an anti–human CXCL12 mAb followed by a FITC-conjugated anti–mouse Ab. The inset summarizes the proportion of CXCL12-containing CD4+ T cells in the 3 patients before and after administration of the first cycle of IL-2.
Loss of membrane CXCR4 expression was accompanied by an abnormal intracellular accumulation of CXCR4 (41.8% ± 11.3% vs 2.0% ± 0.6%, P < .001) and of CXCL12 (39.0% ± 15.9% vs 0%, P < .001) in CD4+ T cells from all patients (Table 1 and Figure 2C). In addition, an abnormal intracellular detection of CXCR4 was found in both naive and memory CD4+ and CD8+ T-cell subsets (Table 1 and supplemental Figure 1). IL-2 therapy was initiated in 4 patients. At the end of the first IL-2 course, surface expression levels of CXCR4 were increased in CD4+ T cells from 3 patients, namely P1, P2, and P6 (Figure 2B). Membrane recovery of CXCR4 was associated with a disappearance of intracellular CXCL12 (Figure 2C) and CXCR4 (data not shown). For P3, membrane expression of CXCR4 remained low after in vivo IL-2 administration, as it had after in vitro IL-2 stimulation (data not shown).
Lack of modulating effects of ICL plasmas on membrane CXCR4 expression
To gain insight into the mechanism underlying the abnormal intracellular accumulation of CXCR4 in ICL T cells, CXCL12 levels were measured in plasmas from healthy and ICL (P1 and P6) subjects by immunoassay. No differences were found between patients and controls (data not shown). As polymorphisms in CXCR4 and CXCL12 genes have been associated with pathologic disorders (reviewed in Busillo and Benovic38 ), we looked for possible mutations associated with ICL. Sequencing of cDNA products amplified from PBMCs (for CXCR4) or BM aspirates (for CXCL12 isoforms) of 2 patients (P1 and P3) did not reveal mutations in the CXCR4 open reading frame or in those of CXCL12-α and -β (data not shown). These findings do not support the notion that anomalies in CXCL12 structure or production account for the sustained CXCR4 internalization and the loss of CXCR4 expression at the surface of ICL CD4+ T cells.
We next looked for the presence of cytokines and chemokines known to modulate CXCR4 expression in the plasma of ICL patients by multiplexed immunoassay. The panel included molecules known to down-regulate CXCR4 (eg, IFN-α, IFN-γ) or to up-regulate CXCR4 (eg, inflammatory cytokines and chemokines, γ-c cytokines) in T lymphocytes.39-42 The levels of 25 analytes measured in P6 were in general within the range of values found in 11 healthy donors (supplemental Table 1). We also investigated whether the plasma of ICL patients could induce CXCR4 down-regulation in normal PBMCs. Membrane CXCR4 expression was measured in healthy donor PBMCs incubated overnight with 30% plasma from ICL or heterologous healthy subjects. We did not detect a major difference between the effects of plasmas from 2 ICL patients (P1 and P6) and 4 healthy donors (supplemental Figure 2). Overall, we have not yet identified a component of ICL plasmas that could account for CXCR4 down-regulation.
Impaired CXCL12-promoted chemotaxis of ICL T lymphocytes
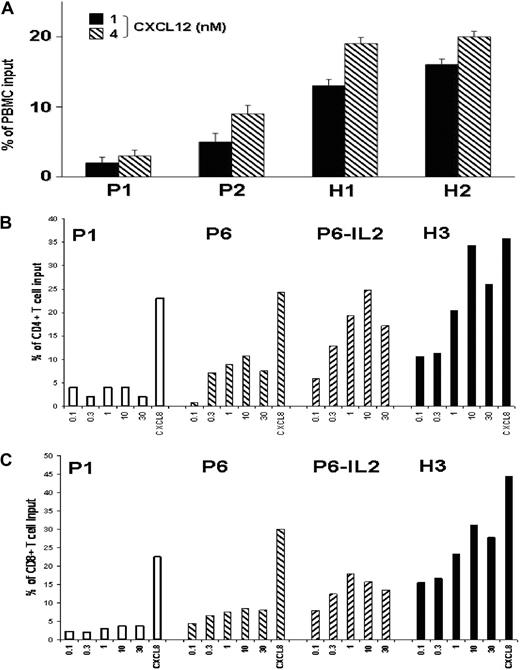
Impaired surface expression of CXCR4 may alter the responsiveness of T lymphocytes to CXCL12. This possibility was investigated in leukocytes from ICL (P1, P2, and P6) and healthy subjects (H1, H2, and H3) using a chemotaxis assay. Addition of CXCL12 resulted in a dose-dependent chemotactic response of control and ICL total lymphocytes (Figure 3A) and CD4+ and CD8+ T cells (Figure 3B-C). However, ICL T cells displayed weaker migratory responses at all concentrations, indicating a lower efficiency of chemotaxis to CXCL12. These findings are consistent with the decreased expression levels of CXCR4 observed in ICL T cells. No alteration of CXCL12-promoted chemotaxis was observed in ICL leukocytes other than T cells (data not shown). Importantly, no impairment in chemotactic response to CXCL8 was detected in ICL leukocytes, independent of the subset considered, including CD4+ or CD8+ T cells (Figure 3B-C), non–T lymphocytes, and monocytes (data not shown), strongly suggesting a defect specific for CXCR4.
Impaired CXCL12-promoted chemotaxis of ICL T lymphocytes. (A) Migration of PBMCs from ICL patients (P1 and P2) and healthy subjects (H1 and H2) in response to 1nM or 4nM CXCL12. (B-C) Migration of PBMCs from ICL patients (P1 and P6) and 1 healthy subject (H3) in response to serial dilutions of CXCL12 (0.1nM to 30nM) or to 30nM CXCL8. PBMCs from P6 were analyzed before or after the course of IL-2 treatment. Transmigrated cells recovered in the lower chamber were counted by flow cytometry after gating on forward and side scatter to select lymphocytes (A) or after gating specifically CD4+ or CD8+ T lymphocytes (B-C) after staining with CD45, CD4, CD8, and CD3 mAbs. Results (medians ± SD for triplicate wells) are expressed as the percentage of input lymphocytes (A), or CD4+ (B) or CD8+ (C) T cells that migrated to the lower chamber.
Impaired CXCL12-promoted chemotaxis of ICL T lymphocytes. (A) Migration of PBMCs from ICL patients (P1 and P2) and healthy subjects (H1 and H2) in response to 1nM or 4nM CXCL12. (B-C) Migration of PBMCs from ICL patients (P1 and P6) and 1 healthy subject (H3) in response to serial dilutions of CXCL12 (0.1nM to 30nM) or to 30nM CXCL8. PBMCs from P6 were analyzed before or after the course of IL-2 treatment. Transmigrated cells recovered in the lower chamber were counted by flow cytometry after gating on forward and side scatter to select lymphocytes (A) or after gating specifically CD4+ or CD8+ T lymphocytes (B-C) after staining with CD45, CD4, CD8, and CD3 mAbs. Results (medians ± SD for triplicate wells) are expressed as the percentage of input lymphocytes (A), or CD4+ (B) or CD8+ (C) T cells that migrated to the lower chamber.
As therapeutic IL-2 effectively restored membrane CXCR4 expression on CD4+ T cells from 3 of 4 patients (Figure 2B), we next investigated whether defective CXCR4-mediated chemotaxis could be normalized upon IL-2 treatment. To address this possibility, PBMCs recovered from P6 immediately after a 5-day course of IL-2 treatment were tested for their ability to migrate toward CXCL12. As shown in Figure 3B-C, both CD4+ and CD8+ T-cell subsets displayed an increase in CXCL12-promoted chemotaxis. Overall, these findings indicate that the defect in membrane CXCR4 expression results in a loss of CXCR4 function in ICL T lymphocytes, which could be improved by IL-2 therapy.
Defective CXCR4 recycling in ICL CD4+ T cells
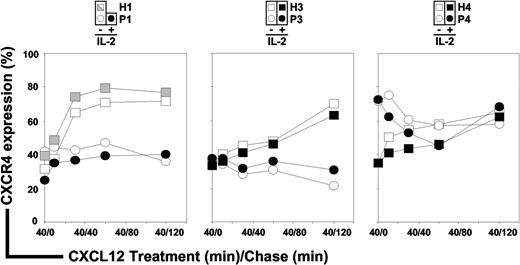
Loss of membrane CXCR4 expression together with abnormal intracellular accumulation of CXCR4 and CXCL12 in ICL CD4+ T cells suggested a defective intracellular routing of the ligand/receptor complex. Processes involved in regulating CXCR4 activity include desensitization, internalization, degradation, and recycling.38,43,44 To investigate the fate of CXCR4 upon CXCL12 exposure, we first set up the experimental conditions that allowed re-expression of CXCR4 at the surface of ICL CD4+ T cells. After overnight culture of PBMCs in complete medium supplemented with 10% FCS, CXCR4 surface expression reached comparable levels in ICL (geometric MFI range: 123 to 276) and control (geometric MFI range: 82 to 275) CD4+ T cells. Of note, interindividual variations in the steady-state levels of membrane CXCR4 expression in healthy donors have been documented.36,45 We then determined that the maximal CXCR4 endocytosis was reached at a concentration of 200nM CXCL12 for control T cells. Surface expression levels of CXCR4 in CD4+ T cells from ICL (P1, P3, and P4) and healthy subjects (H1, H3, and H4) were then analyzed by flow cytometry. After CXCL12 stimulation, the fraction of internalized CXCR4 (∼ 60%) in P1 and P3 CD4+ T cells was comparable with that detected in controls (Figure 4 white symbols, time 40/0 [t40/0]). In contrast, the rate of CXCR4 endocytosis was weaker (∼ 30%) in CD4+ T cells from P4 during the initial 40 minutes of CXCL12 treatment. Thus, CXCL12-promoted internalization of CXCR4 occurred normally in CD4+ T cells from P1 and P3, whereas it was impaired in those from P4.
CXCR4 endocytosis and recycling in ICL CD4+ T cells. PBMCs from 3 independent healthy donors (H1, H3, and H4; □) and 3 ICL patients (P1, P3, and P4; ○) were cultured overnight in complete medium supplemented with 10% FCS, allowing CXCR4 re-expression at the surface of ICL cells, then incubated for 40 minutes with 200nM CXCL12 (treatment, t40/0, CXCR4 endocytosis), and further cultured for up to 120 minutes in the absence of CXCL12 (chase, t40/10 and above, CXCR4 recycling). The protein synthesis inhibitor CHX (50 μg/mL) was present throughout the experiment. Levels of membrane CXCR4 expression were assessed by flow cytometry in CD3+CD4+–gated T cells. Effects of IL-2 on CXCR4 endocytosis and recycling were evaluated in CD4+ T cells from healthy (■) and ICL (●) subjects. The kinetic of CXCR4 down-modulation in PBMCs recovered from P1 immediately after the course of IL-2 treatment was compared with that obtained in cells from H1 recovered the same day and left untreated ( ). Displayed data are means of duplicate determinations and are expressed as percentages of CXCR4 expression (100% corresponding to CXCR4 expression at the surface of CD4+ T cells incubated in medium alone).
). Displayed data are means of duplicate determinations and are expressed as percentages of CXCR4 expression (100% corresponding to CXCR4 expression at the surface of CD4+ T cells incubated in medium alone).
CXCR4 endocytosis and recycling in ICL CD4+ T cells. PBMCs from 3 independent healthy donors (H1, H3, and H4; □) and 3 ICL patients (P1, P3, and P4; ○) were cultured overnight in complete medium supplemented with 10% FCS, allowing CXCR4 re-expression at the surface of ICL cells, then incubated for 40 minutes with 200nM CXCL12 (treatment, t40/0, CXCR4 endocytosis), and further cultured for up to 120 minutes in the absence of CXCL12 (chase, t40/10 and above, CXCR4 recycling). The protein synthesis inhibitor CHX (50 μg/mL) was present throughout the experiment. Levels of membrane CXCR4 expression were assessed by flow cytometry in CD3+CD4+–gated T cells. Effects of IL-2 on CXCR4 endocytosis and recycling were evaluated in CD4+ T cells from healthy (■) and ICL (●) subjects. The kinetic of CXCR4 down-modulation in PBMCs recovered from P1 immediately after the course of IL-2 treatment was compared with that obtained in cells from H1 recovered the same day and left untreated ( ). Displayed data are means of duplicate determinations and are expressed as percentages of CXCR4 expression (100% corresponding to CXCR4 expression at the surface of CD4+ T cells incubated in medium alone).
). Displayed data are means of duplicate determinations and are expressed as percentages of CXCR4 expression (100% corresponding to CXCR4 expression at the surface of CD4+ T cells incubated in medium alone).
We then compared the fate of down-modulated CXCR4 in CD4+ T cells from ICL and healthy persons (t40/10 and above). In control cells, CXCR4 began to recycle to the cell surface immediately after agonist removal and reached 60% to 70% of pretreatment levels within 2 hours. In contrast, the recycling of CXCR4 in CD4+ T cells from P1, P3, and P4 was defective, as we detected no surface reexpression of CXCR4 within the first 30 minutes of chase and no further increase in receptor density for up to 120 minutes. These findings provide evidence for defective CXCR4 recycling in ICL CD4+ T cells. As therapeutic IL-2 effectively restored membrane CXCR4 levels in CD4+ T cells from 3 of 4 patients (Figure 2B), we next studied whether defective CXCR4 recycling could be normalized upon IL-2 treatment. To address this possibility, PBMCs from P3 and P4 incubated overnight with recombinant IL-2 or those recovered from P1 immediately after the course of IL-2 treatment were pulsed and chased with CXCL12. As shown in Figure 4 (black symbols), IL-2 exposure modulated neither CXCR4 endocytosis nor the kinetics of CXCR4 recycling in control and ICL CD4+ T cells.
CD4+ T-cell recovery during IL-2 treatment
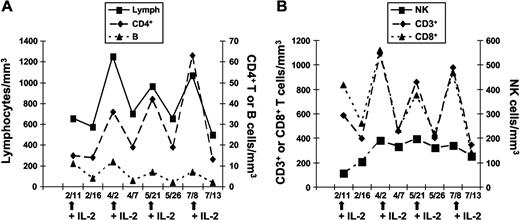
Four patients (P1, P2, P3, and P6) received several courses of recombinant IL-2 for up to 3 years. Treatment efficacy with respect to CD4+ T-lymphocyte recovery was variable, with 1 patient showing a sustained response (P2), 2 displaying transient responses (P1 and P6), and 1 not responding to treatment (P3). Results of immunophenotyping analyses during 4 courses of IL-2 administration are reported for P1 in Figure 5 and for all the 4 treated patients in Table 1. A progressive increase in total lymphocyte number, ascribed to an increase in CD3+ T cells, was observed. Both CD4+ and CD8+ T-cell numbers rose transiently with each IL-2 cure. We did not observe a change in the distribution of naive and memory CD4+ T cells after IL-2 therapy (data not shown). B-cell numbers did not vary significantly over time, although a slow increase followed by a plateau phase was observed for NK cells. Five years later (ie, after 42 IL-2 cycles), P1 is still requiring repeated cytokine administration every 12 weeks, as treatment withdrawal was associated with a drop of CD4+ T cells (< 100 cells/mm3) and reappearance of cryptococcal antigen in serum after sustained negativation for 48 months. After 3 years of IL-2 treatment, P2 presented a sustained CD4+ T-cell count recovery (498 cells/mm3) that was maintained 1 year after IL-2 treatment withdrawal. P6 received 2 cycles of IL-2 therapy in 2005 and 2008, with a sustained CD4+ T-cell recovery after the latest cycle (247 cells/mm3). In contrast, P3 did not exhibit any change in CD4+ T-cell count after IL-2 treatment and developed disseminated M kansasii infection despite the administration of 12 IL-2 courses (data not shown). It is noteworthy that P3 was the patient for whom CXCR4 expression did not improve after in vitro addition of IL-2. These findings suggest an association between the capacity of IL-2 to restore membrane CXCR4 expression and its therapeutic effect on CD4+ T-cell homeostasis.
CD4+ T-cell recovery in ICL patients upon therapeutic administration of IL-2. Changes in absolute leukocyte counts cells in 1 ICL patient (P1) during 4 courses of recombinant IL-2 treatment. (A) Lymphocytes, CD4+ T and B lymphocytes. (B) CD3+ and CD8+ T lymphocytes and NK cells. Arrows indicate IL-2 administration.
CD4+ T-cell recovery in ICL patients upon therapeutic administration of IL-2. Changes in absolute leukocyte counts cells in 1 ICL patient (P1) during 4 courses of recombinant IL-2 treatment. (A) Lymphocytes, CD4+ T and B lymphocytes. (B) CD3+ and CD8+ T lymphocytes and NK cells. Arrows indicate IL-2 administration.
Discussion
In the present study, we provide a novel insight into ICL pathogenesis based on the systematic investigation of 6 patients. We show that ICL, at least in all the patients included in our study, is associated with a profound defect of membrane CXCR4 expression in circulating naive and memory CD4+ T cells, accompanied by an abnormal intracellular accumulation of CXCR4 and of its ligand CXCL12. We did not find evidence for mutations, increased plasma concentration of CXCL12, or changes in cytokine levels in ICL plasmas that could account for CXCR4 down-regulation. This defect seemed to be specific for CXCR4, because expression levels of CCR5 remained within the normal range or was increased depending on the patient. Reduced levels of membrane CXCR4 expression were associated with impaired chemotactic responses to CXCL12, but not to CXCL8, another α-chemokine. These findings suggest that ICL CD4+ T lymphocytes may be defective for CXCR4-specific functions in vivo, including homing, differentiation, and recirculation within tissues. Loss of CXCR4 expression may also disturb T-cell priming within secondary lymphoid organs, as these cells may not encounter their cognate antigen in an environment that would allow efficient antigen presentation and activation. This issue may account for the limited proliferation and cytokine secretion capacity of residual ICL CD4+ T cells, as confirmed here. Given that CXCR4 is the major cognate receptor for CXCL12 and that this interaction is critical for BM homeostasis, lymphocyte homing, and recruitment into inflammatory sites (reviewed in Lataillade21 ; Miyasaka and Tanaka23 ; Ratajczak et al46 ; and Tsutsumi et al47 ), we propose that defective CXCR4 expression contributes to immunodeficiency during ICL. However, considering the variable immunophenotypes reported in ICL,13 we cannot exclude the possibility of a multifactorial etiology of ICL.
CXCL12/CXCR4 interactions have proven a promising target to prevent undesirable cell activation or recruitment in different models of diseases.48-52 Disruption of CXCL12/CXCR4 interactions upon treatment with the selective CXCR4 antagonist AMD3100 leads to a rapid, transient, and reversible mobilization of HPCs as well as mature granulocytes and lymphocytes in the peripheral blood of healthy mice and humans.53 Results from AMD3100 treatment suggest that CXCR4 blockade leads to an increase in circulating CD4+ T cells. However, one should take into account that transient and chronic disruption of the CXCR4/CXCL12 axis appears to have distinct effects on T-cell homeostasis. Permanent intracellular retention of CXCR4 in the CXCL12-intrakine mouse model leads to a severe CD4+ T-cell lymphopenia,29 consistent with our findings in ICL. Thus, CXCR4 dysfunction in ICL may well account for the observed CD4+ T-cell lymphopenia.
The mechanism underlying the defect in membrane CXCR4 expression might involve impaired receptor recycling rather than synthesis. Early steps of CXCR4/CXCL12 interactions appear functional, as physiologic CXCR4 endocytosis is preserved after CXCL12 binding in most ICL CD4+ T cells.37 These findings suggest that, in ICL CD4+ T cells, CXCR4-dependent signaling does occur and leads to phosphorylation events and β-arrestin recruitment required for receptor internalization (reviewed in Busillo and Benovic38 and Moore et al54 ). In contrast, later steps in the intracellular routing process of the receptor appear defective in ICL CD4+ T cells, as CXCR4 persists in intracellular compartments instead of being degraded and/or recycled back to the plasma membrane. The concomitant intracellular accumulation of CXCL12 strongly suggests that CXCR4 fails to dissociate from its cognate ligand. The molecular defect responsible for impaired CXCR4 recycling remains to be elucidated. It may vary according to patients. Indeed, both CXCR4 endocytosis and recycling were affected in CD4+ T cells from P4, whereas only receptor recycling was impaired in those from P1 and P3. The CXCR4 defect appeared restricted to ICL T cells, as membrane CXCR4 expression and function were preserved in monocytes, suggesting a T cell–specific impairment in a gene product selectively involved in the regulation of CXCR4 recycling. Endopeptidases that degrade GPCR ligands within endosomes may play a role, because ligand dissociation is required before receptor recycling.55 Proteins that regulate CXCR4 vesicular trafficking and recycling (eg, synaptotagmin 3 and PIM1) may also contribute to the defective CXCR4 expression in ICL T cells.56,57
The defective membrane CXCR4 expression in ICL T cells is likely to perturb CD4+ T-cell homeostasis at several stages, including thymopoiesis. CXCR4 is believed to orchestrate the localization of early lymphoid progenitors to thymic regions, where lineage commitment and proliferation are controlled.58 Blockade of CXCR4 signaling causes an abnormal retention of thymocytes in the cortex.59 In mice reconstituted with progenitor cells expressing a CXCL12 intrakine, which prevents membrane CXCR4 expression, hematopoiesis is impaired in several compartments, including the thymus, and cell depletion is more pronounced for CD4+ than for CD8+ T cells.29 These findings suggest that the CD4+ T-cell subpopulation is more sensitive to alterations in CXCR4 expression than its CD8+ counterpart. We observed functional defects in residual peripheral ICL CD4+ T cells, which may reflect an activated and/or exhausted status of CD4+ T cells that undergo prolonged homeostatic proliferation and are consistent with the reported increased apoptosis of ICL CD4+ T cells.14
From a therapeutic perspective, IL-2 showed efficacy at restoring membrane CXCR4 expression and function together with CD4+ T-cell counts in a subset of ICL patients. CXCR4 expression is reported to be inducible by cytokines of the γ-c family, including IL-2 and IL-7.40,42,60 Cytokine treatment, as well as triggering of certain surface receptors such as CD62L, can cause the rapid relocalization of CXCR4 intracellular stores to the cell surface.61 In HIV-infected patients, intermittent IL-2 therapy is associated with an increased proliferation of both CD4+ and CD8+ T-cell populations and increased survival of both naive and central memory CD4+ T cells.62 An expansion of naive CD25+ T cells with some, but not all, characteristics of regulatory T cells is also observed.31 Interestingly, IL-2 therapy enhances the number of circulating CD4+ T cells that expressed high levels of CXCR4.62,63 In the present study, 3 of 4 ICL patients responded to IL-2 therapy by an increase in CD4+ T-cell counts, with a sustained response in 1 case (P2) and transient responses in the 2 others (P1 and P6). Strikingly, increased CD4+ T-cell counts correlated with a recovery of membrane CXCR4 expression and function. Furthermore, the only patient (P3) who showed no improvement in CD4+ T-cell count upon therapy did not show membrane CXCR4 re-expression after either in vivo or in vitro IL-2 treatment. These data raise the possibility that in vitro analysis of CXCR4 induction on CD4+ T cells by IL-2 might provide a useful screen to predict the patient's responses to IL-2 immunotherapy. Overall, membrane CXCR4 expression was normalized on CD4+ T cells from 5 of the 6 patients after in vitro IL-2 stimulation, suggesting that IL-2 administration might have a beneficial effect in a significant fraction of ICL patients. This study, together with 2 previous case reports of IL-2 administration10,11 and 1 case report of IFN-γ treatment,12 provide a rationale for furthering cytokine-based immunotherapy in ICL patients. In conclusion, our data indicate that ICL is associated with a defect in membrane CXCR4 expression, which may be reversed by IL-2.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to patients who participated in the study. We thank the medical staff (A. Boibieux, J. Gaillat, B. Ponceau, and M. Vidailhet) for their collaboration and referring their patients to us. We thank E. Cabannes (Inserm U819, Institut Pasteur) for help with genetic studies and are grateful to F. Baleux (Unité de Chimie Organique, Institut Pasteur) for providing us with the synthetic CXCL12. We thank V. Godié (Inserm U996, Université Paris-Sud 11) for technical help and are grateful to D. Emilie (Inserm U996 and Service de Microbiologie-Immunologie Biologique, Hôpital A. Béclère, AP-HP) for supporting funding application (AP-HP, grant number 07018).
This work was supported by Inserm, Institut Pasteur, AP-HP, and the European Union FP6 (INNOCHEM, grant number LSHB-CT-2005-518167).
Authorship
Contribution: D.S.-A. and K.B. designed and performed research, contributed analytic tools, analyzed data, and wrote the paper; L.A.C. performed research, analyzed data, and wrote the paper; L.M. performed research and analyzed data; F.D. designed research, analyzed data, and wrote the paper; C.D. performed research; F.A.-S. contributed analytic tools and analyzed data; and O.L. initiated and coordinated the study, designed and performed research, analyzed data, and was in charge of the writing committee.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olivier Lortholary, Unité de Mycologie Moléculaire, CNRS URA3012, Institut Pasteur, 25 rue du Dr Roux, 75624 Paris cedex 15; e-mail: olortho@pasteur.fr.
References
Author notes
D.S.-A. and K.B. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal